Abstract
Heart failure is a significant global health problem, which is becoming worse as the population ages, and remains one of the biggest burdens on our economy. Despite significant advances in cardiovascular medicine, management and surgery, mortality rates remain high, with almost half of patients with heart failure dying within five years of diagnosis. As a multifactorial clinical syndrome, heart failure still represents an epidemic threat, highlighting the need for deeper insights into disease mechanisms and the development of innovative therapeutic strategies for both treatment and prevention. In this review, we discuss conventional heart failure therapies and highlight new pharmacological agents targeting pathophysiological features of the failing heart, for example, non-coding RNAs, angiotensin receptor-neprilysin inhibitors, cardiac myosin activators, BGP-15 and molecules targeting GRK2 including M119, gallein and paroxetine. Finally, we address the disparity between phase II and phase III clinical trials that prevent the translation of emerging HF therapies into new and approved therapies.
Introduction
Heart failure (HF) is a debilitating disease in which abnormal function of the heart leads to inadequate supply of blood to tissues and organs to meet their metabolic demands. Various factors can contribute to HF pathogenesis, such as myocardial infarction, ischaemia, hypertension or genetic cardiomyopathies. Heart failure is a significant global health problem which is becoming worse as the population ages [1, 2]. Despite significant advances in cardiovascular medicine and management, mortality rates remain high, with almost 50% of HF patients dying within five years of diagnosis [3]. Further, conventional pharmacological treatments largely delay disease progression and death due to HF, but they do not cure HF [4]. As a multifactorial clinical syndrome, HF still represents an epidemic threat, highlighting the need for deeper insights into disease mechanisms and the development of innovative therapeutic strategies. In this review, we will highlight current and new pharmacologic agents for the treatment of heart failure and discuss new therapeutic approaches (e.g., RNA-based therapies, small molecules) with potential to enter clinical trials.
Pathological Cardiac Hypertrophy
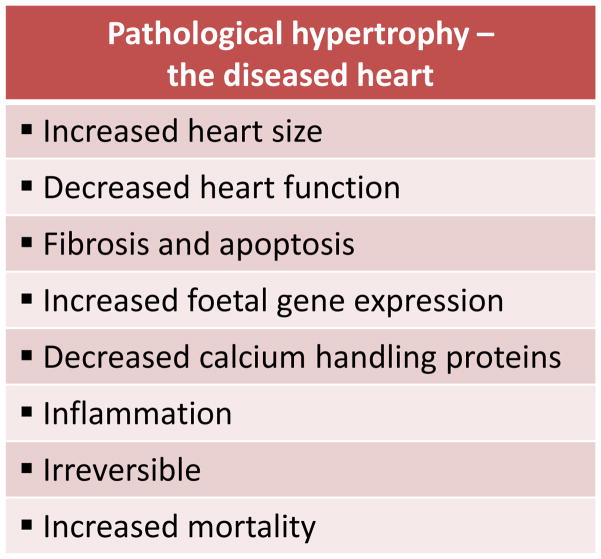
A hallmark of HF development is pathological cardiac hypertrophy, characterised by an increase in cardiomyocyte size and thickening of ventricular walls. It is initially thought to be a compensatory response of the heart to increased workload to maintain heart function. However, with a sustained haemodynamic load, pathological cardiac hypertrophy will proceed, and structural and functional cardiac anomalies develop (reviewed in [5–8]). This is associated with dilation of the ventricle, progressive fibrosis, loss of cardiac myocytes and cardiac dysfunction. At the molecular level, pathological hypertrophy is commonly associated with alterations in cardiac contractile proteins (α-myosin heavy chain and β-myosin heavy chain), increased expression of foetal genes (e.g. atrial natriuretic peptide [ANP], B-type natriuretic peptide [BNP], α-skeletal actin) and down regulation of calcium handling proteins (e.g. sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 2a [SERCA2a]). Other biochemical changes include excessive autophagy, inadequate angiogenesis and chronic inflammation. At the metabolic level, there is a switch from fatty acid to glucose utilisation, although glucose metabolism decreases with the progression to heart failure, thus the heart is unable to produce sufficient energy to meet the body’s metabolic demands. Together, these events lead to impaired contractile performance and contribute to the progression of heart failure (reviewed in [5–8]) (Figure 1).
Figure 1.
Key morphological and functional characteristics of pathological hypertrophy.
The signalling pathways of pathological cardiac hypertrophy are incredibly complex and are reviewed in detail elsewhere [6–8]. In addition, cross-talk between cardiomyocytes and other cardiac cell types (e.g. cardiac fibroblast) occurs that influences cardiac function and pathophysiology [9, 10]. In response to a pathological insult, factors including angiotensin II (Ang II), endothelin 1 (ET-1) and noradrenaline (NE) are released and bind to Gq protein-coupled receptors (GPCR) which in turn activate multiple downstream effectors to stimulate hypertrophy. These downstream signalling effectors of Gq include calcineurin, calcium/calmodulin-dependent protein kinase (CaMK), mitogen activated protein kinases (MAPKs), phospholipase C (PLC), protein kinases (PKC) and histone deacetylases (HDACs) [6–8]. Phosphoinositide 3 kinase (PI3K)[p110γ] is also activated by GPCR pathways and negatively regulates cardiomyocyte contractility by modulating the activity of phosphodiesterases (PDEs) and cAMP [11]. Recent studies have uncovered new findings related to the role of calcineurin and CaMKII in the heart [12], as well as the complexities surrounding activation of extracellular signal-regulated kinases (ERK1/2) at two distinct phosphorylation sites via G protein subunits [13]. Further, some of the molecules implicated in these pathways have been the targets of pharmaceutical development which will be discussed in this review.
Conventional Pharmacological Therapies
The goals for therapy of HF are ultimately to minimise risk factors, reduce symptoms, slow progression of the disease and improve survival. Multiple interventions are available to the clinician, ranging from lifestyle modifications (e.g. exercise) to surgical and device interventions. A host of clinical trials have demonstrated that careful pharmacologic management can achieve these goals in a majority of patients. Conventional pharmacological therapies include beta blockers or diuretics, and a number of agents that inhibit the deleterious effects of the Renin–Angiotensin–Aldosterone–System (RAAS).
Inhibition of the RAAS System
Vasoconstriction, sodium and water retention, aldosterone release, ventricular remodelling, and myocardial hypertrophy are well-known detrimental consequences of excessive circulating angiotensin II. A number of current medications target different points of the RAAS to attenuate these effects, including angiotensin converting enzyme inhibitors (ACE inhibitors), angiotensin II receptor antagonists (ARBs), and mineralocorticoid receptor antagonists (MRAs) (reviewed extensively elsewhere [7, 14–18]). In brief, ACE inhibitors are generally used as first-line therapy for the treatment of a number of cardiovascular and renal diseases. The beneficial effects of ACE inhibitors have been studied in thousands of patients with varying aetiologies and stages of HF (see review [18]). Angiotensin converting enzyme inhibition has repeatedly been shown to attenuate cardiac remodelling and improve heart function in patients with HF and after myocardial infarction. Angiotensin II receptor antagonists are an appealing option in patients who are intolerant to ACE inhibitors. Angiotensin II receptor antagonist therapy is considered an alternative to first line ACE inhibitor therapy due to fewer randomised, controlled trials in patients with HF as well as possible inferiority to ACE inhibitors. Several clinical trials have illustrated a reduction in morbidity and mortality in patients with left ventricular dysfunction (see [14, 18, 19]). Mineralocorticoid receptor antagonists are prescribed in addition to ACE inhibitors, ARBs and β-blockers. The first MRA developed was spironolactone and was shown to reduce hospitalisation and total mortality in patients with severe HF [20]. Further benefit for HF patients was observed with the next generation MRA, eplerenone (Bert Pitt, 2003 NEJM). However, in patients with chronic HF and preserved ejection fraction, spironolactone failed to provide any significant benefit [21].
Beta Blockers
Beta blockers are administered to control HF symptoms (such as shortness of breath, high blood pressure or weakness), which occur due to the release of excess catecholamines. Beta blockers have produced almost uniformly beneficial effects in patients with HF from various causes and in all stages. This is due in large part to their inhibition of the harmful effects of chronic activation of the sympathetic nervous system, which is a hallmark of HF. Significant effects have included improvement in survival, morbidity, ejection fraction, quality of life, remodelling, hospitalisation and incidence of sudden death (see reviews [7, 14, 22]).
Diuretics
Diuretics remain a major component of drug therapy in both hypertension and HF and are combined with an ACE inhibitor, β-blocker or MRA. They are often necessary to combat the water and sodium retention elicited by angiotensin II and aldosterone. Diuretics allow for a rapid improvement in signs and symptoms of HF but their effect on morbidity and mortality remains somewhat unclear [17, 23].
New Pharmacological Agents Targeting Pathophysiological Features of the Failing Heart
Next generation HR therapeutics are required that can improve clinical outcome. Potential pharmacological agents have been identified that target pathophysiological features of the failing heart. Many new medical therapies of heart failure have been extensively reviewed elsewhere (see [15, 16]). Here we focus on two recent therapies that have had positive results from phase II and/or phase III clinical trials.
Dual-action Inhibitor LCZ696
One of the most promising recent developments in pharmacotherapy for HF is the investigational combination drug consisting of valsartan (a RAAS blocker) and the neprilysin inhibitor sacubitril [24]. This combination is often referred to as ARNi (angiotensin receptor-neprilysin inhibitors). Angiotensin receptor-neprilysin inhibitors are designed to inhibit the deleterious effects of RAAS while preventing peptide degradation. The first experimental ARNi drug, LCZ696, was shown to be more effective than the current standard treatment (the ACE inhibitor enalapril) at preventing the progression of HF; sudden death was also reduced [24]. Further, fewer patients receiving LCZ696 were admitted to hospital for HF and had less need for intensive HF treatment compared to patients on enalapril. This study provides strong evidence to use ARNi for the treatment of HF [24], although there are growing concerns regarding under-reported adverse events in the trial.
Finerenone
Finerenone is a nonsteroidal MRA, formally known as BAY94-8862. Results from a phase IIa study in patients with chronic HF with reduced ejection fraction and renal dysfunction demonstrated that finerenone was as effective as spironolactone at decreasing NT-proBNP, but more importantly, lowered the occurrence of hyperkalaemia and worsening renal function [25]. Further, in patients with chronic HF with reduced ejection fraction as well as type 2 diabetes and/or chronic renal disease, finerenone was just as effective as eplerenone at reducing levels of NT-proBNP but also moderately reduced rates of mortality and cardiovascular hospitalisation [26]. A phase III clinical trial is now planned to compare finerenone with eplerenone in chronic HF patients and patients with diabetic kidney disease.
Targeting Cardiac β-adrenergic Signalling through GRK2 Inhibition as a Potential Therapy for HF
Paroxetine
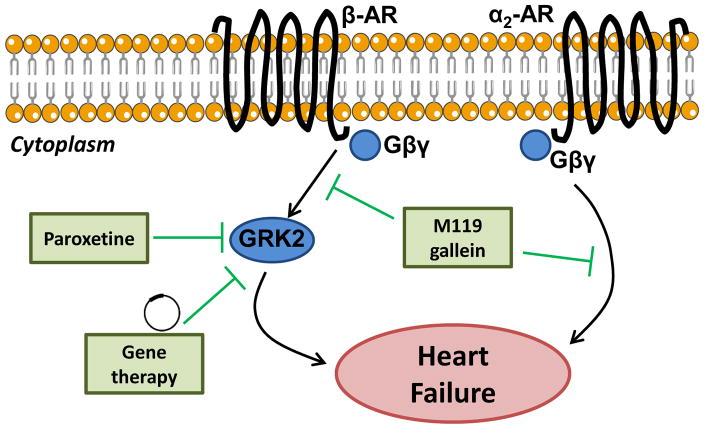
Activity and expression levels of G protein-coupled receptor kinase 2 (GRK2) are elevated in HF, contributing towards HF progression by deactivation and down-regulation of adrenergic signalling, ultimately impairing myocardial contractility and promoting myocyte cell death [27]. Inhibition of GRK2 using a viral mediated gene therapy approach has consistently improved functional and morphological parameters of the failing heart in preclinical animal models [28, 29] (Figure 1). More recently, paroxetine, an antidepressant drug, was shown to inhibit GRK2. Administration of this antidepressant drug in mice two weeks after myocardial infarction was associated with improved cardiac function, decreased fibrosis and preservation of left ventricular structure [30]. Further, paroxetine demonstrated a greater beneficial effect when compared to β-blocker therapy, the current standard-of-care treatment for HF [30]. Finally, two weeks after treatment was stopped, the improvements in cardiac function remained [30]. However, it is unlikely this particular pharmacological agent will be feasible for heart failure patients as the dose required is excessive. Nonetheless, this study demonstrates that small molecule pharmacological inhibitors of GRK2 are a potential therapy for the treatment of HF (Figure 2).
Figure 2. Novel therapies targeting cardiac β-adrenergic signalling.
A schematic representing novel therapies (green) that normalise βAR-Gβγ signalling and cardiac dysfunction.
M119 and Gallein
Other molecules that target GRK2-Gβγ include M119 and gallein [31]. In an acute mouse model of HF due to chronic stimulation of β-ARs via isoproterenol miniosomotic pumps, administration of M119 at the onset of HF was able to improve cardiac function, normalise ventricular wall thickness, decreased cardiac hypertrophy, reduce fibrosis and normalise elevated GRK2 expression [32]. Similarly, the compound gallein, a highly related and more chemically stable analogue to M119, was shown to confer protection and prevent HF progression in two preclinical models of HF [32, 33]. Specifically, the efficacy of gallein was investigated in mice with established HF due to overexpression of calsequestrin or due to pressure overload. Administration of gallein in calsequestrin transgenic mice with established HF showed that treatment prevented the progression of HF, maintained cardiac function, and partially normalised cardiac fibrosis, β-adrenergic receptor expression and HF marker gene expression (ANP, BNP, GRK2) [32]. In a clinically relevant, pressure overload mouse model of HF, gallein treatment (when delivered after the establishment of HF) improved survival, preserved cardiac function, attenuated ventricular hypertrophy and decreased fibrosis, excess adrenal catecholamine release and inflammatory markers [33]. Collectively, these studies demonstrate that small molecule inhibitors of GRK2-Gβγ can improve cardiac function and halt HF progression in small animal models of HF [34] (Figure 2). Before these molecules can proceed to clinical trials, studies in large animal models are required and understanding the systemic effects of GRK2-Gβγ inhibition is important.
Therapies that Target Impaired Cardiac Contractility
Activation of SERCA2a using Viral Vectors
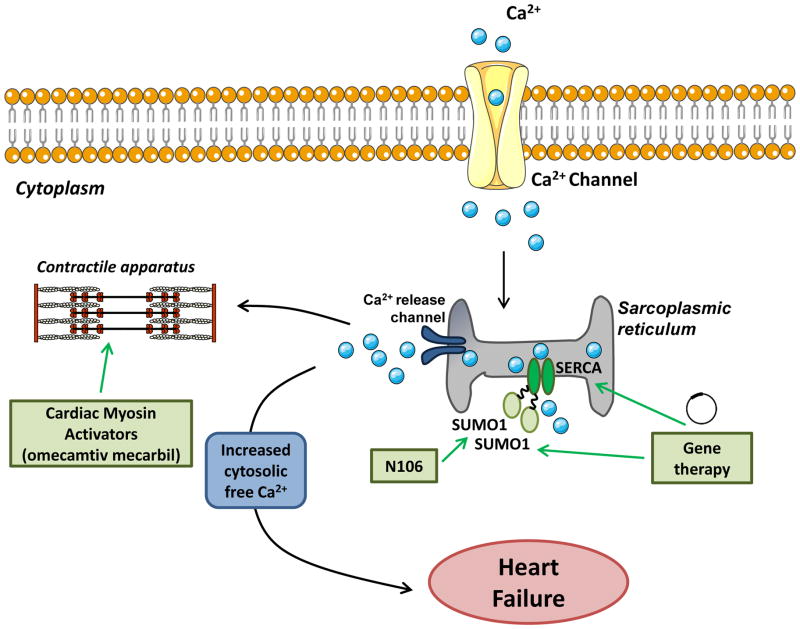
Abnormal handling of calcium ions by cardiomyocytes is a key pathophysiological mechanism in HF. The SERCA2a pump is responsible for calcium re-uptake during excitation–contraction coupling [35]. The importance of SERCA2a has been reflected in numerous studies that demonstrate reduced SERCA2a activity and expression in animal models of HF and in the human failing myocardium. Thus, therapies that can normalise cardiac SERCA2a activity are being investigated (reviewed in [36]). Much research has focussed on a gene therapy approach to deliver the SERCA2a gene to the heart. Preclinical studies in small and large HF animal models have convincingly shown significant improvement in cardiac function and remodelling as a consequence of overexpression of SERCA2a using adenoviral vectors [37–39] (Figure 3). Following successful preclinical studies, clinical trials were conducted and SERCA2a was delivered to the myocardium of patients with HF using an adeno-associated viral (AAV) vector approach. These studies demonstrated that AAV-SERCA2a gene therapy increased SERCA2a protein levels, was safe and improved cardiac function in HF patients [40]. Further, after a 12-month follow-up, patients with advanced HF displayed improved signs and symptoms of HF and cardiac function [41]. More importantly, after a three-year follow-up and long-term treatment of AAV-SERCA2a, no adverse events in patients with HF were reported and SERCA2a vector sequences were present in cardiac tissues from patients for at least 31 months [42]. However, results from a recent international clinical trial (CUPID-2) suggested that AAV-SERCA2a was no different to the placebo when added to a maximal, optimised heart failure drug and device regimen [43]. Several factors may have contributed to the primary endpoint not being achieved in this study. These include dose, less uptake of the vector, patient population, delivery method and the distribution of AAV1 neutralising antibodies is greater in European than USA populations, which can limit effective gene transfer [44]. Second- or third-generation AAV vectors designed using mosaics are being developed, and the therapeutic formulation can be optimised which will be important for future studies [43].
Figure 3. Promising therapies targeting impaired cardiac contractility.
A schematic representing gene-based or small-molecule based therapies that manipulate SERCA2a activity by directly targeting SERCA2a or by SUMOylation. Cardiac myosin activators such as omecamtiv mecarbil targets contractile apparatus of the heart increasing the contracting of the left ventricle of the heart.
Manipulating Serca2a activity by SUMOylation and the Small Molecule Activator N106
An alternate way that SERCA2a activity can be manipulated is through modification of small ubiquitin-like modifier-1 (SUMO1), which is required for preserving SERCA2a function by SUMOylation. Experimental models of HF and primary cardiomyocytes isolated from failing human hearts have decreased SUMO1 protein expression [45]. Gene transfer of SUMO1 in rodents and large animal models of HF improved cardiac function, reduced mortality and restored SERCA2a protein levels [45, 46]. More recently, the small molecule N106, identified from a screening study, was shown to increase SERCA2a SUMOylation [47]. Acute N106 treatment in mice following pressure overload with HF improved LV contractility and increased SUMOlyation of SERCA2a in the hearts of N106 treated mice [47]. Chronic studies will need to be performed to evaluate long-term efficiency and toxicology of N106. Given the recent discovery of N106, this small molecule activator represents a promising novel therapeutic approach for the treatment of HF (Figure 3).
Cardiac Myosin Activators
Decreased cardiac contractility is a central feature of systolic heart failure. Omecamtiv mecarbil is a small molecule, cardiac-specific myosin activator. It speeds up the transition of myosin from a weak actin-binding to a strong actin-binding force generating state, thereby increasing the contraction of the left ventricle [48] (Figure 3). In preclinical studies, cardiac myosin activators increased systolic function, stroke volume and cardiac output without desensitisation or increasing myocardial oxygen consumption [49, 50]. These studies appeared to support advancement of omecamtiv mecarbil into humans. Reports in healthy volunteers established the maximum tolerated dose [51], and in a Phase II trial patients with stable heart failure omecamtiv mecarbil improved cardiac function [52]. However, omecamtiv mecarbil failed to improve symptoms of acute decompensated heart failure in a second Phase II trial (ATOMIC-AHF (Acute Treatment with Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure), raising some concern for future studies, including possible effects of a myosin activator on diastolic function. Combined data from ATOMIC-HF and from the ongoing COSMIC-HF (Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure) will be utilised to determine the whether Phase II clinical trials assessing the effect of omecamtiv mecarbil in patients with acute or chronic heart failure will be pursued.
Targeting Non-coding RNAs for the Treatment of Heart Failure
microRNAs
The multifaceted pathophysiology of heart failure limits the effectiveness of current therapeutics. Thus, fine-tuning gene expression using microRNA-based drugs may represent a novel therapeutic approach for the treatment of HF. microRNAs (miRNAs) are small, non-coding RNA molecules that regulate gene expression by directing their target mRNAs for degradation or translational repression [53, 54]. These tiny RNA molecules, once thought of as “genomic junk”, play crucial roles in health and disease, including the development of cardiac hypertrophy and HF [55–57]. The actions of disease-causing miRNAs can be blocked by synthetic oligonucleotides, some of which have been shown to improve cardiac function and pathology in preclinical rodent models. miRNA replacement therapy using oligonucleotide based miRNA mimics are employed to restore the expression of miRNAs repressed in a setting of disease, but this strategy has received less attention (see review [54]).
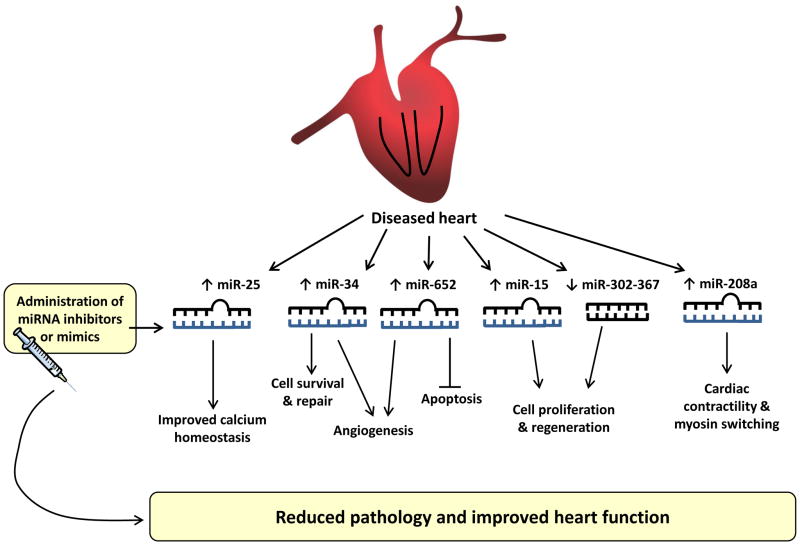
Studies have targeted miRNAs involved in calcium homeostasis, contractility, adaptive heart growth (i.e. cardiac protection) or myocyte proliferation as potential therapies for HF (Figure 4). Defective calcium handling is a key feature of HF. SERCA2a is an important regulator of calcium reuptake in the sarcoplasmic reticulum and is inhibited by miR-25. Blocking miR-25 in a mouse model of HF was associated with improved contractility, survival and restoration of SERCA2a activity [58] (Figure 4). Other studies have investigated the therapeutic potential of miR-208a. miR-208a is encoded by the α-myosin heavy chain gene and regulates the cardiac stress response and contractility. In a rodent model of salt-induced hypertension, inhibition of miR-208a was able to ameliorate hypertrophy, attenuate fibrosis and improve cardiac function [59] illustrating therapeutic potential (Figure 4).
Figure 4. miRNA-based therapies for the treatment of heart failure.
miRNAs implicated in calcium homeostasis, cardiac contractility, adaptive heart growth or myocyte proliferation are dysregulated in the diseased heart. Administration of miRNA inhibitors or mimics in preclinical models of cardiac stress reduced pathology and improved heart function. This demonstrates the therapeutic potential of miRNAs for the treatment of heart failure.
The adult mammalian heart has a limited capacity to regenerate following injury. The loss of cardiomyocytes in adult hearts is a key component of most HF pathogenesis. Thus the potential of applying miRNAs to restore lost cardiomyocytes in the damaged heart would be of considerable therapeutic value. Several studies have sought to identify miRNA regulators of cardiomyocyte proliferation [60–62]. The miR-15 family plays an important role in the cardiac regenerative response by regulating a number of cell-cycle genes including checkpoint kinase 1. Prolonged inhibition of the miR-15 family was able to promote cardiomyocyte proliferation in the infarct region of the heart [61]. The miR-17-92 cluster is among the best-studied miRNA clusters. Expression of this cluster has been shown to promote cell proliferation and suppress apoptosis in cancer cells [63]. Further, this cluster is dysregulated in cardiovascular disease. Cardiac-specific overexpression of the miR-17-92 cluster has been shown to be sufficient to induce cardiomyocyte proliferation in adult hearts and in response to cardiac injury, and this was associated with improved cardiac function [60]. More recently, the miR-302-367 cluster has been shown to be crucial for cardiomyocyte proliferation during development, and functions in part, through repression of Hippo signalling, a pathway that governs cell proliferation and organ size [62]. Transient overexpression of miR-302b/c mimics in adult mice after myocardial infarction led to an increase in cardiomyocyte proliferation and cardiac regeneration, which resulted in decreased fibrosis and improved heart function after injury [62]. Collectively, these studies provide a framework to explore miRNA-based therapies to promote heart repair and regeneration for the treatment of cardiovascular disease (Figure 4).
Whilst most investigators examine the potential of inhibiting miRNAs that have increased expression in a cardiac disease setting (see review [55]), Bernardo and colleagues therapeutically target miRNAs that display differential expression in settings of cardiac stress and protection [64–67]. Inhibition of miR-34, miR-34a, or miR-652 with miRNA inhibitors was associated with reduced pathology and improved cardiac function in mouse models of cardiac disease via mechanisms that are associated with preserved angiogenesis, decreased fibrosis and a more favourable cardiac molecular profile (e.g. attenuation of ANP, BNP gene expression) [64–66]. Further, treatment with these miRNA inhibitors was associated with upregulation of target genes implicated in angiogenesis, contractile function and cell survival [65, 66]. Thus, targeting miRNAs that are differentially regulated in settings of protection and disease represents a promising approach for the development of a novel miRNA-based therapy for the failing heart (Figure 4).
Although these preclinical studies are encouraging, a major obstacle in translating miRNA-based therapeutics into a therapy for HF patients is how to deliver miRNA inhibitor oligonucleotides to the sites where their actions are needed. Dosing, specificity and side effects should be carefully assessed when developing miRNA-based therapies (see review [54]). However, the translational efficacy and safety of miRNA-based therapeutics to patients has been shown in phase IIa clinical trials for the treatment of hepatitis C virus, where results indicate that the treatment was well tolerated in patients[68]. Thus, there is promise for the successful development of miRNA-based therapies for the treatment of HF.
Long Non-coding RNAs
In addition to small non-coding RNAs (ncRNAs), high-throughput analyses of the transcriptome have identified other functional classes of transcripts, which are larger than 200 nucleotides, collectively known as long non-coding RNAs (lncRNAs). There is increasing evidence that lncRNAs have a role in numerous cellular processes, however our understanding of lncRNAs is limited [69, 70]. In the heart, several studies have characterised the expression of lncRNAs involved in cardiac development and disease (see reviews [71–73]). Braveheart (Bvht) and Foxf1 adjacent noncoding developmental regulatory RNA (Fendrr) are two lncRNAs that have been shown to be important in cardiac development [74, 75]. Other studies have demonstrated that lncRNAs are regulated during myocardial infarction (e.g., Novlnc6), arrhythmia (e.g. KCNQ10T1) and heart failure (e.g., Mhrt), whereas others control hypertrophy (e.g. Chrf), mitochondrial homeostasis and apoptosis of cardiomyocytes (e.g. Carl) (see reviews [71–73]). Chemically modified antisense oligonucleotides called “gapmeRs” can be used to downregulate the expression of lncRNAs. As individual lncRNAs have distinct biological functions and target a specific molecular process (e.g. apoptosis, hypertrophy, angiogenesis), inhibition of their function might lead to novel treatments for HF. One of the first pharmacological inhibition studies using gapmeRs for cardiac or vascular disease was performed in an animal model of hind limb ischaemia to inhibit Malat1 [76]. Inhibition of Malat1 decreased vascularisation, suggesting Malat1 as a potential target for angiogeneic processes [76]. Further understanding of the involvement of lncRNAs in cardiac development and disease will aid in the development of new therapeutic strategies for the failing heart.
Other Small Molecule Therapies Showing Therapeutic Promise for the Treatment of HF
BGP15
BGP-15 is a hydroxemic acid derivative and is a co-inducer of the stress-inducible form of heat shock protein 70. BGP-15 has therapeutic potential for HF as it has previously been tested for safety and efficacy in human clinical trials and was shown to have no adverse cardiac effects [77]. Oral administration of BGP-15 in mice with HF and atrial fibrillation was associated with reduced episodes of arrhythmia, improved cardiac function and decreased cardiac fibrosis through increased phosphorylation of the insulin-like growth factor receptor 1 [78]. Given that BGP-15 is safe and well tolerated in humans, it represents a novel small molecule approach for the treatment of HF and atrial fibrillation.
Phosphodiesterase (PDE) Inhibitors
Altered cyclic nucleotide-mediated signalling plays a critical role in the development of cardiovascular pathology, and is regulated by the action or inhibition of PDEs [79]. Altered expression of PDEs and cyclic nucleotide signalling have been implicated in a number of cardiovascular diseases, thus the pharmacological inhibition of PDEs has gained interest as an area for drug development for the treatment of HF. Inhibitors of the cyclic AMP-specific PDE3 (including inotropic drugs such as milrinone, vesnarinone, and enoximone) augment intracellular levels of cyclic AMP, resulting in a subsequent rise in intracellular calcium and have been used to treat congestive heart failure. However, a number of clinical trials have revealed chronic treatment with PDE3 inhibitors in heart failure patients is associated with increased mortality. Thus PDE3 inhibitors are now only used in patients with severe heart failure or those awaiting cardiac transplant (see review [79]). PDE5 expression is highly upregulated in the heart in response to pathological conditions and modulates cyclic GMP activity in the heart. PDE5 inhibitors such as sildenafil have been investigated as potential therapeutic agents in preclinical models (see review [79]). In a mouse model of myocardial pressure overload, inhibition of PDE5 with sildenafil attenuated cardiac hypertrophy and fibrosis, preserved cardiac function, and was associated with deactivation of various hypertrophy signalling cascades [80]. Sildenafil has been tested in a number of clinical trials indicating that PDE5 inhibitor treatment does appear to have beneficial effects on several forms of HF, including improved cardiac performance, reduced levels of inflammatory markers and a tolerable safety profile (see review [79]).
HDAC inhibitors
Both class I and class IIa HDACs have been identified as important regulators of cardiac remodelling and potential therapeutic targets for the treatment of HF [81, 82]. Inhibition of HDACs by administration of pan-HDAC inhibitors (such as trichostatin A or valproic acid), has been shown to prevent, attenuate or reverse LV hypertrophy in preclinical models of load-induced heart growth [83]. More recently, administration of the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA), which has been approved by the FDA for the treatment of lymphoma, improved systolic function in rabbits subjected to ischaemia–reperfusion injury [84]. Given that HDAC inhibitors are capable of blunting adverse remodelling, and several HDAC inhibitors have been approved by the FDA for oncology therapy, HDAC inhibitor therapy may emerge as a novel therapy for targeting ventricular hypertrophy and HF.
Protease-activated Receptor 1 (PAR1) Antagonists
Acute coronary syndrome (ACS) is a term given to a group of heart conditions including MI or unstable angina. These conditions result from reduced blood flow in the coronary arteries, leading to decreased heart function. People with ACS are typically treated with aspirin or clopidogrel to prevent reoccurrence of ischaemic events. However, newer anti-platelet drugs have been developed and tested, such as vorapaxar, which is a PAR1 antagonist. Vorapaxar has been trialled as a dual antiplatelet therapy and found to be effective along with aspirin or clopidogrel in reducing the risk of cardiovascular death, MI or stroke in patients with ACS [85]. However, due to an increased risk in severe bleeding, it is not recommended for patients with a history of stroke. In 2014, U.S. Food and Drug Administration approved vorapaxar for the reduction of thrombotic cardiovascular events in patients with a history of MI or in patients with peripheral arterial disease.
The Disparity between Phase II and Phase III Clinical Trials
A notable feature of drug development programs for the treatment of HF has been the discrepancy between the positive efficacy outcomes from Phase II clinical trials and lack of efficacy and associated safety concerns in Phase III clinical trials [86]. This has been observed for a number of agents including spironolactone, tezosentan, tolvaptan, rolofylline, levosimendan, SERCA2A-AAV and nesiritide [21, 86, 87]. A number of explanations regarding unexpected negative results have been reported. Possible explanations include inadequate dose-response studies; the right patient population, drug and endpoint may not have been carefully selected; suboptimal quality of phase II studies or chance findings in underpowered small trials; overly optimistic interpretation of study results; drug interactions difficult to foresee; and that the pharmacokinetics and pharmacodynamics of new drugs are often investigated in healthy volunteers or in patients with mild HF, but mortality trials are conducted in patients with severe HF in which the drug may act, interact or be metabolised quite differently [86, 88]. A number of new HF therapies are being developed and explored, some which have been discussed in this review. Thus, increased attention to clinical trial development is required. Selection of optimal clinical endpoints and patient population are paramount and understanding the drug’s mechanism(s) of action will hopefully turn potential HF therapies into a growing reality.
Conclusion
Heart failure is the end result of a variety of disease states, including coronary artery disease and hypertension. Heart failure remains a challenge to treat, and the incidence continues to rise with an ageing population. With current HF drugs largely delaying HF progression, some of the new therapeutic approaches discussed in this review may show potential in improving heart function and reversing adverse cardiac remodelling.
Acknowledgments
BC Blaxall is supported by HL129772, HL119810, HL069779-11 and HL132551.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braunwald E. The war against heart failure: the Lancet lecture. The Lancet. 2015;385:812–24. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics—2015 Update: A Report From the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Eisen HJ. Epidemiology of Heart Failure and Scope of the Problem. Cardiol Clin. 2014;32:1–8. doi: 10.1016/j.ccl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Braunwald E. Research Advances in Heart Failure: A Compendium. Circ Res. 2013;113:633–45. doi: 10.1161/CIRCRESAHA.113.302254. [DOI] [PubMed] [Google Scholar]
- 5.Bernardo BC, Ooi JYY, McMullen JR. The yin and yang of adaptive and maladaptive processes in heart failure. Drug Discovery Today: Therapeutic Strategies. 2012;9:e163–e72. [Google Scholar]
- 6.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Tham YK, Bernardo BC, Ooi JY, Weeks KL, McMullen JR. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch Toxicol. 2015;89:1401–38. doi: 10.1007/s00204-015-1477-x. [DOI] [PubMed] [Google Scholar]
- 8.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest. 2013;123:37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin ML, Blaxall BC. Cardiac intercellular communication: are myocytes and fibroblasts fair-weather friends? J Cardiovasc Transl Res. 2012;5:768–82. doi: 10.1007/s12265-012-9404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamo T, Akazawa H, Komuro I. Cardiac Nonmyocytes in the Hub of Cardiac Hypertrophy. Circ Res. 2015;117:89–98. doi: 10.1161/CIRCRESAHA.117.305349. [DOI] [PubMed] [Google Scholar]
- 11.Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, et al. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 2004;118:375–87. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Kreusser MM, Lehmann LH, Keranov S, Hoting MO, Oehl U, Kohlhaas M, et al. Cardiac CaM Kinase II genes delta and gamma contribute to adverse remodeling but redundantly inhibit calcineurin-induced myocardial hypertrophy. Circulation. 2014;130:1262–73. doi: 10.1161/CIRCULATIONAHA.114.006185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruppert C, Deiss K, Herrmann S, Vidal M, Oezkur M, Gorski A, et al. Interference with ERKThr188 phosphorylation impairs pathological but not physiological cardiac hypertrophy. Proc Natl Acad Sci U S A. 2013;110:7440–5. doi: 10.1073/pnas.1221999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falvey J, Blaxall BC. Chapter 7: Pharmacologic Therapy. In: Bisognano JD, Baker ML, Earley MB, editors. Manual of Heart Failure Management. Springer Dordrecht Heidelberg London New York: Springer; 2009. pp. 85–96. [Google Scholar]
- 15.von Lueder TG, Krum H. New medical therapies for heart failure. Nat Rev Cardiol. 2015 doi: 10.1038/nrcardio.2015.137. advance online publication. [DOI] [PubMed] [Google Scholar]
- 16.Tamargo J, Lopez-Sendon J. Novel therapeutic targets for the treatment of heart failure. Nat Rev Drug Discov. 2011;10:536–55. doi: 10.1038/nrd3431. [DOI] [PubMed] [Google Scholar]
- 17.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:1810–52. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 18.Lang CC, Struthers AD. Targeting the renin-angiotensin-aldosterone system in heart failure. Nat Rev Cardiol. 2013;10:125–34. doi: 10.1038/nrcardio.2012.196. [DOI] [PubMed] [Google Scholar]
- 19.Maron BA, Leopold JA. Aldosterone Receptor Antagonists: Effective but Often Forgotten. Circulation. 2010;121:934–9. doi: 10.1161/CIRCULATIONAHA.109.895235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 21.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 22.Frishman WH. Beta-Adrenergic Blockers. Circulation. 2003;107:e117–e9. doi: 10.1161/01.CIR.0000070983.15903.A2. [DOI] [PubMed] [Google Scholar]
- 23.ter Maaten JM, Valente MAE, Damman K, Hillege HL, Navis G, Voors AA. Diuretic response in acute heart failure - pathophysiology, evaluation, and therapy. Nat Rev Cardiol. 2015 doi: 10.1038/nrcardio.2014.215. [DOI] [PubMed] [Google Scholar]
- 24.Packer M, McMurray JJV, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin Receptor Neprilysin Inhibition Compared With Enalapril on the Risk of Clinical Progression in Surviving Patients With Heart Failure. Circulation. 2015;131:54–61. doi: 10.1161/CIRCULATIONAHA.114.013748. [DOI] [PubMed] [Google Scholar]
- 25.Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94–8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34:2453–63. doi: 10.1093/eurheartj/eht187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitt B, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, et al. Rationale and design of MinerAlocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF): a randomized study of finerenone vs. eplerenone in patients who have worsening chronic heart failure with diabetes and/or chronic kidney disease. Eur J Heart Fail. 2015;17:224–32. doi: 10.1002/ejhf.218. [DOI] [PubMed] [Google Scholar]
- 27.Woodall MC, Ciccarelli M, Woodall BP, Koch WJ. G protein-coupled receptor kinase 2: a link between myocardial contractile function and cardiac metabolism. Circ Res. 2014;114:1661–70. doi: 10.1161/CIRCRESAHA.114.300513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raake PW, Schlegel P, Ksienzyk J, Reinkober J, Barthelmes J, Schinkel S, et al. AAV6.betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur Heart J. 2013;34:1437–47. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, et al. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schumacher SM, Gao E, Zhu W, Chen X, Chuprun JK, Feldman AM, et al. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci Transl Med. 2015;7:277ra31. doi: 10.1126/scitranslmed.aaa0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamal FA, Smrcka AV, Blaxall BC. Taking the heart failure battle inside the cell: small molecule targeting of Gbetagamma subunits. J Mol Cell Cardiol. 2011;51:462–7. doi: 10.1016/j.yjmcc.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casey LM, Pistner AR, Belmonte SL, Migdalovich D, Stolpnik O, Nwakanma FE, et al. Small Molecule Disruption of Gβγ Signaling Inhibits the Progression of Heart Failure. Circ Res. 2010;107:532–9. doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamal FA, Mickelsen DM, Wegman KM, Travers JG, Moalem J, Hammes SR, et al. Simultaneous adrenal and cardiac g-protein-coupled receptor-gβγ inhibition halts heart failure progression. J Am Coll Cardiol. 2014;63:2549–57. doi: 10.1016/j.jacc.2014.02.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belmonte SL, Blaxall BC. G protein coupled receptor kinases as therapeutic targets in cardiovascular disease. Circ Res. 2011;109:309–19. doi: 10.1161/CIRCRESAHA.110.231233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lou Q, Janardhan A, Efimov IR. Remodeling of calcium handling in human heart failure. Adv Exp Med Biol. 2012;740:1145–74. doi: 10.1007/978-94-007-2888-2_52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fragoso-Medina J, Zarain-Herzberg A. SERCA2a: its role in the development of heart failure and as a potential therapeutic target. Research Reports in Clinical Cardiology. 2014;5:43–55. [Google Scholar]
- 37.Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15:1550–7. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- 38.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–9. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 39.Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci U S A. 2000;97:793–8. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–81. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–13. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K, Greenberg B, Jessup M, et al. Long-Term Effects of AAV1/SERCA2a Gene Transfer in Patients With Severe Heart Failure: Analysis of Recurrent Cardiovascular Events and Mortality. Circ Res. 2014;114:101–8. doi: 10.1161/CIRCRESAHA.113.302421. [DOI] [PubMed] [Google Scholar]
- 43.Ratner M. Heart failure gene therapy disappoints but experts keep the faith. Nat Biotech. 2015;33:573–4. doi: 10.1038/nbt0615-573a. [DOI] [PubMed] [Google Scholar]
- 44.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide Epidemiology of Neutralizing Antibodies to Adeno-Associated Viruses. J Infect Dis. 2009;199:381–90. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, et al. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477:601–5. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tilemann L, Lee A, Ishikawa K, Aguero J, Rapti K, Santos-Gallego C, et al. SUMO-1 gene transfer improves cardiac function in a large-animal model of heart failure. Sci Transl Med. 2013;5:211ra159. doi: 10.1126/scitranslmed.3006487. [DOI] [PubMed] [Google Scholar]
- 47.Kho C, Lee A, Jeong D, Oh JG, Gorski PA, Fish K, et al. Small-molecule activation of SERCA2a SUMOylation for the treatment of heart failure. Nat Commun. 2015;6 doi: 10.1038/ncomms8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malik FI, Morgan BP. Cardiac myosin activation part 1: From concept to clinic. J Mol Cell Cardiol. 2011;51:454–61. doi: 10.1016/j.yjmcc.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Shen YT, Malik FI, Zhao X, Depre C, Dhar SK, Abarzua P, et al. Improvement of cardiac function by a cardiac Myosin activator in conscious dogs with systolic heart failure. Circ Heart Fail. 2010;3:522–7. doi: 10.1161/CIRCHEARTFAILURE.109.930321. [DOI] [PubMed] [Google Scholar]
- 50.Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–43. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, et al. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet. 2011;378:667–75. doi: 10.1016/S0140-6736(11)61219-1. [DOI] [PubMed] [Google Scholar]
- 52.Cleland JGF, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJV, Lang CC, et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. The Lancet. 2011;378:676–83. doi: 10.1016/S0140-6736(11)61126-4. [DOI] [PubMed] [Google Scholar]
- 53.Bernardo BC, Charchar FJ, Lin RCY, McMullen JR. A MicroRNA Guide for Clinicians and Basic Scientists: Background and Experimental Techniques. Heart, Lung and Circulation. 2012;21:131–42. doi: 10.1016/j.hlc.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Bernardo BC, Ooi JY, Lin RC, McMullen JR. miRNA therapeutics: a new class of drugs with potential therapeutic applications in the heart. Future Med Chem. 2015;7:1751–69. doi: 10.4155/fmc.15.107. [DOI] [PubMed] [Google Scholar]
- 55.Hata A. Functions of MicroRNAs in Cardiovascular Biology and Disease. Annu Rev Physiol. 2013;75:69–93. doi: 10.1146/annurev-physiol-030212-183737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumarswamy R, Thum T. Non-coding RNAs in Cardiac Remodeling and Heart Failure. Circ Res. 2013;113:676–89. doi: 10.1161/CIRCRESAHA.113.300226. [DOI] [PubMed] [Google Scholar]
- 57.Leite-Moreira AM, Lourenco AP, Falcao-Pires I, Leite-Moreira AF. Pivotal role of microRNAs in cardiac physiology and heart failure. Drug Discov Today. 2013;18:1243–9. doi: 10.1016/j.drudis.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 58.Wahlquist C, Jeong D, Rojas-Munoz A, Kho C, Lee A, Mitsuyama S, et al. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature. 2014;508:531–5. doi: 10.1038/nature13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, et al. Therapeutic Inhibition of miR-208a Improves Cardiac Function and Survival During Heart Failure/Clinical Perspective. Circulation. 2011;124:1537–47. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J, Huang ZP, Seok HY, Ding J, Kataoka M, Zhang Z, et al. mir-17–92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ Res. 2013;112:1557–66. doi: 10.1161/CIRCRESAHA.112.300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci U S A. 2013;110:187–92. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian Y, Liu Y, Wang T, Zhou N, Kong J, Chen L, et al. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci Transl Med. 2015;7:279ra38. doi: 10.1126/scitranslmed.3010841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;20:1603–14. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernardo BC, Gao XM, Tham YK, Kiriazis H, Winbanks CE, Ooi JY, et al. Silencing of miR-34a attenuates cardiac dysfunction in a setting of moderate, but not severe, hypertrophic cardiomyopathy. PLoS ONE. 2014;9:e90337. doi: 10.1371/journal.pone.0090337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bernardo BC, Gao XM, Winbanks CE, Boey EJ, Tham YK, Kiriazis H, et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci U S A. 2012;109:17615–20. doi: 10.1073/pnas.1206432109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bernardo BC, Nguyen SS, Winbanks CE, Gao X-M, Boey EJH, Tham YK, et al. Therapeutic silencing of miR-652 restores heart function and attenuates adverse remodeling in a setting of established pathological hypertrophy. FASEB J. 2014;28:5097–110. doi: 10.1096/fj.14-253856. [DOI] [PubMed] [Google Scholar]
- 67.Lin RCY, Weeks KL, Gao X-M, Williams RBH, Bernardo BC, Kiriazis H, et al. PI3K(p110α) protects against myocardial infarction-induced heart failure/Identification of PI3K-regulated miRNAs and mRNAs. Arterioscler Thromb Vasc Biol. 2010;30:724–32. doi: 10.1161/ATVBAHA.109.201988. [DOI] [PubMed] [Google Scholar]
- 68.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 69.Jiang X, Ning Q. The emerging roles of long noncoding RNAs in common cardiovascular diseases. Hypertens Res. 2015;38:375–9. doi: 10.1038/hr.2015.26. [DOI] [PubMed] [Google Scholar]
- 70.Papait R, Kunderfranco P, Stirparo GG, Latronico MV, Condorelli G. Long noncoding RNA: a new player of heart failure? J Cardiovasc Transl Res. 2013;6:876–83. doi: 10.1007/s12265-013-9488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thum T, Condorelli G. Long noncoding RNAs and microRNAs in cardiovascular pathophysiology. Circ Res. 2015;116:751–62. doi: 10.1161/CIRCRESAHA.116.303549. [DOI] [PubMed] [Google Scholar]
- 72.Uchida S, Dimmeler S. Long Noncoding RNAs in Cardiovascular Diseases. Circ Res. 2015;116:737–50. doi: 10.1161/CIRCRESAHA.116.302521. [DOI] [PubMed] [Google Scholar]
- 73.Devaux Y, Zangrando J, Schroen B, Creemers EE, Pedrazzini T, Chang CP, et al. Long noncoding RNAs in cardiac development and ageing. Nat Rev Cardiol. 2015;12:415–25. doi: 10.1038/nrcardio.2015.55. [DOI] [PubMed] [Google Scholar]
- 74.Grote P, Wittler L, Hendrix D, Koch F, Wahrisch S, Beisaw A, et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell. 2013;24:206–14. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–83. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Michalik KM, You X, Manavski Y, Doddaballapur A, Zornig M, Braun T, et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. 2014;114:1389–97. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 77.Literati-Nagy B, Kulcsar E, Literati-Nagy Z, Buday B, Peterfai E, Horvath T, et al. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res. 2009;41:374–80. doi: 10.1055/s-0028-1128142. [DOI] [PubMed] [Google Scholar]
- 78.Sapra G, Tham YK, Cemerlang N, Matsumoto A, Kiriazis H, Bernardo BC, et al. The small-molecule BGP-15 protects against heart failure and atrial fibrillation in mice. Nat Commun. 2014;5:5705. doi: 10.1038/ncomms6705. [DOI] [PubMed] [Google Scholar]
- 79.Knight W, Yan C. Therapeutic potential of PDE modulation in treating heart disease. Future Med Chem. 2013;5:1607–20. doi: 10.4155/fmc.13.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–22. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 81.Lehmann LH, Worst BC, Stanmore DA, Backs J. Histone deacetylase signaling in cardioprotection. Cell Mol Life Sci. 2014;71:1673–90. doi: 10.1007/s00018-013-1516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McKinsey TA, Kass DA. Small-molecule therapies for cardiac hypertrophy: moving beneath the cell surface. Nat Rev Drug Discov. 2007;6:617–35. doi: 10.1038/nrd2193. [DOI] [PubMed] [Google Scholar]
- 83.Kong Y, Tannous P, Lu G, Berenji K, Rothermel BA, Olson EN, et al. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation. 2006;113:2579–88. doi: 10.1161/CIRCULATIONAHA.106.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, et al. Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation. 2014;129:1139–51. doi: 10.1161/CIRCULATIONAHA.113.002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scirica BM, Bonaca MP, Braunwald E, De Ferrari GM, Isaza D, Lewis BS, et al. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2 degrees P-TIMI 50 trial. Lancet. 2012;380:1317–24. doi: 10.1016/S0140-6736(12)61269-0. [DOI] [PubMed] [Google Scholar]
- 86.Vaduganathan M, Greene SJ, Ambrosy AP, Gheorghiade M, Butler J. The disconnect between phase II and phase III trials of drugs for heart failure. Nat Rev Cardiol. 2013;10:85–97. doi: 10.1038/nrcardio.2012.181. [DOI] [PubMed] [Google Scholar]
- 87.Felker GM, Maisel AS. A global rank end point for clinical trials in acute heart failure. Circ Heart Fail. 2010;3:643–6. doi: 10.1161/CIRCHEARTFAILURE.109.926030. [DOI] [PubMed] [Google Scholar]
- 88.van Veldhuisen DJ, Poole-Wilson PA. The underreporting of results and possible mechanisms of ‘negative’ drug trials in patients with chronic heart failure. Int J Cardiol. 2001;80:19–27. doi: 10.1016/s0167-5273(01)00447-8. [DOI] [PubMed] [Google Scholar]