Abstract
OBJECTIVES:
Seventy percent of patients with irritable bowel syndrome (IBS) identify certain foods as triggers for their symptom flare-ups. To help identify potential trigger foods, practitioners often rely on patient food and gastrointestinal (GI) symptom journaling. The aim of the study was to evaluate the feasibility and usability of a novel food and symptom journal app, specifically designed for patients with IBS. Secondary aims were to explore the effect of using the app on GI symptoms and to describe associations between diet and GI symptoms suggested by individual patient data.
METHODS:
The feasibility and usability of the novel app was studied in 11 IBS patients (8 women), aged 21–65 years. Participants were asked to log GI symptoms (abdominal pain, bloating, diarrhea, constipation) using a 100-point color-graded sliding scale (green=none, red=severe) four times a day and to log every meal/snack they ate (at least three times a day) over a 2-week period. The app's feasibility as a data collection tool was evaluated by daily completion, compliance, data hoarding, and fatigability rates. Usability was evaluated with the System Usability Scale (SUS). To explore potential impact of using the app on bowel distress, we compared before and after intervention IBS-Symptom Severity Scale (IBS-SSS) scores. Meal entries were analyzed for nutrients using the Nutrition Data System for Research. Regression analyses were conducted for each participant journal to explore relationships between meal nutrients and subsequent GI symptoms.
RESULTS:
Daily average completion rates of the minimum requested entries for meal and GI symptoms were 112±47% and 78±44%, respectively. Average 24-h compliance rates were 90±19% and 94±12%, respectively. The SUS score was above average (mean 83, range 65–97.5; n=10). Most participants did not have a clinically significant decrease in IBS-SSS. At least one strong association (P≤0.05) between GI symptoms and a meal nutrient was found in 73% of participants. The mean number of associations was 2 (range 0–7; n=11). Patterns of associations differed between individual participants.
CONCLUSIONS:
Our app appeared to be a feasible and usable tool for IBS patients. Our findings are in line with anecdotes that most IBS patients have food triggers and that these vary by individual. Future studies can explore whether individualized dietary changes guided by an app can result in IBS symptom improvement.
INTRODUCTION
Up to 70% of patients with irritable bowel syndrome (IBS) identify certain foods as triggers to their symptom flare-ups.1 Diets eliminating such trigger foods (e.g., high fat, gluten, fermentable oligosaccharides–disaccharides–monosaccharides and polyols) have resulted in significant bowel symptom reductions for some IBS patients.1, 2, 3, 4 In an attempt to identify personalized trigger foods, practitioners commonly rely on patients to collect data on their food and gastrointestinal (GI) symptoms using a paper journal. The utility of such journals in practice is controversial, and their use is variable.
Data collected from paper journals are often unreliable, making it difficult to trust conclusions drawn from them. Reported compliance rates are low, ranging from 11% to 48%.5, 6 Data are frequently incomplete and disorganized and can also be falsified because participants either forward filled or backfilled journal entries.5, 7 Such retrospective journal entries can lead to inaccuracies owing to forgetfulness, selective memory recall, and active memory reconstructions.8 A patient's affect and pain at the time of recording symptoms can also significantly bias recall of past experiences.9, 10
Electronic journals have resulted in improved patient compliance rates, higher quality entries, and more efficient handling of the data compared with paper journals for various medical conditions.7, 11, 12, 13 Compliance rates for electronic journals are much higher compared with paper journals, ranging from 86% to 149%.5, 6, 13, 14 To our knowledge, electronic journals as a potential data collection tool have not been studied in IBS patients.
We therefore developed a food and GI symptom smartphone app for patients with IBS. Although two other food and symptom journal apps were available prior to our app's development (i.e., Doc's Diet Diary (Bearcat Global LLC, San Jose, CA) and GI Monitor from WellApps (Medivo, Inc., New York, NY)), neither was customized for IBS patients and their providers. Doc's Diet Diary was targeted to anybody who wants to “check which foods you are sensitive or allergic to” with non-specific symptom logging via free-text keyboard entry. GI Monitor was designed for patients with inflammatory bowel disease and asked users to track inflammatory bowel disease–specific symptoms not present in IBS, such as bloody and nocturnal bowel movements. To minimize irrelevant or vague app features that may affect an app's feasibility and usability, we felt like it was necessary to develop an IBS-specific food and GI symptom journal app.
Merely logging personal data using electronic journals has led to improvements in health end points for some patient populations, presumably from increased self-awareness. Katz and Nordwall15 delivered daily text messages to patients with Type II diabetes summarizing their 7- and 14-day blood glucose averages, observing significant reductions in glycosylated hemoglobin levels after 3 months of use. Similar health benefit effects were achieved for hypertensive and heart failure patients.14, 16 Therefore, we hypothesized an overall reduction in bowel symptoms in IBS patients adhering to a 2-week period of tracking their food and GI symptoms using our app.
This was a 2-week observational study to test the feasibility and usability of our app in tracking food and GI symptoms in IBS patients. Feasibility was evaluated by average daily completion rates and compliance (i.e., delayed entries, hoarding, and tracking fatigability). Hoarding is a form of non-compliance wherein a participant fails to make timely entries but later backfills any missing data.5 Secondary aims were to explore whether use of the app had any impact on IBS symptom severity and to describe participant experiences using the app. We also conducted exploratory analyses of each participant's journal to describe associations between diet and GI symptoms.
METHODS
Recruitment
Volunteers with IBS were recruited through clinic advertisements, direct physician referrals, and mailings at a single university-based gastroenterology practice located in Stanford, California from 13 October 2012 to 1 May 2013. Human participants' institutional approval was obtained prior to enrolling participants (October 2012).
To be included, men and women had to be between 21 and 65 years of age, had to be comfortable reading and writing in English, had to meet the Rome III criteria for IBS, and had to report current IBS symptoms over the past month.17 Participants also had to have at least 6 months' experience using a smartphone.
Participants were excluded if they had disorders or took medications that might account for GI symptoms, confound the measurement of IBS symptoms, or compromise the participant's ability to complete the study. Participants were excluded if they had a history of coexisting GI pathology (e.g., inflammatory bowel disease, celiac disease) or surgery (e.g., bowel resection), renal or reproductive pathology (e.g., endometriosis, prostate cancer), severe fibromyalgia, Type 1 or 2 diabetes mellitus, infectious diseases (e.g., hepatitis B or C, human immunodeficiency virus), untreated sleep disorders, clinically significant cardiovascular disease in the past 12 months, moderate-to-severe psychiatric conditions (e.g., depression, anxiety, bipolar disorder), moderate-to-severe immunologic diseases (e.g., scleroderma, systemic lupus, arthritis), seizure disorders requiring medications, or current substance abuse. Medications that led to exclusion included the regular use of antibiotics, anticholinergics, and narcotics. Finally, patients were excluded or postponed enrollment if they had any changes in their medications, stressors, travel plans, or non-medical IBS management therapies (e.g., exercise, behavioral therapies) 1 month prior to or anticipated during the study period.
The app
The app's user interface was based on journal templates from the book “Master your IBS: An 8-Week Plan to Control the Symptoms of Irritable Bowel Syndrome”.18 A panel of experts in the field of questionnaire design reviewed the app's wireframes and provided feedback for edits and modifications. We also conducted interviews with five patients with IBS and five IBS providers to provide further feedback on our wireframes for the final edits of our app.
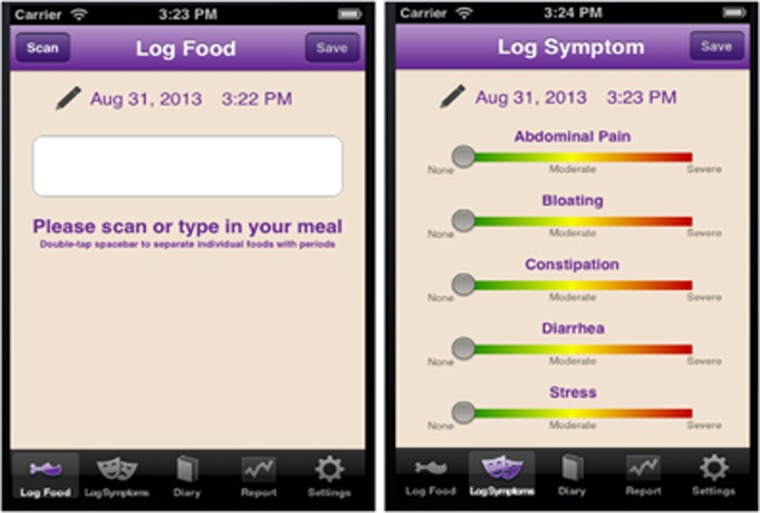
The app was developed using Apple XCode 4.0 (Cupertino, CA). Images of key screens and functionality are shown in Figure 1. For GI symptoms, participants rated the severity of their abdominal pain, bloating, diarrhea, and constipation on a 100-point color-graded sliding scale from green to red (green=none, yellow=moderate, red=severe). For meals, participants were instructed to enter as many details as possible on food product brands, restaurant names, portion sizes, additives (e.g., salt, oil, butter), and food preparation (e.g., grilled, baked, fried). If a meal was home cooked, participants were asked to list all ingredients with portion sizes. Participants could enter these meal details by using a free-text keyboard or a universal product code barcode scanner (Scandit, San Francisco, CA). If a food/drink universal product code barcode was scanned, a picture of the product would appear on the screen and participants could add additional meal details (e.g., portion size, meal preparation) using the free-text keyboard. Participants could chronologically review and edit prior data entries. They could optionally set lock-screen pop-up time reminders to log entries.
Figure 1.
Key wireframes of smartphone app.
Baseline assessment
Participants were assessed for eligibility by a telephone screening and chart review. An in-person session was then conducted during which written consent was obtained, the IBS Symptom Severity Score (IBS-SSS) questionnaire was completed, and a tutorial session on how to use the app was given. The app was available only for iOS, so iPod Touch loaners were provided to participants who owned an Android or a Blackberry smartphone. All app data were protected by a personalized user name and password. All data were transferred via the Internet to a password-protected secure and encrypted website. All statistical analyses were conducted using the R Statistical Software v2.15.2.19 Parking for study-related visits was the only compensation offered to participants.
Intervention phase
For 2 consecutive weeks, participants were instructed to log meal and GI symptom entries at least three and four times a day respectively, which we defined as the “minimum requested entries”. They were also instructed to log meal and GI symptom entries at least 1 h apart from one another. Participants otherwise continued their usual medical care throughout the study.
Follow-up phase
Participants were asked to complete the IBS-SSS and System Usability Scale (SUS) postintervention. Participants gave qualitative feedback about their experience using the app via a semi-structured in-person interview.
Primary outcomes
Feasibility
The feasibility of the app as a meal and GI symptom data collection tool was evaluated by average daily completion rates and compliance measures (i.e., delayed entries, hoarding, and tracking fatigability).
Completion rates
Average daily completion rates for meals and GI symptoms journal entries were calculated by dividing the number of actual over the minimum requested entries. Multiple food or drink items entered within a 1-h time window were considered a single meal entry. Additional symptom descriptions entered as a free-text keyboard entry in the meal section of the app were identified and counted as GI symptom entries. If a participant logged a similar meal or GI symptom entry more than once within a 5-min time window, we assumed this was an erroneous entry and only the latest entry was included for data analysis.
Compliance
Compliance rates were defined as the number of actual journal entries made within a 12- and 24-h window of reported times out of the total number of entries. For example, if a participant used the app at 2000 hours to log breakfast at 0600 hours, the actual vs. reported entry would be 2000 hours and 0600 hours, respectively. This specific entry would be considered compliant within the 24-h but not the 12-h window. Although no standard definitions of compliance currently exist for food journals, the 12- and 24-h time windows were selected based on prior food recall studies.20 We defined hoarding days as days when more than two entries were made within a 15-min window where at least two of these entries were delayed by at least 6 h from reported entry times and were not for the same reported entry times. To evaluate fatigability, daily completion and 24-h compliance rates were compared from week 1 to week 2 for both meal and GI symptom entries as previously used by Stone et al.5
Overall feasibility
Overall feasibility was judged by the fraction of participants obtaining average daily completion and 24-h compliance rates ≥50%. Completion rates ≥50% would provide at least 7 days of food and GI symptom data, the number of journal days typically collected by dietitians.20 The thresholds for compliance rates (24-h window) were set at ≥50% because 24-h and 2-day delays have been validated for food and pain recalls, respectively.8, 20
Usability
The usability of our phone app was measured with the SUS, which consists of 10 statements participants are asked to agree upon using a 5-point scale (1, strongly disagree; 5, strongly agree) (Table 3). It yields a single number representing a composite measure of the overall usability of the system being studied. An SUS score >68 is considered above average.21
Statistical analysis
Welch's unequal variances t-tests were applied to determine any significant differences in primary outcome measures between meal and GI symptom entries, between participants who used their own iPhones vs. iPod Touch loaners, and between participants who used the optional reminder feature vs. not.19
Secondary outcomes
Impact on bowel distress
To explore the potential impact on bowel distress in IBS patients using our app, the IBS-SSS for each participant was compared preintervention and postintervention. The IBS-SSS is an IBS-specific instrument that is sensitive to change in symptoms over time.22 Responders rate retrospectively, for the past 10 days, abdominal pain severity and frequency (separate ratings), bloating severity, dissatisfaction with bowel habits, and life interference from bowel symptoms. These five ratings are totaled to obtain an overall IBS severity score with a maximum score of 500. According to the scale developers, a 50-point or greater change on this scale is considered clinically meaningful.22 Welch's unequal variances t-tests were applied to determine any significant differences in IBS-SSS preintervention and postintervention.
Patterns of meals and GI symptoms
Trained research dietitians collected and analyzed dietary intake data using the Nutrition Data System for Research (NDSR) software version 2013, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN. For specific meal nutrients, refer to Supplementary Appendix S1. For missing information such as portion sizes and/or unfamiliar food products, standard assumptions were made according to NDSR's “Data Entry Rules”.23
For each participant's journal, regression analyses were conducted to examine relationships between GI symptoms and preceding meal nutrients (as recorded within the 4-h window before a recorded symptom). This 4-h window was based on prior IBS patient reports on the timing of symptoms following trigger food ingestion.24 The data matrix for regression analysis consisted of symptom ratings as dependent and independent variables corresponding to a summation of nutrient indices consumed in meals. If more than one meal was made within the 4-h window before a GI symptom recording, we summed the nutrients for all of the meals in that window. We excluded GI symptom entries from our analyses if there was no corresponding meal entry in the 4 h prior to its entry.
Prior to running regression analyses, a feature selection particular to each participant's diet was performed. Food nutrients have a high degree of collinearity owing to both natural co-occurrences (e.g., foods with higher total fat tend to have higher total calories) and personal dietary habits (e.g., some people always drink their caffeinated beverages with milk and a sweetener). However, linear regressions assume a high degree of independence between predictors. Therefore, nutrients that had high pairwise correlations (>0.75) with other nutrients were highlighted, and the nutrient(s) with the highest average correlation of the highly correlated nutrients were removed. Regressions were then performed with these selected nutrient(s). We considered a nutrient to be strongly associated with a GI symptom if the P-value was ≤0.05 and to be very strongly associated if the P-value was ≤0.001.
Semi-structured interview
Qualitative data on participant experiences using our app were obtained using the following open-ended questions: (1) “Which features did you like when using the journal?” (2) “Which features did you not like when using the journal?” Emerging generalized themes from these responses were identified to reflect participant's attitudes by J.Z. Responses were categorized into these theme(s) by a manual indexing system by J.Z. J.S. confirmed the emerging themes and categorizations of each participant's interview transcript. Any disagreements on the themes and/or categorization were resolved by a discussion between J.Z. and J.S.
RESULTS
Participant flow and follow-up
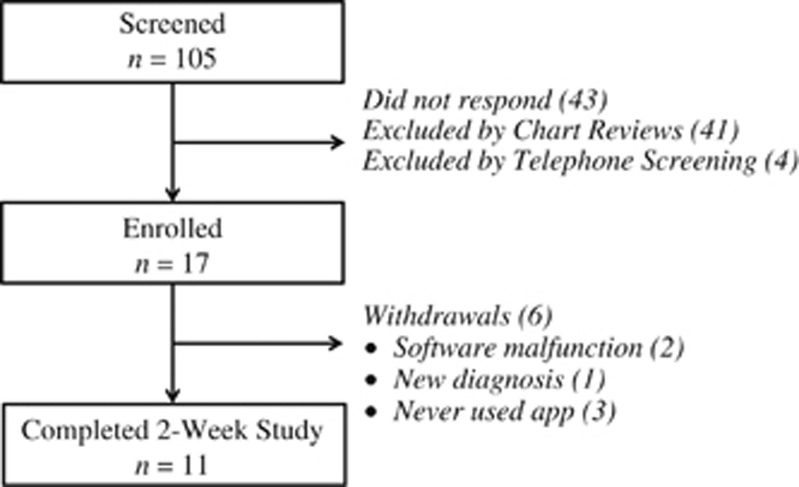
Figure 2 summarizes the participant recruitment pathway from screening to study completion. Two participants discontinued the study prematurely owing to software malfunction. This was corrected prior to enrollment of the remaining study participants, and the data from these two participants was excluded from all analyses. One participant was diagnosed with an infectious gastroenteritis prior to starting the intervention. Three participants never used the app and/or failed to provide data despite enrollment. One participant completed the intervention but failed to follow-up for the exit interview and questionnaires; all data provided by this participant was included in our analyses. Therefore, all secondary outcome data only included 10 participants.
Figure 2.
Participant recruitment pathway and reasons for withdrawals.
Demographics and baseline clinical characteristics are provided in Table 1. Participants were mainly female (N=8, 78%) and White (N=8, 78%), with moderate severity scoring on the IBS-SSS (M=260, s.d.=40). All participants were well educated with at least a college education (N=11, 100%). Most participants owned an iPhone (N=9, 82%) and used the optional reminder feature (N=8, 73%).
Table 1. Baseline demographics and clinical characteristics.
| Demographics | |
| Age, mean (s.d.) | 35 (11) |
| Gender, female, % (n) | 73% (8) |
| Race, White, % (n) | 73% (8) |
| College educated or above, % (n) | 100% (11) |
| IBS characteristics | |
| Years since IBS diagnosis, mean (s.d.) | 6.5 (5.6) |
| Predominant bowel patterna | |
| IBS-Subtype, diarrhea, % (n) | 55% (6) |
| IBS-Subtype, constipation, % (n) | 9% (1) |
| IBS-Subtype, mixed, % (n) | 36% (4) |
| IBS baseline severity based on IBS-SSSb | |
| IBS-SSS baseline score, mean (s.d.) | 259.5 (39.6) |
| Mild, % (n) | 0% (0) |
| Moderate, % (n) | 100% (10) |
| Severe, % (n) | 0% (0) |
Primary outcomes
Feasibility
Table 2 summarizes the average daily journal entries, daily completion rates, and compliance rates (12 and 24 h) for meal and GI symptom entries. There was a trend of higher daily completion rates for meal than GI symptom journal entries (P=0.09). The meal completion rates exceeded 100% for six participants (55%). Two participants (18%) added GI symptom entries as a free-text meal entry.
Table 2. Feasibility of app: average number of daily diary entries, completion rates, and compliance rates.
| Type of diary entry | Daily diary entries, number (s.d.) | Daily completion rate,a % (s.d.) |
Compliance rate,b
% (s.d.) |
|
|---|---|---|---|---|
| 12-h | 24-h | |||
| Meals | 3.4 (1.4) | 112 (47) | 83 (25) | 90 (19) |
| GI symptoms | 3.1 (1.7) | 78 (44) | 88 (17) | 94 (12) |
GI, gastrointestinal.
Daily completion rate percent and the number of actual over minimum requested number of daily diary entries averaged over the 2-week study period. The minimum requested number of diary entries for meals and GI symptoms was 3 and 4, respectively.
Compliance rate percent and the number of actual diary entries made with a 12- and 24-h window of reported times.
There were no statistically significant differences in compliance rates (i.e., 12 and 24 h) or hoarding days between meal and GI symptom journal entries. Only one participant (9%) forward filled the journal for four entries. All other non-compliant entries were backfilled entries. During the 2-week study period, eight participants (73%) had at least one hoarding day (average 6 days; range 2–13; n=11).
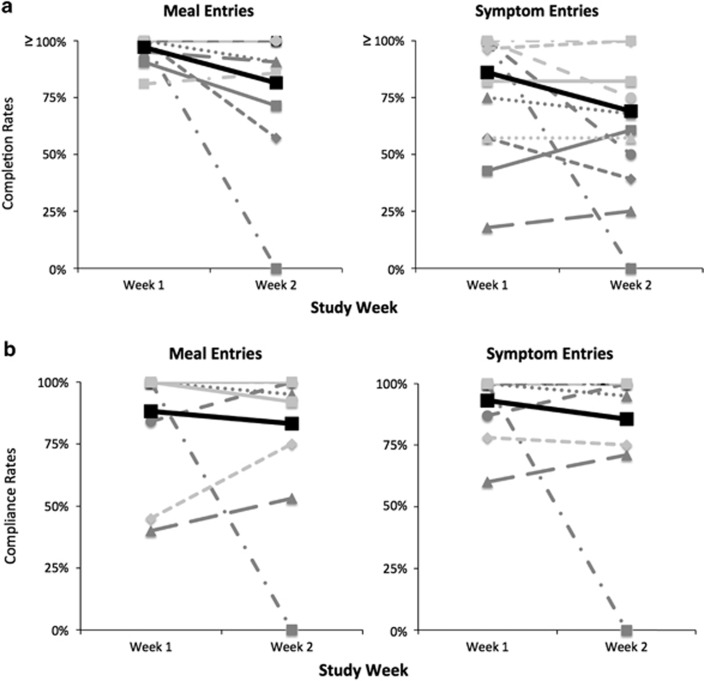
Figure 3 compares the average daily completion and 24-h compliance rates for each participant from week 1 to week 2 of the study, respectively. The mean decrease in daily completion rates was 25% (s.d.=39%) and 17% (s.d.=42%) for meal and GI symptom entries, respectively. The mean decrease in 24-h compliance rates was 5% (s.d.=33%) and 8% (s.d.=31%) for meal and GI symptom entries, respectively.
Figure 3.
Tracking fatigability. (a) Average daily completion rates and (b) compliance rates (24-h) from week 1 to week 2 for meal and symptom diary entries of each participant (n=11). The bolded black thick line represents the mean. Please note completion rates were calculated from minimum requested entries and thus exceeded 100% for some participants.
Seventy-three percent (8/11) of participants met our app's feasibility thresholds: daily completion and 24-h compliance rates ≥50% for both meal and GI symptom journal entries. Of the two participants (18%) who did not meet the completion rate threshold, they both failed to meet this threshold for GI symptom entries. Only one participant (9%) failed to meet the compliance rate threshold (meal entry).
Usability
The average SUS score was 83 (range 65–97.5; n=10). Table 3 displays the mean responses to individual SUS statements.
Table 3. Responses to individual statements in the System Usability Scale.
| Statement | Mean (s.d.) |
|---|---|
| 1. I think that I would like to use this system frequently | 3.1 (1.1) |
| 2. I found the system unnecessarily complex | 1.1 (0.3) |
| 3. I thought the system was easy to use | 4.4 (0.8) |
| 4. I think that I would need the support of a technical person to be able to use this system | 1.0 (0) |
| 5. I found the various functions in this system were well integrated | 3.6 (1.0) |
| 6. I thought there was too much inconsistency in this system | 1.7 (1.1) |
| 7. I would imagine that most people would learn this system very quickly | 4.7 (0.5) |
| 8. I found the system very cumbersome | 1.9 (1.3) |
| 9. I felt very confident using the system | 4.3 (1.1) |
| 10. I needed to learn a lot before I could get going with this system | 1.2 (0.4) |
Scoring on the System Usability Scale ranged from 1=totally disagree to 5=totally agree.
N=10; one participant failed to provide postintervention follow-up data.
Effect of phone ownership and reminders on primary outcomes
Participants who used the optional reminder reported a higher SUS score (M=92, s.d.=3.8) than those who did not (M=79, s.d.=11.7) (P=0.03). There was also a trend for higher symptom daily completion rates in participants who used their personal iPhone (M=85%, s.d.=38%) compared with those who used an iPod Touch loaner (M=35%, s.d.=19%) (P=0.07). No significant differences were otherwise seen for meal daily completion rates, compliance rates (12 h, 24 h), or hoarding days between participants who used their own phones vs. an iPod Touch loaner or between participants who used an optional app reminder feature vs. not.
Secondary outcomes
Impact on bowel distress
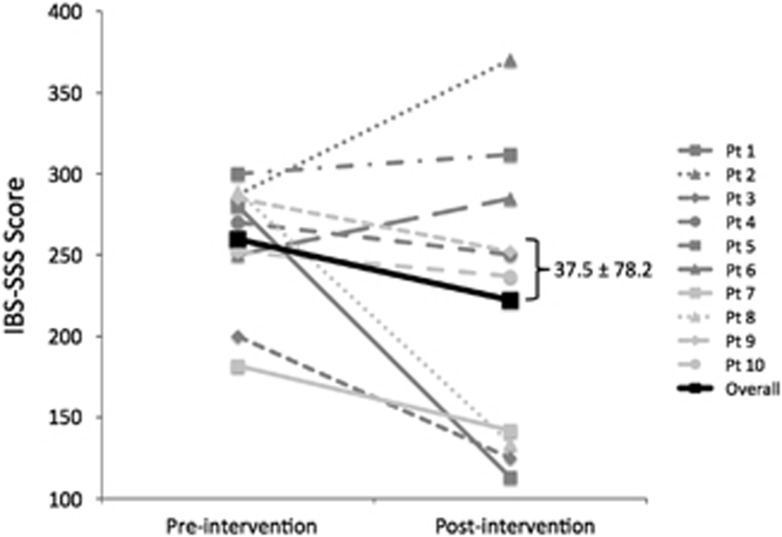
Figure 4 displays the change in IBS-SSS preintervention and postintervention for each participant. The mean change in IBS-SSS was 37.5 (s.d.=78.2), but this was not statistically significant. Three participants (30%) had a clinically significant IBS-SSS score decrease (≥50 points).
Figure 4.
Change in irritable bowel syndrome Symptom Severity Score (IBS-SSS) preintervention and postintervention. Each line represents an enrolled participant (n=10; one participant failed to provide postintervention follow-up data). The bolded black thick line represents the mean.
Patterns of meals and GI symptoms
Eight participants (73%), all of our female and none of our male participants, demonstrated at least one strong association (P≤0.05) between a GI symptom and meal nutrient. Patterns of association differed among individual participants (Table 4). The mean number of associations was 2 (range 0–7; n=11).
Table 4. Individual relationships between GI symptoms and preceding meal nutrients using regression analyses.
| Participant | Symptom | Directionality | Nutrient | Correlated nutrientsa |
|---|---|---|---|---|
| 1F | Bloating | Improving | Galactose* | None |
| Worsening | Caffeine* | Fructose | ||
| Total protein* | Total calories, total fat, magnesium, potassium | |||
| Diarrhea | Worsening | Caffeine* | Fructose | |
| 2F | Diarrhea | Worsening | Soluble dietary fiber* | Total carbohydrates, total dietary fiber, starch |
| 3F | Abdominal pain | Worsening | Total sugars* | Total calories, fructose, total carbohydrates |
| Diarrhea | Worsening | Total protein* | Sodium | |
| 4F | Constipation | Worsening | Galactose* | None |
| 5F | Diarrhea | Improving | Percentage of fat* | None |
| Fructose* | None | |||
| Starch** | Total calories, total carbohydrates, sodium | |||
| Magnesium** | Potassium, total dietary fiber, insoluble fiber | |||
| Worsening | Soluble dietary fiber* | Total dietary fiber | ||
| Lactose* | Galactose, total protein | |||
| Total fat | Total protein, potassium | |||
| 9F | Bloating | Worsening | Percentage of fat* | None |
| Magnesium* | Total carbohydrates, sodium, potassium, total dietary fiber, soluble dietary fiber, insoluble dietary fiber | |||
| 10F | Bloating | Worsening | Mannitol* | None |
| 11F | Abdominal pain | Worsening | Sorbitol** | None |
| Constipation | Improving | Sorbitol* | None | |
| Starch* | Total calories, total carbohydrates, total protein | |||
| Diarrhea | Worsening | Caffeine* | None | |
| 6M, 7M, 8M | No significant associations found |
F, female; GI, gastrointestinal; M, male. *P<0.05, **P<0.001.
Prior to running regression analyses, a feature selection particular to each participant's diet was performed. Nutrients that had high pairwise correlations (>0.75) with other nutrients are in bold and the nutrient(s) with the highest average correlation of the highly correlated were removed and listed in this column.
Open-ended responses
Our app resulted in a sense of greater self-awareness for all responding participants. One participant commented that the app “forced [her] to analyze what [she] was eating and to be more conscious of [her] symptoms”. Accountability was also a common theme. “If I didn't write it down, I didn't care. The app helped me realize when I ate too much”. The app was generally considered easy to use and not embarrassing. Participants appreciated the reminder feature.
However, most participants wished that the app provided them with “answers” or at least better representations of their data with “some sort of analysis”. They wanted guidance on how to change their current eating behaviors. They also wanted to customize their symptom input. Finally, participants found use of the app time-consuming, both in its daily commitment and total duration.
DISCUSSION
Patient compliance with data collection has been shown to be superior when using electronic over paper journals for a variety of conditions.5, 6, 13, 14 Improved compliance leads to more reliable and accurate data collection, which is essential in reaping the benefits of journal data. We therefore developed a novel food and GI symptom journal app for IBS patients. To our knowledge, this is the first study investigating the feasibility and usability of an electronic journal for data collection in IBS patients.
Based on this pilot study, our novel phone food and GI symptom journal app promises feasibility as a usable data collection tool for IBS patients for at least a 2-week period. Preliminary analyses of individual journal data demonstrated unique patterns of associations between GI symptoms and meal nutrients, supporting the anecdote that most IBS patients not only have food trigger(s) but also individualized trigger(s). However, a 2-week period of tracking one's meals and GI symptoms using our app did not appear to affect an IBS patient's overall bowel symptoms, at least not immediately postintervention and based on a small sample size.
Daily completion rates far exceeded our expected thresholds, especially for meal entries. Higher meal vs. symptom entry completion rates are likely due to the following reasons. First, the number of minimum requested entries were lower for meals than symptoms. Second, the act of eating or drinking served as a concrete reminder to log food/drinks. Third, eating or drinking is an objective event making its details easier to recall than symptoms.
Based on this rationale, one might have also expected higher compliance rates for meal than symptom entries, but this was not the case. There were no significant differences in any of our measures of compliance (i.e., compliance rates, hoarding, and tracking fatigability) between meal and symptom entries. This was likely counterbalanced by the increased likelihood to log symptoms in real time. Unlike eating and/or drinking, experiencing symptoms is an abstract event, the details of which are harder to recall as more time passes.
Overall, participants found our app to be usable for at least a 2-week period. The optional reminder feature possibly enhanced the app's usability by minimizing the burden associated with remembering to log. The SUS statement least in favor of the app discussed how cumbersome the app was, elaborated during the open-ended responses as being time-consuming. Participants were neutral on whether they would use our app frequently. This corresponds to prior studies where patients were more likely to continue using an app if it was easy to use and non-burdensome.25, 26 Perhaps our participants were also unwilling to use our app frequently because they were unclear on how to use it to better manage their IBS, especially because most did not see an immediate improvement in their IBS symptoms. Many participants wanted more from the app such as data analysis and/or advice.
In this study, we performed regression analyses to discover possible relationships between GI symptoms and meal nutrients using food and symptom journals. In all, 73% of participants had at least one suspected food trigger, similar to the 70% of IBS patients attributing food as a possible symptom trigger.1 This observation could possibly be inflated given the inherent bias on who chose to participate in our study and/or the “faulty” associations concluded by our analyses (discussed below in “Limitations”). However, this could be balanced by the possibility that some IBS patients have undiscovered food triggers.
Our compliance rates were comparable to prior studies investigating electronic journals for active data collection.5, 6, 13, 14 However, given the heterogeneity of these studies, we make this comparison cautiously. Depending on the health condition and measure(s) being tracked, there was wide variance in the frequency, duration, and acceptable delays of data collection.5, 6 Authors of these studies often provided limited explanations on how they arrived at these study specifics or, similar to our study, needed to make assumptions based on weak evidence.
We anticipated the data entry fatigue observed in this study based on prior studies requiring daily active health tracking.14, 20 In past dietary studies, as the number of consecutive recording days increased, so did the number of incomplete and retrospective entries.20 Participant open-ended responses indicated that they found this app time-consuming, and a recent survey study by Zia et al.26 found that gastroenterology patients were only willing to spend up to 5 min a day using a health-related app. This likely contributed to the data entry fatigability we observed in this study. Although not specific to health-related self-tracking apps, 90% of all downloaded apps are used only once and eventually deleted by users.27
A 2-week period of tracking one's meals and GI symptoms had no perceived impact in overall bowel symptoms, at least not immediately postintervention. This lack of clinical improvement did not correlate with prior studies, where the act of self-tracking alone has led to improvement of health measures for several medical problems.14, 15, 16 Our sample size was likely too small and not powered enough to draw any valid conclusions on our app's effect on GI symptoms. In addition, unlike these other health conditions, IBS does not have established, universal, and effective treatment strategies. Diabetics track their blood sugars to maximize known effective treatment strategies (e.g., insulin dosing, reduced dietary carbohydrates). People with IBS, on the other hand, track their food and GI symptoms in hopes of identifying effective treatment strategies (e.g., elimination diets). The clinical efficacy of apps for patients with IBS should therefore be evaluated differently from other self-tracking studies. Perhaps this could be determined by its ability to identify potential trigger food(s) and/or its symptom impact after participants are given the opportunity to eliminate potential trigger food(s) identified.
Finally, Kueper et al.28 used similar statistical analyses to identify problem foods from food and symptom journals of 164 patients with chronic medical problems, such as headaches, fatigue, congestion, abdominal pain, and sinus problems. Their method was also able to identify a unique set of trigger foods for most of their participants. Most importantly, the results helped reduce symptoms for 75% of their participants when used as a guide for personalized elimination diets.28 It is our hope that such clinical utility will also result from the ongoing development and refinement of our app. We envision our app to be used with a medical provider and/or dietitian but recognize the need to improve patient access to dietary counseling, a service that is not always covered by insurance plans. Our app will therefore also provide some basic dietary counseling.
Limitations
Our study has several limitations. First, the results of this study might not be applicable to all IBS patients, especially given our small sample size. Our participants were likely more educated and technologically savvy than the general IBS population. Not only were participants recruited from a high-technology region, our inclusion criteria also required smartphone experience. Patients less motivated to keep a journal might have also been less inclined to participate in this study, further inflating the feasibility of our app.
Second, the criteria used to evaluate our app's feasibility were based on numerous assumptions, often made by limited evidence and/or expert opinion. Changes to any of these assumptions (e.g., number of expected journal entries, compliance rate time windows, feasibility thresholds) could significantly alter our feasibility results. For example, delayed entries up to 24 h might not accurately capture GI symptom fluctuations in IBS patients. Even subtle changes in the design of our app interface and/or symptom choices could affect our app's feasibility. Future research should continue to assess how much data is necessary to provide meaningful guidance to patients with IBS, as well as how accurately recorded that data must be.
Third, the regression analyses we used to describe participant patterns of GI symptoms and meal nutrients was based on multiple assumptions and did not account for potential confounders (e.g., total calories, time of day, day of the week, stress levels, medications). Our analyses assumed that our app obtained complete and accurate enough journal entries and that symptom levels were related to the sum of meal nutrients. We also excluded nutrients that had high pairwise correlations with other nutrients. One of these excluded nutrients, or some combination of these nutrients, could be the “true” underlying trigger. Furthermore, our analyses assumed that culprit foods triggered most GI symptoms within a 4-h window. Given the multiple mechanisms by which foods generate GI symptoms (e.g., osmotic, chemical, mechanical, neuroendocrine, microbiome, bacterial fermentation), our analyses might not capture the effects from all of these mechanisms.
At this stage of development, our analyses are unable to distinguish the directionality of these associations (e.g., sorbitol in food causing constipation vs. constipation causing participants to eat more foods with sorbitol). Our analyses also excluded all GI symptoms entered without a meal entry within the prior 4 h, making the assumption that these symptoms were unrelated to food. However, we would like to emphasize that developing these analyses was exploratory and attempting to address one of the themes from participants' open-ended responses: a need for the app to analyze journal data in order to provide some guidance. Further development and validation of the clinical efficacy of our analyses still need to be conducted.
Electronic food and symptom journals, such as the one designed and evaluated in this study, appear to be feasible and usable data collection tools for patients with IBS, at least for short-term periods. They offer patients an alternative to paper journals and may better support automated or expert analysis of these journals. During this study, we also performed preliminary analyses to help IBS patients identify potential food triggers using their journal data. Initial results from these analyses support the anecdote that most IBS patients have food triggers and that these triggers vary for each individual. In the future, we hope to validate the food triggers identified by our analyses and for our app to provide customized dietary guidance for effective IBS symptom management.
Study Highlights

Acknowledgments
We thank our team of engineers: Raymond Bonneau, MS, MBA and David Camarillo, PhD, who helped us with the conception and design of our phone app and express our gratitude to the patients who volunteered to participate.
Guarantor of the article: Jasmine Zia, MD.
Specific author contributions: Conception and design: P.B., M.H., U.L., L.N., and J.Z.; acquisition of data: J.Z.; analysis and interpretation of data: J.F., J.S., and J.Z.; drafting of the manuscript: J.Z.; critical revision of the manuscript for important intellectual content: P.B., J.F., M.H., U.L., S.M., L.N., J.S., and J.Z.; statistical analysis: J.F., J.S., and J.Z.; obtaining funding: J.Z.; administrative, technical, material support: JZ; supervision: M.H. and U.L.
Financial support: This work was supported by the American College of Gastroenterology.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol 2012; 107: 657–666; quiz 667. [DOI] [PubMed] [Google Scholar]
- Halmos EP, Power VA, Shepherd SJ et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014; 146: 67– 75.e5. [DOI] [PubMed] [Google Scholar]
- Biesiekierski JR, Newnham ED, Irving PM et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol 2011; 106: 508–514; quiz 515. [DOI] [PubMed] [Google Scholar]
- Staudacher HM, Whelan K, Irving PM et al. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet 2011; 24: 487–495. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S, Schwarts JE et al. Patient compliance with paper and electronic diaries. Control Clin Trials 2003; 24: 182–199. [DOI] [PubMed] [Google Scholar]
- Walker I, Sigouin C, Sek J et al. Comparing hand-held computers and paper diaries for haemophilia home therapy: a randomized trial. Haemophilia 2004; 10: 698–704. [DOI] [PubMed] [Google Scholar]
- Jhaveri M, Lee E. Performance of electronic diaries in diabetes clinical trials measured through overall satisfaction of site coordinators. J Diabetes Sci Technol 2007; 1: 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Wang W, Potts SL et al. Reliability and validity of individual and composite recall pain measures in patients with cancer. Pain Med 2012; 13: 1284–1291. [DOI] [PubMed] [Google Scholar]
- Eich E, Reeves J, Jaeger B et al. Memory for pain: relation between past and present pain intensity. Pain 1985; 223: 375–379. [DOI] [PubMed] [Google Scholar]
- Goodwin A, Sher K. Effects of induced mood on diagnostic interviewing: evidence for a mood and memory effect. Psychol Assess 1993; 5: 197–202. [Google Scholar]
- Palmblad M, Tiplady B. Electronic diaries and questionnaires: designing user interfaces that are easy for all patients to use. Qual Life Res 2004; 13: 1199–1207. [DOI] [PubMed] [Google Scholar]
- Heinonen R, Luoto R, Lindfors P et al. Usability and feasibility of mobile phone diaries in an experimental physical exercise study. Telemed J E Health 2012; 18: 115–119. [DOI] [PubMed] [Google Scholar]
- Lauritsen K, Degl' Innocenti A, Hendel L et al. Symptom recording in a randomised clinical trial: paper diaries vs. electronic or telephone data capture. Control Clin Trials 2004; 25: 585–597. [DOI] [PubMed] [Google Scholar]
- Logan AG, McIsaac WJ, Tisler A et al. Mobile phone-based remote patient monitoring system for management of hypertension in diabetic patients. Am J Hypertens 2007; 20: 942–948. [DOI] [PubMed] [Google Scholar]
- Katz DL, Nordwall B. Novel interactive cell-phone technology for health enhancement. J Diabetes Sci Technol 2008; 2: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E, Leonard KJ, Masino C et al. Attitudes of heart failure patients and healthcare providers towards mobile phone-based remote monitoring. J Med Internet Res 2010; 12: e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth GF, Thompson WG, Chey WD et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- Barney P, Weisman P, Jarrett M, et al. Master Your IBS: An 8-Week Plan to Control the Symptoms of Irritable Bowel Syndrome. AGA Press: Bethesda, MD, 2010.
- Team RC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2010.
- Thompson FE, Subar AF. Dietary Assessment Methodology. Nutrition in the Prevention and Treatment of Disease. 3rd edn. Academic Press: San Diego, California, 2013, pp. 5–46..
- Brooke J.. Usability Evaluation In Industry: Technology & Engineering. Taylor and Francis: London, UK, 1996.
- Francis CY, Morris J, Whorwell P. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997; 11: 395–402. [DOI] [PubMed] [Google Scholar]
- Austin M, Harnack L, Bhaskarani J, et al. Nutrition Data System for Research User Manual. Regents of the University of Minnesota: Minneapolis, MN, 2013.
- Eswaran S, Tack J, Chey WD. Food: the forgotten factor in the irritable bowel syndrome. Gastroenterol Clin North Am 2011; 40: 141–162. [DOI] [PubMed] [Google Scholar]
- Dennison L, Morrison L, Conway G et al. Opportunities and challenges for smartphone applications in supporting health behavior change: qualitative study. J Med Internet Res 2013; 15: e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zia JK, Le T, Munson S et al. Download alert: understanding gastroenterology patients' perspectives on health-related smartphone apps. Clin Transl Gastroenterol 2015; 6: e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez S. Users have low tolerance for buggy apps - only 16% will try a failing app more than twice. TechCrunch 2013; http://techcrunch.com/2013/03/12/users-have-low-tolerance-for-buggy-apps-only-16-will-try-a-failing-app-more-than-twice/.
- Kueper T, Martinelli D, Konetzki W et al. Identification of problem foods using food and symptom diaries. Otolaryngol Head Neck Surg 1995;112:412–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.