Abstract
HSV type-1 and -2 are widespread pathogens producing lifelong infection with multiple sequelae, including oral, ocular and genital disease. The process of herpesvirus entry is a highly complex process involving numerous viral and cellular factors. Entry begins with attachment of virus to the cell surface followed by interactions between viral glycoproteins and cellular receptors to facilitate capsid penetration. The nucleocapsid is then transported along microtubules to the nuclear membrane, where viral DNA is released for replication in the nucleus. The work reviewed here comprises the most recent advancements in our understanding of the mechanism involved in the herpesvirus entry process.
Keywords: attachment, cell-to-cell spread, endocytosis, entry, fusion, glycoproteins, herpesviruses, HSV
HSVs belong to the Herpesviridae family of over 100 viruses, each consisting of an enveloped icosahedral capsid containing a proteinaceous tegument and a linear dsDNA genome [1]. Of these, only eight are capable of infecting human hosts. HSV type-1 and -2 are members of the Alphaherpesvirinae subfamily, and most commonly cause vesicular eruptions of epithelial cells at various body sites. Symptomatic disease due to HSV-1 is typically limited to cold sores of the orolabial region and keratitis of the cornea, while HSV-2 is mostly responsible for genital lesions [1]. However, both viruses are capable of causing lesions at either of these locations. HSV infections are most commonly acquired through direct contact with sites of viral shedding, or via exposure to mucocutaneous fluids, which may also carry the virus [2]. Recent studies have indicated that HSV infection, particularly HSV-2, may also be transmitted from mother to child, through contact with an infected birth canal [3].
Aside from causing direct cell death in a lytic infection, a number of Herpesviridae are capable of establishing latency in diverse cell types, allowing for evasion of the host immune system and reactivation of viral replication later in life [4]. In the case of HSV-1 and HSV-2, sensory neurons and ganglia serve as the reservoir for latent virus, however, little is known about the mechanism of reactivation in these cell types. The ability of the virus to exist in the latent phase may account for some of the inefficacy of the most commonly prescribed treatments, acyclovir and its analogs, in controlling HSV infections [5].
Defining mechanisms of viral entry into cells has been an objective of basic science research for years, with an ultimate goal of developing novel prophylactics or therapeutics to prevent initial infection or cell-to-cell spread. It is known that the four viral glycoproteins (gB, gD, gH and gL) are required and sufficient for HSV-1 and -2 entry into host cells. However, the dynamics of the interactions of these molecules with cellular surface proteins remains to be elucidated. In the process of a lytic infection, viral particles become loosely attached to the cellular surface through association of viral glycoproteins gB and gC with attachment receptor heparan sulfate (HS). At this point, virions are capable of ‘surfing’ along cellular filopodia, slender plasma membrane projections, to reach the cell body or surrounding cells [6,7]. High-affinity binding of gD to one or more of its receptors on the cell surface induces a conformational change promoting recruitment of gB, gH and gL as components of a fusion complex. Together this complex of glycoproteins and cellular receptors mediates membrane fusion and penetration of the viral capsid into the host cell. This review will outline the major players involved in the process of HSV entry, and examine recent developments in the field.
Viral attachment to cells
The initial binding of virus to cells is mediated through association of viral glycoprotein(s) gB and/or gC with HS proteoglycans (HSPG)s present on the cell surface. Although gC is not required for entry, gC-deficient virions display decreased overall binding to cell surfaces [1]. gC makes the first contact with HSPGs on the cell surface, but in the absence of gC, gB can take over this function.
HSV-1 has been reported to attach to cells by multiple methods. In some cases, the virus binds initially to filopodia, and migrates toward the cell body to initiate entry, in a process termed ‘viral surfing’ [6]. In this process, viral particles travel along extracellular filopodial surfaces through the interaction of gB, HSPGs and Rho GTPases, with the latter involved in directing actin remodeling [7]. PI3K has also been implicated in this process as the PI3K inhibitor LY294002 was shown to prevent filopodia formation and viral entry [8]. Fluorescence labeling studies demonstrated that HSPG expression is higher along filopodia, while expression of viral fusion receptors, like nectin-1, is limited to the cell body [6]. Furthermore, exposure to HSV-1 induces increased formation of filopodia, enhancing chances of viral attachment and providing a mechanism for targeted delivery of viral particles to the cell body for entry [7]. Viral surfing has also been observed for vaccinia virus, HPV type 16, HCV and HIV [9], suggesting that manipulation of the actin cytoskeleton may be a general mechanism of virus targeting and entry to the cell body.
Efforts to further understand the viral attachment process recently led to the development of a potential therapeutic consisting of zinc oxide tetrapods. These filopodia-like nanostructures effectively increase the surface area of negatively charged molecules, thereby mimicking the overall negative charge of HS to bind up positively charged viral attachment glycoproteins and decrease cell–virus interactions. These compounds have been shown to be effective in diminishing infectivity of both HSV-1 [10] and HSV-2 [11] in in vitro and in vivo models of infection. Trapping viral particles in this way to hamper initial infection and prevent cell-to-cell spread could prove a valuable prophylactic and therapeutic in humans.
Viral glycoproteins & membrane fusion
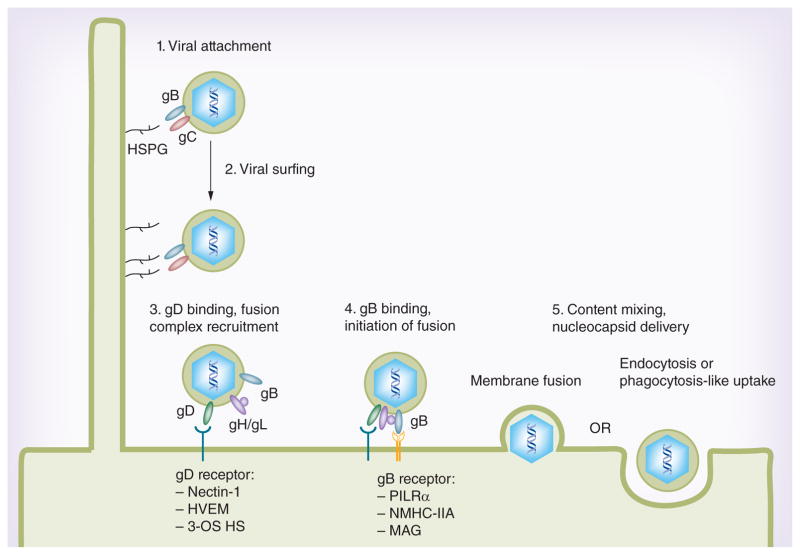
Fusion of the viral envelope with the plasma membrane is a pH-independent process that requires gB, gD, gH and gL, along with cellular receptors for gD, such as nectin-1, herpes virus entry mediator (HVEM) or 3-O-sulfated HS (3-OS HS) (Figure 1). gD binding to one of its receptors triggers a conformational change in this glycoprotein’s structure that drives recruitment of a fusion complex including glycoproteins gB, gH and gL. Ultimately this fusion complex results in merging of the lipid bilayers and content mixing with eventual release of the viral nucleocapsid and tegument proteins into the host cytoplasm. Much of the work in recent years has aimed to understand the mechanism of interactions between these glycoproteins and elucidate the steps required for successful fusion of the viral envelope with the plasma membrane.
Figure 1. Molecular interactions mediating HSV entry.
Initial attachment to cells is facilitated by binding of HSPGs by HSV glycoproteins gC and/or gB. Virions are then transported along cellular filopodia toward the cell body in a process termed viral surfing. Recruitment of a fusion complex comprising gB, gD and gH/gL is initiated by gD binding to one of its receptors, nectin-1, herpes virus entry mediator or 3-O-sulfated heparan sulfate. Binding of gB to one of its receptors, PILRα, NMHC-IIA or MAG, is then required for delivery of the viral nucleocapsid to the cytoplasm, accomplished either by membrane fusion or endocytosis/phagocytosis-like uptake. 3-OS HS: 3-O-sulfated heparan sulfate; HVEM: Herpes virus entry mediator.
Along these lines, in the last decade substantial evidence for alternate entry mechanism(s) involving endocytosis and/or a phagocytosis-like uptake has come to light [7,12]. This apparently atypical endocytosis is unique in that it is not mediated by clathrin-coated pits or caveolae and it may or may not be pH-dependent. Interestingly, the phagocytosis-like uptake requires massive cytoskeletal rearrangement and activation of Rho GTPases. However, similar to endocytosis, clathrin-coated pits play no detectable role in virus trafficking as Eps15 dominant-negative mutants do not block HSV-1 uptake via the phagocytosis-like mechanism [7]. Irrespective of the mechanism of uptake (endocytosis or phagocytosis-like), the viral envelope eventually fuses with the vesicular membrane. It is proposed that interactions similar to the one described above for the plasma membrane facilitate fusion with the vesicular membrane and result in the release of viral capsid into the cytoplasm [7,12]. A gD receptor has been colocalized with endosomal markers and electron micrographs show the fusion and exit of nucleocapsids from the intracellular vesicles [7]. The choice between endocytosis and fusion at the plasma membrane appears to be cell type-dependent. In Vero and HEp-2 cells, fusion at the plasma membrane is likely preferred; however, in cell types such as HeLa, retinal pigment epithelial cells, human epidermal keratinocytes and human conjunctival epithelial cells, evidence of HSV endocytosis has been reported [12,13]. It is speculated that one or more envelope glycoproteins and the availability of a unique set of corresponding host receptors dictate the mode of entry.
gD
A widely accepted model of HSV fusion contends that gD binding to one of its cellular receptors triggers a change in gD’s conformation, an event that serves as a signal for assembly of a fusion complex required for viral entry. Coprecipitation studies showed that gD promotes fusion through its interaction with the gH/gL heterodimer [14]. Previous studies had characterized a ‘profusion domain’ of gD [15], and recent experiments provide evidence that this domain enables a functional interaction between gD and gH/gL, which is required for fusion [16]. Furthermore, it was recently observed that mutational displacement of the C-terminal portion of gD is sufficient to induce fusion in the absence of receptor binding, in a cell-based fusion assay [17]. This led to the understanding that displacement of the C-terminus of gD is the physical mechanism linking gD receptor binding to the uncovering of motifs required for activation of fusion.
gH/gL
Further studies support the belief that gH/gL provides the signal required for activation of gB and viral fusion with the plasma membrane. The gH/gL complex was originally postulated to function as a viral fusogen, as the complex contains heptad-repeat and fusion peptides that were predicted to impart fusogenic properties [18–20]. Crystal structure information obtained from HSV-2 gH/gL showed that gH/gL does not resemble any known viral fusogen, and that these peptides are mostly buried in the core of the protein complex [21]. This study also found that the structures of gH and gL are intertwined in such a way that they require one another for proper folding. Additional support for this theory came from a study that showed that all four glycoproteins are required for lipid mixing [22]. In the absence of gB, the gH/gL complex is insufficient to establish hemifusion, a previously described intermediate in which the two membrane bilayers have merged, but a viral pore allowing mixing of viral and cellular contents has not yet formed [23]. Moreover, bimolecular complementation assays showed that an interaction between gH/gL and gB is required for fusion [24].
gB
Recent studies have shown that the gB trimer has multiple fusogenic domains, and this glycoprotein was recently categorized as a class III fusogen [25,26]. As a charter member of this novel hybrid group of fusogenic proteins, gB was found to possess features of both class I and class II fusogens. Class I proteins contain hairpin trimers with N-terminal hydrophobic membrane-penetrating peptides and centrally located α-helical coiled-coils. By contrast, class II proteins contain β-structures with internally located fusion domains [27]. Similar to class I, the gB trimer contains a central α-helical core, but similar to class II, the fusion loops are part of elongated β-hairpins [25]. A recent study has put forth that gB’s ectodomain contains a membrane-proximal region (MPR) and two fusion loops; the MPR serves to cover the fusion loops from interacting with membrane lipids while gB is in its prefusion form, providing a regulation of the fusion process [28]. Several other studies provide evidence suggesting the gB is the sole fusogen used by HSV in penetrating host cell membranes, with gD and gH/gL serving as regulators of this process [21,22,29]. Atanasiu et al. also showed in a cell-based fusion assay that gD and gH/gL can act in trans (i.e., from different cells) to activate gB in conjunction with one of its receptors [29].
gD receptors
It is believed that the role of gD as a catalyst in the entry process involves interactions with specific receptors at the cellular surface, inducing a conformational change that allows it to form a complex with the other fusion-related glycoproteins [30,31]. These receptors for gD include nectin-1, HVEM and 3-OS HS, with varying cell types relying on different receptors for HSV entry.
Nectin-1 & -2
Nectin-1 and nectin-2 are members of the immunoglobulin superfamily involved in cell adhesion, with nectin-1 serving as the major functional HSV-1 gD receptor in cells of epithelial or neuronal origin [32,33]. HSV-2 entry can be mediated by nectin-1 or nectin-2, but nectin-2 does not function in the entry of wild-type HSV-1 [34]. Recent work has shown that gD binds to the homodimerization interface of nectin-1, thereby preventing its normal function in cell adhesion [35].
Herpes virus entry mediator (HVEM)
HVEM is a member of the tumor necrosis factor receptor superfamily, and while its tissue expression profile is not well understood, it is known to aid HSV entry into T lymphocytes and several types of ocular epithelial cells. Interestingly, gD’s modes of binding to nectin-1 and HVEM are completely distinct, however, because a common set of residues are required for binding to each of these receptors, gD cannot simultaneously bind these two receptors. Furthermore, Zhang et al. found that soluble nectin-1 blocks entry into HVEM-transfected Chinese hamster ovary (CHO) cells, and vice versa [35]. A role for HVEM in viral latency-reactivation has recently been suggested, as it was found that presence of latency-associated transcript (LAT) in mouse ocular infection increased expression of HVEM but not of other gD receptors [36]. HSV-1 latency-reactivation was also significantly reduced in HVEM-knockout mice, indicating that LAT can enhance latency and reactivation through upregulation of HVEM expression [37].
3-OS HS
HS represents a ubiquitously expressed, structurally diverse family of polysaccharides with a shared backbone containing various modifications that impart specific functions to different types of chains [38]. It serves as an important attachment receptor for many pathogenic viruses, including all human herpesviruses except Epstein–Barr virus [1]. In the unique case of HSV-1, however, HS also serves as a fusion receptor after it has been modified by members of the 3-OST family, producing 3-OS HS capable of binding gD [39–42]. These enzymes have distinct cell and tissue expression profiles, suggesting their roles in cell-specific regulation of HS functions, but their individual physiological functions are not yet well known. However, an important role for 3-OS HS in infection of a target cell type has been demonstrated. It is known to mediate HSV-1 entry into primary cultures of corneal fibroblasts [43], which lack nectin-1 expression and demonstrate very low levels of HVEM on the cell surface. Recent studies have shown that zebrafish expresses multiple 3-OST isoforms, and many of those generate HSV-1 entry receptors [44]. To add further credence to its role as an HSV-1 receptor, a soluble form of 3-OS HS is known to trigger entry in CHO cells, an infection-resistant cell line [45].
gB receptors
Paired immunoglobulin-like receptor
Recent studies have also identified specific receptors that bind gB independently of HS to drive viral fusion. The first of these to be recognized was paired immunoglobulin-like receptor (PILRα), an inhibitory receptor found on monocytes, macrophages and dendritic cells [46]. PILRα contains a tyrosine-based motif capable of delivering inhibitory signals to the host cell; by interacting with this receptor, gB may also allow HSV-1 to evade immune activation. In cells expressing both HVEM and PILRα, PILRα-blocking antibodies resulted in decreased viral entry, indicating a separate need for the two receptors [46]. In characterizing the mechanism of receptor binding, it was observed that the Ig-like V-type domain of PILRα, and more specifically the tryptophan-139 residue, is required for gB binding [47]. Mutation of gB’s O-glycosylation sites (threonine-53 and threonine-480), which serve as PILRα-binding sites, decrease PILRα-dependent viral binding and pathogenesis in mice [48]. The significance of this receptor in the fusion process remains to be elucidated, but interestingly, it was found that amino acid differences in gB of McKrae and F HSV-1 strains are responsible for the increased ability of the McKrae strain to enter PILRα-expressing cells [49].
Myelin-associated glycoprotein
Myelin-associated glycoprotein (MAG) was recently identified as a gB receptor; however, its significance in the viral entry process is not fully understood. It is predominantly expressed on glial cells, suggesting its importance in regulating myelin-axon interactions. MAG associates with gB from HSV-1 and Varicella Zoster virus (VZV) [50], although since MAG is not naturally expressed in epithelial and neuronal cells, MAG is not thought to serve as a major receptor for these viruses. However, as both HSV-1 and VZV infect glial cells during the acute phase of infection, MAG may be important in development of the neurological disorders associated with HSV-1 and VZV.
Non-muscle myosin heavy chain-IIA
Non-muscle myosin heavy chain (NMHC)-IIA was also found to be a receptor for HSV-1 gB [51]. NM-II is a key protein in the control of actin cross-linking, cell contractility, migration and adhesion. NM-II is composed of two heavy chains, two regulatory light chains and two essential light chains. While expression of PILRα and MAG is limited to certain types of cells, NMHC-IIA is ubiquitously expressed in human tissues, suggesting its important role as a functional gB receptor. A second isoform NMHC-IIB was also recently reported to qualify as a gB receptor [52], although the significance of this finding remains to be seen.
gH/gL receptors
αvβ6-, αvβ8-integrins
Recent work indicates that αvβ6- and αvβ8-integrins are receptors for gH/gL, specifically promoting viral entry by the endocytic pathway [53]. Based on this work, the prevailing theory is that these receptors provide an additional mechanism of viral entry, and cannot substitute in the absence of gD or one of its receptors. Several studies have also found that gH/gL can trigger NF-κB activation and innate immune responses through αvβ3-integrin or toll-like receptor 2 binding [54,55], but the significance of these findings remains unknown.
Viral spread
HSV-1 is capable of using multiple modes of spread to transfer from infected to uninfected cells and increase the number of its progeny virions. Elucidating the mechanism of viral dissemination and subsequent entry into neighboring cells is important to understand the infection process. Several viral glycoproteins are reported to be required for successful release of virions from parent cells. For instance, the HSV-1 glycoprotein heterodimer gE/gI accumulates in the trans-Golgi network early in infection and redistributes to cell junctions in order to promote cell-to-cell spread [56]. It was also recently shown that gE/gI promotes capsid transport from neuronal cell bodies into initial axon segments, with the authors predicting that this heterodimer functions to maintain interactions between viral protein complexes and kinesin motor proteins [57]. Howard et al. later showed that gE/gI was required for spread from neuronal cell bodies to epithelial cells in a coculture system, suggestive of a role in reactivation from viral latency [58]. Another HSV-1 glycoprotein, gK, was found to be required for spread between corneal and neuronal cells [59,60]. Treatment of mice with gK-derived peptides before ocular infection resulted in increased viral replication and pathogenicity [61]. Furthermore, mutation of the N-terminus of gK prevented HSV-1 infection of mouse trigeminal ganglia after corneal infection [62], indicating that gK may also be involved in establishment of viral latency.
The mechanisms of virion egress from epithelial cells and spread to neurons and vice versa remain poorly understood. Upon reactivation from viral latency, newly replicated virions in sensory ganglia undergo anterograde transport along microtubules to reach epithelial cells [63]. A recent study employing immunogold labeling indicated that after replication in neuronal growth cones and varicosities, viral particles used the large vesicle pathway of exocytosis [64]. The authors also showed that several known neurosecretory proteins colocalized with HSV-1 antigens in human fetal dorsal root ganglia, further suggesting a targeted exocytotic mechanism in viral egress from neurons. Another group used live-cell fluorescence imaging to show that most incidences of anterograde-directed spread from neurons to epithelial cells involve only a single viral capsid [65]. This bottleneck in transmission from neuronal to epithelial cells would prevent complementation or gene transfer between different viral genomes, presenting a potential to limit spread of viral drug resistance.
A recent study from the authors’ laboratory indicates that the host-encoded enzyme heparanase-1 (HPSE) is required for HSV release from cells [66]. Similarly to influenza viruses, which use virally encoded neuraminidase to cleave sialic acid residues used for virus attachment, herpes viruses depend on a host enzyme to be released from their attachment receptor HS. With progression of infection, HPSE is upregulated, effectively shifting the cell from a viral attachment mode to a viral detachment mode, thus allowing newly produced virions to escape the cell unimpeded. We also showed that knockdown of HPSE resulted in decreased HSV-1 spread and keratitis severity in a corneal infection model. Interestingly, HPSE has been heavily implicated in other studies in the development of multiple inflammatory conditions and metastatic disease, making this an attractive target to pursue further. We believe these findings represent an important transition in our understanding of how viruses can seize host cell machinery to promote replication and spread.
Viral entry inhibitors
Entry into cells is the first essential step in the viral replicative cycle, and therefore, blocking of viral entry into target cells by small molecules, peptides or nanoparticles can provide not only new tools to study infection but also the lead candidates for the suppression of viral infectivity. The latter could impart an attractive antiviral strategy, and many such tools are already being investigated. A series of small cationic peptides has been identified that interfere with HSV-1 attachment to cells and block viral entry [67–69]. The cationic peptides likely bind the negatively charged HS moieties on cell surfaces and competitively inhibit viral attachment. Quite interestingly, a similar peptide, G2, is known to bind 3-OS HS and shows the ability to block both viral binding and cell fusion [70]. A fluorescent version of G2 peptide demonstrates strong diagnostic potential for the analysis of 3-OS HS expression on cell surfaces [70]. Along the same lines, Yasin et al. have described the antiviral effect of synthetic theta defensins, specifically RC-2, on inhibiting HSV entry via gB binding [71]. Similarly, a few different nanoparticles have been identified that block virus binding to cell surfaces. These include zinc oxide micro-nanoparticles and nanowires [11,72]. The latter demonstrate the added ability to block membrane fusion as well, suggesting their ability to interfere with cell-to-cell adherence and fusion pore formation. Recently, a small molecule, Epigallocatechin gallate, was described as a new broad-spectrum HSV-1 attachment inhibitor. Epigallocatechin gallate competes with HS for virus binding and its effect can be seen with other nonrelated viruses as well [73]. A similar small molecule HS inhibitor, surfen, was also shown to block HSV-1 infection [74]. Another new approach has involved the use of RNA aptamers that bind with high affinity to gD and block infection, representing a novel promising candidate as a future antiviral therapy [75]. Thus, with new understanding of the HSV-1 entry process, many innovative strategies and tools now show the strong promise for development as antiviral drugs; their highest potential will be realized as a new class of prophylactic agents that can preemptively control recurrent episodes of herpetic diseases.
Conclusion & future perspective
The herpes entry field has made unprecedented progress in the last two decades. It is now very clear that precise mechanisms of binding, fusion and subsequent cell-to-cell spread facilitate the initial steps of the HSV replicative cycle. More accurate roles for viral glycoproteins in entry are now defined. Binding can be initiated on actin-rich membrane projections or filopodia via the interaction of gC and gB. Subsequent fusion and capsid penetration utilize gB, gD, gH and gL. Depending on the cell types, entry can directly occur at the plasma membrane or the virions can be taken up by membrane projections resembling a phagocytosis-like mechanism [7]. Fusion is mediated by the fusogenic regions of gB that are surface exposed after the interaction of gD with one of its receptors and subsequent interaction of gB with a host partner. It is also now clear that cell-to-cell HSV spread utilizes gD receptors, all essential fusion glycoproteins, the gE/gI heterodimer and gK. Crystal structures of many entry glycoproteins and receptors have been solved and new pieces of information generated on the protein complexes needed to facilitate entry. While the mechanism of entry is largely understood, a few areas still remain poorly defined. For example, the role of cellular signaling in entry is weakly defined. Activation of Rho signaling has been demonstrated [6,7], as has the involvement of PKC [8] and calcium signaling pathways [76], yet a complete picture is far from clear. It is also unclear what additional factors are needed to support endocytosis as opposed to fusion at the plasma membrane. The ultimate question of molecular factors that dictate HSV tissue tropism still remains unanswered. On a brighter note, the advances in understanding entry mechanisms, especially in lieu of a protective vaccine, have driven many investigators to develop efficient entry-based microbicides or prophylactics. Many such small molecules, antiviral peptides and nanoparticles are in the pipeline for development as new antivirals. Cell-to-cell spread is an important part of the virus life cycle, but blocking transfer of virions between cells has thus far been difficult to study. In this review we have addressed several promising agents that prevent viral fusion with the host cell membrane; however, further studies will be needed to determine the efficacy of these agents in clinical situations. In summary, HSV entry is an important research area with strong new promise for identifying alternate strategies to prevent HSV infection and preemptively control recurrent diseases.
EXECUTIVE SUMMARY.
Molecular basis of HSV entry
Two HSV envelope glycoproteins (gB and gC) support attachment to cells.
Four envelope glycoproteins (gB, gD, gH, gL) are essential for membrane penetration.
Crystal structures have been elucidated for gB, gD and gH-gL.
A multiprotein complex with essential glycoproteins and host receptors facilitates membrane fusion.
Host receptors for entry
A number of host receptors are required for entry.
Heparan sulfate moieties facilitate viral attachment to cells.
Nectin-1, HVEM or 3-O-sulfated heparan sulfate is needed for interaction with gD.
PILRα, NMHC-IIA or MAG is needed for interaction with gB.
The glycoprotein gH engages integrins during entry.
Structural requirements for entry
Cytoskeletal changes are required for entry.
Membrane processes such as filopodia facilitate viral transport to cell bodies.
Cell signaling mediated by Rho GTPases and PKC is needed for the alterations.
Unique modes of entry
One unique mode involves pH-independent fusion of the viral envelope at the plasma membrane.
Another mode of entry is pH-dependent or -independent endocytosis.
Phagocytosis-like uptake involves cytoskeletal rearrangement and eventual fusion of the viral envelope with a vesicular membrane.
The mode of entry is dependent on cell type.
Entry inhibitors as novel tools for research & antiviral therapy
Cationic peptides act as promising antivirals.
ZnO nanoparticles and nanowires act as prophylactic agents.
Small-molecule inhibitors of viral attachment also provide a novel tool for research and antiviral therapy.
Footnotes
Financial & competing interests disclosure
This work was supported by NIH grant EY024710 (D Shukla), a core grant (EY001792) and unrestricted funds from Research to Prevent Blindness Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
For reprint orders, please contact: reprints@futuremedicine.com
References
Papers of special note have been highlighted as:
• of interest; • of considerable interest
- 1.Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001;108(4):503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fatahzadeh M, Schwartz RA. Human HSV infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57(5):737–763. doi: 10.1016/j.jaad.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Anzivino E, Fioriti D, Mischitelli M, et al. HSV infection in pregnancy and in neonate: status of art of epidemiology, diagnosis, therapy and prevention. Virol J. 2009;6(1):40. doi: 10.1186/1743-422X-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitley RJ, Roizman B. HSV infections. Lancet. 2001;357(9267):1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 5.James SH, Prichard MN. Current and future therapies for HSV infections: mechanism of action and drug resistance. Curr Opin Virol. 2014;8:54–61. doi: 10.1016/j.coviro.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Oh M, Akhtar J, Desai P, Shukla D. A role for heparan sulfate in viral surfing. Biochem Biophys Res Commun. 2010;391(1):176–181. doi: 10.1016/j.bbrc.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yue BYJT, Shukla D. A novel role for phagocytosis-like uptake in HSV entry. J Cell Biol. 2006;174(7):1009–1021. doi: 10.1083/jcb.200509155. Shows that endocytosis of HSV particles is an important mechanism of viral entry, and that modulation of cellular actin networks is critical in this process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiwari V, Shukla D. Phosphoinositide 3 kinase signalling may affect multiple steps during HSV type-1 entry. J Gen Virol. 2010;91(12):3002–3009. doi: 10.1099/vir.0.024166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spear M, Wu Y. Viral exploitation of actin: force-generation and scaffolding functions in viral infection. Virol Sin. 2014;29(3):139–147. doi: 10.1007/s12250-014-3476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra YK, Adelung R, Rohl C, Shukla D, Spors F, Tiwari V. Virostatic potential of micro-nano filopodia-like ZnO structures against HSV-1. Antiviral Res. 2011;92(2):305–312. doi: 10.1016/j.antiviral.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antoine TE, Mishra YK, Trigilio J, Tiwari V, Adelung R, Shukla D. Prophylactic, therapeutic and neutralizing effects of zinc oxide tetrapod structures against HSV type-2 infection. Antiviral Res. 2012;96(3):363–375. doi: 10.1016/j.antiviral.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicola AV, Straus SE. Cellular and viral requirements for rapid endocytic entry of HSV. J Virol. 2004;78(14):7508–7517. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akhtar J, Shukla D. Viral entry mechanisms: cellular and viral mediators of HSV entry. FEBS J. 2009;276(24):7228–7236. doi: 10.1111/j.1742-4658.2009.07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gianni T, Amasio M, Campadelli-Fiume G. HSV gD forms distinct complexes with fusion executors gB and gH/gL in part through the C-terminal profusion domain. J Biol Chem. 2009;284(26):17370–17382. doi: 10.1074/jbc.M109.005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocchi F, Fusco D, Menotti L, et al. The soluble ectodomain of HSV gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc Natl Acad Sci USA. 2004;101(19):7445–7450. doi: 10.1073/pnas.0401883101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan Q, Longnecker R, Connolly SA. Substitution of HSV 1 entry glycoproteins with those of saimiriine herpesvirus 1 reveals a gD–gH/gL functional interaction and a region within the gD profusion domain that is critical for fusion. J Virol. 2014;88(11):6470–6482. doi: 10.1128/JVI.00465-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher JR, Saw WT, Atanasiu D, Lou H, Eisenberg RJ, Cohen GH. Displacement of the C Terminus of HSV gD is sufficient to expose the fusion-activating interfaces on gD. J Virol. 2013;87(23):12656–12666. doi: 10.1128/JVI.01727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galdiero S, Vitiello M, D’Isanto M, et al. Analysis of synthetic peptides from heptad-repeat domains of HSV type 1 glycoproteins H and B. J Gen Virol. 2006;87(Pt. 5):1085–1097. doi: 10.1099/vir.0.81794-0. [DOI] [PubMed] [Google Scholar]
- 19.Gianni T, Fato R, Bergamini C, Lenaz G, Campadelli-Fiume G. Hydrophobic alpha-helices 1 and 2 of HSV gH interact with lipids, and their mimetic peptides enhance virus infection and fusion. J Virol. 2006;80(16):8190–8198. doi: 10.1128/JVI.00504-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian RP, Geraghty RJ. HSV type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc Natl Acad Sci USA. 2007;104(8):2903–2908. doi: 10.1073/pnas.0608374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. Crystal structure of the conserved herpesvirus fusion regulator complex gH–gL. Nat Struct Mol Biol. 2010;17(7):882–888. doi: 10.1038/nsmb.1837. Shows gH/gL does not resemble any known fusion proteins, and provides evidence that gH/gL may function as a regulator of gB activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson JO, Longnecker R. Reevaluating HSV Hemifusion. J Virol. 2010;84(22):11814–11821. doi: 10.1128/JVI.01615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chernomordik LV, Kozlov MM. Membrane hemifusion: crossing a chasm in two leaps. Cell. 2005;123(3):375–382. doi: 10.1016/j.cell.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 24••.Atanasiu D, Whitbeck JC, de Leon MP, et al. Bimolecular complementation defines functional regions of HSV gB that are involved with gH/gL as a necessary step leading to cell fusion. J Virol. 2010;84(8):3825–3834. doi: 10.1128/JVI.02687-09. Demonstrates that a sequential interaction between gB, gD and gH/gL is required for cell fusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Crystal structure of glycoprotein B from HSV 1. Science. 2006;313(5784):217–220. doi: 10.1126/science.1126548. Describes structure of HSV-1 gB, providing first evidence that gB is a viral fusogen. [DOI] [PubMed] [Google Scholar]
- 26.Backovic M, Jardetzky TS. Class III viral membrane fusion proteins. Curr Opin Struct Biol. 2009;19(2):189–196. doi: 10.1016/j.sbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podbilewicz B. Virus and cell fusion mechanisms. Annu Rev Cell Dev Biol. 2014;30:111–139. doi: 10.1146/annurev-cellbio-101512-122422. [DOI] [PubMed] [Google Scholar]
- 28.Shelly SS, Cairns TM, Whitbeck JC, et al. The membrane-proximal region (MPR) of HSV gB regulates association of the fusion loops with lipid membranes. mBio. 2012;3(6) doi: 10.1128/mBio.00429-12. pii: e00429–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. Cascade of events governing cell–cell fusion induced by HSV glycoproteins gD, gH/gL, and gB. J Virol. 2010;84(23):12292–12299. doi: 10.1128/JVI.01700-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spear PG. HSV: receptors and ligands for cell entry. Cell Microbiol. 2004;6(5):401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 31.Carfi A, Willis SH, Whitbeck JC, et al. HSV glycoprotein D bound to the human receptor HveA. Mol Cell. 2001;8(1):169–179. doi: 10.1016/s1097-2765(01)00298-2. [DOI] [PubMed] [Google Scholar]
- 32.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaher-pesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280(5369):1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 33.Warner MS, Geraghty RJ, Martinez WM, et al. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of HSV type 1, HSV type 2, and pseudorabies virus. Virology. 1998;246(1):179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 34.Krummenacher C, Baribaud F, Ponce de Leon M, et al. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of HSV. Virology. 2004;322(2):286–299. doi: 10.1016/j.virol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 35•.Zhang N, Yan J, Lu G, et al. Binding of HSV glycoprotein D to nectin-1 exploits host cell adhesion. Nat Commun. 2011;2:577–510. doi: 10.1038/ncomms1571. Reports structure of gD in complex with nectin-1 or herpes virus entry mediator, defining glycoprotein regions required for binding these receptors. [DOI] [PubMed] [Google Scholar]
- 36.Allen SJ, Mott KR, Ghiasi H. Overexpression of HSV glycoprotein K (gK) alters expression of HSV receptors in ocularly-infected mice. Invest Ophthalmol Vis Sci. 2014;55(4):2442–2451. doi: 10.1167/iovs.14-14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen SJ, Rhode-Kurnow A, Mott KR, et al. Interactions between herpesvirus entry mediator (TNFRSF14) and latency-associated transcript during HSV 1 latency. J Virol. 2014;88(4):1961–1971. doi: 10.1128/JVI.02467-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindahl U, Kusche-Gullberg M, Kjellen L. Regulated diversity of heparan sulfate. J Biol Chem. 1998;273(39):24979–24982. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- 39.O’Donnell CD, Tiwari V, Oh M, Shukla D. A role for heparan sulfate 3-O-sulfotransferase isoform 2 in HSV type 1 entry and spread. Virology. 2006;346(2):452–459. doi: 10.1016/j.virol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Xu D, Tiwari V, Xia G, Clement C, Shukla D, Liu J. Characterization of heparan sulphate 3-O-sulphotransferase isoform 6 and its role in assisting the entry of HSV type 1. Biochem J. 2005;385(Pt 2):451–459. doi: 10.1042/BJ20040908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiwari V, Clement C, Duncan MB, Chen J, Liu J, Shukla D. A role for 3-O-sulfated heparan sulfate in cell fusion induced by HSV type 1. J Gen Virol. 2004;85(Pt. 4):805–809. doi: 10.1099/vir.0.19641-0. [DOI] [PubMed] [Google Scholar]
- 42.Xia G, Chen J, Tiwari V, et al. Heparan sulfate 3-O-sulfotransferase isoform 5 generates both an antithrombin-binding site and an entry receptor for HSV, type 1. J Biol Chem. 2002;277(40):37912–37919. doi: 10.1074/jbc.M204209200. [DOI] [PubMed] [Google Scholar]
- 43.Tiwari V, Clement C, Xu D, et al. Role for 3-O-sulfated heparan sulfate as the receptor for HSV type 1 entry into primary human corneal fibroblasts. J Virol. 2006;80(18):8970–8980. doi: 10.1128/JVI.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yakoub AM, Rawal N, Maus E, Baldwin J, Shukla D, Tiwari V. Comprehensive analysis of HSV 1 (HSV-1) entry mediated by zebrafish 3-O-sulfotransferase isoforms: implications for the development of a zebrafish model of HSV-1 infection. J Virol. 2014;88(21):12915–12922. doi: 10.1128/JVI.02071-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiwari V, O’donnell C, Copeland RJ, Scarlett T, Liu J, Shukla D. Soluble 3-O-sulfated heparan sulfate can trigger HSV type 1 entry into resistant Chinese hamster ovary (CHO-K1) cells. J Gen Virol. 2007;88(Pt. 4):1075–1079. doi: 10.1099/vir.0.82476-0. [DOI] [PubMed] [Google Scholar]
- 46•.Satoh T, Arii J, Suenaga T, et al. PILRalpha is a HSV-1 entry coreceptor that associates with glycoprotein B. Cell. 2008;132(6):935–944. doi: 10.1016/j.cell.2008.01.043. Establishes that binding of gB to a cellular receptor is required for HSV-1 entry into cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan Q, Longnecker R. The Ig-like V-type domain of paired Ig-like type 2 receptor alpha is critical for HSV type 1-mediated membrane fusion. J Virol. 2010;84(17):8664–8672. doi: 10.1128/JVI.01039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arii J, Wang J, Morimoto T, et al. A single-amino-acid substitution in HSV 1 envelope glycoprotein B at a site required for binding to the paired immunoglobulin-like type 2 receptor (PILRα) abrogates PILRα-dependent viral entry and reduces pathogenesis. J Virol. 2010;84(20):10773–10783. doi: 10.1128/JVI.01166-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chowdhury S, Naderi M, Chouljenko VN, Walker JD, Kousoulas KG. Amino acid differences in glycoproteins B (gB), C (gC), H (gH) and L(gL) are associated with enhanced HSV type-1 (McKrae) entry via the paired immunoglobulin-like type-2 receptor α. Virol J. 2012;9(1):112. doi: 10.1186/1743-422X-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suenaga T, Satoh T, Somboonthum P, Kawaguchi Y, Mori Y, Arase H. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc Natl Acad Sci USA. 2010;107(2):866–871. doi: 10.1073/pnas.0913351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arii J, Goto H, Suenaga T, et al. Non-muscle myosin IIA is a functional entry receptor for HSV-1. Nature. 2010;467(7317):859–862. doi: 10.1038/nature09420. [DOI] [PubMed] [Google Scholar]
- 52.Arii J, Hirohata Y, Kato A, Kawaguchi Y. Nonmuscle myosin heavy chain IIB mediates HSV 1 Entry. J Virol. 2015;89(3):1879–1888. doi: 10.1128/JVI.03079-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gianni T, Salvioli S, Chesnokova LS, Hutt-Fletcher LM, Campadelli-Fiume G. αvβ6- and αvβ8-integrins serve as interchangeable receptors for HSV gH/gL to promote endocytosis and activation of membrane fusion. PLoS Pathog. 2013;9(12):e1003806. doi: 10.1371/journal.ppat.1003806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gianni T, Leoni V, Campadelli-Fiume G. Type I interferon and NF-κB activation elicited by HSV gH/gL via αvβ3 integrin in epithelial and neuronal cell lines. J Virol. 2013;87(24):13911–13916. doi: 10.1128/JVI.01894-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leoni V, Gianni T, Salvioli S, Campadelli-Fiume G. HSV glycoproteins gH/gL and gB bind toll-like receptor 2, and soluble gH/gL is sufficient to activate NF-κB. J Virol. 2012;86(12):6555–6562. doi: 10.1128/JVI.00295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farnsworth A, Johnson DC. HSV gE/gI must accumulate in the trans-Golgi network at early times and then redistribute to cell junctions to promote cell–cell spread. J Virol. 2006;80(7):3167–3179. doi: 10.1128/JVI.80.7.3167-3179.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howard PW, Howard TL, Johnson DC. HSV membrane proteins gE/gI and US9 act cooperatively to promote transport of capsids and glycoproteins from neuron cell bodies into initial axon segments. J Virol. 2012;87(1):403–414. doi: 10.1128/JVI.02465-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howard PW, Wright CC, Howard T, Johnson DC. HSV gE/gI Extracellular domains promote axonal transport and spread from neurons to epithelial cells. J Virol. 2014;88(19):11178–11186. doi: 10.1128/JVI.01627-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.David AT, Baghian A, Foster TP, Chouljenko VN, Kousoulas KG. The HSV type 1 (HSV-1) glycoprotein K(gK) is essential for viral corneal spread and neuroinvasiveness. Curr Eye Res. 2008;33(5):455–467. doi: 10.1080/02713680802130362. [DOI] [PubMed] [Google Scholar]
- 60.David AT, Saied A, Charles A, Subramanian R, Chouljenko VN, Kousoulas KG. A HSV 1 (McKrae) mutant lacking the glycoprotein K gene is unable to infect via neuronal axons and egress from neuronal cell bodies. mBio. 2012;3(4):e00144–12. doi: 10.1128/mBio.00144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mott KR, Chentoufi AA, Carpenter D, BenMohamed L, Wechsler SL, Ghiasi H. The role of a glycoprotein K (gK) CD8+ T-cell epitope of HSV on virus replication and pathogenicity. Invest Ophthalmol Vis Sci. 2009;50(6):2903–2912. doi: 10.1167/iovs.08-2957. [DOI] [PubMed] [Google Scholar]
- 62.Saied AA, Chouljenko VN, Subramanian R, Kousoulas KG. A replication competent HSV-1(McKrae) with a mutation in the amino-terminus of glycoprotein K (gK) is unable to infect mouse trigeminal ganglia after cornea infection. Curr Eye Res. 2014;39(6):596–603. doi: 10.3109/02713683.2013.855238. [DOI] [PubMed] [Google Scholar]
- 63.Kramer T, Enquist LW. Directional spread of alphaherpesviruses in the nervous system. Viruses. 2013;5(2):678–707. doi: 10.3390/v5020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miranda-Saksena M, Boadle RA, Aggarwal A, et al. HSV utilizes the large secretory vesicle pathway for anterograde transport of tegument and envelope proteins and for viral exocytosis from growth cones of human fetal axons. J Virol. 2009;83(7):3187–3199. doi: 10.1128/JVI.01579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor MP, Kobiler O, Enquist LW. Alphaherpesvirus axon-to-cell spread involves limited virion transmission. Proc Natl Acad Sci USA. 2012;109(42):17046–17051. doi: 10.1073/pnas.1212926109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Hadigal SR, Agelidis AM, Karasneh GA, et al. Heparanase is a host enzyme required for HSV-1 release from cells. Nat Commun. 2015;6:6985. doi: 10.1038/ncomms7985. Demonstrates that upregulation of a host protein is required to drive efficient release of HSV-1 progeny from parent cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tiwari V, Liu J, Valyi-Nagy T, Shukla D. Anti-heparan sulfate peptides that block HSV infection in vivo. J Biol Chem. 2011;286(28):25406–25415. doi: 10.1074/jbc.M110.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaishankar D, Yakoub AM, Bogdanov A, Valyi-Nagy T, Shukla D. Characterization of a proteolytically stable d-Peptide that suppresses HSV 1 infection: implications for the development of entry-based antiviral therapy. J Virol. 2015;89(3):1932–1938. doi: 10.1128/JVI.02979-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jose GG, Larsen IV, Gauger J, et al. A cationic peptide, TAT-Cd0, inhibits HSV type 1 ocular infection in vivo. Invest Ophthalmol Vis Sci. 2013;54(2):1070–1079. doi: 10.1167/iovs.12-10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ali MM, Karasneh GA, Jarding MJ, Tiwari V, Shukla D. A 3-O-sulfated heparan sulfate binding peptide preferentially targets HSV 2-infected cells. J Virol. 2012;86(12):6434–6443. doi: 10.1128/JVI.00433-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yasin B, Wang W, Pang M, et al. Theta defensins protect cells from infection by HSV by inhibiting viral adhesion and entry. J Virol. 2004;78(10):5147–5156. doi: 10.1128/JVI.78.10.5147-5156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trigilio J, Antoine TE, Paulowicz I, Mishra YK, Adelung R, Shukla D. Tin oxide nanowires suppress HSV-1 entry and cell-to-cell membrane fusion. PLoS ONE. 2012;7(10):e48147. doi: 10.1371/journal.pone.0048147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colpitts CC, Schang LM. A small molecule inhibits virion attachment to heparan sulfate- or sialic acid-containing glycans. J Virol. 2014;88(14):7806–7817. doi: 10.1128/JVI.00896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schuksz M, Fuster MM, Brown JR, et al. Surfen, a small molecule antagonist of heparan sulfate. Proc Natl Acad Sci USA. 2008;105(35):13075–13080. doi: 10.1073/pnas.0805862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gopinath SCB, Hayashi K, Kumar PKR. Aptamer that binds to the gD protein of HSV 1 and efficiently inhibits viral entry. J Virol. 2012;86(12):6732–6744. doi: 10.1128/JVI.00377-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheshenko N, Trepanier JB, Gonzalez PA, Eugenin EA, Jacobs WR, Herold BC. HSV Type 2 glycoprotein H interacts with integrin αvβ3 to facilitate viral entry and calcium signaling in human genital tract epithelial cells. J Virol. 2014;88(17):10026–10038. doi: 10.1128/JVI.00725-14. [DOI] [PMC free article] [PubMed] [Google Scholar]