Summary
Background
With continued roll-out of antiretroviral therapy (ART) in resource-limited settings, evidence is emerging of increasing levels of transmitted drug-resistant HIV. We aimed to compare the effectiveness and cost-effectiveness of different potential public health responses to substantial levels of transmitted drug resistance.
Methods
We created a model of HIV transmission, progression, and the effects of ART, which accounted for resistance generation, transmission, and disappearance of resistance from majority virus in the absence of drug pressure. We simulated 5000 ART programmatic scenarios with different prevalence levels of detectable resistance in people starting ART in 2017 (t0) who had not previously been exposed to antiretroviral drugs. We used the model to predict cost-effectiveness of various potential changes in policy triggered by different prevalence levels of resistance to non-nucleoside reverse transcriptase inhibitors (NNRTIs) measured in the population starting ART.
Findings
Individual-level resistance testing before ART initiation was not generally a cost-effective option, irrespective of the cost-effectiveness threshold. At a cost-effectiveness threshold of US$500 per quality-adjusted life-year (QALY), no change in policy was cost effective (ie, no change in policy would involve paying less than $500 per QALY gained), irrespective of the prevalence of pretreatment NNRTI resistance, because of the increased cost of the policy alternatives. At thresholds of $1000 or higher, and with the prevalence of pretreatment NNRTI resistance greater than 10%, a policy to measure viral load 6 months after ART initiation became cost effective. The policy option to change the standard first-line treatment to a boosted protease inhibitor regimen became cost effective at a prevalence of NNRTI resistance higher than 15%, for cost-effectiveness thresholds greater than $2000.
Interpretation
Cost-effectiveness of potential policies to adopt in response to different levels of pretreatment HIV drug resistance depends on competing budgetary claims, reflected in the cost-effectiveness threshold. Results from our model will help inform WHO recommendations on monitoring of HIV drug resistance in people starting ART.
Funding
WHO (with funds provided by the Bill & Melinda Gates Foundation), CHAIN (European Commission).
Introduction
Of about 10 million people on antiretroviral therapy (ART) worldwide, most are on first-line non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimens.1 Drug resistance associated with virological failure of such regimens will gradually emerge, which has the potential to lead to extensive transmission of drug-resistant HIV, compromising the efficacy of future treatment.2 The fact that ART is currently delivered in most settings without regular testing of viral load to detect virological failure increases this concern. WHO has developed surveillance strategies for monitoring levels of transmitted drug resistance,3–5 and evidence suggests that levels of transmitted drug resistance are rising, albeit slowly.5–7 For reasons of feasibility and public health relevance, WHO now recommends surveys of drug resistance in populations starting ART to estimate the prevalence of pretreatment drug resistance. In the surveys, previous exposure to antiretroviral drugs is assessed at the time of treatment initiation, and resistance prevalence is calculated among populations with no previous exposure to antiretroviral drugs. Since the population assessed in WHO surveys is most probably ART-naive, any pretreatment resistance identified is probably transmitted drug resistance rather than resistance acquired from drug exposure.
When substantial levels of resistance to NNRTIs are detected in HIV-positive populations beginning ART with no previous exposure to antiretroviral drugs, several potential policy responses are available. First is to change the recommended national standard first-line regimen to a boosted protease inhibitor-based regimen. Second is to introduce individual-level pretreatment resistance testing to optimise selection of initial ART regimens on a case-by-case basis. Third is to begin routine monitoring of viral load for people on ART (eg, 6 months after initiation of ART and every 12 months thereafter, as recommended by WHO);8 this approach is currently not widely adopted in most low-income and middle-income countries. Measurement of viral load allows earlier detection of virological failure than is the case with CD4 cell count or clinical monitoring, thus allowing a more prompt switch to second-line ART without unnecessary accumulation of drug resistance. Moreover, patients found to have a non-suppressed viral load can undergo targeted adherence interventions.9,10 A fourth policy option, which would be cheaper and perhaps more feasible than regular viral load testing, could be to measure viral load at the 6 month timepoint after ART initiation, with a confirmatory second test at 1 year after treatment initiation if the 6 month viral load is more than 1000 copies per mL, but with monitoring of CD4 cell count thereafter. This strategy would allow detection of virological failure that has happened early after ART initiation (eg, resulting from the presence of pretreatment resistance) allowing a prompt switch to a second-line regimen.
Here, we use an individual-based simulation model to compare the effectiveness and cost-effectiveness of the different public health responses described above when triggered by different prevalence levels of pretreatment resistance.
Methods
HIV Synthesis transmission model
HIV Synthesis is an individual-based stochastic model of heterosexual transmission, progression, and treatment of HIV infection within a southern African context, which has been described previously.11–13 Resistance is modelled in terms of the presence or absence of specific mutations, with consideration given to the effect of such mutations on virus susceptibility to specific drugs. Distinction is made for every mutation as to whether it is only present in minority virus (assumed not transmissible) or if it is present in majority virus (assumed transmissible). Additional results and methods used for this analysis are described in the appendix (pp 2–5). A detailed description of the model is available else where (appendix pp 9–12).12 The model has been used to contribute to several HIV Modelling Consortium joint modelling projects.14
Programmatic scenarios modelled
Every simulation run of our model generates an HIV epidemic with specific programmatic characteristics. To use our model to simulate a range of programmatic scenarios with various levels of ongoing transmitted drug resistance at t0 (the timepoint at which the policy decision is being made, arbitrarily designated as the year 2017), every time the model was run we simultaneously varied model parameters (such as the population adherence profile) that determine the level of transmitted drug resistance present in a population. By doing this process 5000 times, we generated a range of programmatic scenarios (table 1). To ensure that we included scenarios with a high prevalence of transmitted drug resistance, we used a distribution of adherence patterns, which includes some with a high proportion of people with poor adherence.
Table 1.
Characteristics of simulated modelled HIV programmatic scenarios at t0
| Median (90% range) over programmatic scenarios | |
|---|---|
| HIV prevalence (age 15–65 years) | 18% (14–22) |
|
| |
| HIV incidence (per 100 person-years) | 1·1 (0·7–1·6) |
|
| |
| People starting ART | |
| NNRTI resistance* | 11% (5–22) |
| NNRTI resistance, excluding women who previously took antiretroviral drugs for prevention of mother-to-child transmission* | 11% (4–22) |
| Any resistance, excluding women who previously took antiretroviral drugs for prevention of mother-to-child transmission and resistance* | 12% (5–24) |
|
| |
| New HIV infections | |
| With transmitted drug resistance* | 29% (12–49) |
| With NNRTI resistance* | 26% (11–46) |
|
| |
| HIV-positive people | |
| Diagnosed | 84% (80–86) |
| Started on ART | 54% (42–62) |
| Currently on ART | 46% (34–54) |
|
| |
| People in need of ART, according to criteria for need of ART based on CD4 count <350 cells per μL | |
| On ART | 60% (47–68) |
| Ever started ART | 73% (61–79) |
|
| |
| People in need of ART, according to criteria for need of ART based on CD4 count <500 cells per μL | |
| On ART | 51% (39–60) |
| Ever started ART | 63% (50–70) |
|
| |
| Of people remaining on ART 1 year after starting ART, proportion with viral suppression† | 69% (42–83) |
|
| |
| Of people on ART, proportion with viral suppression† | 76% (54–85) |
|
| |
| Of people on ART, proportion now on second-line (bPI) regimen | 8% (6–11) |
|
| |
| Of all people with HIV, proportion with unsuppressed viral load‡ | 63% (53–81) |
| Of all people with unsuppressed viral load, proportion with resistance*§ | 23% (13–39) |
|
| |
| People with fewer than three fully active drugs¶ when starting ART | 14% (6–28) |
Data are median proportion (90% range), unless otherwise stated. 5000 programmatic scenarios were generated. ART=antiretroviral treatment. bPI=ritonavir-boosted protease inhibitor. NNRTI=non-nucleoside reverse transcriptase inhibitor.
In the majority virus.
<500 copies per mL.
Irrespective of whether diagnosed and in care. The number of adults alive in 2017 in the population is a median of 36 500 (90% range 35 500–37 500).
Whether diagnosed or not.
A drug was not fully active if a resistance mutation to that drug was present in the minority or majority virus.
In our model, we assumed that ART had been monitored up to t0 by use of the CD4 cell count. We also assumed that the rate of switching to second-line regimens in patients who fulfilled the treatment failure definition increased at t0 from 0·03 per 3 months (reflecting that switching to second-line regimens has been slow to take place in many settings) to 0·20 per 3 months, to ensure that the comparison of strategies is done in the context of them being implemented.
At t0, for every programmatic scenario, we predicted outcomes over 15 years from 2017, for the following policy options, according to the current level of NNRTI resistance in people starting ART without previous exposure to antiretroviral drugs (so, for example, women who received antiretroviral drugs previously for the prevention of mother-to-child transmission would not be included). The first option was no change in policy (ie, NNRTI-based first-line ART and routine monitoring of CD4 cell count). Second was a change of the standard NNRTI-based regimen to a boosted protease inhibitor-based first-line regimen, with use of a different boosted protease inhibitor for second-line treatment and replacement of tenofovir with zidovudine, or vice versa). The third option was individual-level resistance testing before ART initiation to detect key NNRTI mutations, to inform whether use of an NNRTI-based or boosted protease inhibitor-based regimen would be best as first-line treatment. Fourth was introduction of routine monitoring of viral load (6 months and 12 months after initiation of ART and then every year) and concomitant cessation of CD4 cell count monitoring. The final option was to test viral load 6 months after ART initiation, with a confirmatory second test at 12 months if viral load was greater than 1000 copies per mL, with CD4 cell count monitoring thereafter. In this final scenario, if the viral load was greater than 1000 copies per mL on the confirmatory test, patients were switched to a second-line regimen.
Economic analysis
All the evaluated alternatives for programmatic intervention have different implications in terms of health benefits and costs, taking a public health systems perspective. We estimated health benefits on the basis of quality-adjusted life-years (QALYs). We based cost estimates (in US$) on resource use in delivery of the policies and associated unit costs (all unit costs include supply chain, transport, human resources, etc, as relevant), with both costs and health benefits discounted to their present value with a 3·5% yearly rate. We compared expected costs and health outcomes (QALYs) associated with every policy alternative to ascertain the alternative that was likely to represent the best value from available resources. The net monetary benefit represents a way to summarise in one measure the benefits and costs of a given policy by putting both on a single scale, that of costs. Net monetary benefit is expressed as QALYs resulting from a policy multiplied by a cost-effectiveness threshold (thus, converting QALYs into costs by using the cost-effectiveness threshold) less the costs resulting from that policy. The cost-effectiveness threshold represents the opportunity costs of resources needed to fund the intervention, in terms of the health gains those resources could generate if used for alternative purposes.14,15 To summarise results, we indicated the policy option that (over the programmatic scenarios for the given prevalence of NNRTI resistance at t0 in people starting ART and for a given cost-effectiveness threshold) most often generated the highest net monetary benefit and that was expected to maximise health gains in the population.16,17
A central concern to informing efficient allocation of resources to the policy alternatives is what the cost-effectiveness threshold should be in particular countries, with differing levels of resource availability and varying claims on limited budgets. Ultimately, the policy decision needs assessment of how resources can otherwise generate health gains in the population. Guidance from WHO recommends a threshold equal to a country’s gross domestic product (GDP) per person.18 However, other analysts have suggested that this amount is too high and risks diverting resources away from greater priorities (eg, continued expansion of ART coverage).14,19,20 The most thorough estimation of a threshold for a particular country comes from the UK and suggests a level equivalent to 0·52 of GDP per person.21 For more poorly resourced health systems in sub-Saharan Africa, a cost-effectiveness threshold of $500 or lower is probably realistic, since many interventions offering health gains at this amount or less remain unfunded.14,22 We did several sensitivity analyses around costing of viral load, resistance testing, and second-line regimens of boosted protease inhibitors.
Role of the funding source
This work was done as a collaborative exercise led by WHO. As such, colleagues at WHO were closely involved in all aspects of the design of the investigation and interpretation of results. WHO themselves received a 7 year grant from the Bill & Melinda Gates Foundation to support a wide range of activities; the work described here is one of the many deliverables under this grant. This work was not commissioned or influenced by the Foundation but was proposed and initiated by WHO independently. The European Commission had no role in the study. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
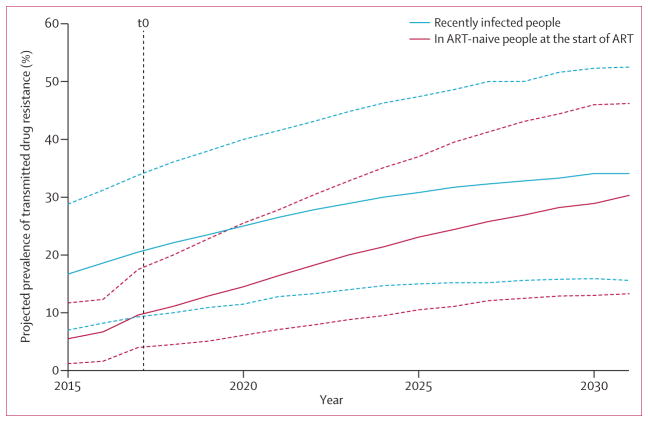
Table 1 presents data for the range of generated programmatic scenarios at t0. The change in HIV prevalence predicted over the 15 year period is shown in the appendix (p 8). At t0, the median proportion of people recently infected with NNRTI-resistant HIV is 26% (90% range across programmatic scenarios, 11–46), whereas in people starting ART the proportion with NNRTI resistance in majority virus is lower (11%, 5–22), because people starting ART have generally been infected some years before, when levels of transmitted drug resistance were lower and because drug-resistance mutations do not persist indefinitely in majority virus. These relatively high average levels of resistance are attributable to the fact that we are considering a range of potential future scenarios, including those in which levels of transmitted drug resistance have reached very high levels. Figure 1 shows the projected increase in transmitted drug resistance, with a higher level of drug resistance at infection than is present at the start of ART, but with this difference closing over time.
Figure 1. Projected trend over time in transmitted drug resistance to NNRTIs.
Projected trend in prevalence of transmitted drug resistance to NNRTIs over 15 years in recently infected people (blue lines) and in ART-naive people at the start of treatment (red lines) with no change in policy, restricted to programmatic scenarios in which t0 levels of NNRTI resistance in ART-naive people at the start of treatment are less than 10%. The t0 level of NNRTI resistance in people starting ART is calculated as the mean over all four quarters of the year 2016. The plotted values are the median (solid line) and 90% range (dotted line) over all quarters and all programmatic scenarios for every given year (which is why the upper 90% limit is higher than 10% at t0). ART=antiretroviral treatment. NNRTI=non-nucleoside reverse transcriptase inhibitor.
Table 2 shows the range of outcomes 15 years after t0 of the potential new policies, according to the level of NNRTI resistance at t0 in people starting ART. In view of our assumed rate of switch to a second-line boosted protease inhibitor-based regimen after first-line failure (0·2 per 3 months), the proportion of people on ART who are on a boosted protease inhibitor is projected to rise to 18%, even for programmatic scenarios in which the prevalence of NNRTI resistance at t0 in people starting ART is less than 5%, and to much higher levels for situations in which the prevalence of NNRTI resistance at t0 is 5% or greater in people starting ART. As expected, the proportion of people using a boosted protease inhibitor is highest when a change to this regimen is recommended as standard first-line treatment. The policy that most effectively curbs the increase in NNRTI resistance is to change to a boosted protease inhibitor-based regimen as standard first-line treatment (although the difference is less striking when considering all drug resistance, not only resistance to NNRTIs); the effect of other policies is fairly modest. With respect to viral load suppression (at a threshold of 500 copies per mL), the proportion of people who have viral suppression 1 year after starting ART is projected to fall over time with the current policy, because of the cumulative effects of rising transmitted drug resistance. Policies that entail monitoring of viral load have only a small beneficial effect on this outcome, whereas the policy to change to a boosted protease inhibitor-based regimen as standard first-line treatment and the policy to test for resistance before starting ART have a substantial positive effect (ie, the proportion with viral suppression after 1 year from start of ART is projected to rise). Considering the proportion of all people on ART with viral suppression 15 years after t0, similar results were achieved by implementing routine monitoring of viral load or by changing to a boosted protease inhibitor-based standard first-line regimen.
Table 2.
Outcomes after 15 years from t0 of the five policy options, according to prevalence of NNRTI resistance at t0 among people starting ART
| t0 (2017) | 15 years after t0 (2032)
|
|||||
|---|---|---|---|---|---|---|
| Current policy | bPI as first-line regimen* | Pre-ART resistance testing† | Viral load monitoring‡ | Single viral load test at 6 months§ | ||
| Proportion on a bPI–based regimen¶ | ||||||
|
| ||||||
| <5% | 7% (6–8) | 18% (16–20) | 59% (57–61) | 22% (19–25) | 17% (14–21) | 19% (16–21) |
| 5% to <10% | 7% (6–9) | 19% (17–23) | 61% (58–64) | 24% (21–30) | 20% (16–26) | 21% (18–26) |
| 10% to <15% | 8% (7–10) | 23% (19–27) | 63% (60–66) | 30% (25–35) | 25% (20–32) | 25% (21–31) |
| 15% to <20% | 9% (7–11) | 26% (21–33) | 66% (61–71) | 35% (29–43) | 31% (24–40) | 30% (24–38) |
| 20% to <25% | 10% (9–11) | 29% (24–34) | 69% (63–72) | 40% (34–45) | 37% (30–42) | 35% (29–40) |
| ≥25% | 10% (9–11) | 31% (27–35) | 70% (66–73) | 43% (39–46) | 39% (35–43) | 36% (33–41) |
|
| ||||||
| Of people starting ART, proportion having NNRTI resistance (in majority virus)|| | ||||||
|
| ||||||
| <5% | 4% (2–5) | 25% (15–36) | 11% (5–19) | 22% (13–33) | 21% (11–29) | 24% (14–35) |
| 5% to <10% | 8% (5–10) | 32% (20–44) | 14% (7–23) | 28% (18–40) | 27% (16–38) | 31% (19–42) |
| 10% to <15% | 12% (10–15) | 41% (29–51) | 17% (9–26) | 36% (26–46) | 35% (24–45) | 39% (27–49) |
| 15% to <20% | 17% (15–20) | 48% (37–58) | 20% (11–31) | 44% (33–54) | 42% (32–52) | 46% (36–56) |
| 20% to <25% | 22% (20–24) | 54% (44–63) | 22% (12–32) | 49% (40–57) | 47% (36–56) | 51% (40–59) |
| ≥25% | 26% (25–29) | 57% (51–65) | 24% (14–34) | 52% (44–60) | 50% (41–55) | 53% (44–61) |
|
| ||||||
| Of people starting ART, proportion having any resistance (in majority virus)||** | ||||||
|
| ||||||
| <5% | 5% (3–6) | 28% (17–41) | 20% (10–30) | 26% (16–38) | 25% (14–36) | 28% (16–40) |
| 5% to <10% | 8% (6–11) | 36% (23–49) | 26% (14–38) | 33% (22–46) | 32% (20–45) | 35% (22–48) |
| 10% to <15% | 13% (11–16) | 45% (33–57) | 33% (20–45) | 43% (30–54) | 41% (29–53) | 44% (32–55) |
| 15% to <20% | 19% (16–22) | 53% (42–64) | 40% (27–53) | 51% (39–63) | 49% (37–61) | 52% (41–63) |
| 20% to <25% | 24% (22–26) | 60% (49–70) | 45% (32–58) | 58% (47–66) | 56% (43–65) | 58% (46–68) |
| ≥25% | 28% (26–32) | 64% (56–73) | 49% (37–60) | 60% (51–72) | 59% (51–68) | 61% (51–69) |
|
| ||||||
| Of people on ART 1 year after starting ART, proportion with viral suppression | ||||||
|
| ||||||
| <5% | 80% (73–86) | 69% (60–78) | 83% (75–89) | 80% (71–87) | 71% (62–80) | 70% (60–79) |
| 5% to <10% | 77% (64–84) | 63% (49–74) | 81% (69–89) | 77% (64–85) | 66% (52–76) | 65% (51–75) |
| 10% to <15% | 67% (46–78) | 52% (35–64) | 74% (58–84) | 68% (49–79) | 55% (39–67) | 54% (37–66) |
| 15% to <20% | 49% (40–70) | 36% (26–54) | 61% (51–78) | 53% (43–73) | 40% (30–58) | 40% (30–57) |
| 20% to <25% | 45% (39–52) | 31% (25–39) | 58% (51–69) | 49% (42–59) | 35% (28–46) | 34% (28–44) |
| ≥25% | 44% (38–49) | 30% (25–35) | 59% (53–64) | 50% (44–57) | 34% (27–41) | 33% (27–38) |
|
| ||||||
| Of people on ART, proportion with viral suppression | ||||||
|
| ||||||
| <5% | 84% (80–86) | 83% (79–86) | 88% (86–90) | 86% (83–88) | 87% (83–90) | 84% (80–86) |
| 5% to <10% | 82% (73–85) | 81% (74–85) | 87% (82–90) | 84% (79–88) | 85% (79–89) | 82% (75–85) |
| 10% to <15% | 74% (56–82) | 75% (61–81) | 83% (69–88) | 80% (66–85) | 81% (70–85) | 77% (65–82) |
| 15% to <20% | 56% (53–76) | 62% (56–77) | 70% (67–84) | 67% (63–82) | 71% (66–82) | 66% (61–78) |
| 20% to <25% | 54% (52–58) | 59% (55–64) | 68% (67–70) | 65% (63–68) | 69% (66–74) | 64% (60–68) |
| ≥25% | 54% (52–56) | 58% (55–62) | 68% (67–69) | 65% (63–67) | 69% (65–72) | 64% (61–67) |
|
| ||||||
| Death rate | ||||||
|
| ||||||
| <5% | 2·8 (2·2–3·7) | 2·5 (2·0–3·1) | 2·2 (1·6–2·7) | 2·3 (1·8–3·0) | 2·4 (1·8–3·0) | 2·4 (1·9–3·0) |
| 5% to <10% | 3·1 (2·3–4·6) | 2·7 (2·0–3·6) | 2·2 (1·7–2·9) | 2·4 (1·9–3·3) | 2·5 (1·9–3·3) | 2·6 (2·0–3·5) |
| 10% to <15% | 4·1 (2·8–7·4) | 3·1 (2·4–4·9) | 2·4 (1·9–3·4) | 2·8 (2·2–4·4) | 2·8 (2·2–4·0) | 2·9 (2·3–4·4) |
| 15% to <20% | 7·0 (3·6–8·5) | 4·5 (2·7–6·1) | 3·1 (2·1–4·2) | 4·0 (2·4–5·3) | 3·6 (2·4–4·9) | 3·8 (2·5–5·2) |
| 20% to <25% | 7·6 (6·0–8·7) | 5·0 (3·7–6·0) | 3·4 (2·6–4·1) | 4·4 (3·4–5·3) | 3·9 (3·2–4·9) | 4·3 (3·4–5·2) |
| ≥25% | 7·7 (6·4–8·5) | 5·0 (4·4–5·6) | 3·4 (2·9–4·1) | 4·4 (3·6–5·2) | 3·8 (3·3–4·8) | 4·0 (3·6–4·8) |
Data are median proportion (90% range), or death rate per 100 person-years (90% range). Results are based on 5000 programmatic scenarios, according to prevalence of NNRTI resistance at t0 in people starting ART, as follows: <5%, n=451; 5% to <10%, n=1800; 10% to <15%, n=1518; 15% to <20%, n=807; 20% to <25%, n=376; ≥25%, n=48. ART=antiretroviral therapy. bPI=boosted protease inhibitor. NNRTI=non-nucleoside reverse transcriptase inhibitor. NRTI=nucleoside reverse transcriptase inhibitor.
Change of the standard NNRTI-based regimen to a bPI-based first-line regimen.
Individual-level resistance testing before ART initiation to detect key NNRTI mutations to inform whether use of an NNRTI-based or bPI-based regimen is optimal as first-line treatment.
Introduction of routine (6 month, 12 month, then annual) viral load monitoring, replacing 6 monthly CD4 cell count monitoring.
A single routine measurement of viral load 6 months after start of ART, with routine CD4 cell count monitoring. In this last scenario, if viral load is >1000 copies per mL, the test is repeated 6 months later; if viral load remains at >1000 copies per mL, a switch to second-line treatment is effected.
First-line or second-line.
Including women who have taken antiretroviral drugs for the prevention of mother-to-child transmission.
Including NNRTI, NRTI, or PI resistance.
Changing to a boosted protease inhibitor-based standard first-line regimen led to the lowest death rate in people on ART (table 2), but generally, death rates follow a similar pattern to that for the proportion of people on ART with viral suppression. The death rate generally rises with increasing levels of NNRTI resistance at t0 in people starting ART, mainly because the underlying adherence pattern is a determinant of both the level of transmitted drug resistance (appendix p 7) and of the effectiveness of the treatment, in terms of achieving viral suppression and increases in CD4 cell count.
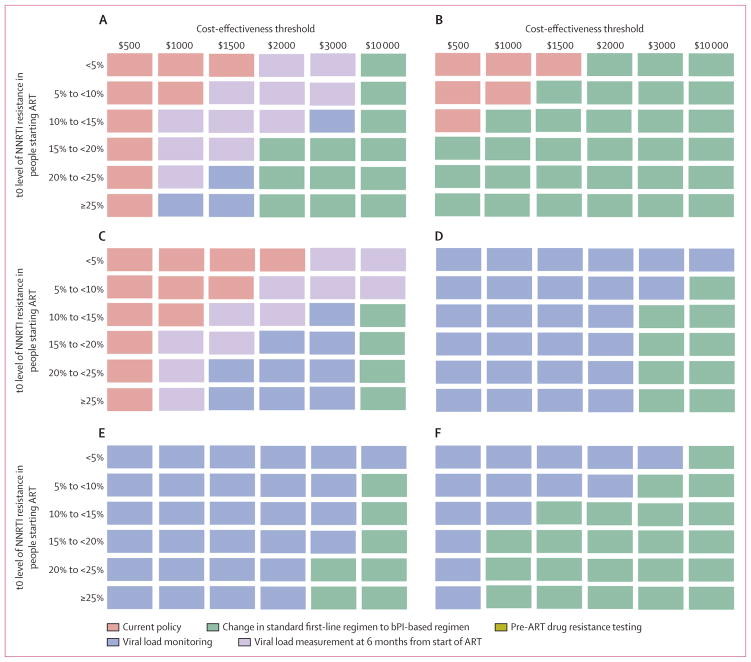
Table 3 shows costs according to the policy option at t0. The main differences between policy options are associated with choice of ART, viral load monitoring ($45), and resistance tests ($250). Costs are highest for the option to change to a protease inhibitor-based standard first-line regimen because prices of these antiretroviral drugs are high (eg, $219 per year for atazanavir; appendix p 13). Figure 2A shows the most cost-effective policy option for various cost-effectiveness thresholds and prevalence levels of NNRTI resistance at t0 in people starting ART (increments in costs and QALYs compared with the current policy are presented in the appendix p 6). At a cost-effectiveness threshold of $500 per QALY, no change in policy is cost effective, even if the prevalence of NNRTI resistance at t0 in people starting ART is higher than 25%, because of the increased expense of the alternative policies. At higher cost-effectiveness thresholds, adoption of a policy of a single viral load measurement 6 months after initiation of ART becomes cost effective if the t0 level of NNRTI resistance is above 10% for a cost-effectiveness threshold of $1000 (although an increase in overall spend is needed compared with the current policy), and single viral load measurement at 6 months is cost effective at a t0 level of resistance higher than 5% for a cost-effectiveness threshold of $1500. For a cost-effectiveness threshold of $2000 or higher, a change to current policy is indicated irrespective of the level of NNRTI resistance at the start of ART. In our main analysis, changing the national standard first-line regimen from NNRTIs to a boosted protease inhibitor-based regimen was the most cost-effective action when the t0 level of NNRTI resistance in people starting ART was 15% or higher; when the cost-effectiveness threshold was $10 000, this policy was cost effective at any level of resistance.
Table 3.
Distribution of annual discounted costs per person for five different policy options, over 15 years from t0 (2017 to 2032)
| Current policy | bPI as first-line regimen* | Pre-ART resistance testing† | Viral load monitoring‡ | Single viral load test at 6 months§ | |
|---|---|---|---|---|---|
| ART | 9·43 | 13·60 | 10·09 | 9·85 | 9·71 |
| CD4 cell count | 0·83 | 0·87 | 0·85 | 0·12 | 0·81 |
| Viral load | 0 | 0 | 0 | 2·22 | 0·24 |
| Resistance test | 0 | 0 | 1·22 | 0 | 0 |
| Treatment for WHO stage 4 conditions | 1·26 | 1·16 | 1·21 | 1·20 | 1·23 |
| TB treatment | 0·41 | 0·38 | 0·39 | 0·38 | 0·40 |
| Treatment for WHO stage 3 conditions | 0·47 | 0·43 | 0·45 | 0·44 | 0·46 |
| Co-trimoxazole | 0·15 | 0·14 | 0·14 | 0·16 | 0·15 |
| HIV testing | 3·38 | 3·37 | 3·38 | 3·38 | 3·38 |
| Clinic visits | 5·17 | 5·24 | 5·20 | 5·23 | 5·21 |
| Total | 21·1 | 25·2 | 22·9 | 23·0 | 21·6 |
Data are mean discounted costs (US$) per adult aged 15–65 years in the population (including both HIV-positive and HIV-negative people) per year. Results are based on 5000 programmatic scenarios. Facility costs are apportioned to resource inputs. Full unit costs are provided in the appendix (p 13). ART=antiretroviral therapy. bPI=boosted protease inhibitor. NNRTI=non-nucleoside reverse transcriptase inhibitor. NRTI=nucleoside reverse transcriptase inhibitor.
Change of the standard NNRTI-based regimen to a bPI-based first-line regimen.
Individual-level resistance testing before ART initiation to detect key NNRTI mutations to inform whether use of an NNRTI-based or bPI-based regimen is optimum as first-line treatment.
Introduction of routine (6 month, 12 month, then annual) viral load monitoring, replacing 6 monthly CD4 cell count monitoring.
A single routine measurement of viral load 6 months after start of ART, with routine CD4 cell count monitoring. In this last scenario, if viral load is >1000 copies per mL, the test is repeated 6 months later; if viral load remains at >1000 copies per mL, a switch to second-line treatment is effected.
Figure 2. Most cost-effective policy according to the prevalence of NNRTI resistance at the start of ART and the cost-effectiveness threshold.
5000 programmatic scenarios shown, divided according to NNRTI resistance at t0: <5%, n=451; 5% to <10%, n=1800; 10% to <15%, n=1518; 15% to <20%, n=807; 20% to <25%, n=376; and ≥25%, n=48. For every programmatic scenario, the policy with the highest net monetary benefit was ascertained and the policy that most frequently had the highest value was indicated as the most cost-effective policy. Pre-ART resistance testing was not cost effective in any scenario. (A) Most cost-effective policy according to the prevalence of NNRTI resistance when ART is initiated at t0 and the cost-effectiveness threshold per QALY. Cost of second-line boosted protease inhibitor (atazanavir), $219 per year; cost of viral load testing, $45; and unit cost of resistance testing, $250. These costs include commodities and programme costs for genotyping. (B) As for (A) but with the cost of boosted protease inhibitors reduced by 50%. (C) As for (A) but with the cost of boosted protease inhibitors increased by 50%. (D) As for (A) but with the cost of viral load testing cut to $15. (E) As for (A) but with unit cost of resistance testing reduced to $100 and viral load testing cut to $15. (F) As for (A) but with cost of boosted protease inhibitors reduced by 50% and viral load testing cut to $15. ART=antiretroviral treatment. NNRTI=non-nucleoside reverse transcriptase inhibitor. QALY=quality-adjusted life-year.
In sensitivity analyses in which we varied the cost of boosted protease inhibitors (figures 2B and 2C), a policy to change to first-line boosted protease inhibitors was not cost effective at thresholds up to and including $3000 when the cost of boosted protease inhibitors was increased by 50%, irrespective of the t0 level of NNRTI resistance in people starting ART (figure 2C). However, if the cost of boosted protease inhibitors was halved then a change to a national standard first-line boosted protease inhibitor-based regimen was the most cost-effective policy in almost all situations (figure 2B). In further sensitivity analyses, in which the cost of viral load testing was cut to $15 from $45 (figures 2D and 2E; in figure 2E, the cost of resistance test was also lowered from $250 to $100), introduction of viral load monitoring every 6 months is almost universally the most cost-effective policy, irrespective of the cost-effectiveness threshold and level of resistance at t0. If the viral load cost was $15 and cost of boosted protease inhibitors was halved then a change of the national standard first-line regimen to a boosted protease inhibitor-based regimen is the most cost-effective approach at high cost-effectiveness thres holds and at a higher t0 level of NNRTI resistance in people starting ART (figure 2F).
Discussion
For a given prevalence of resistance to NNRTIs in people starting ART, the most cost-effective policy was dependent on the cost-effectiveness threshold for the setting; in general, when the cost-effectiveness threshold was $500 per QALY, no change in policy was cost effective. At thresholds of $1000, $1500, and $2000, a policy of a single viral load measurement 6 months after the start of ART became cost effective once the proportion of people with NNRTI resistance was higher than 10%, 5%, and 0%, respectively. Changing the standard first-line regimen to a boosted protease inhibitor-based regimen was generally predicted to be the most effective policy, and can become a cost-effective option at the highest cost-effectiveness thresholds, depending on the level of NNRTI resistance in people starting ART. If the current cost of boosted protease inhibitors were to be reduced by 50%, and if the level of NNRTI resistance in populations starting ART were above 15%, a change to a boosted protease inhibitor-based regimen as standard first-line treatment would be a cost-effective new policy, even if the cost-effectiveness threshold were as low as $500. However, if the cost of boosted protease inhibitors in a country were 50% higher than the value we used, then a change of the standard first-line regimen to a boosted protease inhibitor-based regimen was not cost effective, emphasising the importance of fully understanding cost implications before making such a change.
In the face of variable and occasionally low adherence, boosted protease inhibitor-based regimens are likely to result in better long-term outcomes than are NNRTI-based regimens, irrespective of the prevalence of transmitted drug resistance, because resistance to boosted protease inhibitors is slow to accumulate, even when adherence is poor.23–25 Additionally, HIV with resistance mutations to protease inhibitors generally replicates poorly,26 the decline in CD4 cell count is less rapid in the presence of virological failure with a boosted protease inhibitor-based regimen than it is with an NNRTI-based regimen,27–29 and risk of death could be higher in patients whose treatment is failing because of NNRTI resistance compared with protease inhibitor resistance.26 Although findings of clinical trials comparing outcomes between first-line NNRTIs and boosted protease inhibitors show small differences in viral load,30,31 these studies typically include people who are more likely to adhere to ART, and our modelling suggests that among less adherent people, the long-term outcomes of boosted protease inhibitor-based regimens will be superior. To date, scant data are available for the rate at which mutations to protease inhibitors emerge in people maintained on a boosted protease inhibitor-based regimen in the face of virological failure without options to switch.
As ART coverage increases, prevalence of transmitted drug resistance in people starting ART will almost inevitably rise. Countries must minimise the rate at which this escalation takes place, to sustain the huge population benefits of ART. Ensuring that a high proportion of patients adhere to—and are retained on—ART, and maximising prevention of HIV transmission, are key to achievement of this goal. Individual-level genotyping is not generally cost effective at this stage. Although our findings provide a broad overview of potential policy directions, as far as is feasible, countries should develop and analyse their own country-specific models to evaluate potential policy changes. In particular, this approach might be helpful for countries transitioning from generalised to concentrated epidemics. Further work is also needed to understand what cost-effectiveness threshold should be used in a given country. With a cut in the cost of viral load testing to $15, a reduction that could well take place in the future in view of developments in this area, measurement of viral load becomes the most cost-effective policy out of those we considered. Previous modelling (similarly using a viral load cost of $45) suggests that a country should aim to introduce viral load monitoring once it has sustained close to full coverage of ART for those in need.14 Although one benefit of viral load monitoring is that evidence of viraemia can be used to provide targeted counselling to those most in need, which can boost adherence,9,10 our analysis does not include the additional cost of targeted counselling explicitly, and this expense will need to be considered if the cost is substantial. Moreover, our analysis assumes that adherence counselling is offered to all patients with a detectable viral load, yet this might not be the case in many settings. Furthermore, our modelling assumes an ideal scenario in which implementation of viral load testing is done at the appropriate time and results are obtained and acted on, if necessary. Further work is needed to assess the effect of failures in viral load measurement and return of results, and of other implementation realities, including reduced accuracy of viral load if measured on dried blood-spot samples and the possibilities for simplified clinic visits for people with known long-term viral suppression.
Although the implications of transmission of drug-resistant HIV have been considered in several models,32–37 only one (to our knowledge) has previously addressed the question of whether a policy change in the face of a given level of transmitted drug resistance is cost effective (panel). Walensky and colleagues38 investigated whether changing to a boosted protease inhibitor-based standard first-line regimen was cost effective in Côte d’Ivoire; they reported that such a policy change was not favourable.
Panel. Research in context.
Systematic review
We searched Web of Knowledge on Feb 12, 2014, and on Sept 13, 2014, with the search terms: “HIV*” AND “resistance” AND “cost-effective*”. We identified only one report directly relevant to the cost-effectiveness of different ART approaches in response to levels of transmitted drug resistance.38 In this report from Côte d’Ivoire, a change to a boosted protease inhibitor-based standard first-line regimen was not cost effective. We have considered this policy change and other options in the context of a dynamic transmission model that accounts for transmission of specific mutations to specific drugs and use of second-line regimens.
Interpretation
Results from our model will inform WHO recommendations on monitoring of HIV drug resistance in people starting ART. Cost-effectiveness of potential policies to adopt in response to different prevalence levels of pretreatment HIV drug resistance depends on competing budgetary claims, reflected in the cost-effectiveness threshold.
An issue we did not consider is that among women receiving antiretroviral drugs for prevention of mother-to-child transmission, viral suppression must be achieved to reduce the risk of transmission to the child. Changes to regimens for pregnant women might be needed, even when the prevalence of resistance in people starting ART is below the level to be cost effective when accounting for the whole adult population on ART. Further modelling is needed to assess these issues. For a particular cost-effectiveness threshold, we have provided some indication of the policy alternative likely to be cost effective for a given level of resistance to NNRTIs in populations initiating first-line ART. Surveillance of transmitted drug resistance in people ostensibly starting ART for the first time will include some people who have previous undeclared ART use. Thus, estimates of the prevalence of resistance before initiation of ART thought to have arisen because of transmitted drug resistance could be overestimated by such surveys. Policy makers should consider this possibility and whether any change in policy proposed would still be indicated if the true level of transmitted drug resistance in people starting ART for the first-time were somewhat lower than that estimated in surveys.
This modelling analysis has entailed drawing on knowledge acquired within the area of HIV on sexual behaviour, HIV transmission, progression of untreated infection, and effects of treatment.11–13 Even if most of these aspects are generally well described, any model is, at best, an approximation to reality. Although this limitation should be borne in mind, the fact that our model is mechanistic and tries to capture the underlying processes entailed, in terms of variables that are measured (eg, CD4 cell count, viral load, presence of specific resistance mutations) and the ultimate endpoint of length of life, means that extensive scope exists to compare the model outputs with recorded data, which should improve the ability to approximate reality.
The results from our model will help to inform WHO recommendations on monitoring of HIV drug resistance in people initiating ART. Additional modelling is required to inform specific country-level policy actions.
Supplementary Material
Acknowledgments
This study was supported by WHO (with funds provided by the Bill & Melinda Gates Foundation, grant number 38180) and CHAIN (Co-ordination and Harmonisation of Advanced e-Infrastructures for Research and Education Data Sharing; with funds provided by the European Commission). We acknowledge use of the UCL Legion High Performance Computing Facility (Legion@UCL) and associated support services in completion of this work. MRJ is supported by CFAR (grant number 1P30A142853). PR is supported by funding from the Department for International Development (DFID) for the Lablite project; Lablite is funded by DFID for the benefit of developing countries. The views expressed here are not necessarily those of DFID.
Footnotes
See Comment page e56
Contributors
All authors contributed to the idea for this modelling cost-eff ectiveness analysis; provided input into the implementation of the analysis, the underlying assumptions, and the interpretation; and helped write the report. ANP, VC, and FN programmed and did the modelling.
Declaration of interests
ANP has received funding for consultancy work from Gilead Sciences, GlaxoSmithKline, and Abbvie. ADL has received funding for research, personal fees, or travel grants from Abbvie, Siemens, Gilead Sciences, Janssen, Bristol-Myers Squibb, and ViiV Healthcare. All other authors declare no competing interests.
Contributor Information
Andrew N Phillips, Research Department of Infection and Population Health, University College London, London, UK.
Valentina Cambiano, Research Department of Infection and Population Health, University College London, London, UK.
Alec Miners, London School of Hygiene & Tropical Medicine, London, UK.
Paul Revill, University of York, York, UK.
Deenan Pillay, Africa Centre, KwaZulu Natal, South Africa.
Jens D Lundgren, Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Diane Bennett, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Elliott Raizes, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Fumiyo Nakagawa, Research Department of Infection and Population Health, University College London, London, UK.
Andrea De Luca, University Division of Infectious Diseases, Siena University Hospital, Siena, Italy.
Marco Vitoria, World Health Organization, Geneva, Switzerland.
Jhoney Barcarolo, World Health Organization, Geneva, Switzerland.
Joseph Perriens, World Health Organization, Geneva, Switzerland.
Michael R Jordan, Tufts University School of Medicine and Tufts Medical Center, Boston, MA, USA.
Silvia Bertagnolio, World Health Organization, Geneva, Switzerland.
References
- 1.UNAIDS. [accessed Sept 25, 2014];UNAIDS report on the global AIDS epidemic 2013. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
- 2.Hamers RL, Schuurman R, Sigaloff KCE, et al. for the PharmAccess African Studies to Evaluate Resistance (PASER) Investigators. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multicentre cohort study. Lancet Infect Dis. 2012;12:307–17. doi: 10.1016/S1473-3099(11)70255-9. [DOI] [PubMed] [Google Scholar]
- 3.Bennett DE, Myatt M, Bertagnolio S, Sutherland D, Gilks CF. Recommendations for surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antivir Ther. 2008;13:25–36. [PubMed] [Google Scholar]
- 4.Jordan MR, Bennett DE, Wainberg MA, et al. Update on World Health Organization HIV drug resistance prevention and assessment strategy: 2004–2011. Clin Infect Dis. 2012;54:S245–49. doi: 10.1093/cid/cis206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. [accessed Sept 25, 2014];WHO HIV drug resistance report 2012. 2012 Jul; http://apps.who.int/iris/bitstream/10665/75183/1/9789241503938_eng.pdf.
- 6.Gupta RK, Jordan MR, Sultan BJ, et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet. 2012;380:1250–58. doi: 10.1016/S0140-6736(12)61038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frentz D, Boucher CAB, van de Vijver DAMC. Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world. AIDS Rev. 2012;14:17–27. [PubMed] [Google Scholar]
- 8.WHO. [accessed Sept 25, 2014];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2013 Jun 30; http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. [PubMed]
- 9.Bonner K, Mezochow A, Roberts T, et al. Viral load monitoring as a tool to reinforce adherence: a systematic review. J Acquir Immune Defic Syndr. 2013;64:74–78. doi: 10.1097/QAI.0b013e31829f05ac. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann CJ, Charalambous S, Sim J, et al. Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa. Clin Infect Dis. 2009;49:1928–35. doi: 10.1086/648444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips AN, Pillay D, Garnett G, et al. Effect on transmission of HIV-1 resistance of timing of implementation of viral load monitoring to determine switches from first to second-line antiretroviral regimens in resource-limited settings. AIDS. 2011;25:843–50. doi: 10.1097/QAD.0b013e328344037a. [DOI] [PubMed] [Google Scholar]
- 12.Cambiano V, Bertagnolio, Jordan M, et al. Transmission of drug resistant HIV and its potential impact on mortality and treatment outcomes in resource-limited settings. J Infect Dis. 2013;207:S57–62. doi: 10.1093/infdis/jit111. [DOI] [PubMed] [Google Scholar]
- 13.Cambiano V, Bertagnolio S, Jordan MR, et al. Predicted levels of HIV drug resistance in South Africa: potential impact of expanding diagnosis, retention, and eligibility criteria for antiretroviral therapy initiation. AIDS. 2014;28(suppl 1):S15–23. doi: 10.1097/QAD.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 14.Keebler D, Revill P, Braithwaite S, et al. Cost-effectiveness of different strategies to monitor adults on antiretroviral treatment: a combined analysis of three mathematical models. Lancet Glob Health. 2014;2:e35–43. doi: 10.1016/S2214-109X(13)70048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claxton K, Walker S, Palmer S, Sculpher M. [accessed Sept 25, 2014];Appropriate perspectives for health care decisions: CHE research paper 54. 2010 Jan; http://www.york.ac.uk/media/che/documents/papers/researchpapers/rp54_appropriate_perspectives_for_health_care_decisions.pdf.
- 16.Phelps CE, Muslin AI. On the (near) equivalence of cost-effectiveness and cost-benefit analyses. Int J Technol Assess Health Care. 1991;95:394–97. doi: 10.1017/s0266462300004803. [DOI] [PubMed] [Google Scholar]
- 17.Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18(suppl):S68–80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- 18.WHO. [accessed Sept 25, 2014];Cost effectiveness and strategic planning (WHO-CHOICE): cost-effectiveness thresholds. http://www.who.int/choice/costs/CER_thresholds/en/
- 19.Kessler J, Braithwaite RS. Modelling the cost effectiveness of HIV treatment: how to buy ‘health’ when resources are limited. Curr Opin HIV AIDS. 2013;8:544–49. doi: 10.1097/COH.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Revill P, Sculpher M. Cost effectiveness of interventions to tackle non-communicable diseases. BMJ. 2012;344:e609. doi: 10.1136/bmj.d7883. [DOI] [PubMed] [Google Scholar]
- 21.Claxton K, Martin S, Soares M, et al. [accessed Sept 25, 2014];Methods for the estimation of the NICE cost effectiveness threshold: CHE Research Paper 81. 2013 Nov; http://www.york.ac.uk/media/che/documents/papers/researchpapers/CHERP81_methods_estimation_NICE_costeffectiveness_threshold_(Nov2013).pdf.
- 22.Bowie C, Mwase T. Assessing the use of an essential health package in a sector wide approach in Malawi. Health Res Policy Syst. 2011;9:4. doi: 10.1186/1478-4505-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta R, Hill A, Sawyer AW, Pillay D. Emergence of drug resistance in HIV type 1-infected patients after receipt of first-line highly active antiretroviral therapy: a systematic review of clinical trials. Clin Infect Dis. 2008;47:712–22. doi: 10.1086/590943. [DOI] [PubMed] [Google Scholar]
- 24.Wallis CL, Mellors JW, Venter WDF, Sanne I, Stevens W. Protease inhibitor resistance is uncommon in HIV-1 subtype C infected patients on failing second-line lopinavir/r-containing antiretroviral therapy in South Africa. AIDS Res Treat. 2011;2011:769627. doi: 10.1155/2011/769627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston V, Cohen K, Weisner L, et al. Viral suppression following switch to second-line antiretroviral therapy: associations with nucleoside reverse transcriptase inhibitor resistance and subtherapeutic drug concentrations prior to switch. J Infect Dis. 2014;209:711–20. doi: 10.1093/infdis/jit411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoddart CA, Liegler TJ, Mammano F, et al. Impaired replication of protease inhibitor-resistant HIV-1 in human thymus. Nat Med. 2001;7:712–18. doi: 10.1038/89090. [DOI] [PubMed] [Google Scholar]
- 27.Ledergerber B for the PLATO Collaboration. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364:51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 28.Ledergerber B for The Pursuing Later Treatment Option II (PLATO II) project team of the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Predictors of CD4+ T-cell counts of HIV type 1-infected persons after virologic failure of all 3 original antiretroviral drug classes. J Infect Dis. 2013;207:759–67. doi: 10.1093/infdis/jis752. [DOI] [PubMed] [Google Scholar]
- 29.Mocroft A, Phillips AN, Ledergerber B, et al. for the EuroSIDA Study Group. Estimated average annual rate of change of CD4(+) T-cell counts in patients on combination antiretroviral therapy. Antivir Ther. 2010;15:563–70. doi: 10.3851/IMP1559. [DOI] [PubMed] [Google Scholar]
- 30.Lockman S, Hughes M, Sawe F, et al. Nevirapine- versus lopinavir/ritonavir-based initial therapy for HIV-1 infection among women in Africa: a randomized trial. PLoS Med. 2012;9:e1001236. doi: 10.1371/journal.pmed.1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill A, McBride A, Sawyer AW, Clumeck N, Gupta RK. Resistance at virological failure using boosted protease inhibitors versus non-nucleoside reverse transcriptase inhibitors as first-line antiretroviral therapy: implications for sustained effcacy of ART in resource-limited settings. J Infect Dis. 2013;207:S78–84. doi: 10.1093/infdis/jit112. [DOI] [PubMed] [Google Scholar]
- 32.Blower S, Ma L, Farmer P, Koenig S. Predicting the impact of antiretrovirals in resource-poor settings: preventing HIV infections whilst controlling drug resistance. Curr Drug Targets Infect Disord. 2003;3:345–53. doi: 10.2174/1568005033480999. [DOI] [PubMed] [Google Scholar]
- 33.Vardavas R, Blower S. The emergence of HIV transmitted resistance in Botswana: “when will the WHO detection threshold be exceeded?”. PLoS One. 2007 doi: 10.1371/journal.pone.0000152. published online Jan 17. http://dx.doi.org/10.1371/journal.pone.0000152. [DOI] [PMC free article] [PubMed]
- 34.Baggaley RF, Garnett GP, Ferguson NM. Modelling the impact of antiretroviral use in resource-poor settings. PLoS Med. 2006;3:e124. doi: 10.1371/journal.pmed.0030124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blower S, Bodine E, Kahn J, McFarland W. The antiretroviral rollout and drug-resistant HIV in Africa: insights from empirical data and theoretical models. AIDS. 2005;19:1–14. doi: 10.1097/00002030-200501030-00001. [DOI] [PubMed] [Google Scholar]
- 36.Blower S, Farmer P. [accessed Oct 2, 2014];Predicting the public health impact of antiretrovirals: preventing HIV in developing countries. 2003 http://aidscience.org/Articles/aidscience033.asp.
- 37.Hoare A, Kerr SJ, Ruxrungtham K, et al. Hidden drug resistant HIV to emerge in the era of universal treatment access in southeast Asia. PLoS One. 2010 doi: 10.1371/journal.pone.0010981. published online June 8. http://dx.doi.org/10.1371/journal.pone.0010981. [DOI] [PMC free article] [PubMed]
- 38.Walensky RP, Weinstein MC, Yazdanpanah Y, et al. HIV drug resistance surveillance for prioritizing treatment in resource-limited settings. AIDS. 2007;21:973–82. doi: 10.1097/QAD.0b013e328011ec53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.