Abstract
Objectives: Serum adiponectin and leptin levels have been shown to be related to obesity, insulin resistance and cardiovascular diseases (CVD). Opium addiction has a positive association with endocrine system disorders. The relationship between adipokines and opium addiction is unclear. In the present study, we aimed to determine serum adiponectin and leptin levels in opium addicted subjects.
Methods: 176 men, 88 opium addicts and 88 non- addicts were randomly selected from subjects who participated in Kerman Coronary Artery Disease Risk factors Study (KERCADRS); a population-based study. Serum adiponectin and leptin levels were measured using ELISA and compared between two groups. We adjusted the effect of some confounding factors such as the patients' demographic, clinical and medical history in multivariate analysis model.
Results: The serum level of adiponectin in opium addicts was significantly lower than control group (6.5±3.6 vs. 9.8±8.1 µg/ml, P<0.001). There was no significant difference in serum leptin level between two groups (11.8±10.3 ng/ml in control group vs. 11.5±10.8 ng/ml in opium addicts, p = 0.80). In the multivariate analysis, after adjusting for age, cigarette smoking, body mass index, type 2 diabetes, hypertension, cholesterol, triglyceride and high and low density lipoproteins, the negative association between opium addiction and decreased adiponectin level was still present (β = -0.144, P value = 0.005).
Conclusions: The results showed that opium addiction reduces serum adiponectin level. Since adiponectin has been shown to have anti-diabetic and anti-atherogenic effects, its reduction may account for increase in the risk of metabolic disorders such as insulin resistance and CVD amongst opium addicted patients.
Keywords: opium addiction, leptin, adiponectin, cardiovascular diseases
Introduction
Opium addiction is one of the social and economic problems that many societies in the world are dealing with, especially countries in the Middle East (UNODC, 2010[25]). The drug most abused in Western countries is heroine and most researchers have focused on the effect of heroine on body systems. In Iran, opium which contains many alkaloids along with morphine is the most common drug abused (UNODC, 2010[25]). Opioid compounds affect a variety of hormones and their functions and this has been termed opioid endocrinopathy. There are many investigations suggesting opioids can modulate the endocrine system (Benyamin et al., 2008[5]; Hejazian et al., 2007[8]; Katz, 2005[11]; Rhodin et al., 2010[24]). Opioids can bind to opioid receptors in the hypothalamus, pituitary and testes and can cause reduction in the plasma level of testosterone, estrogen, cortisol, luetenizing hormone (LH) and gonadotropin releasing hormone (GnRH) (Katz, 2005[11]; Benyamin et al., 2008[5]).
Khademi et al. (2012[12]) showed that opium usage increases risk of mortality. The relationship between opium consumption and risk of coronary artery disease and its risk factors have been shown in previous studies (Masoumi et al., 2010[15]; Najafipour et al., 2010[21], 2012[22]). Leptin and adiponectin are secreted along with some other hormones such as apelin, tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) from adipocytes in response to specific changes in certain body metabolic and physiologic conditions (Zhang et al., 1994[27]; Maeda et al., 1996[13]). Leptin regulates energy homeostasis, appetite and metabolism of glucose and lipids (Havel, 2004[7]; Meier, 2004[19]). The level of leptin increases in some metabolic disorders such as obesity, insulin resistance and dyslipidemia (Matsubara et al., 2000[17]) which are cardiovascular disease (CVD) risk factors as well. Leptin enhances the calcification of vascular cells of artery wall (Parhami et al., 2001[23]) leading to atherosclerosis.
Adiponectin is present in large amounts in plasma and accounts for 0.01 percent of human total plasma protein (Arita et al., 1999[2]). Several studies have previously shown that adiponectin level decreases in metabolic disorders and related diseases including obesity, type 2 diabetes mellitus (T2DM), insulin resistance, dyslipidemia and CVD (Matsubara et al., 2002[18]; Margoni et al., 2011[14]). It has also been mentioned in some reviews that adiponectin has remarkable anti-diabetic, anti-atherogenic and anti-inflammatory roles (Meier, 2004[19]; Kadowaki et al., 2006[10]).
Serum glucose has been shown to increase after opium administration in rats (Asadikaram et al., 2008[3]). Housova et al. (2005[9]) showed that serum leptin and adiponectin levels decreased in heroin addicts and methadone therapy for a year normalized leptin level.
To the best of our knowledge, there is no report regarding the effect of opium addiction on serum levels of leptin and adiponectin. As opium addiction is a CVD risk factor and adiponectin and leptin are important hormones influencing CVD risk factors, the present study was performed to investigate the effect of opium addiction on serum leptin and adiponectin levels in order to test the hypothesis that opium may worsen CVD diseases by causing changes in the production of these hormones.
Patients and Methods
Participants
The study subjects were randomly selected from participants in a population-based study, Kerman Coronary Artery Disease Risk factors Study (KERCADRS), in Kerman province of Iran in which 5900 of 15 to 75 year old city residents were recruited (Najafipour et al., 2012[22]). For each group of opium addicts and non-addicts (healthy subjects), the sample size was calculated to be 88. As the prevalence of opium addiction is much higher in male subjects and menstrual cycle may affect the serum concentration of hormones, only male subjects were included in the study. This study was approved by the Ethic Committee of Kerman University of Medical Sciences (permission No 90/399 KA). Informed consent was obtained from all subjects participating in the study.
Opium addiction definition
Opium addiction was defined according to the DSM-IV criteria (American Psychiatric Association, 2000[1]). A physician, after medical examination and taking fasting blood sample, asked the participants to disclose whether they had ever used any type of drug. She/he assured them that the information will be analyzed anonymously and used only for research purposes. Almost all people in Iran trust on medical doctors in disclosing their confidential health related information. The opium addicted group were participants who disclosed that they were addicted to opium; therefore, there was no need to confirm their addiction by laboratory tests. Serum levels of adiponectin and leptin were measured by ELISA method (LaborDiagnostika Nord GmbH & Co. KG Leptin ELISA and Bender MedSystems GmbH eBioscience Adiponectin ELISA).
Demographic and clinical variables
The following variables were recorded for all participants: age, BMI [(defined as weight (kg) divided by the square of height (m2)]. BMI > 25 kg/m2 was considered abnormal. Cigarette smoking was categorized as current smoker and non-smoker. Other variables measured were hypertension (BP>140/90 mmHg), T2DM (Fasting blood glucose (FBS) ≥126 mg/dl measured twice) and dyslipidemia (serum cholesterol >240 mg/dl, high density lipoprotein (HDL) <35 mg/dl, low density lipoprotein (LDL) >130 mg/dl and serum triglyceride (TG) >200 mg/dl (NCEP III, 2002). LDL was calculated based on Friedewald formula: LDL = Total cholesterol - (HDL + TG/5) (Friedewald et al., 1972[6]).
Statistical analysis
Data are presented as mean ± SD for quantitative variables and as absolute and relative frequencies for categorical variables. Normal distribution of data was assessed by Kolmogorov-Smirnov test. Serum adiponectin and leptin levels were logarithmically transformed to correct the skewness. Comparison between the two groups of the opium addict and non-addict subjects was analyzed using student's t-test for quantitative variables and chi square test for categorical variables. For adjusting some potential variables such as age, cigarette smoking, BMI, T2DM, hypertension, cholesterol, TG, HDL and LDL, multivariate linear regression was used to determine association between opium addiction and serum adiponectin and leptin levels. Then, in order to find this association, we formed five linear regression models including Model 1) simple or unadjusted, Model 2) adjusted for age and BMI, Model 3) adjusted for cigarette smoking, Model 4) adjusted for clinical variables including T2DM, hypertension, cholesterol, TG, HDL and LDL, and finally Model 5) adjusted for all variables as final model. The two dependent variables, adiponectin and leptin were not normally distributed, therefore we transformed them to logarithmical values. Then log-transformed data was used regarding adiponectin and leptin in the linear regression models. Statistical analyses were performed by STATA software, version 10 and P<0.05 was considered as statistically significant.
Results
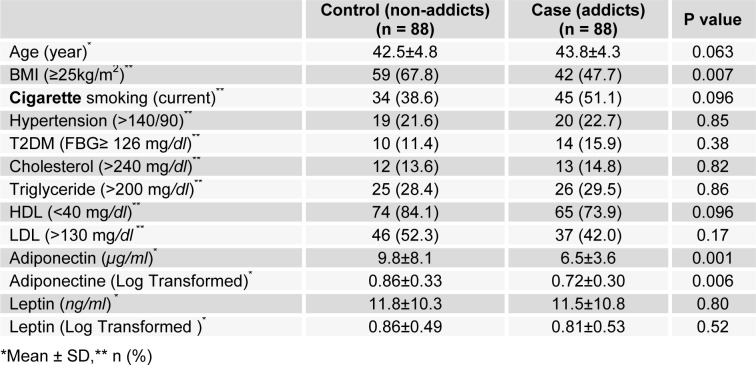
A total of 176 subjects consisting of 88 cases (addicted) and 88 controls (non-addicted) men were enrolled in the study. A summary of descriptive and clinical characteristics of participants is presented in Table 1(Tab. 1). (The complete individual data are presented in the form of two supplementary tables included in the electronic version of the MS). The two groups were only significantly different regarding abnormal BMI (≥ 25 kg/m2) so that 47.7 % of addicts compared to 67.8 % of non-addicts were overweight or obese (P value = 0.007). Distribution of age and frequency of cigarette smoking, hypertension, T2DM, having high cholesterol, TG and LDL and low HDL were similar between the two groups (P > 0.05).
Table 1. Comparison of descriptive and clinical characteristics in addicted and non-addicted groups.
Mean and standard deviations for adiponectin and leptin levels accompanied by their log-transformed distributions are also presented in Table 1(Tab. 1). The adiponectin concentration in addicted subjects was significantly lower than it was in non-addicts (P = 0.001). However, regarding leptin level, there was no statistically significant difference between the two groups (P = 0.80).
Association between adiponectin and addiction
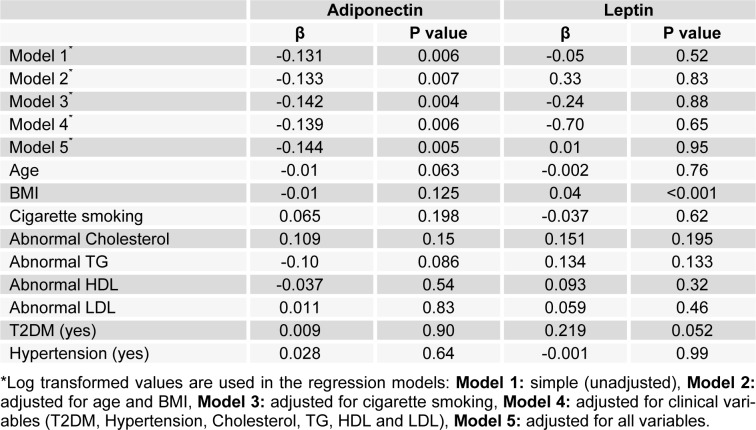
According to the linear regression models in Table 2(Tab. 2), by entering log-transformed values in the models, in the simple (unadjusted) regression model, adiponectin concentration was significantly negatively associated with opium addiction (β = -0.131, P = 0.006).The model adjusted by all potential confounding variables (Model 5), consisting of age, cigarette smoking, BMI, T2DM, Hypertension, Cholesterol, TG, HDL and LDL, also produced results similar to the unadjusted model (β = -0.144, P = 0.005). This means that there was still a negative significant association between addiction and decreased adiponectin level. When we fitted the regression model for the original data without log-transformed values, the similar results were obtained (β = -3014.1, P value = 0.001). Hence addiction, independent of other potential confounding variables had a significantly negative effect on the adiponectin level. The interaction between addiction and cigarette smoking on adiponectin concentration was not statistically significant (β = 0.13, P value = 0.184). None of the variables had a significant effect on adiponectin, based on the final regression model (Table 2(Tab. 2)).
Table 2. Association between adiponectin and leptin with opium addiction.
Association between leptin and addiction
In simple linear regression analyses (Model 1 in Table 2(Tab. 2)), no statistically significant association was found between serum leptin concentration and addiction status (β = -0.05, P = 0.52). After adjusting for potential confounding variables (Model 2 to 5), there was still no association between addiction and leptin (β = 0.01, P = 0.95).
By using the original data without being log-transformed, similar results were produced (β = 0.70, P value = 0.67, not shown in the Table). Interaction between addiction and cigarette smoking on leptin concentration was not statistically significant (β = -0.15, P = 0.32). The results of multivariate model (Model 5) demonstrated that except for BMI, which had significant positive effect (β = 0.04, P<0.001), none of other variables had a considerable effect on the serum leptin level.
Discussion
The main finding of this study was that adiponectin level in opium addicts (even after adjusting for some potential confounding variables) was lower than it was in non- addicts; while there was no statistically significant difference between the two groups regarding leptin level. In fact, there was a negative association between opium addiction and serum adiponectin concentration.
It has been shown that adiponectin level increases in lean and decreases in obese subjects and that there is an inverse correlation between adiponectin level and BMI (Weyer et al., 2001[26]; Margoni et al., 2011[14]). Here we showed that frequency of abnormal BMI is significantly lower in opium addicts; and adiponectin concentration is also significantly lower in the addicts. Thus, due to the inverse association of adiponectin with BMI, lower adiponectin in opium addicts cannot be inferred to their BMI (Meier, 2004[19]; Kadowaki et al., 2006[10]). On the other hand adiponectin is inversely correlated with obesity, but here in control group with higher BMI, adiponectin level was also significantly higher. Therefore, BMI is not the reason for lower adiponectin in addicted subjects either, and opium addiction should be the main reason for the reduction in adiponectin level. As adiponectin has shown anti-diabetic and anti-atherogenic effects (Meier, 2004[19]; Kadowaki et al., 2006[10]), its reduction may account for the increased risk of CAD and metabolic disorders in opium users. In accordance with this finding, Mohammadi et al. (2009[20]) reported that oral opium administration speeds up atherosclerosis formation in hypercholesterolemic rabbits. Interestingly, Najafipour et al. (2010[21]) reported that opium, even in passive smoking form, in long term increases blood pressure and plasma lipids in rabbits. Two other studies have reported that current opium users have a higher risk of CAD (Masoumi et al., 2010[16]) and that opium consumption can increase the risk of heart attack and stroke (Asgary et al., 2008[4]).
In contrast to adiponectin, the results showed that serum leptin levels in opium addicts and healthy subjects did not differ. Serum leptin level is increased in subjects with metabolic disorders such as obesity and insulin resistance (Matsubara et al., 2007). We expected that leptin level in control group to be higher due to higher BMI. Housova et al. (2005[9]) has shown that serum leptin level is lower in heroin addicts and becomes normal after one year of methadone therapy. Moreover, positive correlation of leptin level with BMI was lost in addicted group in their study. It has been well proven that circulating leptin increases in obesity (Matsubara et al., 2000[17]). Our findings also support the fact that leptin level rises with increment of BMI (Table 2(Tab. 2)); other factors did not show any significant effects on leptin levels. The difference in the results of the two studies may be due to the effect of constituents other than morphine, present in opium. This hypothesis needs more investigation to be proven.
In this study we excluded female subjects from the study. In our society, due to cultural reasons, the prevalence of opium addiction is much less (one fifth) in women in comparison to men (the prevalence in women was found to be 1.8 percent in KERCADR study (unpublished observations). Therefore, in addition to the changes in hormonal concentrations due to menstrual cycle in women, which might affect the leptin and adiponectin level, there was not enough opium addicted female samples to enter in the study.
Conclusion
Opium addiction may reduce adiponectin levels. Since adiponectin has shown anti-diabetic and anti-atherogenic effects, its reduction may account for increased risk of CVD and metabolic syndrome in opium users. As opium contains many alkaloids other than morphine, it seems necessary to conduct more investigations on the effect of opium components on leptin and adiponectin levels.
Acknowledgement
This research was financially supported by Physiology Research Center of Kerman University of Medical Sciences.
Conflict of interest
The authors declare that there is no conflict of interest in this study.
Supplementary Material
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV-TR. 4th. Washington, DC: American Psychiatric Publ.; 2000. [Google Scholar]
- 2.Arita Y, Kihara O, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;83:257–279. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 3.Asadikaram GH, Rashidinejad HR, Aghaee MM, Ahmadi J, Rahmani MR, Mahmoodi M, et al. Opium can differently alter blood glucose, sodium and potassium in male and female rats. Pak J Pharm Sci. 2008;21:180–184. [PubMed] [Google Scholar]
- 4.Asgary S, Sarrafzadegan N, Naderi GA, Rozbehani R. Effect of opium addiction on new and traditional cardiovascular risk factors: do duration of addiction and route of administration matter? Lipids Health Dis. 2008;7:42. doi: 10.1186/1476-511X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. 2008;11:105–120. [PubMed] [Google Scholar]
- 6.Friedewald WT, Levi RI, Fredrickson DS. Estimation of low density lipoprotein cholesterol in plasma without use of the ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 7.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53:143–151. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 8.Hejazian SH, Dashti MH, Rafati A. The effect of opium on serum LH, FSH and testosterone concentration in addicted men. Iran J Reproduct Med. 2007;1:35–38. [Google Scholar]
- 9.Housova J, Wilczek H, Haluzik MM, Kremen J, Krizova J, Haluzik M. Adipocyte-derived hormones in heroin addicts: the influence of methadone maintenance treatment. Physiol Res. 2005;54:73–78. doi: 10.33549/physiolres.930568. [DOI] [PubMed] [Google Scholar]
- 10.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz N. The impact of opioids on endocrine system. Pain Management Rounds. 2005;1:9. [Google Scholar]
- 12.Khademi H, Malekzadeh R, Pourshams A, Jafari E, Salahi R, Semnani S, et al. Opium use and mortality in Golestan cohort study: prospective cohort study of 50000 adults in Iran. BMJ. 2012;344:e2502. doi: 10.1136/bmj.e2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apMI (adipose most abundant gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–9. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 14.Margoni A, Perrea DN, Vlachos I, Prokopaki G, Pantopoulou A, Fotis L, et al. Serum leptin, adiponectin and tumor necrosis factor (TNF)-α in hyperlipidemic rats with/ without concomitant diabetes mellitus. Mol Med. 2011;17:36–40. doi: 10.2119/molmed.2010.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masoumi M, Ramezani MA, Karimzadeh H. The relationship of opium addiction with coronary artery disease. Int J Prev Med. 2010;1:182–186. [PMC free article] [PubMed] [Google Scholar]
- 16.Masoumi M, Shahesmaeili A, Mirzazadeh A, Tavakoli M, Ali AZ. Opium addiction and severity of coronary artery disease: a case-control study. J Res Med Sci. 2010;15:27–32. [PMC free article] [PubMed] [Google Scholar]
- 17.Matsubara M, Chiba H, Maruoka S, Katayose S. Elevated serum leptin concentrations in women with components of multiple risk factors clustering syndrome. J Atheroscler Thromb. 2000;7:231–7. doi: 10.5551/jat1994.7.231. [DOI] [PubMed] [Google Scholar]
- 18.Matsubara M, Marouka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002;147:173–180. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]
- 19.Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 20.Mohammadi A, Darabi M, Nasry M, Sabet-Jahromi MJ, Malekpour-Afshar R, Sheibani H. Effect of opium addiction on lipid profile and atherosclerosis formation in hypercholesterolemic rabbits. Exp Toxicol Pathol. 2009;61:145–9. doi: 10.1016/j.etp.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Najafipour H, Joukar S, Malekpour-Afshar R, Mirzaeipour F, Nasri HR. Passive opium smoking does not have beneficial effect on plasma lipids and cardiovascular indices in hypercholesterolemic rabbits with ischemic and non-ischemic hearts. J Ethnopharmacol. 2010;127:257–263. doi: 10.1016/j.jep.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Najafipour H, Mirzazadeh A, Haghdoost AA, Shadkam M, Afshari M, Moazenzadeh M, et al. Coronary artery disease risk factors in an urban and pri-urban setting (KERCADR Study): Methodology and preliminary report. Iran J Public Health. 2012;41(9):86–92. [PMC free article] [PubMed] [Google Scholar]
- 23.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circulation Res. 2001;88:954–60. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 24.Rhodin A, Stridsberg M, Gordh T. Opioid endocrinopathy: a clinical problem in patients with chronic pain and long-term oral opioid treatment. Clin J Pain. 2010;26:374–380. doi: 10.1097/AJP.0b013e3181d1059d. [DOI] [PubMed] [Google Scholar]
- 25.UNODC, United Nations Office on Drugs and Crime. World drug report. 2010. [Google Scholar]
- 26.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.