Abstract
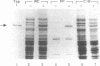
An Escherichia coli protease designated Tsp (tail-specific protease) has been purified, and its gene has been cloned and sequenced. Tsp specifically degrades a variant of the N-terminal domain of lambda repressor in which the five C-terminal residues, which are polar in wild type, have been replaced by nonpolar residues. This substrate specificity in vitro parallels the previously reported selective degradation in vivo of N-terminal-domain variants with nonpolar C-terminal residues. The gene sequence and N-terminal protein sequence of Tsp predict a protein of 660 amino acids. The deduced protein sequence of Tsp shows no significant homology to known protease sequences but does show sequence similarity to the human and bovine interphotoreceptor retinoid-binding proteins, which bind hydrophobic ligands.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bowie J. U., Sauer R. T. Identification of C-terminal extensions that protect proteins from intracellular proteolysis. J Biol Chem. 1989 May 5;264(13):7596–7602. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Gribskov M., McLachlan A. D., Eisenberg D. Profile analysis: detection of distantly related proteins. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4355–4358. doi: 10.1073/pnas.84.13.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H., Yamamoto Y., Higashitani A., Suzuki H., Nishimura Y. Cloning, mapping, and characterization of the Escherichia coli prc gene, which is involved in C-terminal processing of penicillin-binding protein 3. J Bacteriol. 1991 Aug;173(15):4799–4813. doi: 10.1128/jb.173.15.4799-4813.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kornitzer D., Teff D., Altuvia S., Oppenheim A. B. Isolation, characterization, and sequence of an Escherichia coli heat shock gene, htpX. J Bacteriol. 1991 May;173(9):2944–2953. doi: 10.1128/jb.173.9.2944-2953.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lim W. A., Sauer R. T. The role of internal packing interactions in determining the structure and stability of a protein. J Mol Biol. 1991 May 20;219(2):359–376. doi: 10.1016/0022-2836(91)90570-v. [DOI] [PubMed] [Google Scholar]
- Liou G. I., Geng L., Baehr W. Interphotoreceptor retinoid-binding protein: biochemistry and molecular biology. Prog Clin Biol Res. 1991;362:115–137. [PubMed] [Google Scholar]
- Nagasawa H., Sakagami Y., Suzuki A., Suzuki H., Hara H., Hirota Y. Determination of the cleavage site involved in C-terminal processing of penicillin-binding protein 3 of Escherichia coli. J Bacteriol. 1989 Nov;171(11):5890–5893. doi: 10.1128/jb.171.11.5890-5893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H., Lee Y. S., Chung C. H., Goldberg A. L. Purification and characterization of protease Re, a cytoplasmic endoprotease in Escherichia coli. J Bacteriol. 1988 Feb;170(2):921–926. doi: 10.1128/jb.170.2.921-926.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell D. A., Silber K. R., Sauer R. T. Carboxy-terminal determinants of intracellular protein degradation. Genes Dev. 1990 Feb;4(2):277–286. doi: 10.1101/gad.4.2.277. [DOI] [PubMed] [Google Scholar]
- Roseman J. E., Levine R. L. Purification of a protease from Escherichia coli with specificity for oxidized glutamine synthetase. J Biol Chem. 1987 Feb 15;262(5):2101–2110. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Weiss M. A., Karplus M., Sauer R. T. 1H NMR aromatic spectrum of the operator binding domain of the lambda repressor: resonance assignment with application to structure and dynamics. Biochemistry. 1987 Feb 10;26(3):890–897. doi: 10.1021/bi00377a033. [DOI] [PubMed] [Google Scholar]