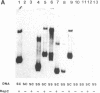
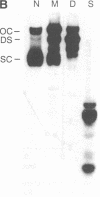
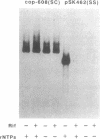
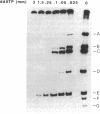
Abstract
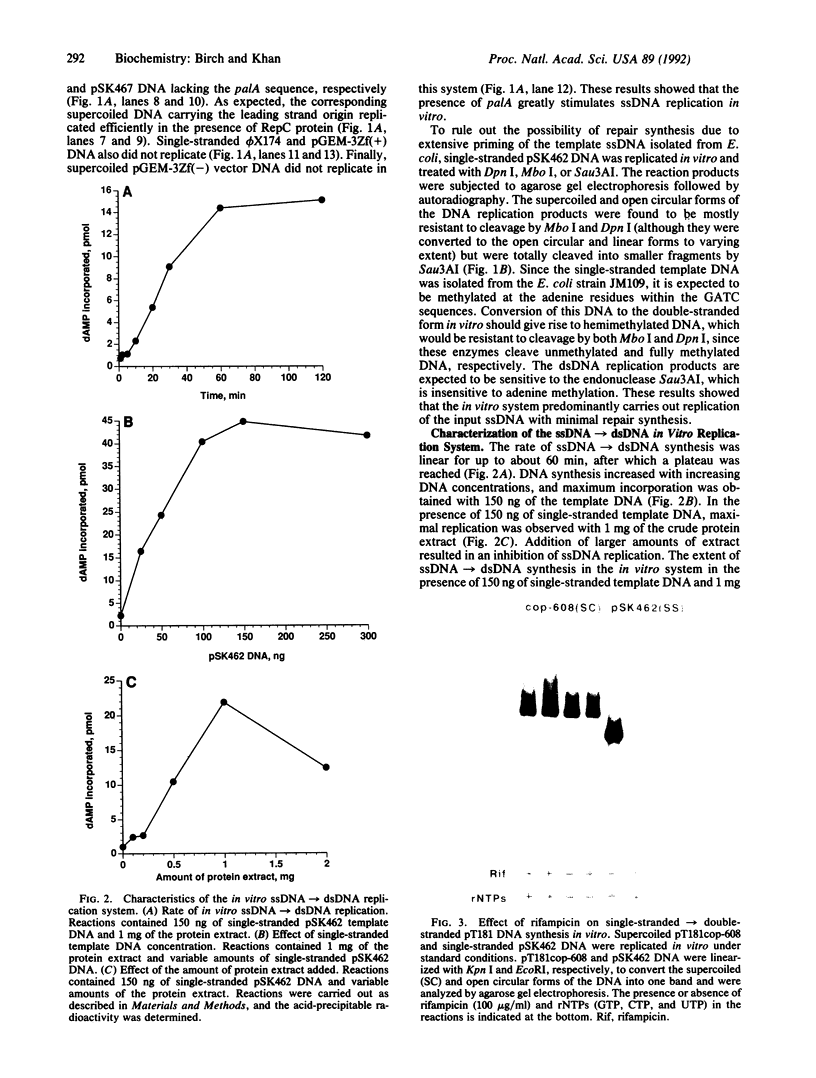
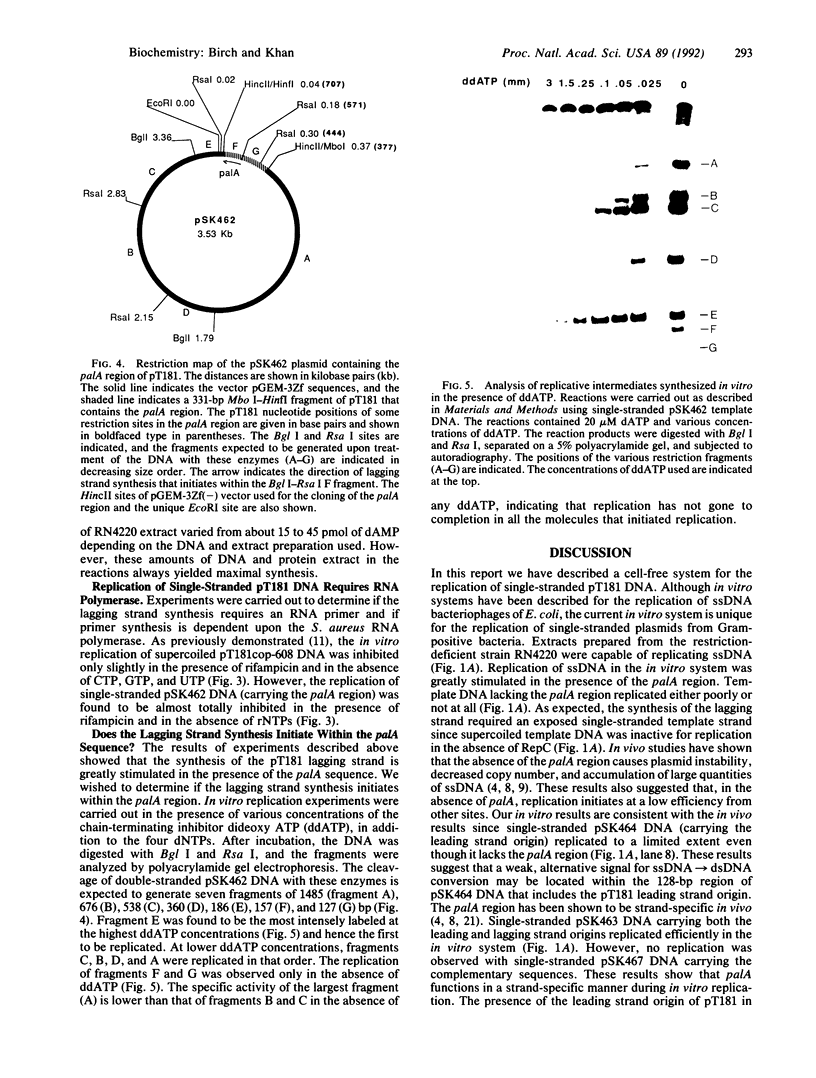
Plasmid pT181 is a 4437-base-pair, multicopy plasmid of Staphylococcus aureus that encodes tetracycline resistance. The replication of the leading strand of pT181 DNA initiates by covalent extension of a site-specific nick generated by the initiator protein at the origin of replication and proceeds by an asymmetric rolling circle mechanism. The origin of the leading strand synthesis also serves as the site for termination of replication. Replication of pT181 DNA in vivo and in vitro has been shown to generate a single-stranded intermediate that corresponds to the leading strand of the DNA. In vivo results have suggested that a palindromic sequence, palA, located near the leading strand termination site acts as the lagging strand origin. In this paper we report the development and characterization of an in vitro system for the replication of single-stranded pT181 DNA. Synthesis of the lagging strand of pT181 proceeded in the absence of the leading strand synthesis and did not require the pT181-encoded initiator protein, RepC. The replication of the lagging strand required RNA polymerase-dependent synthesis of an RNA primer. Replication of single-stranded pT181 DNA was found to be greatly stimulated in the presence of the palA sequence. We also show that palA acts as the lagging strand origin and that DNA synthesis initiates within this region.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boe L., Gros M. F., te Riele H., Ehrlich S. D., Gruss A. Replication origins of single-stranded-DNA plasmid pUB110. J Bacteriol. 1989 Jun;171(6):3366–3372. doi: 10.1128/jb.171.6.3366-3372.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché J. P., Rowen L., Kornberg A. The RNA primer synthesized by primase to initiate phage G4 DNA replication. J Biol Chem. 1978 Feb 10;253(3):765–769. [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Scott J. F., Kronberg A. Enzymatic replication of phiX174 duplex circles: continuous synthesis. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):295–302. doi: 10.1101/sqb.1979.043.01.036. [DOI] [PubMed] [Google Scholar]
- Geider K., Beck E., Schaller H. An RNA transcribed from DNA at the origin of phage fd single strand to replicative form conversion. Proc Natl Acad Sci U S A. 1978 Feb;75(2):645–649. doi: 10.1073/pnas.75.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro M. L., Iordanescu S., Novick R. P., Murray R. W., Steck T. R., Khan S. A. Functional organization of the plasmid pT181 replication origin. J Mol Biol. 1989 Jan 20;205(2):355–362. doi: 10.1016/0022-2836(89)90346-x. [DOI] [PubMed] [Google Scholar]
- Gruss A. D., Ross H. F., Novick R. P. Functional analysis of a palindromic sequence required for normal replication of several staphylococcal plasmids. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2165–2169. doi: 10.1073/pnas.84.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss A., Ehrlich S. D. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989 Jun;53(2):231–241. doi: 10.1128/mr.53.2.231-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanescu S., Projan S. J. Replication termination for staphylococcal plasmids: plasmids pT181 and pC221 cross-react in the termination process. J Bacteriol. 1988 Aug;170(8):3427–3434. doi: 10.1128/jb.170.8.3427-3434.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A., Adler G. K., Novick R. P. Functional origin of replication of pT181 plasmid DNA is contained within a 168-base-pair segment. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4580–4584. doi: 10.1073/pnas.79.15.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A., Carleton S. M., Novick R. P. Replication of plasmid pT181 DNA in vitro: requirement for a plasmid-encoded product. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4902–4906. doi: 10.1073/pnas.78.8.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A., Novick R. P. Complete nucleotide sequence of pT181, a tetracycline-resistance plasmid from Staphylococcus aureus. Plasmid. 1983 Nov;10(3):251–259. doi: 10.1016/0147-619x(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Koepsel R. R., Khan S. A. Cleavage of single-stranded DNA by plasmid pT181-encoded RepC protein. Nucleic Acids Res. 1987 May 26;15(10):4085–4097. doi: 10.1093/nar/15.10.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsel R. R., Murray R. W., Rosenblum W. D., Khan S. A. Purification of pT181-encoded repC protein required for the initiation of plasmid replication. J Biol Chem. 1985 Jul 15;260(14):8571–8577. [PubMed] [Google Scholar]
- Koepsel R. R., Murray R. W., Rosenblum W. D., Khan S. A. The replication initiator protein of plasmid pT181 has sequence-specific endonuclease and topoisomerase-like activities. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6845–6849. doi: 10.1073/pnas.82.20.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- McMacken R., Ueda K., Kornberg A. Migration of Escherichia coli dnaB protein on the template DNA strand as a mechanism in initiating DNA replication. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4190–4194. doi: 10.1073/pnas.74.10.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. W., Koepsel R. R., Khan S. A. Synthesis of single-stranded plasmid pT181 DNA in vitro. Initiation and termination of DNA replication. J Biol Chem. 1989 Jan 15;264(2):1051–1057. [PubMed] [Google Scholar]
- Novick R. P. Staphylococcal plasmids and their replication. Annu Rev Microbiol. 1989;43:537–565. doi: 10.1146/annurev.mi.43.100189.002541. [DOI] [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. An Escherichia coli replication protein that recognizes a unique sequence within a hairpin region in phi X174 DNA. Proc Natl Acad Sci U S A. 1980 Feb;77(2):799–803. doi: 10.1073/pnas.77.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J., Dressler D. Site-specific initiation of a DNA fragment: nucleotide sequence of the bacteriophage G4 negative-strand initiation site. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3094–3098. doi: 10.1073/pnas.75.7.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Zinder N. D., Horiuchi K. Multiregulatory element of filamentous bacteriophages. Microbiol Rev. 1985 Jun;49(2):101–106. doi: 10.1128/mr.49.2.101-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H., Michel B., Ehrlich S. D. Are single-stranded circles intermediates in plasmid DNA replication? EMBO J. 1986 Mar;5(3):631–637. doi: 10.1002/j.1460-2075.1986.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H., Michel B., Ehrlich S. D. Single-stranded plasmid DNA in Bacillus subtilis and Staphylococcus aureus. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2541–2545. doi: 10.1073/pnas.83.8.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]