Abstract
Background
The effect of moderately elevated blood glucose levels among non-diabetic subjects on cancer prognosis is not well described. The goal of this study was to examine the association of elevated random blood glucose (RBG) levels in non-diabetic breast cancer patients with overall survival (OS) and time to tumor recurrence (TTR).
Results
Forty-nine deaths and 32 recurrences occurred among 148 eligible study subjects during 855.44 person-years of follow-up, with median follow-up of 5.97 years. We observed that patients with elevated RBG levels experienced significantly shorter OS (hazard ratio [HR], 3.01; 95 % confidence interval [CI] (1.70–5.33); P < 0.001) and shorter TTR (HR, 2.08; CI (1.04–4.16); P = 0.04) as compared to patients with non-elevated RBG levels. After controlling for tumor grade, tumor stage, race, and BMI, elevated RBG continued to display high and statistically significant association with shorter OS (HR, 3.50; CI (1.87–6.54); P < 0.001). Adjustment for age, race, and BMI strengthened HR of RBG for TTR. The association of RGB with TTR lost its borderline statistical significance upon controlling for both tumor grade and stage.
Conclusions
The data suggest that elevated blood glucose is associated with poor prognosis of breast cancer patients. Given the potential clinical implication, these findings warrant further investigation.
Keywords: Breast cancer, Blood glucose, Patient survival
Background
Numerous epidemiologic studies indicate that type 2 diabetes is associated with an increased risk of breast cancer [1–6]. Several large studies that compared cancer-related mortality between subjects with and without diabetes suggested an association between increased mortality and diabetes for breast cancer [1, 7–9]. However, the contribution of other factors including the delay in diagnosis, lower use of effective adjuvant therapies, diabetes-prescribed drug use, and diabetes-related comorbidities on observed association between diabetes and breast cancer is not clearly addressed [9–20]. To manage the unpredictable impact of the confounding factors on the observational studies, Boyle et al. [21] investigated the association of elevated blood glucose and breast cancer risk in women without diabetes and reported a small increase in breast cancer risk in a meta-analysis study. With regard to the published data, studying breast cancer outcome among non-diabetic subjects with elevated blood glucose levels is clearly needed.
Random blood glucose (RBG) values ≥120 mg/dL have been shown to have 90–92 % specificity for detection of any glucose intolerance that include patients with pre-diabetes and undiagnosed diabetes [22, 23]. We adopted RBG values of 120 mg/dL or higher to be an indication of disorders in glucose metabolism [22, 24], and performed a retrospective cohort study to evaluate the relationship between elevated blood glucose levels and survival of non-diabetic breast cancer subjects. We observed that elevated RBG levels correlate with poor prognosis in breast cancer patients independently of age, tumor characteristics, race, and BMI.
Methods
Study design and characteristics of patient population
This was a retrospective chart-review cohort study that was performed according to a protocol approved by the UAMS Institutional Review Board (IRB). The UAMS IRB determined the study to met the criteria for exempt status per 45 CFR 46, meaning there was no requirement to obtain informed consent from the study subjects. A cohort was formed by including subjects diagnosed with breast cancer from 1995 to 2000. African-American (AA) and European-American (EA) women who were ≥18 years of age at diagnosis, and who had at least one RBG measurement from 1 year before cancer diagnosis to the time of diagnosis, were included. Patients with a diagnosis of diabetes at any time before cancer diagnosis to the last follow-up date were excluded. Patients with documented disease stages I, II, and III were included, whereas patients with stage IV (metastatic) disease were excluded. A total of 148 patients were identified, all of whom were treated at the hospital of University Arkansas for Medical Sciences (UAMS). The dates of diagnosis, tumor recurrence, and last follow-up, vital status, and disease status at the last follow-up, age, race, tumor stage and grade were provided by the UAMS tumor registry. RBG, height, and weight were extracted from the medical-record charts.
Outcome and predictor variables
There were two measures of outcome: overall survival (time to death by any reason with censoring occurring at last contact when patient was still alive) and time to tumor recurrence (with censoring at death or last contact). Measured RBG levels were classified as “high” or “elevated” if ≥120 mg/dL versus “low” or “non-elevated” if <120 mg/dL, and this dichotomous classification was used as the main predictor variable. The RBG measurements evaluated in this study included data collected within a 1-year period prior to the diagnosis, and were averaged together if multiple numbers were recorded to reach a single measurement for each patient. Height, weight, age, and race were recorded at the time of diagnosis. BMI (trichotomized as ≥30, <30, and unknown), race (AA vs. EA), age (as a continuous variable), tumor grade (as a categorical variable), and stage (as a categorical variable) were used as covariates.
Statistical analysis
Survival and time to tumor recurrence in the high and low RBG groups were visualized using Kaplan-Meier curves and compared for differences with the log-rank test. After confirming the validity of the proportional-hazards assumption, outcomes were analyzed for covariate associations using univariate and multivariate Cox-regression analyses. In order to avoid overfitting the multivariate Cox models, we required every model to have a minimum of 10 observed events per covariate [25]. This meant that the maximum number of predictor variables in a multivariate Cox model was five for analysis of overall survival (OS) and three for analysis of time to tumor recurrence (TTR). Hazard ratios (HRs) with 95 % confidence intervals were calculated for each covariate. All hypothesis tests were two-sided; all P values were reported numerically, and evaluated using an alpha = 0.05 significance level. IBM SPSS Statistics Version 22 (IBM Corp., Armonk, NY) software was used for statistical analyses.
Results
Characteristics of patient population
Forty-nine deaths and 32 recurrences occurred among 148 eligible study subjects during 855.44 person-years of follow-up, with median follow-up of 5.97 years. Distributions of selected variables in studied population are summarized in Table 1. Of the total number of the patients, 120 (81 %) were EA and 28 (19 %) were AA women. Most patients (123, 83 %) were diagnosed with stage II breast cancer, although 20 (14 %) and 5 (3 %) of total eligible subjects were respectively diagnosed with stages I and III disease. Sixty-eight patients (46 %) were diagnosed with pathological grade III tumors versus 54 (36 %) with grade II and 26 (18 %) with grade I. Age was distributed with a minimum, median, and maximum age of 28, 53, and 89 years, respectively.
Table 1.
Characterization of the studied population
| Characteristics | RBG < 120 | RBG ≥ 120 | Total | P value |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||
| All patients | 95 (64 %) | 53 (36 %) | 148 (100 %) | |
| Age | 0.02 | |||
| Min | 29 | 28 | 28 | |
| Max | 89 | 85 | 89 | |
| Mean (SD) | 53.3 (13.3) | 57.4 (12.9) | 54.80 (13.3) | |
| Median | 50 | 56 | 53.0 | |
| <50 | 45 (47 %) | 15 (28 %) | 60 (41 %) | |
| ≥50 | 50 (53 %) | 38 (72 %) | 88 (59 %) | |
| Tumor grade | 0.14 | |||
| Grade I | 21 (22 %) | 5 (9 %) | 26 (18 %) | |
| Grade II | 34 (36 %) | 20 (38 %) | 54 (36 %) | |
| Grade III | 40 (42 %) | 28 (53 %) | 68 (46 %) | |
| Tumor stage | 0.19 | |||
| Stage I | 16 (17 %) | 4 (8 %) | 20 (14 %) | |
| Stage II | 75 (79 %) | 48 (91 %) | 123 (83 %) | |
| Stage III | 4 (4 %) | 1 (2 %) | 5 (3 %) | |
| Race | 0.05 | |||
| EA | 82 (86 %) | 38 (72 %) | 120 (81 %) | |
| AA | 13 (14 %) | 15 (28 %) | 28 (19 %) | |
| BMI | 0.1 | |||
| Min | 17 | 21 | 17 | |
| Max | 54 | 43 | 54 | |
| Mean (SD) | 28.5 (6.8) | 30.3 (6.3) | 29.1 (6.6) | |
| Median | 27 | 29 | 27 | |
| <30 | 61 (64 %) | 25 (47 %) | 49 (33 %) | |
| ≥30 | 28 (29 %) | 21 (40 %) | 86 (58 %) | |
| Unknown | 6 (6 %) | 7 (13 %) | 13 (9 %) | |
| Death | 20 (41 %) | 29 (59 %) | 49 (100 %) | |
| Recurrence | 16 (50 %) | 16 (50 %) | 32 (100 %) | |
| Median follow-up (years) | 6.27 | 5.14 | 5.97 |
Elevated RBG correlates with poor prognosis and shorter time to tumor recurrence
We adopted RBG values of 120 mg/dL or higher to be an indication of disorders in carbohydrate metabolism [22, 24]. An RBG cutoff value of 120 mg/dL has been shown to have 90–92 % specificity in the detection of any glucose intolerance, which includes patients with pre-diabetes and undiagnosed diabetes [22, 23]. We performed survival analysis to evaluate the association of high RBG, a likely representative of pre-diabetes, with patients’ survival and time to tumor recurrence. Patients with elevated RBG had significantly shorter overall survival and time to tumor recurrence (Fig. 1). Subjects in elevated RBG group had a median ± SE of 6.05 ± 0.65 for OS. Median OS was not reached for subjects with low RBG. Neither of the study subpopulations reached median TTR.
Fig. 1.
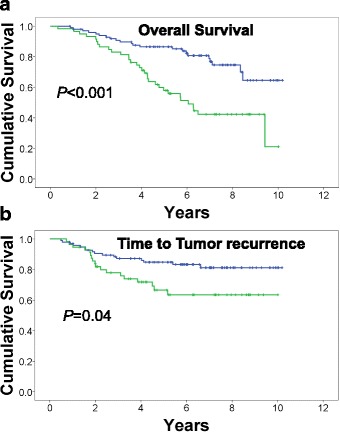
Kaplan-Meier analysis of RBG and patients’ survival. High RBG levels are associated with shorter overall survival (a) and shorter time to tumor recurrence (b). RGB values of ≥120 mg/dL were considered high. P values were estimated using log-rank test. Green, RBG ≥ 120; blue, RBG < 120. Plus signs indicate censoring
Association of RBG with survival persists after controlling for tumor characteristics, age, BMI, and race
Tumor grade and tumor stage are the common prognostic factors that correlate with recurrence and survival endpoints. Race also affects survival, since AA breast cancer patients experience shorter survival time than their EA counterparts [26]. RBG levels may associate with BMI. Therefore, the association of RBG with both OS and TTR was evaluated after controlling for age, tumor grade, tumor stage, BMI, and race (Table 2). RBG level of ≥120 mg/dL was associated with a shorter OS (hazard ratio [HR] = 3.01; 95 % confidence interval [CI], (1.70–5.33); P < 0.001), and a shorter time to tumor recurrence (HR, 2.08; CI (1.04–4.16), P = 0.039). In univariate analysis, none of the other variables showed significant association with OS, and only age displayed a significant association with TTR (HR, 0.96; CI (0.94–0.99), P = 0.01).
Table 2.
Hazard ratios for the association between RBG (≥120 vs. <120) and breast cancer outcomes
| Overall survival | HR (95 % CI) | P value |
| Crude | 3.01 (1.70–5.33) | <0.001 |
| Adjusted for age | 2.99 (1.68–5.33) | <0.001 |
| Adjusted for age group (≥50 vs. <50) | 3.07 (1.72–5.48) | <0.001 |
| Adjusted for grade | 3.08 (1.71–5.57) | <0.001 |
| Adjusted for stage | 3.09 (1.73–5.53) | <0.001 |
| Adjusted for age, tumor grade and tumor stage | 3.19 (1.74–5.86) | <0.001 |
| Adjusted for age, tumor grade, tumor stage, and race | 3.24 (1.75–5.99) | <0.001 |
| Adjusted for age group (≥50 vs. <50), tumor grade, tumor stage, and race | 3.40 (1.83–6.33) | <0.001 |
| Adjusted for BMI | 3.24 (1.78–5.89) | <0.001 |
| Adjusted for tumor grade, tumor stage, race, and BMI | 3.50 (1.87–6.54) | <0.001 |
| Time to tumor recurrencea | ||
| Crude | 2.08 (1.04–4.16) | 0.039 |
| Adjusted for age | 2.59 (1.27–5.26) | 0.009 |
| Adjusted for age group (≥50 vs. <50) | 2.65 (1.29–5.44) | 0.008 |
| Adjusted for grade | 2.06 (1.01–4.18) | 0.046 |
| Adjusted for stage | 2.01 (1.00–4.04) | 0.051 |
| Adjusted for grade and stage | 2.00 (0.97–4.09) | 0.059 |
| Adjusted for race | 2.17 (1.08–4.38) | 0.030 |
| Adjusted for BMI | 2.37 (1.16–4.83) | 0.017 |
| Adjusted for BMI and grade | 2.32 (1.13–4.73) | 0.021 |
| Adjusted for BMI and stage | 2.32 (1.13–4.76) | 0.022 |
aFive out of 148 (3.4 %) subjects who never became disease free were removed from TTR analyses
Controlling for tumor grade, tumor stage, or age did not appreciably change the HR value of RBG for OS (Table 2). However, adjustment for these three covariates combined led to a 6 % increase in the HR value of RBG for OS, from 3.01 to 3.19 (Table 2). The addition of race to this model (adjusting for age, tumor grade, tumor stage, and race) barely affected the associations of RBG with OS. Controlling for BMI improved association of RBG with OS (HR increased from 3.01 to 3.24, an 8 % increase). After adjusting for tumor grade, tumor stage, race, and BMI, the HR value of RBG for OS increased by 16 % (HR, 3.50; CI (1.87–6.54); P < 0.001). With respect to TTR, controlling for age led to a 25 % increase in the HR value of RBG compared to its value from univariate analysis (Table 2). Adjusting for both tumor grade and tumor stage slightly weakened RBG’s association with TTR, as the HR dropped from 2.08 before adjustment to 2.00 (a 4 % decrease) after adjustment, but the weakening was enough to render the HR statistically insignificant. Adjusting for BMI strengthened the association of RBG and TTR where HR increased to 2.37 from 2.08 (a 14 % increase). The HR of TTR with RBG showed consistent increases over the crude estimate after controlling for either BMI and grade or BMI and stage (Table 2). These data suggest that RBG levels in breast cancer patients correlate with patients’ survival and probably recurrence of the disease.
Discussion
Our study revealed that breast cancer patients with elevated RBG levels have shorter OS and TTR. Epidemiological studies strongly suggest an association between high blood glucose and mortality in breast cancer patients [9]. In a study examining the general population, Saydah et al. [27] classified the subjects as having either diagnosed diabetes, undiagnosed diabetes, impaired glucose tolerance, or normal glucose tolerance, and went on to observe that individuals with impaired glucose tolerance had the highest adjusted relative hazard of cancer mortality compared to the reference group with normal glucose tolerance. Consistently, our data suggest that RBG, which is a non-fasting blood glucose measurement, has a significant association with breast cancer outcomes, particularly with OS.
Controlling for tumor grade, tumor stage, and age suggests that RBG can be considered as an independently significant factor that correlates with overall survival. Age displayed a strong confounding effect on the RBG association with TTR, such that controlling for age accentuated the RBG association by 25 %. On the other hand, the prognostic significance of RBG for TTR, which was already at borderline, crossed the border into insignificance upon controlling for tumor grade and stage. Strengthening of the HR of RBG for OS after adjusting for race or BMI suggests that these two covariates confound the association of RBG with patients’ survival. A similar phenomenon was observed regarding the association of RBG with TTR. Therefore, race and BMI should be considered as major confounders in the design and conduct of future studies.
A diabetic population is a heterogeneous group to be studied for cancer mortality for at least three reasons. First, clinical diagnosis of diabetes often leads to treatment with anti-diabetic medications that reduce blood glucose, thus reducing the impact of diabetes on the tumor, and potentially introducing a source of variability when a given anti-diabetes drug may directly affect the tumor [12, 18–20, 28]. Second, there may be disease-dependent variation in glucose and insulin levels in diabetics. Third, there is a complex set of relationships between diabetes and cancer, and an incomplete understanding of underlying biological mechanisms associating these two health issues. Similar factors as above may contribute to heterogeneity of the grouping and play well into low power of the analyses. The fact that we observed a strong association between RBG and survival may suggest that RBG screening may have significant clinical implications. Pre-diabetes is a condition that occurs when a person’s blood glucose levels are higher than normal, though not high enough for a diagnosis of diabetes. People with pre-diabetes develop insulin resistance and are at an increased risk of type 2 diabetes. Measuring RBG levels has been proposed as an easy-to-measure and reliable predictor of pre-diabetes and early diabetes [22, 24]. Screening for high RBG may pick patients with undiagnosed early diabetes or in the pre-diabetes stage. Therefore, lack of drug use and being early in the process of development of the disease makes such a group of patients a relatively homogenous one, allowing to reduce confounders and improve confidence levels compared to cancer studies on diabetics or obese populations.
High glucose levels may be accompanied with high insulin levels. Insulin enhances cell growth and promotes cell proliferation due to its mitogenic effects [29]. High levels of insulin were associated with distant recurrence and poor survival [30–32]. We were not able to separate the impact of blood glucose and insulin on the survival endpoints. The possibility that breast cancer further increased the levels of RBG cannot be excluded, which is a limitation of this study. Moreover, treatment for breast cancer may increase RBG further, and adversely affect survival [33]. For example, the glucocorticoid Dexamethasone, which is widely used to prevent side effects during chemotherapy, is associated with elevation of blood glucose [34]. The use of this drug may unevenly affect patients with elevated RGB than those with low RGB levels. We were not able to address this issue, and further studies are required to explore these possibilities.
The hormone-receptor details needed to identify basal-like disease were not available for this study, and, therefore, we were not able to rule in or out any contribution for the breast cancer subtypes in the observed association between survival and RBG. Therefore, we cannot exclude the possibility that differences in the prevalence of triple-negative cancers contributed to the poorer survival among patients with high blood glucose. Other limitations of this study are small sample size and lack of cancer-specific mortality data that potentially can affect the outcome of this study. Availability of pre-diagnosis blood sugar measurement, while could have increased homogeneity may have also introduced unknown factors in selection of the patient population. Therefore, these results should be interpreted with caution.
Conclusions
This study suggests that hyperglycemia, a component of pre-diabetes and undiagnosed early diabetes, affects survival of breast cancer patients. Therefore, a routine measurement of blood glucose may potentially give guidance on mortality risk for breast cancer patients. As the prevalence of disorders in carbohydrate metabolism grows, the population of breast cancer patients with this disorder is also growing, and our study suggests that these patients tend to experience shorter survival than patients with normal blood glucose levels. Our findings could represent a great public health issue and therefore warrant further investigation in larger cohorts.
Acknowledgements
The project described was supported by the Translational Research Institute, grant UL1TR000039 through the NIH National Center for Research Resources and National Center for Advancing Translational Sciences to BMK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding agency did not have any role in the design, collection of the data, analysis or interpretation of the data.
Abbreviations
- AA
African American
- BMI
body mass index
- CI
confidence interval
- EA
European American
- HR
hazard ratio
- IRB
Institutional Review Board
- OS
overall survival
- RBG
random blood glucose
- TTR
time to tumor recurrence
- UAMS
University of Arkansas for Medical Sciences
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BMK initiated the study and was in charge of the protocol and initial accruing of the data. He led the data collection, management, and analysis. He finalized statistical analysis and drafted the manuscript. RG, VK, and SM worked on initial cohort data, identified the subjects, extracted the data from various databases and medical records, and validated the data. FJ worked on data preparation and participated in some initial analyses of the data. ERS participated in the statistical analysis and guiding through statistical approaches, he also participated in drafting and writing the manuscript. BJF reviewed and improved the manuscript and guided through some advanced analyses. AMS, LFH, and TKE participated in design and conduction of the study and in interpretation of the results and helped draft the manuscript. All authors included deserve the authorship right and they read and approved the final manuscript.
Contributor Information
Behjatolah Monzavi-Karbassi, Phone: (501) 526-7874, Email: karbassi@uams.edu.
Rhonda Gentry, Email: GentryRhondaW@uams.edu.
Varinder Kaur, Email: Varinder.Kaur@bccancer.bc.ca.
Eric R. Siegel, Email: SiegelEricR@uams.edu
Fariba Jousheghany, Email: jousheghanyfariba@uams.edu.
Srikanth Medarametla, Email: SMedarametla@uams.edu.
Barbara J. Fuhrman, Email: BJFuhrman@uams.edu
A. Mazin Safar, Email: SafarAhmedM@uams.edu.
Laura F. Hutchins, Email: HutchinsLauraF@uams.edu
Thomas Kieber-Emmons, Email: tke@uams.edu.
References
- 1.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159(12):1160–7. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 2.Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE. Diabetes mellitus and breast cancer: a retrospective population-based cohort study. Breast Cancer Res Treat. 2006;98(3):349–56. doi: 10.1007/s10549-006-9172-5. [DOI] [PubMed] [Google Scholar]
- 3.Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE. Increased prevalence of prior breast cancer in women with newly diagnosed diabetes. Breast Cancer Res Treat. 2006;98(3):303–9. doi: 10.1007/s10549-006-9166-3. [DOI] [PubMed] [Google Scholar]
- 4.Liao S, Li J, Wei W, Wang L, Zhang Y, Li J, et al. Association between diabetes mellitus and breast cancer risk: a meta-analysis of the literature. Asian Pac J Cancer Prev. 2011;12(4):1061–5. [PubMed] [Google Scholar]
- 5.Cleveland RJ, North KE, Stevens J, Teitelbaum SL, Neugut AI, Gammon MD. The association of diabetes with breast cancer incidence and mortality in the Long Island Breast Cancer Study Project. Cancer Causes Control. 2012;23(7):1193–203. doi: 10.1007/s10552-012-9989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HF, Liu MD, Chen P, Chen LH, Chang YH, Wen PC, et al. Risks of breast and endometrial cancer in women with diabetes: a population-based cohort study. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0067420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verlato G, Zoppini G, Bonora E, Muggeo M. Mortality from site-specific malignancies in type 2 diabetic patients from Verona. Diabetes Care. 2003;26(4):1047–51. doi: 10.2337/diacare.26.4.1047. [DOI] [PubMed] [Google Scholar]
- 8.Erickson K, Patterson RE, Flatt SW, Natarajan L, Parker BA, Heath DD, et al. Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol. 2011;29(1):54–60. doi: 10.1200/JCO.2010.29.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011;29(1):40–6. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckman TJ, Cuddihy RM, Scheitel SM, Naessens JM, Killian JM, Pankratz VS. Screening mammogram utilization in women with diabetes. Diabetes Care. 2001;24(12):2049–53. doi: 10.2337/diacare.24.12.2049. [DOI] [PubMed] [Google Scholar]
- 11.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29(2):254–8. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 12.Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007;25(12):1476–81. doi: 10.1200/JCO.2006.07.2777. [DOI] [PubMed] [Google Scholar]
- 13.Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE. The impact of diabetes on survival following breast cancer. Breast Cancer Res Treat. 2008;109(2):389–95. doi: 10.1007/s10549-007-9654-0. [DOI] [PubMed] [Google Scholar]
- 14.Fierz Y, Novosyadlyy R, Vijayakumar A, Yakar S, LeRoith D. Insulin-sensitizing therapy attenuates type 2 diabetes-mediated mammary tumor progression. Diabetes. 2010;59(3):686–93. doi: 10.2337/db09-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowker SL, Yasui Y, Veugelers P, Johnson JA. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia. 2010;53(8):1631–7. doi: 10.1007/s00125-010-1750-8. [DOI] [PubMed] [Google Scholar]
- 16.Baur DM, Klotsche J, Hamnvik OP, Sievers C, Pieper L, Wittchen HU, et al. Type 2 diabetes mellitus and medications for type 2 diabetes mellitus are associated with risk for and mortality from cancer in a German primary care cohort. Metabolism. 2011;60(10):1363–71. doi: 10.1016/j.metabol.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Bo S, Ciccone G, Rosato R, Villois P, Appendino G, Ghigo E, et al. Cancer mortality reduction and metformin: a retrospective cohort study in type 2 diabetic patients. Diabetes Obes Metab. 2012;14(1):23–9. doi: 10.1111/j.1463-1326.2011.01480.x. [DOI] [PubMed] [Google Scholar]
- 18.Currie CJ, Peyrot M, Morgan CL, Poole CD, Jenkins-Jones S, Rubin RR, et al. The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care. 2012;35(6):1279–84. doi: 10.2337/dc11-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranc K, Jorgensen ME, Friis S, Carstensen B. Mortality after cancer among patients with diabetes mellitus: effect of diabetes duration and treatment. Diabetologia. 2014;57(5):927–34. doi: 10.1007/s00125-014-3186-z. [DOI] [PubMed] [Google Scholar]
- 20.Wu JW, Boudreau DM, Park Y, Simonds NI, Freedman AN. Commonly used diabetes and cardiovascular medications and cancer recurrence and cancer-specific mortality: a review of the literature. Expert Opin Drug Saf. 2014;13(8):1071–99. doi: 10.1517/14740338.2014.926887. [DOI] [PubMed] [Google Scholar]
- 21.Boyle P, Koechlin A, Pizot C, Boniol M, Robertson C, Mullie P, et al. Blood glucose concentrations and breast cancer risk in women without diabetes: a meta-analysis. Eur J Nutr. 2013;52(5):1533–40. doi: 10.1007/s00394-012-0460-z. [DOI] [PubMed] [Google Scholar]
- 22.Ziemer DC, Kolm P, Foster JK, Weintraub WS, Vaccarino V, Rhee MK, et al. Random plasma glucose in serendipitous screening for glucose intolerance: screening for impaired glucose tolerance study 2. J Gen Intern Med. 2008;23(5):528–35. doi: 10.1007/s11606-008-0524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolka DB, Narayan KM, Thompson TJ, Goldman D, Lindenmayer J, Alich K, et al. Performance of recommended screening tests for undiagnosed diabetes and dysglycemia. Diabetes Care. 2001;24(11):1899–903. doi: 10.2337/diacare.24.11.1899. [DOI] [PubMed] [Google Scholar]
- 24.Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Caudle JM, et al. Age, BMI, and race are less important than random plasma glucose in identifying risk of glucose intolerance: the Screening for Impaired Glucose Tolerance Study (SIGT 5) Diabetes Care. 2008;31(5):884–6. doi: 10.2337/dc07-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somorjai RL, Dolenko B, Baumgartner R. Class prediction and discovery using gene microarray and proteomics mass spectroscopy data: curses, caveats, cautions. Bioinformatics. 2003;19(12):1484–91. doi: 10.1093/bioinformatics/btg182. [DOI] [PubMed] [Google Scholar]
- 26.Hunt BR, Whitman S, Hurlbert MS. Increasing Black:White disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol. 2014;38(2):118–23. doi: 10.1016/j.canep.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidemiol. 2003;157(12):1092–100. doi: 10.1093/aje/kwg100. [DOI] [PubMed] [Google Scholar]
- 28.Srokowski TP, Fang S, Hortobagyi GN, Giordano SH. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27(13):2170–6. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chappell J, Leitner JW, Solomon S, Golovchenko I, Goalstone ML, Draznin B. Effect of insulin on cell cycle progression in MCF-7 breast cancer cells. Direct and potentiating influence. J Biol Chem. 2001;276(41):38023–8. doi: 10.1074/jbc.M104416200. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20(1):42–51. doi: 10.1200/JCO.20.1.42. [DOI] [PubMed] [Google Scholar]
- 31.Borugian MJ, Sheps SB, Kim-Sing C, Van Patten C, Potter JD, Dunn B, et al. Insulin, macronutrient intake, and physical activity: are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomarkers Prev. 2004;13(7):1163–72. [PubMed] [Google Scholar]
- 32.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101(1):48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji GY, Jin LB, Wang RJ, Bai Y, Yao ZX, Lu LJ, et al. Incidences of diabetes and prediabetes among female adult breast cancer patients after systemic treatment. Med Oncol. 2013;30(3):687. doi: 10.1007/s12032-013-0687-4. [DOI] [PubMed] [Google Scholar]
- 34.Hickish T, Astras G, Thomas P, Penfold S, Purandare L, Hickish TF, et al. Glucose intolerance during adjuvant chemotherapy for breast cancer. J Natl Cancer Inst. 2009;101(7):537. doi: 10.1093/jnci/djp025. [DOI] [PubMed] [Google Scholar]


