Abstract
In this study, we investigated the relationship between target of rapamycin (TOR) and H2O2-induced hormetic response in the budding yeast Saccharomyces cerevisiae grown on glucose or fructose. In general, our data suggest that: (1) hydrogen peroxide (H2O2) induces hormesis in a TOR-dependent manner; (2) the H2O2-induced hormetic dose–response in yeast depends on the type of carbohydrate in growth medium; (3) the concentration-dependent effect of H2O2 on yeast colony growth positively correlates with the activity of glutathione reductase that suggests the enzyme involvement in the H2O2-induced hormetic response; and (4) both TOR1 and TOR2 are involved in the reciprocal regulation of the activity of glucose-6-phosphate dehydrogenase and glyoxalase 1.
Keywords: hormesis, hydrogen peroxide, glutathione reductase, monosaccharides, Saccharomyces cerevisiae, TOR1, TOR2
Introduction
Based on its intensity, stress can be considered as either beneficial or harmful.1–5 Mild stress can stimulate organisms’ biological functions and result in an acquisition of their resistance to high doses of the same stressor (preadaptation) as well as other stressing factors (cross-protection or cross-adaptation).1,6–9 This phenomenon also known as hormesis is observed in a variety of organisms responding to a wide range of chemical, physical, and biological stressors. A hormetic response is usually limited to the 30% to 60% increase in biological function under mild stress conditions.3
It was recently found that hydrogen peroxide (H2O2) plays a crucial role in the induction of hormesis.6 Stimulation of the reproductive potential through hormesis-induced compounds, like H2O2, appears to be an effective approach to improve yeast survival and cross-adaptation to different stressors.7,8 In turn, the physiological state of organism is critical in the mobilization of stress-responsive defenses. As reported earlier, fructose-grown yeast in the exponential phase (short-term model) exposed to H2O2 demonstrated higher survival than glucose-grown cells.10 The phenomenon was explained by the protective role of fructose, in spite of its more potent ability to produce reactive oxygen and carbonyl species compared to glucose.11–13 Short-term application of fructose has been suggested to induce a mild carbonyl/oxidative stress stimulating defense mechanisms responsible for cell survival under lethal H2O2 stress: a reduced level of reactive oxygen species in fructose-grown cells after exposure to H2O2 was consistent with a broad peak of superoxide dismutase and catalase activation by H2O2. At the same time, cells grown on glucose demonstrated an increase in the level of reactive oxygen species and a sharp rise in the antioxidant enzyme activities followed by their rapid inactivation.10 Unlike short-term model, in the stationary phase (long-term model), a higher level of carbonyl/oxidative stress markers has been detected, which was correlated with a higher aging and mortality rate of fructose- compared to glucose-grown yeast.14
The specificity of stress response is also determined by the nature of the stressor and the variety of downstream effectors involved.15,16 A hormesis suggests the existence of complex mechanisms which sense and respond to different kinds of stress. As one of the main signaling mechanisms, the target of rapamycin (TOR) pathway that regulates cell growth and metabolism is involved in cellular responses to many types of stress.6,17–20 In spite of the apparent and sometimes hotly debated contradictions between the hormesis and TOR hypothesis of aging and stress resistance, both are applied to explain some common molecular mechanisms.21,22
Target of rapamycin promotes cell growth in response to nutrient availability.23 Most studies on nutrient-mediated activation of TOR were focused on nitrogen sources,20,24,25 but little attention was paid to carbohydrates. In addition, TOR, as a central controller of cell growth, may respond to different types of stress and play an important role under stressful conditions other than nutrient limitation.24
Here we used TOR-deficient strains grown on glucose or fructose to study the effects of different concentrations of H2O2 on yeast. It was shown that H2O2 induced the hormetic dose–response in Saccharomyces cerevisiae in a TOR-dependent manner. The effect was also dependent on the type of carbohydrate in growth medium. The potential role of glutathione reductase (GR), glucose-6-phosphate dehydrogenase (G6PDH), and glyoxalase 1 (GLO1), which are functionally associated with metabolism of carbohydrates and H2O2,13,26–28 is discussed.
Materials and Methods
Yeast Strains and Chemicals
The S. cerevisiae strains used were as follows: JK9-3da (wild type MAT a leu2–3, 112 ura3–52 rme1 trp1 his4 HML a)29 and its derivatives MH349-3d (JK9-3da, tor1:: LEU2-4),30 SH121 (JK9-3da, ade2 tor2:: ADE2-3/YCplac111:: tor2-21ts),31 and SH221 (JK9-3da, ade2 his3 HIS4 tor1:: HIS3 tor2:: ADE2-3/YCplac111:: tor2-21ts),32 kindly provided by Prof. Michael Hall (University of Basel, Switzerland). Chemicals were obtained from Sigma-Aldrich Chemical Co. (USA) and Fluka (Germany). All chemicals were of analytical grade.
Growth Conditions, Stress Induction, and Cell Extracts
Yeast cells were grown at 28°C with shaking at 175 rpm in a liquid medium containing 1% yeast extract, 2% peptone, and 2% glucose (YPD) for 24 hours. For experiments, the obtained cultures were diluted to about 75 × 106 cells/mL in the same medium. Aliquots of the main culture after 24 hours growth were exposed to different concentrations of H2O2 followed by their incubation at 28°C for 1 hour. Control cells were incubated under the same conditions but without H2O2. After incubation, cells from experimental or control cultures were collected by centrifugation (5 minutes, 8000g) and washed with 50 mmol/L potassium phosphate (K-phosphate) buffer (pH 7.0). The yeast pellets were resuspended in lysis buffer (50 mmol/L K-phosphate buffer, 1 mmol/L phenylmethylsulfonyl chloride, and 0.5 mmol/L EDTA). Cell extracts were prepared by vortexing yeast suspensions with glass beads (0.5 mm) as described earlier33 and kept on ice for immediate use.
Reproductive Ability
Yeast reproductive ability was analyzed by plating in triplicate on YPD agar after proper dilution. The plates were incubated at 28°C for 3 days and the colony-forming units counted.34 Reproductive ability was expressed as percentage of total amount of cells plating on YPD agar.
Enzymatic Activity Measurements
The parameters were measured spectrophotometrically with a Spekol 211 spectrophotometer (Carl Zeiss, Germany) and CΦ-46 (ЛOMO, USSR).
The activity of GR and G6PDH was measured by following the consumption or production of nicotinamide adenine dinucleotide phosphate reduced form (NADPH), respectively, at 340 nm. The extinction coefficient for the coenzyme of 6.22 mmol/L−1·cm−1 was used for calculations.33
The activity of GLO1 was measured by following the formation of S-D-lactoylglutathione at 240 nm. The extinction coefficient for S-D-lactoylglutathione of 3.1 mmol/L−1·cm−1 was used for calculations.35
One unit of the activity of the enzymes was defined as the amount of supernatant protein that produces or utilizes 1 μmol of product or substrate per minute. The activities were measured at 25°C and expressed per milligram of soluble protein in supernatant.
Protein Concentration Measurement and Statistical Analysis
Protein concentration was determined by the Coomassie brilliant blue G-250 dye-binding method36 with bovine serum albumin as the standard. Experimental data are expressed as the mean value of 3 to 7 independent experiments ± the standard error of the mean, and statistical testing was carried out used Student t test.
Results
Hormetic Effect of H2O2 Depends on TOR and Type of Carbohydrate in Yeast Growth Medium
According to the data on the vital effect of reducing monosaccharides, glucose and fructose have various influences on cell survival under stress conditions.12,37–41 Recently, we have found that glucose- and fructose-supplemented growth differently affected S. cerevisiae YPH250 survival under oxidative stress: after treatment with low concentrations of H2O2, fructose-grown yeast in the exponential phase demonstrated higher reproductive ability compared to glucose-grown yeast.10 In the present study, we used S. cerevisiae JK9-3da and its TOR mutants grown to early stationary phase on glucose or fructose in order to study the potential role of TOR in yeast resistance to H2O2.
Figure 1 demonstrates the influence of different concentrations of H2O2 on the yeast reproductive ability. The biphasic dose–response dependence, characterized by low-concentration stimulation and high-concentration inhibition of yeast colony growth, has been observed in all cases, except fructose-grown TOR1 and TOR2 single mutants. As seen in Figure 1A, both the studied wild-type cell groups (glucose- and fructose-grown) demonstrated the peak hormetic response at 25 mmol/L and 50 mmol/L H2O2, respectively. At the hormetic concentrations of H2O2, yeast grown in glucose- and fructose-containing medium showed about 155% and 130% of the initial reproductive ability, respectively. However, at the highest H2O2 concentration used (100 mmol/L), both the studied wild-type cell groups demonstrated significantly lower colony growth as compared with control (without H2O2) cells (54% and 33% of the initial reproductive ability of glucose- and fructose-grown yeast, respectively).
Figure 1.
Effect of hydrogen peroxide on reproductive ability of Saccharomyces cerevisiae JK9-3da wild type (A) and its mutants defective in target of rapamycin (TOR): TOR1 (B), TOR 2 (C), and TOR1 TOR2 (D). Results are shown as the mean ± standard error of the mean (SEM; n = 4-8).
Figure 1B and C presents the reproductive ability of the TOR1 and TOR2 single knockouts under the same experimental conditions as for the wild type (Figure 1A). For both single mutants, the peak hormetic response was seen after stress induction by 2.5 mmol/L H2O2 in the case of glucose-supplemented yeast cultivation (122% and 136% of the control reproductive ability for the TOR1 and TOR2 single knockouts, respectively). In fructose-grown TOR1 or TOR2 mutant cells, H2O2 did not demonstrate a hormetic effect at any concentration used; moreover, 2.5 mmol/L H2O2 decreased the reproductive ability of the yeast by 20% compared to the untreated control cells. Independent of the type of monosaccharide in the growth medium, both single mutants demonstrated the lowest reproductive ability at the highest H2O2 concentration used (100 mmol/L), but fructose- compared to glucose-grown single mutants showed higher colony growth (∼30% and ∼2% of the initial reproductive ability, respectively).
Exposure of the TOR1 TOR2 double mutant to H2O2 resulted in virtually the same patterns of reproduction in both the studied cell groups (glucose- and fructose-grown; Figure 1D). The parameter increased significantly and reached a maximum value (130% of the control reproductive ability) in the presence of 5 mmol/L H2O2. At the higher H2O2 concentrations used, reproductive ability of the double mutant decreased by 50% compared to the control untreated cells. Thus, in S. cerevisiae H2O2 can induce monosaccharide- and TOR-dependent hormetic dose–response.
Glutathione Reductase is Involved in Hormetic Effect of H2O2
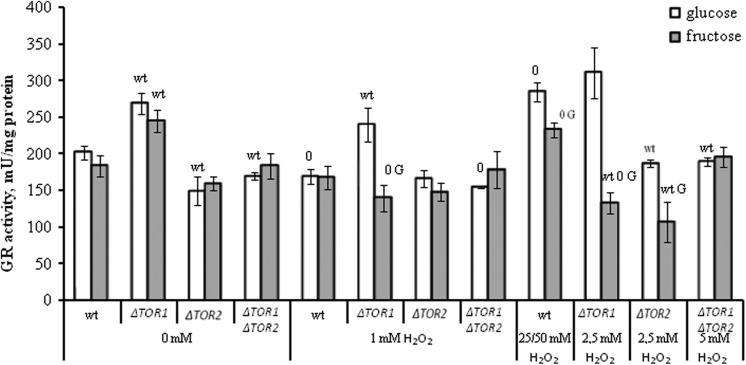
Next we examined the potential role of GR, G6PDH, and GLO1 in H2O2-induced hormesis using glucose- and fructose-grown S. cerevisiae JK9-3da and its TOR mutants. The enzymes are important in the metabolism of carbohydrates and H2O2 13,27,28,42,43; however, little is known about their role in hormesis and functional relationship between TOR and these enzymes. The activity of GR as well as the other 2 enzymes was measured in the 4 strains under control conditions (without H2O2) and after treatment with H2O2. The data are shown in Figure 2.
Figure 2.
The activity of glutathione reductase in wild-type and target of rapamycin (TOR)-deficient Saccharomyces cerevisiae incubated with hydrogen peroxide. Results are shown as the mean ± standard error of the mean ( SEM; n = 3-6). Significantly different from respective values wtfor wild type with P < .05, Gfor cells growing on glucose with P < .05, and 0for corresponding control (without hydrogen peroxide [H2O2]) cells growing on respective monosaccharide with P < .05.
In general, the activity of GR was not changed or somewhat decreased during incubation of the yeast strains with as little as 1 mmol/L H2O2 compared to the control untreated cells. At the same time, the hormetic concentrations of H2O2 (25 mmol/L and 50 mmol/L H2O2 for glucose- and fructose-grown wild type, respectively) increased GR activity by about 1.4-fold in the parental strain. Hormetic concentration of H2O2 for glucose-grown cells lacking TOR1 or TOR2 (2.5 mmol/L H2O2) in most cases did not change significantly the parameter in the 2 single mutants grown on neither glucose nor fructose. However, in fructose-grown TOR1 single mutant H2O2 caused the decline in GR activity by about 2-fold. In the double mutant, GR activity was not changed in both the studied cell groups (glucose- and fructose-grown) after their exposure to the hormetic concentration of H2O2 (5 mmol/L H2O2).
When compared the activities of GR in the parental and mutant strains, it should be noted that under control conditions the parameter was higher by 1.3-fold in the TOR1 single knockout than the wild type in both the studied cell groups (glucose- and fructose-grown). After exposure to H2O2, the activity in the TOR1 single mutant remained higher than in the parental strain grown in glucose-supplemented medium, whereas fructose-grown TOR1 single mutant demonstrated GR activity lower than that in the wild type. At the same time, glucose-grown TOR2 single and TOR1 TOR2 double mutants in most cases demonstrated GR activity lower than wild-type strain. Fructose-grown TOR2 single mutant, the TOR1 TOR2 double knockout, and wild type did not differ significantly from each other in their GR activities, except the TOR2 mutant cells treated with the hormetic concentration of H2O2.
Comparing the 2 studied cell types (glucose- and fructose-grown), no significant difference was found between them under control conditions (without H2O2) and at 1 mmol/L H2O2 in all cases, except the TOR1 mutant (1.7-fold higher in glucose-grown cells). At the respective hormetic concentrations of H2O2, GR activity was higher by 1.2- to 1.8-fold in glucose- than fructose-grown wild-type, TOR1 and TOR2 mutant strains.
Interestingly, H2O2 affected both the yeast colony growth (Figure 1) and the GR activity (Figure 2) in a similar way under all experimental conditions used in this study that suggested the involvement of GR in hormetic effect of H2O2.
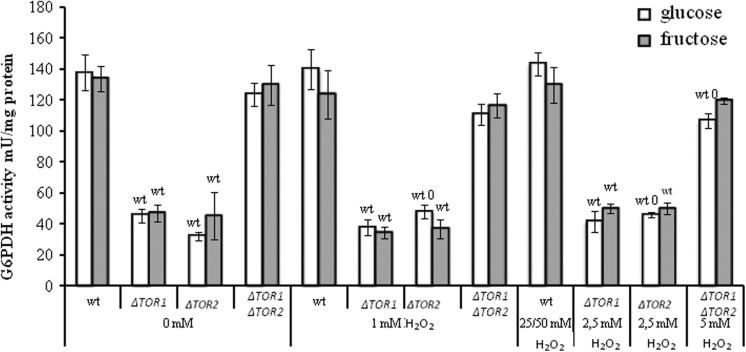
Mutation in Either TOR1 or TOR2 Genes Causes Decline in the Activity of Glucose-6-Phosphate Dehydrogenase
Like GR, G6PDH is involved in the stabilization of the pool of reduced glutathione, important antioxidant and antiglycation agent in the cell.28,42 Under stress conditions, GR and G6PDH were found to demonstrate a strong relationship,33,44 however in the present study the enzymes showed different behaviors in the yeast treated with H2O2 (Figures 2 and 3). Unlike GR, the activity of G6PDH was not affected by H2O2 in neither wild type nor TOR1 single knockout (both glucose- and fructose-grown). In the TOR2 single mutant, H2O2 at the 2 concentrations used increased G6PDH activity by 1.5-fold in glucose-supplemented medium, whereas did not affect the parameter in fructose-grown TOR2 mutant cells. The activity of G6PDH was slightly decreased in the TOR1 TOR2 double mutant after incubation with the 2 concentrations of H2O2 (1 mmol/L and 5 mmol/L H2O2), independent of the type of carbohydrate in the growth medium.
Figure 3.
The activity of glucose-6-phosphate in wild type and target of rapamycin (TOR)-deficient Saccharomyces cerevisiae incubated with hydrogen peroxide. Results are shown as the mean ± standard error of the mean (SEM; n = 3-7). Significantly different from respective values wtfor wild type with P < .05, Gfor cells growing on glucose with P < .05, and 0for corresponding control (without hydrogen peroxide [H2O2]) cells growing on respective monosaccharide with P < .05.
Comparison of G6PDH activities in the parental and mutant strains clearly indicated that the parental strain had the highest value, while both the single knockouts had the lowest values (3-4-fold lower than wild type), independent of the experimental conditions. The double knockout and wild type did not differ significantly from each other in their G6PDH activities under control conditions, however the TOR1 TOR2 mutant had somewhat lower activity of G6PDH than that in the wild type after treatment with 5 mmol/L H2O2.
When compared the 2 studied cell groups (glucose- and fructose-grown), no significant difference was observed between them in all strains and under all experimental conditions used in this study.
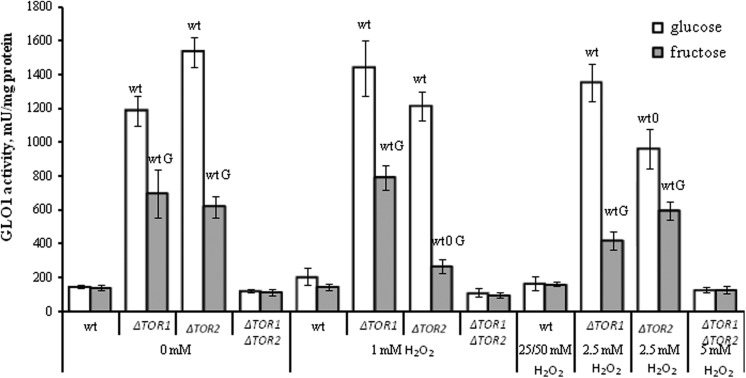
Mutation in Either TOR1 or TOR2 Genes Activates Glyoxalase 1
Glyoxalase 1 is functionally associated with GR and G6PDH via reduced glutathione, since the latter is used by GLO1 to detoxify the reactive carbonyl metabolites produced during carbohydrate metabolism.27,28 Figure 4 shows that H2O2 did not change GLO1 activity under any experimental conditions used in all strains studied, except the TOR2 mutant where the parameter was overall decreased by 1.3- to 2.3-fold in H2O2-treated cells compared to the respective controls.
Figure 4.
The activity of glyoxalase 1 in wild-type and target of rapamycin (TOR)-deficient Saccharomyces cerevisiae incubated with hydrogen peroxide. Results are shown as the mean ± standard error of the mean (SEM; n = 3-6). Significantly different from respective values wtfor wild type with P < .05, Gfor cells growing on glucose with P < .05, and 0for corresponding control (without hydrogen peroxide [H2O2]) cells growing on respective monosaccharide with P < .05.
Unlike G6PDH, GLO1 activity in the parental strain demonstrated the lowest value, while both the single knockouts had the highest parameters (2- to 11-fold higher than that in the wild type), independent of the experimental conditions. Like G6PDH, GLO1 activities did not differ significantly in both the wild type and the double knockout of all experimental groups of cells.
Comparing the 2 studied cell groups (glucose- and fructose-grown), no significant difference was observed between them in the parental and double mutant strains, independent of H2O2 concentrations used. At the same time, both the single mutants demonstrated GLO1 activity higher by 1.6- to 3.2-fold in glucose- than fructose-supplemented medium at all H2O2 concentrations used.
Discussion
Recent studies strongly support the notion that H2O2 plays a dual role in biological systems.6,21,45,46 Its effects can be considered as either beneficial or harmful, because at high concentrations H2O2 causes a stress, when oxidative damage to cell structures occurs, whereas at low concentrations it is a part of many cellular signaling systems. At low concentrations, H2O2 also plays a crucial role in the induction of hormesis.6,7,21,47 The potential effect of H2O2 depends not only on its concentration but also on the physiological state of the cell. Using the model organism S. cerevisiae, we have recently shown that fructose-grown yeast exposed to low concentrations of H2O2 demonstrated higher reproductive ability than glucose-grown cells.10
The experiment mentioned earlier has been conducted with an exponential culture of S. cerevisiae YPH250, whereas in this study, we used S. cerevisiae JK9-3da and its derivatives TOR mutants grown to early stationary phase. Although different strains may have various sensitivities to oxidants, and stationary culture is more resistant to stress than exponential one,33,48 the present study is consistent with the previous work10 and extends it with the first report on the monosaccharide- and TOR-dependent H2O2-induced hormetic response.
The findings of the present study demonstrate that both the cell types studied (glucose- and fructose-grown) respond differently to exogenous H2O2 (Figure 1A). Although in both cases we observe typical biphasic concentration–response curve, exhibiting a hormetic effect of H2O2, H2O2 triggers the hormetic response in the 2 cell groups at different concentrations. While the reproductive ability of cells grown on glucose reached a maximum value after yeast incubation with 25 mmol/L H2O2, in fructose-grown cells the peak hormetic response shifted to a higher concentration of 50 mmol/L H2O2. This is consistent with our previous suggestion on the ability of fructose to induce a mild carbonyl/oxidative stress stimulating cellular defensive mechanisms responsible for cell survival under more severe stress.10,41 The last mechanism can be posited from the in vitro and in vivo studies reporting that fructose is a more potent glycoxidation agent, than glucose, and therefore capable of producing greater amounts of reactive species.11–14
The oxidative stress response in S. cerevisiae has been analyzed in detail at the transcriptome, posttranscriptome, proteome, and postproteome levels. H2O2 induces the yeast stimulon of at least 115 genes and suppresses another 52 genes.26 Cellular functions affected by H2O2 are quite different: from enzymes involved in the carbohydrate metabolism to heat shock proteins and proteases.26,49 The antioxidant enzymes induced by H2O2 in S. cerevisiae at the transcription and translation levels are Cu, Zn-, and Mn-containing superoxide dismutases; cytosolic catalase T; cytochrome c peroxidase; GR; G6PDH; and so on.26,49–51 Antiglycation GLO1 enzyme has not been found among the members of H2O2 regulon in S. cerevisiae; moreover, inhibition of GLO1 by exogenous and endogenous H2O2 has been reported in several experimental models.52,53
An increasing number of studies have reported a lack of correlation between gene expression at different levels of cellular organization.50,51,54–56 The weak elevation in antioxidant enzyme activities in yeast treated with sublethal concentrations of H2O2 10,33,48,57 did not correspond to reported earlier high level of synthesis of the respective enzyme molecules.26,58 Exposure of microorganisms to low hormetic concentrations of H2O2 leads to the acquisition of cellular resistance to a subsequent lethal stress.7,8,59 We may suppose a rapid reorganization of gene expression and accumulation of nonactive synthesized de novo stress-protectant molecules in yeast cells exposed to low hormetic doses of H2O2, since a sudden increase in the antioxidant activities could dramatically disturb the intracellular redox homeostasis leading to other kinds of stress, for instance reductive stress.42 Nevertheless, such accumulation provides the cells with the capability to respond quickly and survive consequent lethal challenge via rapid posttranslational activation of synthesized de novo nonactive proteins.
Manipulation of the reproductive potential through hormesis-stimulating compounds, such as H2O2, appears to be an effective approach to improve yeast survival and cross-adaptation to different stresses.7,8 As one of the main signaling mechanisms, the TOR pathway by regulating cell growth and metabolism is involved in cellular response to many types of stress.6,17–20 In spite of the apparent and sometimes hotly debated contradictions between the hormesis and TOR theories, both are applied to explain some common mechanisms of aging as well as stress resistance.21,22 Our study demonstrates the involvement of the Tor1 and Tor2 proteins in the hormetic effect of H2O2 (Figure 1). The mutations in either TOR1 (Figure 1B) or TOR2 (Figure 1C) genes make yeast more susceptible to H2O2 that is reflected by the shift of the peak of the hormetic curve to a lower H2O2 concentration (glucose-grown cells) or even the disappearance of the peak (fructose-grown cells). Fructose- compared to glucose-cultivated single knockouts, like the parental strain, are more resistant to high concentrations of H2O2 that corresponds well to the previous data on the protective role of fructose against exogenous stress.10,12,37–41 Similar behavior of the single mutants under the same experimental conditions (Figure 1B and C) can be explained by the fact that TOR1 and TOR2 share common functions and therefore can compensate in some way the loss of each other.19,60,61
Since for the double mutant (Figure 1D) the peak hormetic response is found in the both studied cell groups (glucose- and fructose-grown) at somewhat higher concentration (5 mmol/L H2O2) than for the respective single mutants (2.5 mmol/L H2O2), a simultaneous loss of both TOR1 and TOR2 genes can be suggested to activate some additional compensatory mechanism(s). One obvious candidate for compensation for the inactivation of TOR signaling is the protein kinase Sch9p that has been found to act downstream or in parallel with the TOR pathway.60–62 Previous data suggest that having TOR-dependent and -independent roles in the general stress response Sch9 is essential for yeast adaptation to stress conditions when TOR is inactive.62,63 When active, TOR as well as Sch9 inhibits the Rim15p kinase that is found to activate its downstream target Msn2/4p, which in turn induces expression of common effectors of stress tolerance.17 Besides TOR and Sch9, the activity of Rim15p can be controlled by at least such highly conserved pathways as RAS and PKA.17,64 The pathways mentioned earlier can be involved as compensatory mechanisms for each other deficiency.
General mechanisms of yeast adaptation to stress and hormetic response to H2O2 can be suggested to involve enzymes such as GR, G6PDH, and GLO1. Glutathione reductase plays a crucial role in maintaining the proper range of such important low-weight antioxidant agents as reduced glutathione. The thiol group of reduced glutathione can directly or in concert with other antioxidants reduce and detoxify reactive oxygen species.28,65 Glucose-6-phosphate dehydrogenase produces the reduced equivalents NADPH, which are used by GR to reduce oxidized glutathione, and therefore G6PDH also plays a significant role in cell protection against H2O2 as well as other reactive species. Besides its antioxidant role, reduced glutathione is an important part of antiglycation system that eliminates reactive carbonyl metabolites, which continuously produced during metabolism of carbohydrates.13,27,43 Especially, utilization of fructose can lead to high generation of reactive metabolites.11,12,14 For instance, reduced glutathione is consumed in the reaction catalyzed by GLO1, the fundamental function of which is the metabolism of reactive carbonyl species to less reactive products.27,28 In general, the enzymes mentioned previously are important in the metabolism of carbohydrates as well as H2O2,13,27,42,43 however little is known about their role in hormetic response as well as functional connection with TOR.
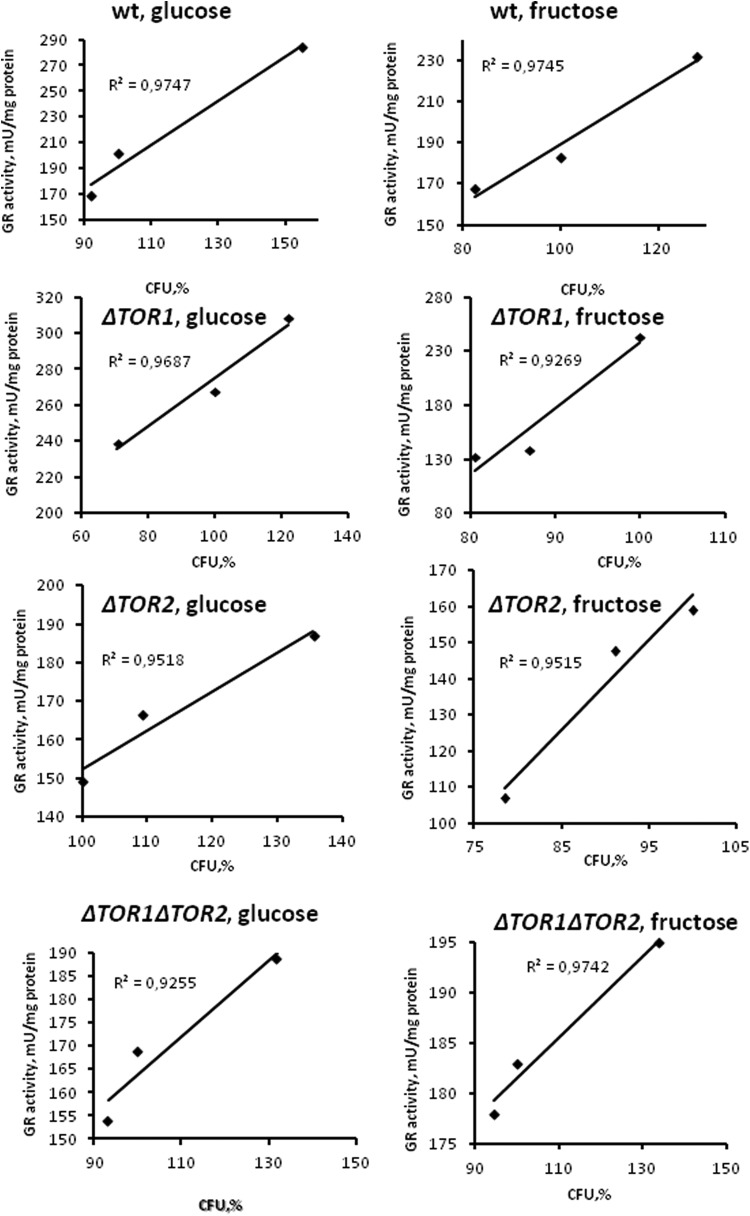
Since GR is a member of H2O2 stimulon in S. cerevisiae,26,50,51 it is not surprising that the hormetic concentrations of H2O2 increase the enzyme activity (Figure 2). Interestingly, the GR activation accompanies the hormetic increase in the yeast reproductive ability (Figure 1). In all experimental cell types, including fructose-grown TOR1 and TOR2 single mutants (no H2O2-induced hormesis), a strong positive correlation between GR activity and reproductive ability is found (Figure 5). These data suggest an important role of GR in the overall H2O2-induced stress response and hormetic response in yeast.
Figure 5.
Correlation analysis of data obtained with wild type and target of rapamycin (TOR)-deficient Saccharomyces cerevisiae strains treated with different hydrogen peroxide (H2O2) concentrations: correlating between yeast reproductive ability and glutathione reductase activity.
Activity of G6PDH, another member of H2O2-stimulon in S. cerevisiae,26,50,51 usually tightly correlates with GR activity under stress conditions.33,44 However, in the present study, the enzymes show different behaviors. Unlike GR (Figure 2), the activity of G6PDH was not affected by H2O2 (Figure 3). One more interesting fact can be observed when compared the activities of G6PDH in the strains used in this study—the TOR1 and TOR2 single knockouts have G6PDH activity significantly lower than that in the parental strain, independent of the experimental conditions used. Our current data on the reduced activity of G6PDH in the cells lacking either TOR1 or TOR2 genes correspond well to recent studies reported suppressed G6pd gene induction as a result of TOR inhibition in mammalian cells.66,67 Using a combination of genomic, metabolomic, and bioinformatic analysis, Düvel and coworkers found that mammalian target of rapamycin (mTOR) activation stimulated a gene network controlling specific metabolic pathways such as lipid biosynthesis, glycolysis, and pentose phosphate pathway, whereas rapamycin treatment decreased the expression levels of these genes.66 The mTOR signaling has been shown to regulate the pentose phosphate pathway by controlling the expression of the G6pd gene as well as the posttranscriptional levels of G6PDH.66,67 The authors have demonstrated that cell treatment with rapamycin stimulated 75 genes (mTORC1 repressed) and inhibited 130 other genes (mTORC1 induced). It was recently found that rapamycin and other TOR inhibitors changed the level of 24 metabolites in S. cerevisiae.68 Metabolic intermediates involved in the pentose phosphate pathway were strongly downregulated in response to TOR2 inhibition by rapamycin. Since the activity of G6PDH in the TOR1 TOR2 double mutant does not differ from that in the wild type (Figure 3), it is likely that some compensatory mechanisms help to correct G6PDH activity and compensate for simultaneous loss of both TOR1 and TOR2 genes in the double knockout.
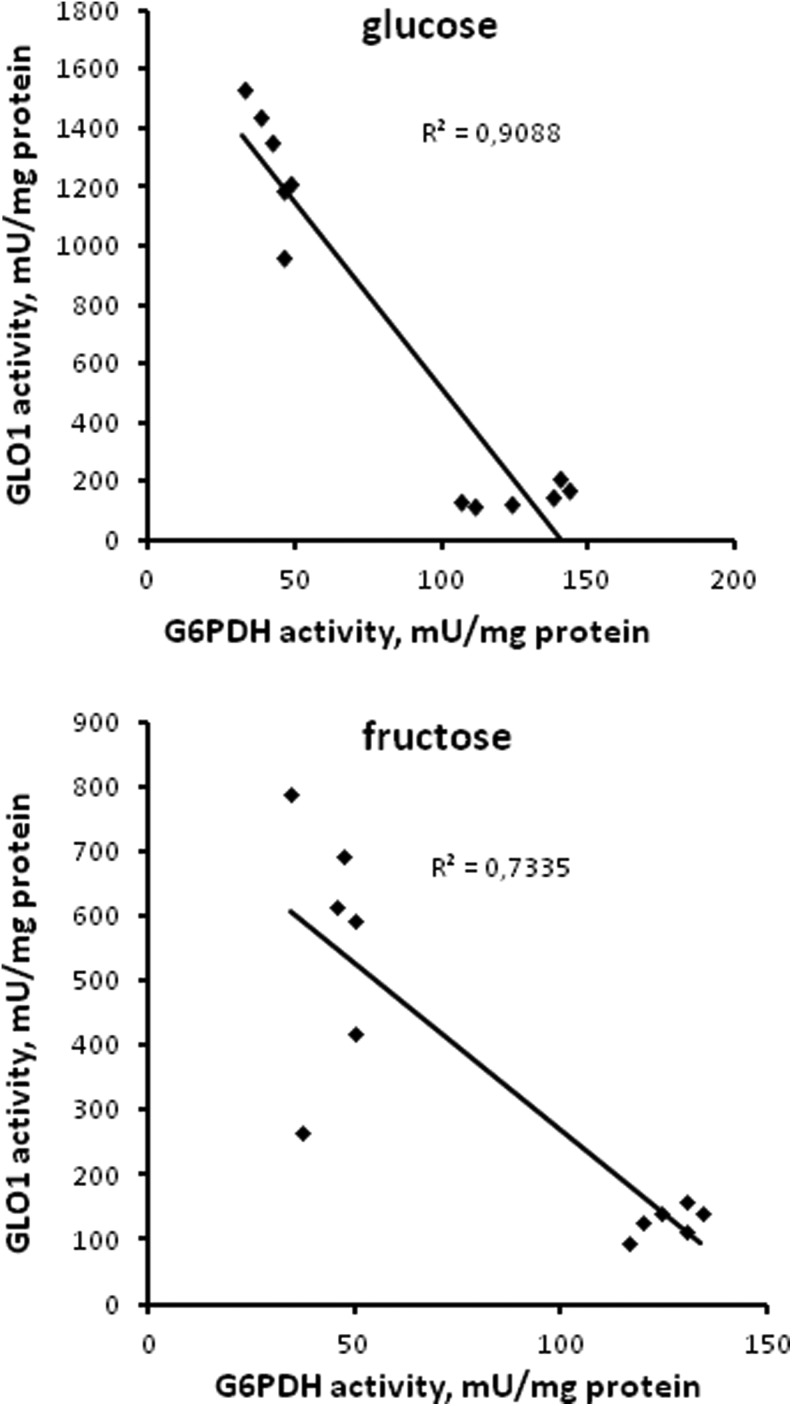
In spite of the functional relationship between GR, G6PDH, and GLO1, the last one demonstrates a pattern of the activity (Figure 4) quite different from GR (Figure 2) and G6PDH (Figure 3). Moreover, when GLO1 activity was plotted against the activity of G6PDH, a strong negative correlation between the parameters can be seen (Figure 6). Unlike G6PDH, GLO1 activity in both TOR1 and TOR2 single knockouts was significantly higher than that in the parental strain and the double knockout. From the previous studies, it is known that glucose and other nutrients activate Sch9-, Ras-, PKA-, and TOR-dependent pathways that, in turn, inhibit the protein kinase Rim15 and prevent Rim15-dependent induction of many important genes, including those involved in the carbohydrate metabolism.17,69,70 Earlier it was also shown that the basal level of GLO1 messenger RNA was about 3-fold lower in ▵rim15 mutant cells.71 Therefore, it can be suggested that in the wild-type cells, active TOR suppresses Rim15-dependent activity of GLO1, whereas loss and inhibition of TOR1 or TOR2 lead to GLO1 activation. However, it is not case when both TOR1 and TOR2 are depleted simultaneously.
Figure 6.
Correlation analysis of data obtained with wild type and target of rapamycin (TOR)-deficient Saccharomyces cerevisiae strains treated with different hydrogen peroxide (H2O2) concentrations: correlating between glucose-6-phosphate dehydrogenase and glyoxalase 1 activity.
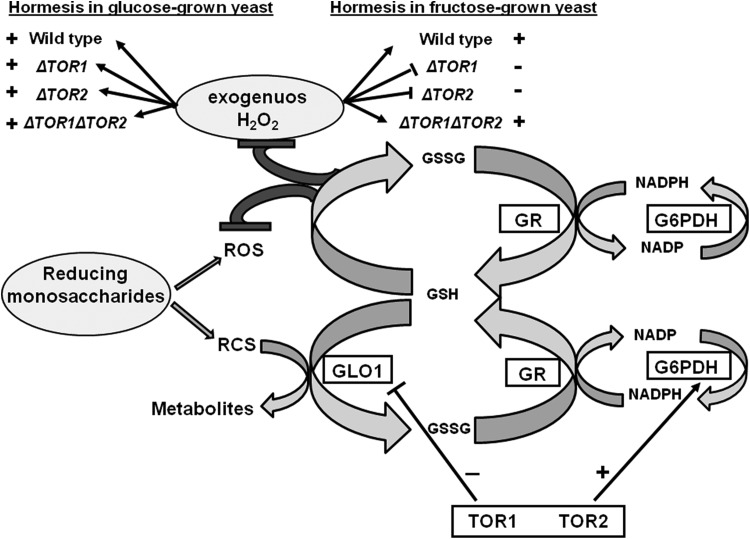
In summary (Figure 7), several interesting conclusions can be drawn: (1) H2O2-induced hormetic response in S. cerevisiae depends on the type of carbohydrate in the growth medium; (2) H2O2 induces hormesis in a TOR-dependent manner; (3) concentration-dependent effect of H2O2 on yeast colony growth positively correlates with GR activity that suggests the enzyme involvement in overall H2O2-induced stress response and hormetic response in yeast; and (4) TOR1 and TOR2 are involved in the positive regulation of G6PDH and the negative regulation of GLO1.
Figure 7.
Relationship between hydrogen peroxide (H2O2)-induced hormetic response in Saccharomyces cerevisiae, target of rapamycin (TOR) proteins, and enzymes involved in detoxification of either exogenous or produced during carbohydrate metabolism reactive species. G6PDH indicates glucose-6-phosphate dehydrogenase; GLO1, glyoxalase 1; GR, glutathione reductase; GSH, reduced glutathione; GSSG, oxidized glutathione; RCS, reactive carbonyl species; ROS, reactive oxygen species.
Acknowledgments
The authors are grateful to Prof Michael Hall for providing the yeast strains and Dr Liudmyla Lozinska for critical reading of the manuscript. H.S. would like to express sincere gratitude and appreciation to Hmeleva Humanitarian-Investment Project and Mr Vladyslav Kyrychenko for support and providing creative work atmosphere, in which the manuscript has been prepared. Two anonymous referees are gratefully acknowledged for their highly professional and helpful work improving the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Martins I, Galluzzi L, Kroemer G. Hormesis, cell death and aging. Aging (Albany NY). 2011;3(9):821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calabrese EJ. Toxicology rewrites its history and rethinks its future: giving equal focus to both harmful and beneficial effects. Environ Toxicol Chem. 2011;30(12):2658–2673. [DOI] [PubMed] [Google Scholar]
- 3. Calabrese V, Cornelius C, Cuzzocrea S, Iavicoli I, Rizzarelli E, Calabrese EJ. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol Aspects Med. 2011;32(4-6):279–304. [DOI] [PubMed] [Google Scholar]
- 4. Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224c:164–175. [DOI] [PubMed] [Google Scholar]
- 5. Lushchak VI. Dissection of the hormetic curve: analysis of components and mechanisms. Dose Response. 2014;12(3):466–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ludovico P, Burhans WC. Reactive oxygen species, ageing and the hormesis police. FEMS Yeast Res. 2014;14(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Semchyshyn H. Hormetic concentrations of hydrogen peroxide but not ethanol induce cross-adaptation to different stresses in budding yeast. Int J Microbiol. 2014;2014:485792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Semchyshyn HM, Abrat OB, Miedzobrodzki J, Inoue Y, Lushchak VI. Acetate but not propionate induces oxidative stress in bakers’ yeast Saccharomyces cerevisiae. Redox Rep. 2011;16(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calabrese EJ. Hormesis within a mechanistic context. Homeopathy. 2015;104(2):90–96. [DOI] [PubMed] [Google Scholar]
- 10. Semchyshyn HM, Lozinska LM. Fructose protects baker’s yeast against peroxide stress: potential role of catalase and superoxide dismutase. FEMS Yeast Res. 2012;12(7):761–773. [DOI] [PubMed] [Google Scholar]
- 11. Sakai M, Oimomi M, Kasuga M. Experimental studies on the role of fructose in the development of diabetic complications. Kobe J Med Sci. 2002;48(5-6):125–136. [PubMed] [Google Scholar]
- 12. Spasojević I, Bajić A, Jovanović K, Spasić M, Andjus P. Protective role of fructose in the metabolism of astroglial C6 cells exposed to hydrogen peroxide. Carbohydr Res. 2009;344(13):1676–1681. [DOI] [PubMed] [Google Scholar]
- 13. Semchyshyn H, Miedzobrodzki J, Bayliak M, Lozinska L, Homza B. Fructose compared with glucose is more a potent glycoxidation agent in vitro, but not under carbohydrate-induced stress in vivo: potential role of antioxidant and antiglycation enzymes. Carbohydr Res. 2014;384:61–69. [DOI] [PubMed] [Google Scholar]
- 14. Semchyshyn HM, Lozinska LM, Miedzobrodzki J, Lushchak VI. Fructose and glucose differentially affect aging and carbonyl/oxidative stress parameters in Saccharomyces cerevisiae cells. Carbohydr Res. 2011;346(7):933–938. [DOI] [PubMed] [Google Scholar]
- 15. Rattan SI, Demirovic D. Hormesis can and does work in humans. Dose Response. 2009;8(1):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Torelli NQ, Ferreira-Júnior JR, Kowaltowski AJ, da Cunha FM. RTG1- and RTG2-dependent retrograde signaling controls mitochondrial activity and stress resistance in Saccharomyces cerevisiae . Free Radic Biol Med. 2015;81:30–37. [DOI] [PubMed] [Google Scholar]
- 17. Weinberger M, Mesquita A, Caroll T, et al. Growth signaling promotes chronological aging in budding yeast by inducing superoxide anions that inhibit quiescence. Aging (Albany NY). 2010;2(10):709–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Milisav I, Poljsak B, Šuput D. Adaptive response, evidence of cross-resistance and its potential clinical use. Int J Mol Sci. 2012;13(9):10771–10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Betz C, Hall MN. Where is mTOR and what is it doing there? J Cell Biol. 2013;203(4):563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Afanas’ev I. Signaling and damaging functions of free radicals in aging-free radical theory, hormesis, and TOR. Aging Dis. 2010;1(2):75–88. [PMC free article] [PubMed] [Google Scholar]
- 22. Blagosklonny MV. Hormesis does not make sense except in the light of TOR-driven aging. Aging (Albany NY). 2011;3(11):1051–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103(2):253–262. [DOI] [PubMed] [Google Scholar]
- 24. Crespo JL, Hall MN. Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae . Microbiol Mol Biol Rev. 2002;66(4):579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab. 2009;296(4):e592–e602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Godon C, Lagniel G, Lee J, et al. The H2O2 stimulon in Saccharomyces cerevisiae . J Biol Chem. 1998;273(35):22480–22489. [DOI] [PubMed] [Google Scholar]
- 27. Inoue Y, Maeta K, Nomura W. Glyoxalase system in yeasts: structure, function, and physiology. Semin Cell Dev Biol. 2011;22(3):278–284. [DOI] [PubMed] [Google Scholar]
- 28. Townsend DM, Lushchak VI, Cooper AJ. A comparison of reversible versus irreversible protein glutathionylation. Adv Cancer Res. 2014;122:177–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253(5022):905–909. [DOI] [PubMed] [Google Scholar]
- 30. Helliwell SB, Wagner P, Kunz J, Deuter-Reinhard M, Henriquez R, Hall MN. TOR1 and TOR2 are structurally and functionally similar but not identical phosphatidylinositol kinase homologues in yeast. Mol Cell Biol. 1994;5(1):105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmidt A, Kunz J, Hall MN. TOR2 is required for organization of the actin cytoskeleton in yeast. Proc Natl Acad Sci U S A. 1996;93(24):13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Helliwell SB, Howald I, Barbet N, Hall MN. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics. 1998;148(1):99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bayliak M, Semchyshyn H, Lushchak V. Effect of hydrogen peroxide on antioxidant enzyme activities in Saccharomyces cerevisiae is strain-specific. Biochemistry (Moscow). 2006;71(9):1013–1020. [DOI] [PubMed] [Google Scholar]
- 34. Conconi A, Jager-Vottero P, Zhang X, Beard BC, Smerdon MJ. Mitotic viability and metabolic competence in UV-irradiated yeast cells. Mutat Res. 2000;459(1):55–64. [DOI] [PubMed] [Google Scholar]
- 35. Bhor VM, Raghuram N, Sivakami S. Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabetic rats. Int J Biochem Cell Biol. 2004;36(1):89–97. [DOI] [PubMed] [Google Scholar]
- 36. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 37. Valeri F, Boess F, Wolf A, Göldlin C, Boelsterli UA. Fructose and tagatose protect against oxidative cell injury by iron chelation. Free Radic Biol Med. 1996;22(1-2):257–268. [DOI] [PubMed] [Google Scholar]
- 38. Frenzel J, Richter J, Eschrich K. Fructose inhibits apoptosis induced by reoxygenation in rat hepatocytes by decreasing reactive oxygen species via stabilization of the glutathione pool. Biochim Biophys Acta. 2002;1542(1-3):82–94. [DOI] [PubMed] [Google Scholar]
- 39. Bogdanović J, Mojović M, Milosavić N, Mitrović A, Vucinić Z, Spasojević I. Role of fructose in the adaptation of plants to cold-induced oxidative stress. Eur Biophys J. 2008;37(7):1241–1246. [DOI] [PubMed] [Google Scholar]
- 40. MacAllister SL, Choi J, Dedina L, O’Brien PJ. Metabolic mechanisms of methanol/formaldehyde in isolated rat hepatocytes: carbonyl-metabolizing enzymes versus oxidative stress. Chem Biol Interact. 2011;191(1-3):308–314. [DOI] [PubMed] [Google Scholar]
- 41. Semchyshyn HM. Fructation in vivo: detrimental and protective effects of fructose. BioMed Res Int. 2013;2013:343914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lushchak VI. Adaptive response to oxidative stress: bacteria, fungi, plants and animals. Comp Biochem Physiol C Toxicol Pharmacol. 2011;153(2):175–190. [DOI] [PubMed] [Google Scholar]
- 43. Semchyshyn HM, Lushchak VI. Interplay between oxidative and carbonyl stresses: molecular mechanisms, biological effects and therapeutic strategies of protection In: Lushchak VI, Semchyshyn HM. (eds.), Oxidative Stress – Molecular Mechanisms and Biological Effects. Rijeka, Croatia: InTech; 2012: 15–46. [Google Scholar]
- 44. Bolin AP, Guerra BA, Nascimento SJS, Otton R. Changes in lymphocyte oxidant/antioxidant parameters after carbonyl and antioxidant exposure. Int Immunopharmacol. 2012;14(4):690–697. [DOI] [PubMed] [Google Scholar]
- 45. Ruttkay-Nedecky B, Nejdl L, Gumulec J, et al. The role of metallothionein in oxidative stress. Int J Mol Sci. 2013;14(3):6044–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bleier L, Wittig I, Heide H, Steger M, Brandt U, Dröse S. Generator-specific targets of mitochondrial reactive oxygen species. Free Radic Biol Med. 2015;78:1–10. [DOI] [PubMed] [Google Scholar]
- 47. Mesquita A, Weinberger M, Silva A, et al. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc Natl Acad Sci U S A. 2010;107(34):15123–15128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bayliak M, Semchyshyn H, Lushchak V. Possible accumulation of non-active molecules of catalase and superoxide dismutase in S. cerevisiae cells under hydrogen peroxide induced stress. Cent Eur J Biol. 2007;2(3):326–336. [Google Scholar]
- 49. Vandenbroucke K, Robbens S, Vandepoele K, Inzé D, Van de Peer Y, Van Breusegem F. Hydrogen peroxide-induced gene expression across kingdoms: a comparative analysis. Mol Biol Evol. 2008;25(3):507–516. [DOI] [PubMed] [Google Scholar]
- 50. Semchyshyn H. Hydrogen peroxide-induced response in E. coli and S. cerevisiae: different stages of the flow of the genetic information. Cent Eur J Biol. 2009;4(2):142–153. [Google Scholar]
- 51. Lushchak VI. Oxidative stress in yeast. Biochemistry (Moscow). 2010;75(3):281–296. [DOI] [PubMed] [Google Scholar]
- 52. Okado-Matsumoto A, Fridovich I. The role of alpha, beta-dicarbonyl compounds in the toxicity of short chain sugars. J Biol Chem. 2000;275(45):34853–34857. [DOI] [PubMed] [Google Scholar]
- 53. Antognelli C, Gambelunghe A, Talesa VN, Muzi G. Reactive oxygen species induce apoptosis in bronchial epithelial BEAS-2B cells by inhibiting the antiglycation glyoxalase I defence: involvement of superoxide anion, hydrogen peroxide and NF-κB. Apoptosis. 2014;19(1):102–116. [DOI] [PubMed] [Google Scholar]
- 54. Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19(3):1720–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cyrne L, Martins L, Fernandes L, Marinho HS. Regulation of antioxidant enzymes gene expression in the yeast Saccharomyces cerevisiae during stationary phase. Free Radic Biol Med. 2003;34(3):385–393. [DOI] [PubMed] [Google Scholar]
- 56. Holcik M, Sonenberg N. Translational control in stress apoptosis. Mol Cell Biol. 2005;6(4):318–327. [DOI] [PubMed] [Google Scholar]
- 57. Martins V, Manfredini V, Benfato MS. High levels of catalase in SOD mutants of Saccharomyces cerevisiae in high aeration conditions. Braz J Microbiol. 2005;36(4):347–351. [Google Scholar]
- 58. Lee J, Godon C, Lagniel G, et al. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J Biol Chem. 1999;274(23):16040–16046. [DOI] [PubMed] [Google Scholar]
- 59. Collinson LP, Dawes IW. Inducibility of the response of yeast cells to peroxide stress. J Gen Microbiol. 1992;138(2):329–335. [DOI] [PubMed] [Google Scholar]
- 60. Shertz CA, Bastidas RJ, Li W, Heitman J, Cardenas ME. Conservation, duplication, and loss of the Tor signaling pathway in the fungal kingdom. BMC Genomics. 2010;11:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189(4):1177–11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smets B, De Snijder P, Engelen K, et al. Genome-wide expression analysis reveals TORC1-dependent and -independent functions of Sch9. FEMS Yeast Res. 2008;8(8):1276–1288. [DOI] [PubMed] [Google Scholar]
- 63. Pascual-Ahuir A, Proft M. Control of stress-regulated gene expression and longevity by the Sch9 protein kinase. Cell Cycle. 2007;6(20):2445–2447. [DOI] [PubMed] [Google Scholar]
- 64. Welch AZ, Gibney PA, Botstein D, Koshland DE. TOR and RAS pathways regulate desiccation tolerance in Saccharomyces cerevisiae. Mol Biol Cell. 2013;24(2):115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med. 1999;27(3-4):322–328. [DOI] [PubMed] [Google Scholar]
- 66. Düvel K, Yecies JL, Menon S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39(2):171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tsouko E, Khan AS, White MA, et al. Regulation of the pentose phosphate pathway by an androgen receptor-mTOR-mediated mechanism and its role in prostate cancer cell growth. Oncogenesis. 2014;3:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kliegman JI, Fiedler D, Ryan CJ, et al. Chemical genetics of rapamycin-insensitive TORC2 in S. cerevisiae . Cell Rep. 2013;5(6):1725–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Swinnen E, Wanke V, Roosen J, et al. Rim15 and the crossroads of nutrient signalling pathways in Saccharomyces cerevisiae. Cell Div. 2006;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang N, Jian W, Oliver SG. Gis1 is required for transcriptional reprogramming of carbon metabolism and the stress response during transition into stationary phase in yeast. Microbiology. 2009;155(pt 5):1690–1698. [DOI] [PubMed] [Google Scholar]
- 71. Cameroni E, Hulo N, Roosen J, Winderickx J, De Virgilio C. The novel yeast PAS kinase Rim 15 orchestrates G0-associated antioxidant defense mechanisms. Cell Cycle. 2004;3(4):462–468. [PubMed] [Google Scholar]