Abstract
We developed a novel PCR-based pre-amplification (PreAmp) technology that can increase the abundance of over 350 target genes one million-fold. To assess potential bias introduced by PreAmp we utilized ERCC RNA reference standards, a model system that quantifies measurement error in RNA analysis. We assessed three types of bias: amplification bias, dynamic range bias and fold-change bias. We show that our PreAmp workflow introduces only minimal amplification and fold-change bias under stringent conditions. We do detect dynamic range bias if a target gene is highly abundant and PreAmp occurred for 16 or more PCR cycles; however, this type of bias is easily correctable. To assess PreAmp bias in a gene expression profiling experiment, we analyzed a panel of genes that are regulated during differentiation using the NTera2 stem cell model system. We find that results generated using PreAmp are similar to results obtained using standard qPCR (without the pre-amplification step). Importantly, PreAmp maintains patterns of gene expression changes across samples; the same biological insights would be derived from a PreAmp experiment as with a standard gene expression profiling experiment. We conclude that our PreAmp technology can facilitate analysis of extremely limited samples in gene expression quantification experiments.
Abbreviations: PreAmp, pre-amplification; ERCC, external RNA controls consortium; NIST, National Institute of Standards and Technology; NT2, NTera2; RA, retinoic acid
Keywords: Pre-amplification, PreAmp, Gene expression profiling, Bias, PCR, ERCC
1. Introduction
Pre-amplification (PreAmp) of nucleic acid is a powerful technique that allows for the analysis of large numbers of target genes from limiting samples. PreAmp can be achieved through whole transcriptome amplification [1] or at the level of targeted gene panels using PCR-based methodology [2], [3]. However, there is a legitimate concern that PreAmp might change a sample to an extent that results generated from it are misleading or inaccurate. Better understanding of the limitations of a PreAmp-based workflow is necessary to ensure the reliability of research results.
To address such concerns, the National Institute of Standards and Technology (NIST), in conjunction with the External RNA controls consortium (ERCC) developed a set of reference standards to evaluate the performance of RNA quantification systems and workflows [4], [5]. The ERCC standards are mixtures of up to 96 synthetic RNAs that are spiked into an RNA sample and processed and quantified along with the natural RNAs. The amount of each ERCC RNA in a mixture is precisely defined; the performance of an RNA quantification workflow/platform is determined by comparing the measured amount with the actual, defined amount of each ERCC control RNA. In addition, by spiking two sets of ERCC standards (with defined ratios of each ERCC target) into two different biological samples, the accuracy in quantifying gene expression differences between samples can be determined. ERCC standards have been used to assess qPCR, digital PCR, microfluidic qPCR, microarray and RNA-seq platforms for their precision, accuracy and detection limits in RNA quantification [6], [7], [8], [9], [10].
There are two fundamental challenges in PreAmp reactions because multiple targets are amplified simultaneously. The first challenge is increasing the capacity of the amplification reaction to allow targets of vastly different starting quantity to be efficiently amplified through every PCR cycle. The second challenge is maintaining target amplification specificity in the presence of large numbers of primers. Unless both challenges are addressed in a PreAmp reagent, the probability of having biased pre-amplification will be high. We developed a PreAmp reagent that utilizes an engineered DNA polymerase with improved binding affinity to DNA and, consequently, dramatically increased processivity. This results in improved PreAmp reaction capacity which ensures that all targets are efficiently amplified [11]. We also focused our PreAmp reagent formulation efforts to mediate extremely stringent specificity of primer annealing.
To provide a stringent assessment of our new PreAmp reagent, we used the ERCC controls to quantify bias in a gene expression profiling experiment involving stem cell differentiation. We then used the ERCC information to discriminate regulated genes from background noise. Our findings, from analysis of ERCC standards and natural target genes, demonstrate that our novel PreAmp reagent will provide accurate results in gene expression profiling experiments.
2. Materials and methods
2.1. Cell culture and sample processing
NTera2 cells (NT2) were obtained from the American Type Culture Collection (Manassas, VA) and cultured as directed. To induce differentiation, cells were treated with 10 μM all-trans-Retinoic acid (Cat# R2625, Sigma–Aldrich, St. Louis MO) for 0, 1, 2, 3, 4 or 7 days. RNA was isolated using the Aurum Total RNA Mini Kit which incorporates an on-column genomic DNA clearance step (Cat# 7326820, Bio-Rad Laboratories, Hercules, CA), quantified with a NanoDrop ND-1000 spectrophotometer (Thermo Fischer Scientific, Waltham, MA) and stored at −80 °C. ERCC ExFold RNA Spike-in Mixes (Cat# 4456739, Thermo Fischer Scientific, Waltham, MA) were diluted and spiked into RNA samples which were then reverse transcribed using iScript Reverse Transcription Supermix as directed by the manufacturer (Cat# 1708849, Bio-Rad Laboratories, Hercules, CA) to make stock cDNA samples which were stored at −20 °C. Stock cDNA samples were pre-amplified for 10–20 cycles using SsoAdvanced PreAmp Supermix as directed by the manufacturer (Cat# 1725160, Bio-Rad Laboratories, Hercules, CA) diluted at least 5-fold and stored at −20 °C. PreAmp was performed with panels of assays which include PrimePCR PreAmp assays (Bio-Rad Laboratories, Hercules, CA), assays culled from public databases, assays designed using online software tools and assays from published manuscripts (Supplemental Fig. 1); primers were at a final concentration of 50 nM each. Target genes were quantified by qPCR using SsoAdvanced Universal SYBR Green Supermix (Cat# 1725270, Bio-Rad Laboratories, Hercules, CA) and CFX96 or CFX384 Real-Time PCR Detection Systems (Bio-Rad Laboratories, Hercules, CA) using 20 μl or 10 μl reaction volumes respectively. Genomic DNA contamination was assessed using the PrimePCR DNA Contamination Control (Cat# 10025352, Bio-Rad Laboratories, Hercules, CA). As a no-PreAmp control, stock cDNA was used to quantify gene targets directly by standard qPCR using SsoAdvanced Universal SYBR Green Supermix. qPCR conditions were: 98 °C for 2 min; 40 cycles of (98 °C, 5 s; 60 °C, 30 s); melt curve analysis, 65–98 °C, 0.5 °C increments, 5 s hold. CFX Manager version 3.1 was used for qPCR analysis (Bio-Rad Laboratories, Hercules, CA). A synthetic DNA interplate calibrator was used to set the Cq baseline.
2.2. Measurement of fold-change bias
The two ERCC ExFold RNA Spike-in mixes contain in-vitro transcribed RNA transcripts in 4 sub-pools that have set ratios between the mixes (1:1, 1:1.5, 1:2 and 4:1). To quantify fold-change bias, ExFold mixes were spiked into RNA samples and processed as described above; the amount of each ERCC target in each sample was then determined by qPCR. The 1:1 sub-pool was used as a calibrator to normalize the Mix 1 and Mix 2 results. To visualize bias the ΔCq value between the Mix 1 and Mix 2 samples was determined for each target and plotted against the average no PreAmp Cq value. Fold-change bias was calculated as the difference between the observed ΔCq and the theoretical ΔCq for each ERCC target. The theoretical ΔCq is 0, 0.585, 1.0 and 2.0 for the 1:1, 1:1.5, 1:2 and 4:1 sub-pools, respectively. Variation in fold-change measurement error was calculated using the equivalence test from JMP statistical software version 12.1.0 (SAS, Cary, NC), the practical difference threshold was specified as the standard deviation of the no PreAmp fold change measurement error.
2.3. Limits-of-detection of fold-change measurement
Fold-change bias 95% confidence intervals were determined using Microsoft Excel 2010 for the ΔΔCq values illustrated in Fig. 1B and rounded-up to the closest 0.5Cq. We analyzed three different ranges of target abundance, average no PreAmp Cq value less than 30, between 30 and 32.5, and greater than 32.5. The limit of detection was estimated as 2-fold the 95% confidence interval. For example, if the fold-change 95% confidence interval for targets between 30 and 32.5 was calculated as 1.0Cq, then 2-fold this value is 2.0Cq, which represents a 4-fold change. Therefore, targets with a no PreAmp Cq value between 30 and 32.5 have a 4-fold change limit of detection.
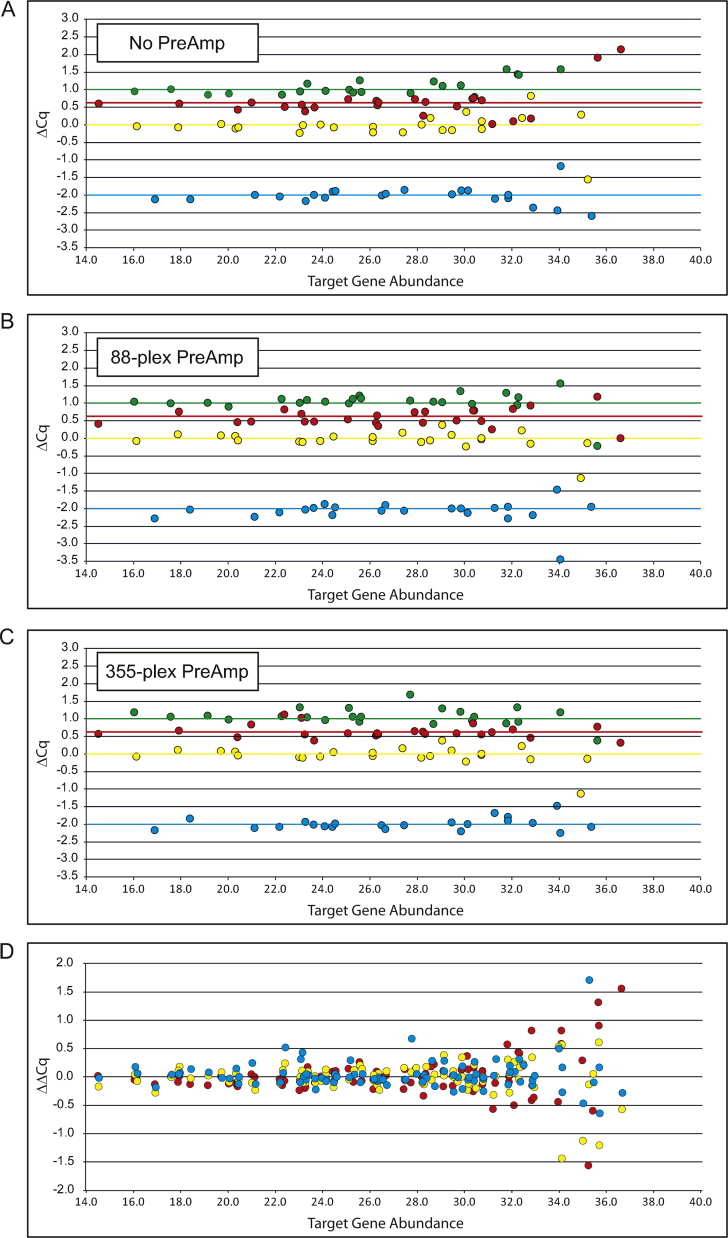
Fig. 1.
Fold-change bias of ERCC standards. ExFold spike in-mixtures were added to 10 ng NT2 RNA, reverse transcribed and pre-amplified for 14 PCR cycles. ERCC targets were quantified by qPCR using three technical replicates, the experiment was performed one time. Fold-change measurements for each ERCC target are plotted against target abundance. Results show the measured difference, between samples, of the amount of each ERCC target (colored circles) and the actual difference that should be detected (Fig. 1A–C; colored lines at 1.0, 0.585, 0 and −2.0 ΔCq units representing RNA abundance ratios of 2–1, 1.5–1, 1–1 and 1–4 respectively). (A) Analysis of samples that were not pre-amplified. (B) Analysis of samples in which 88 ERCC targets were pre-amplified. (C) Analysis of samples in which 355 targets were pre-amplified. (D) Overall fold-change bias for 88 ERCC targets. No PreAmp samples (red circles), 88-plex PreAmp samples (yellow circles), 355-plex PreAmp samples (blue circles).
2.4. Measurement of amplification bias
Analysis of the stock cDNA samples by PreAmp and by standard qPCR was performed three times independently. PreAmp amplification bias was calculated as “Amplification bias = (Cq Target (no-PreAmp) − Cq Target (PreAmp)) − 7.03” where 7.03 is a constant derived from 14 PreAmp cycles and 5-fold sample dilution, which reflects the theoretical ΔCq between the no-PreAmp and PreAmp target gene Cq values if PreAmp is 100% efficient; a PreAmp bias score of 0 indicates that target gene PreAmp is 100% efficient.
3. Results
3.1. Analysis of fold-change bias using ERCC controls
To assess bias introduced by PreAmp we used the ERCC ExFold RNA spike-in control product; this product consists of two tubes that contain the same ERCC RNAs at precisely defined amounts and ratios. Analysis of the ERCC standards spiked into two different biological samples reveals the ability of a workflow to quantify differences between samples [10]. We added the ExFold mixtures to two RNA samples and converted them to cDNA. We then pre-amplified the cDNA samples for 14 cycles using PreAmp primer panels targeting either 88 ERCC RNAs only (88-plex PreAmp) or the ERCC RNAs plus an additional 267 target genes (355-plex PreAmp, see Supplemental Table 1 for gene targets and assay information). We then quantified each ERCC transcript in the PreAmp’d samples as well as in the original, non-amplified cDNA samples.
Our results show the measured difference versus the actual pre-defined difference of each ERCC RNA spiked into the two samples (Fig. 1A–C). We find that fold-change measurement bias appears similar when comparing the no PreAmp, 88-plex PreAmp and 355-plex PreAmp results. In Fig. 1D we show the measurement error in fold-change results; no measurement error would have a ΔΔCq value of 0, a ΔΔCq value of 1 indicates a measurement error of 100%. We observe that measurement error increases as the amount of ERCC target RNA decreases. This finding is expected and is consistent with stochastic partitioning of a small number of RNA molecules in the qPCR reactions. We then quantified the fold-change measurement error associated with RNA abundance ranges (Table 1). We find that for RNAs with moderate to high abundance (Cq < 30) fold-change measurement error is, on average, only about 0.1Cq representing 7% error. For low abundance RNAs (Cq between 30 and 32.5) measurement error averages 20%, for trace RNAs (Cq > 32.5) 75% average measurement error is observed. Importantly, a test of statistical equivalence indicates that PreAmp (88-plex or 355-plex) has only a slight effect on fold-change measurement error relative to the no PreAmp samples (only the trace RNAs in the 88-plex PreAmp sample has a p-Value above the 0.05 threshold that indicates a PreAmp bias, the p-value is 0.0515). These findings are consistent with a previous report [6] and demonstrates that our PreAmp workflow only has low fold-change bias when analyzing trace expression target genes.
Table 1.
Fold-change measurement error.
| Target gene expression level (Cq) |
||||
|---|---|---|---|---|
| <30 | 30–32.5 | >32.5 | ||
| No PreAmp | Average fold change measurement error (Cq) | 0.093 | 0.265 | 0.804 |
| Average fold change measurement error (percent) | 7% | 20% | 75% | |
| 88-plex PreAmp | Average fold change measurement error (Cq) | 0.094 | 0.162 | 0.578 |
| Average fold change measurement error (percent) | 7% | 12% | 49% | |
| Statistical equivalence with no PreAmp. p-value (upper threshold) | <0.0001a | 0.0121a | 0.0515 | |
| Statistical equivalence with no PreAmp. p-value (lower threshold) | 0.0021a | 0.0003a | <0.0001a | |
| 355-plex PreAmp | Average fold change measurement error (Cq) | 0.108 | 0.119 | 0.380 |
| Average fold change measurement error (percent) | 8% | 9% | 30% | |
| Statistical equivalence with no PreAmp. p-value (upper threshold) | <0.0001a | 0.0010a | 0.0074a | |
| Statistical equivalence with no PreAmp. p-value (lower threshold) | 0.0205a | 0.0050a | 0.0002a | |
| Estimated fold-change limits-of-detection | 2-fold | 4-fold | 8-fold | |
Statistically equivalent with no PreAmp.
Our results also imply that the ability to detect a meaningful difference in RNA expression between two samples by RT-qPCR is a function of RNA abundance. Using the ERCC RNAs as an internal control we estimated that the limits-of-detection of fold-change measurement is 2-fold for moderately or highly expressed RNA targets, 4-fold for RNAs with low expression, and, at best, 8-fold for RNAs with trace expression (Table 1).
3.2. Analysis of fold-change bias using regulated genes
Our ERCC-based work demonstrates that even high multiplex PreAmp does not introduce appreciable bias to fold-change measurements. However, the ERCC model system is based on spike-in of in-vitro transcribed RNAs, not natural RNA transcripts. To determine if the PreAmp workflow can accurately quantify gene expression changes of natural targets, we analyzed RNA samples purified from NT2 cells, an established model system of human stem cell behavior. When treated with retinoic acid (RA), NT2 cells differentiate into neurons [12], [13]; during differentiation the expression of stem cell-specific biomarkers decrease while the expression of neuron-specific biomarkers increase [14], [15], [16], [17]. To identify genes regulated during differentiation we applied the fold-change limits-of-detection criteria established in the previous section (Table 1) to 267 genes involved in cell maintenance, cell fate and adaptation. We identified 34 regulated genes, which include well known stem cell biomarkers such as NANOG and neural cell biomarkers such as NEUROD1 (Table 2). Most of these genes were previously characterized to be involved in NT2 differentiation [14], [15], [16], [17], which is confirmatory of our approach.
Table 2.
Genes regulated by retinoic acid.
| Target gene | No PreAmp Avg Cq | % Expression in RA treated cells relative to control cells |
|---|---|---|
| T (Brachyury) | 34.1 | 1% |
| OTX2 | 27.4 | 6% |
| DKK1 | 32.2 | 10% |
| NANOG | 23.7 | 18% |
| DPPA3 | 29.7 | 22% |
| MIXL1 | 30.0 | 23% |
| TDGF1 | 24.6 | 23% |
| RARG | 29.4 | 23% |
| PRDM14 | 26.3 | 24% |
| CD44 | 28.6 | 25% |
| PMAIP1 | 25.6 | 25% |
| POU5F1 | 21.0 | 26% |
| SOX9 | 27.6 | 27% |
| CA12 | 29.8 | 28% |
| THY1 | 24.5 | 47% |
| DLL3 | 28.8 | 307% |
| SNCG | 26.8 | 404% |
| GABRB2 | 29.1 | 493% |
| CRABP2 | 20.0 | 831% |
| POU3F2 | 29.7 | 1280% |
| CDX1 | 30.1 | 1801% |
| MEIS1 | 28.6 | 1879% |
| RARB | 28.6 | 1884% |
| HOXD4 | 29.3 | 3074% |
| ASCL1 | 28.6 | 3141% |
| HNF4A | 31.5 | 3507% |
| LEFTY2 | 25.1 | 6656% |
| NEUROG2 | 33.2 | 8626% |
| FOXA1 | 33.3 | 9277% |
| HOXA4 | 33.5 | 10550% |
| PAX6 | 27.7 | 13467% |
| NEUROD1 | 35.2 | 13703% |
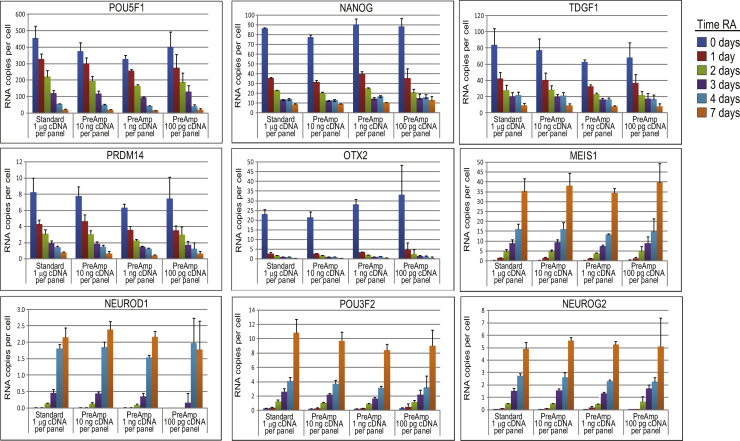
We analyzed a panel of 100 genes that are involved in lineage determination (Supplemental Table 1). We pre-amplified and analyzed varied amounts of cDNA (100 pg, 1 ng and 10 ng) derived from NT2 cells treated with RA for 0, 1, 2, 3, 4 and 7 days; as a no-PreAmp control, 1 μg cDNA was used to quantify gene targets directly by standard qPCR (10 ng/target, 1 μg per 100 target panel). We show data for several representative regulated target genes, gene transcript levels are expressed as “RNA copies per cell” after normalizing with TBP and assuming that there are 10 TBP copies per cell [18]; this method of normalization provides information on the relative level of target gene expression as well as fold-change of RNA abundance (Fig. 2). Data presented in Supplemental Fig. 1 shows that TBP expression is relatively stable over the time course of RA exposure changing less than 0.5Cq (25%), this justifies its use as a normalization gene. The assumption that there are 10 TBP copies per cell does not affect fold-change results; it is used because it provides a relative estimate of target gene levels in a biologically relevant context.
Fig. 2.
Fold-change bias of native target genes with limited sample. cDNA derived from NT2 cells treated with RA for 0–7 days were pre-amplified for 10 PCR cycles and used to quantify native target genes by qPCR; 100 pg, 1 ng and 10 ng cDNA was used in the PreAmp reactions. 1 μg cDNA that was not pre-amplified was also used to quantify target gene expression by standard qPCR (10 ng per target). The level of expression of each target gene was determined and expressed as “RNA copies per cell” after normalizing with TBP and assuming that there are 10 TBP copies per cell. The experiment was performed three times, each with two technical replicates, the average values are reported. Error bars represent standard deviation. Data for selected target genes that show RA-induced changes are shown.
We find that the gene expression profiling results obtained from the PreAmp’d samples show similar trends as those derived from the original cDNA samples, even though up to 10,000-fold less cDNA is analyzed. This implies that PreAmp can analyze limited samples effectively and that the same biological insights and conclusions would be derived from an experiment using PreAmp and a standard gene expression profiling experiment. Importantly, the changes in gene expression observed over the NT2 differentiation time course are consistent with previously published results [14], [15], [16], [17] suggesting that our results are accurate.
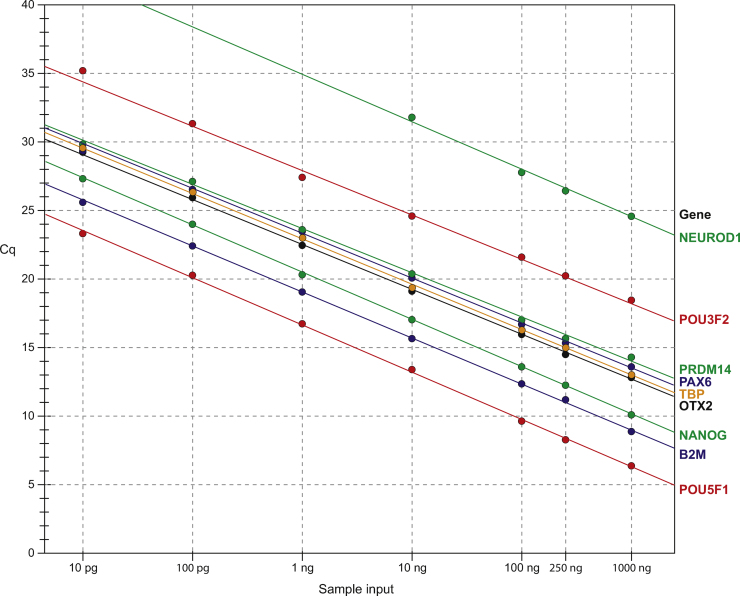
To further explore the dynamic range of sample input, we pre-amplified varied amounts of cDNA, from 10 pg to 1 μg, and then used the samples to analyze gene targets that span a wide expression range. Our results show a linear relationship between sample input and target amount (Fig. 3). This demonstrates that our PreAmp reagent has the capacity to analyze samples with a 6-log input range (10 pg to 1 μg) and faithfully maintain the relative quantity of gene targets over a wide expression level range (e.g. a span of 18Cq from POU5F1 to NEUROD1).
Fig. 3.
Effect of sample input. Titrating amounts of NT2 cDNA, from 10 pg to 1 μg, were pre-amplified for 12 PCR cycles; natural target genes encompassing a wide expression range were quantified by qPCR using three technical replicates, the experiment was performed one time. Results show the relationship between sample input and target gene abundance (Cq).
3.3. Analysis of dynamic range bias
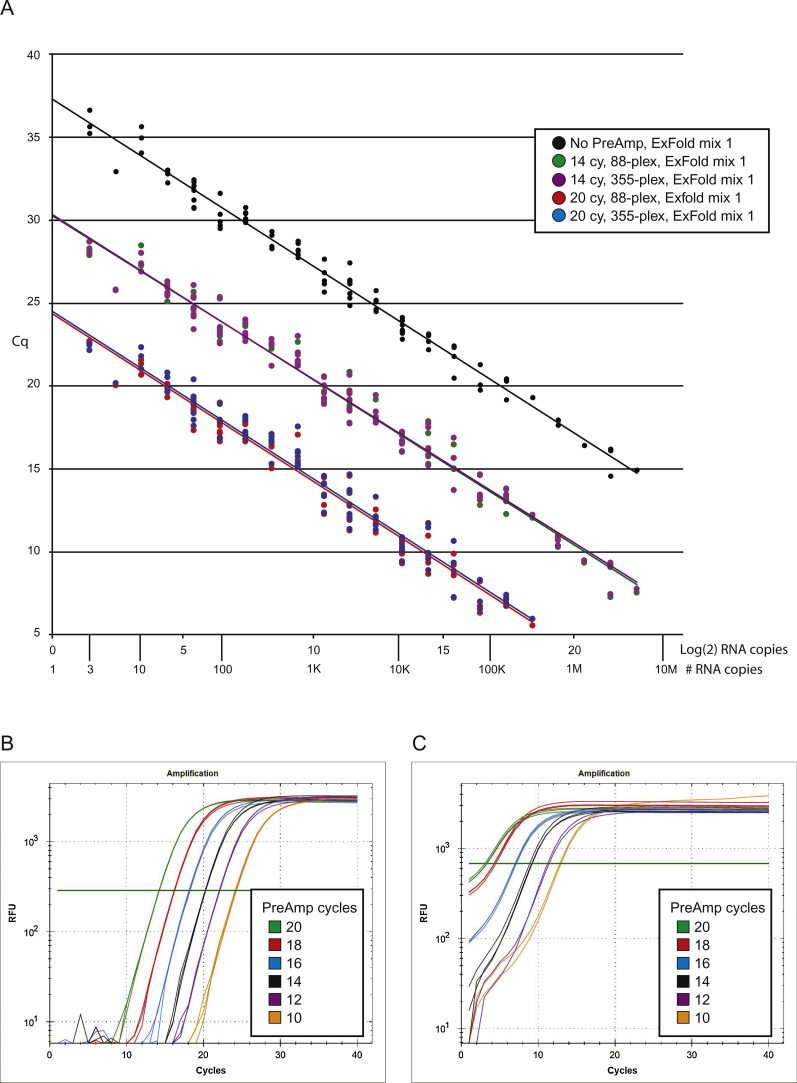
An important consideration in a PreAmp experiment is the number of amplification cycles. Typically, qPCR platforms require 10–14 PreAmp cycles for single copy detection. However, microfluidic-based qPCR instruments have a nanoliter scale interrogation volume and often require up to 20 PreAmp cycles to achieve single copy detection [19]. To assess bias associated with PreAmp cycle number we used the ERCC controls which have wide range of RNA abundance [10]. We diluted the ExFold mixtures such that all targets should be present in a PreAmp reaction (2.5 copies to 5.6 million copies). We then pre-amplified the samples using standard (88-plex) and high multiplex (355-plex) conditions for 10–20 PreAmp cycles; this reflects a target amplification range of 103–106-fold. The amount of ERCC target detected, as a function of RNA abundance, is shown in Fig. 4A. We find that all ERCC targets can be detected in the no PreAmp sample and in the samples processed for 14 PreAmp cycles. This implies that a moderate number of PreAmp cycles will not bias the 6-log dynamic range of target detection. However, for samples that underwent 20 PreAmp cycles, quantification of targets in the 1 million copy range, corresponding to a Cq value less than 5, are of unreliable value (see Fig. 4C).
Fig. 4.
Dynamic range bias. ExFold spike in-mixtures were added to 10 ng NT2 RNA, reverse transcribed and pre-amplified for 10–20 PCR cycles; ERCC targets were quantified by qPCR using two technical replicates, the experiment was performed one time. (A) The measured amount of ERCC target gene is plotted as a function of actual RNA abundance. All ERCC targets can be detected in the no PreAmp sample and in the samples processed for 14 PreAmp cycles. For samples processed for 20 PreAmp cycles, quantification of targets in the 1 million copy range, have a Cq value less than 5 and are of unreliable value. (B) qPCR results for ERCC-00144, which contained 5,513 copies in the PreAmp reaction, show good results over the entire amplification range. (C) qPCR results for ERCC-00074, which contained 2,822,879 copies in the PreAmp reaction, show poor results when amplified for 18 or 20 PCR cycles.
To illustrate the dynamic range bias associated with a highly expressed target we show selected qPCR results. Fig. 4B shows the qPCR traces for the moderate abundance target, ERCC-00144, which is present at ∼5,500 copies in the PreAmp reaction. We find that amplification efficiency, with respect to cycle number, is linear and close to 100%. However, for the high abundance target ERCC-00074, which is present at over 2.8 million copies in the PreAmp reaction, high numbers of PreAmp cycles results in poor target quantification, thus exhibiting dynamic range bias (Fig. 4C). We conclude that high numbers of PreAmp cycles could bias results for very highly expressed genes and, therefore, recommend using the fewest number of PreAmp cycles required to achieve robust single copy target gene detection. Supplementary Table 2 lists the amplification efficiency and dynamic range bias of all ERCC targets; the results demonstrate that our PreAmp workflow supports quantitative target gene amplification up to 1 million-fold under both standard and high multiplex conditions.
The data presented in Fig. 4A was also analyzed quantitatively to delineate salient properties of the PreAmp reaction (Table 3). We find that PreAmp amplifies targets at all levels of abundance with similar efficiency; this is reflected by a slope close to 1. We find that PreAmp decreases the Cq value associated with single copy detection (X-intercept) by the expected 2 cycles for every 2 cycle increase in PreAmp cycle number; this reflects linear amplification over 10–20 PreAmp cycles. We also observe a slight decrease in the R2 values when using 18–20 PreAmp cycles, likely reflecting unreliable qPCR quantification of targets with extremely highly abundance (see Fig. 4C).
Table 3.
Effect of PreAmp cycles on RNA detection.
| PreAmp cycles | ERCC mix | PreAmp plex | Slope | R2 | X-int (Cq) |
|---|---|---|---|---|---|
| 0 | Mix 1 | NA | 1.005 | 0.983 | 37.3 |
| 0 | Mix 2 | NA | 0.988 | 0.984 | 36.9 |
| 10 | Mix 1 | 88 | 0.995 | 0.979 | 34.4 |
| 10 | Mix 1 | 355 | 0.994 | 0.978 | 34.5 |
| 10 | Mix 2 | 88 | 0.987 | 0.979 | 34.1 |
| 10 | Mix 2 | 355 | 0.985 | 0.980 | 34.1 |
| 12 | Mix 1 | 88 | 0.996 | 0.979 | 32.3 |
| 12 | Mix 1 | 355 | 0.993 | 0.980 | 32.4 |
| 12 | Mix 2 | 88 | 0.989 | 0.978 | 32.0 |
| 12 | Mix 2 | 355 | 0.983 | 0.979 | 32.4 |
| 14 | Mix 1 | 88 | 0.996 | 0.977 | 30.3 |
| 14 | Mix 1 | 355 | 0.991 | 0.975 | 30.3 |
| 14 | Mix 2 | 88 | 1.000 | 0.977 | 30.3 |
| 14 | Mix 2 | 355 | 0.987 | 0.978 | 30.1 |
| 16 | Mix 1 | 88 | 1.009 | 0.976 | 28.5 |
| 16 | Mix 1 | 355 | 0.994 | 0.972 | 28.4 |
| 16 | Mix 2 | 88 | 0.992 | 0.978 | 28.2 |
| 16 | Mix 2 | 355 | 0.994 | 0.979 | 28.2 |
| 18 | Mix 1 | 88 | 0.998 | 0.969 | 26.3 |
| 18 | Mix 1 | 355 | 0.999 | 0.966 | 26.4 |
| 18 | Mix 2 | 88 | 0.997 | 0.969 | 26.2 |
| 18 | Mix 2 | 355 | 1.000 | 0.971 | 26.2 |
| 20 | Mix 1 | 88 | 1.013 | 0.964 | 24.3 |
| 20 | Mix 1 | 355 | 1.010 | 0.958 | 24.5 |
| 20 | Mix 2 | 88 | 1.010 | 0.960 | 24.4 |
| 20 | Mix 2 | 355 | 1.021 | 0.960 | 24.1 |
3.4. Analysis of amplification bias
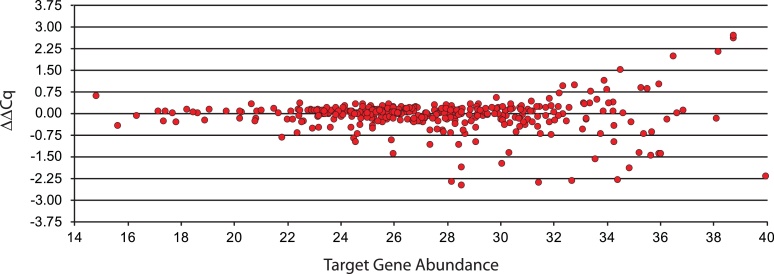
Amplification bias is the difference between the measured amount of a target gene after pre-amplification, relative to the theoretical amount. In practice, the theoretical target gene amount can be estimated from the amount of target detected in the non-amplified cDNA sample and the number of PreAmp cycles (see Section 2). To assess amplification bias, we compared target gene levels in cDNA samples before and after 14 PreAmp cycles. For each target gene we calculated the amplification bias introduced by PreAmp and plotted it against the level of target gene expression in the original sample (Fig. 5). We considered gene targets that have a Cq ≥ 40 in the original sample to be not expressed and excluded them from analysis. We observe that for 319 (91%) of the 346 expressed gene targets, PreAmp did not change the expression level by more than 1.0Cq.
Fig. 5.
Amplification bias. ExFold spike-in mixture 1 was added to 10 ng NT2 RNA, reverse transcribed and pre-amplified for 14 PCR cycles in a 355-plex PreAmp reaction. All 355 targets were quantified by qPCR using two technical replicates, the experiment was performed one time. Amplification bias was determined and plotted against target gene expression.
4. Discussion
We developed a novel PreAmp reagent that features an engineered DNA polymerase designed to improve amplification capacity and efficiency. Here we report an in-depth analysis of the bias our PreAmp reagent introduces into a gene expression profiling experiment. We analyzed three types of bias: amplification bias, dynamic range bias and fold-change bias.
Amplification bias reflects PreAmp PCR efficiency and is a commonly used parameter to measure PreAmp performance [1], [2], [3]. Specifically, amplification bias measures whether each target is amplified at 100% efficiency throughout the PCR cycling. We find that our PreAmp reagent performs well on this metric, even under high multiplex conditions (Fig. 5).
In a previous report the amplification bias of 194 genes was analyzed using standard qPCR; 80% of targets were not altered by more than 1.0 ΔΔCq and about 91% of targets were not altered by more than 1.5 ΔΔCq [2]. Our results show less amplification bias when analyzing 346 genes; 91% of targets were not altered by more than 1.0 ΔΔCq. This difference may reflect differences in the samples and/or target genes analyzed, it may also reflect differences in the reagents and overall workflow used in the analysis.
Interestingly however, even if a target gene exhibits amplification bias, as long as it is biased to the same extent in all samples, relative gene expression results will still be accurate. Thus, amplification bias reproducibility is a key requirement for accurate gene expression profiling results. Not surprisingly, given our gene expression profiling data (Fig. 2), we find that amplification bias standard deviation is extremely low, averaging 0.06Cq when analyzing technical replicates and averaging 0.17Cq when analyzing multiple samples (Supplemental Fig. 2). These results reflect the precision associated with our PreAmp-based gene expression profiling analysis.
Dynamic range bias measures the ability to accurately quantify target genes over a wide range of abundance. Our standard, no PreAmp gene expression profiling workflow can quantify the entire 6-log dynamic range of the ERCC controls. Pre-amplification for up to 14 PCR cycles can also accurately quantify the entire ERCC assay set and, thus, does not introduce dynamic range bias. However, in instances of high target gene expression coupled with a high number of PreAmp cycles (e.g. 20 cycles) target gene quantification may be compromised. Dynamic range bias was previously reported in a microfluidic-based qPCR study and is quite consistent with our findings [3]; a high number of PreAmp cycles biased the quantification of high abundance target genes. We believe that dynamic range bias will not be an issue in standard qPCR experiments. Such bias is apparent in the qPCR traces (Fig. 4C), and can be alleviated through sample dilution or use of fewer PreAmp cycles. For applications that require high levels of PreAmp, such as microfluidic-based qPCR, our conclusions may not apply. More work is necessary to better quantify the various types of bias associated with high PCR cycle PreAmp.
Fold-change measurement is fundamental to gene expression profiling experiments; it allows for the comparison of target gene expression levels across samples. To our knowledge, this is the first report that analyses fold-change bias using RNA reference standards and natural target genes. Using the ERCC standards we quantified fold-change measurement bias and found that our PreAmp reagent does not introduce bias for target genes expressed above trace levels (Cq < 32.5); for target genes that have trace expression (Cq > 32.5) we do observe low bias and suggest that only 8-fold and higher gene expression differences are detectable (Table 1).
There are limitations to our PreAmp workflow; it has limited use in detecting gene expression changes of less than 2-fold (Table 1). To detect small changes a different platform, such as digital PCR, is likely more appropriate [20].
We also analyzed the expression of a panel of regulated target genes across six samples; here we found that the PreAmp results are similar to the results using standard methodology (Fig. 2). This finding is significant and implies that PreAmp can be used in gene expression profiling experiments without compromising results. Indeed, we show that PreAmp faithfully maintains patterns of relative gene expression changes (Fig. 2); the same insights and conclusions would be drawn from a PreAmp-based experiment and a standard gene expression profiling experiment.
Our novel PreAmp technology amplifies over 350 gene targets up to 1 million-fold. The high level of multiplex allows for the incorporation of ERCC standards into a PreAmp-based workflow as an internal control. We find that this control is valuable because it both validates PreAmp performance and establishes screening specifications to identify regulated genes with significant expression changes (Table 1, Table 2). We envision that such a workflow can facilitate experimentation with very limited samples and lead to advances in fields requiring sensitive and accurate gene expression analysis, such as single cell biology.
Conflict of interest
The authors are employees and shareholders of Bio-Rad Laboratories Inc.
Funding
No funding outside of Bio-Rad Laboratories was used in this research.
Author contributions
S.O. and Y.W conceived the project. S.O., M.K., and Y.W. designed the experiments. S.O. and M.K. executed the experiments and S.O., M.K., H.S. and Y.W analyzed the data. S.O. wrote the first draft of the manuscript. H.S. and Y.W edited the manuscript.
Disclosure
The authors are employees and shareholders of Bio-Rad Laboratories Inc.
Acknowledgements
We thank Xiao-Song Gong, Evan Bursey, Elizabeth Jordan, Sonia Streng and Paul Streng for their advice and assistance on this project. No external funding was used in this work.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bdq.2015.12.001.
Contributor Information
Steven T. Okino, Email: steven_okino@bio-rad.com.
Michelle Kong, Email: michelle_kong@bio-rad.com.
Haya Sarras, Email: haya_sarras@bio-rad.com.
Yan Wang, Email: yan_wang@bio-rad.com.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Li J., Smyth P., Cahill S., Denning K., Flavin R., Aherne S., Pirotta M., Guenther S.M. Improved RNA quality and TaqMan Pre-amplification method (PreAmp) to enhance expression analysis from formalin fixed paraffin embedded (FFPE) materials. BMC Biotechnol. 2008;8:10. doi: 10.1186/1472-6750-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermeulen J., Derveaux S., Lefever S., De Smet E., De Preter K., Yigit N., De Paepe A., Pattyn F. RNA pre-amplification enables large-scale RT-qPCR gene-expression studies on limiting sample amounts. BMC Res. Notes. 2009;2:235. doi: 10.1186/1756-0500-2-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korenkova V., Scott J., Novosadova V., Jindrichova M., Langerova L., Svec D., Sidova M., Sjoback R. Pre-amplification in the context of high-throughput qPCR gene expression experiment. BMC Mol. Biol. 2015;16:5. doi: 10.1186/s12867-015-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The ERCC Consortium Proposed methods for testing and selecting the ERCC external RNA controls. BMC Genomics. 2005;6:150. doi: 10.1186/1471-2164-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker S.C., Bauer S.R., Beyer R.P., Brenton J.D., Bromley B., Burrill J., Causton H., Conley M.P. The external RNA controls consortium: a progress report. Nat. Methods. 2005;2:731–734. doi: 10.1038/nmeth1005-731. [DOI] [PubMed] [Google Scholar]
- 6.Devonshire A.S., Elaswarapu R., Foy C.A. Applicability of RNA standards for evaluating RT-qPCR assays and platforms. BMC Genomics. 2011;12:118. doi: 10.1186/1471-2164-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devonshire A.S., Elaswarapu R., Foy C.A. Evaluation of external RNA controls for the standardisation of gene expression biomarker measurements. BMC Genomics. 2010;11:662. doi: 10.1186/1471-2164-11-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang L., Schlesinger F., Davis C.A., Zhang Y., Li R., Salit M., Gingeras T.R., Oliver B. Synthetic spike-in standards for RNA-seq experiments. Genome Res. 2011;21:1543–1551. doi: 10.1101/gr.121095.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders R., Mason D.J., Foy C.A., Huggett J.F. Evaluation of digital PCR for absolute RNA quantification. PLoS One. 2013;8:e75296. doi: 10.1371/journal.pone.0075296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu L., Lemire A., Lea K., Batten D., Jian Gu S., Whitley P., Bramlett K. Development of ERCC RNA Spike-In Control Mixes. J. Biomol. Tech. 2011;22(Suppl):S46. [Google Scholar]
- 11.Wang Y., Prosen D.E., Mei L., Sullivan J.C., Finney M., Vander Horn P.B. A novel strategy to engineer DNA polymerases for enhanced processivity and improved performance in vitro. Nucleic Acids Res. 2004;32:1197–1207. doi: 10.1093/nar/gkh271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews P.W. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev. Biol. 1984;103:285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- 13.Pleasure S.J., Lee V.M. NTera 2 cells: a human cell line which displays characteristics expected of a human committed neuronal progenitor cell. J. Neurosci. Res. 1993;35:585–602. doi: 10.1002/jnr.490350603. [DOI] [PubMed] [Google Scholar]
- 14.Przyborski S.A., Smith S., Wood A. Transcriptional profiling of neuronal differentiation by human embryonal carcinoma stem cells in vitro. Stem Cells. 2003;21:459–471. doi: 10.1634/stemcells.21-4-459. [DOI] [PubMed] [Google Scholar]
- 15.Coyle D.E., Li J., Baccei M. Regional differentiation of retinoic acid-induced human pluripotent embryonic carcinoma stem cell neurons. PLoS One. 2011;6:e16174. doi: 10.1371/journal.pone.0016174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elizalde C., Campa V.M., Caro M., Schlangen K., Aransay A.M., dM Vivanco M., Kypta R.M. Distinct roles for Wnt-4 and Wnt-11 during retinoic acid-induced neuronal differentiation. Stem Cells. 2011;29:141–153. doi: 10.1002/stem.562. [DOI] [PubMed] [Google Scholar]
- 17.Deb-Rinker P., Ly D., Jezierski A., Sikorska M., Walker P.R. Sequential DNA methylation of the Nanog and Oct-4 upstream regions in human NT2 cells during neuronal differentiation. J. Biol. Chem. 2005;280:6257–6260. doi: 10.1074/jbc.C400479200. [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi K., Kajiyama T., Kambara H. Quantitative analysis of gene expression in a single cell by qPCR. Nat. Methods. 2009;6:503–506. doi: 10.1038/nmeth.1338. [DOI] [PubMed] [Google Scholar]
- 19.Livak K.J., Wills Q.F., Tipping A.J., Datta K., Mittal R., Goldson A.J., Sexton D.W., Holmes C.C. Methods for qPCR gene expression profiling applied to 1440 lymphoblastoid single cells. Methods. 2013;59:71–79. doi: 10.1016/j.ymeth.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whale A.S., Cowen S., Foy C.A., Huggett J.F. Methods for applying accurate digital PCR analysis on low copy DNA samples. PLoS One. 2013;8:e58177. doi: 10.1371/journal.pone.0058177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.