Abstract
Background & objectives:
Postmenopausal women constitute an ideal model for studying the extent of hypothalamo-pituitary gonadal (HPG) axis suppression in critical illness as the gonadotropins are normally high and non-cyclical in them. The objective was to assess the impact of acute severe illness in postmenopausal women on the HPG axis and the activities of the hypothalamo-pituitary-adrenal (HPA), the hypothalamo- pituitary-thyroid (HPT) axes; and levels of serum prolactin, by comparison between critically ill postmenopausal women and otherwise healthy postmenopausal women.
Methods:
Thirty five consecutive postmenopausal women older than 60 yr admitted to medical intensive care with a Simplified Acute Physiology Score II (SAPS II) more than 30 were included. On day five of their in-hospital stay, blood samples were collected for oestradiol, luteinizing hormone (LH), follicle stimulating hormone (FSH), cortisol, androstenedione, prolactin and thyroid profile. Thirty five apparently healthy postmenopausal women were selected as controls.
Results:
Levels of LH, FSH, thyrotropin, free thyroxin (fT4) and free tri-iodothyronine (fT3) were lower while oestradiol, cortisol and dehydroepiandrosterone were higher among patients in comparison to healthy controls. Prolactin levels were similar in patients and controls. Among sick patients both FSH and fT4 showed a negative correlation (P<0.05) with the SAPS II score.
Interpretation & conclusions:
In critically ill postmenopausal women, paradoxically elevated oestrogen levels despite gonadotropin suppression suggests a non-ovarian origin. Prolactin remained unaltered in patients despite their illness, possibly reflecting atrophy of lactotrophs in menopause.
Keywords: Critical illness, estradiol, gonadotropins, postmenopausal, prolactin
Acute severe illness causes a number of hormonal alterations aimed at directing available resources towards combating disease and ensuring the survival of the individual at the cost of other less essential functions such as reproduction. Studies in both men and women, suffering from acute illness or stress, showed low circulating sex steroid hormone levels (estradiol and testosterone in females and males, respectively) associated with inappropriately low gonadotropin levels consistent with a transient suppression of the hypothalamo-pituitary-gonadal (HPG) axis1,2,3,4,5. This is associated with simultaneous activation of the hypothalamo-pituitary adrenal (HPA) axis with consequent elevation of the serum cortisol levels6,7. The hypothalamo-pituitary-thyroid (HPT) axis in turn undergoes a series of complex changes during acute illness known as non-thyroidal illness or the sick euthyroid syndrome8,9,10. Initially, there is an impaired conversion of thyroxin (T4) to tri-iodothyronine (T3) leading on to elevated serum levels of T4 and depressed levels of T3. In more severe illness the T4 returns to normal or even may become low while the T3 remains low throughout and may even become undetectable. All these changes occur in the face of an inappropriately low thyrotropin (TSH) thereby signifying a transient functional suppression of the HPT axis10.
In postmenopausal women the gonadotropin levels are normally grossly elevated. It is, therefore, easy in this model to demonstrate the suppression of gonadotropin levels due to acute illness. Only a few studies3,5,11,12,13 have evaluated the gonadotropin levels in this population during acute severe illness or have endeavored to correlate the suppression of HPG axis with the activity of other relevant axes such as HPA12,13 and HPT axis11 or with the severity of illness12. This study was planned to simultaneously evaluate all the three axes (HPT, HPA and HPG) and also prolactin in severe acute illness in postmenopausal women with focus on the HPG axis and to correlate the observations with the severity of illness using the Simplified Acute Physiology Score II (SAPS II)14.
Material & Methods
Consecutive postmenopausal women were recruited according to the following inclusion and exclusion criteria: Cases were postmenopausal women aged more than 60 yr of age who were admitted to the intensive care unit (ICU) in the department of Medicine, Sri Venkateswara Institute of Medical Sciences, Tirupati, India, with a variety of acute, clinically severe, systemic illnesses and having a SAPS II score on the day of admission more than 30 (consistent with a predicted mortality >10%). Patients receiving postmenopausal hormone replacement therapy with estrogens or selective estrogen receptor modulators (SERMS) or those who had received glucocorticoids during the current admission or in the recent past were excluded from the study. Likewise patients undergoing treatment for or known to be having hypothyroidism or hyperthyroidism or those known to be having any pituitary disease were not included. Patients not surviving upto the fifth day of in-hospital admission and also those who withheld consent to participate were also excluded from the study. Apparently healthy, ambulatory, non-hospitalized, postmenopausal women aged more than 60 yr and not currently suffering from any acute intercurrent illness were included as controls.
The recruitement period was from March 2012 to February 2013 over a duration of one year. Informed written consent was obtained from each participant. The study was approved by the institutional ethics committee.
Data and sample collection: Relevant demographic, clinical, laboratory and imaging data were collected to identify the diagnosis in the given patient. The severity of the illness was assessed by calculating SAPS II on the day of admission14. In all patients, on the fifth day of admission to medical ICU peripheral venous blood was collected starting at 0800 h in plain vials as three equal aliquots taken 20 min apart from each other. The three aliquots were then pooled for hormone analysis. Serum was separated and stored at -40°C till processing. In controls, sampling was done at 0800 h am in a similar fashion. The following hormones were estimated in all these samples: luteinizing hormone (LH), follicular stimulating hormone (FSH), oestradiol (E2), free triodothyronine (fT3), free thyroxin (fT4), thyroid stimulating hormone (TSH), cortisol, prolactin, dehydroepiandrosterone (DHEA), androstenedione and sex hormone binding globulin (SHBG).
Hormone assays: LH was measured by immunoradiometric assay (IRMA) using kits (IM1381-IM3302) from Beckman Coulter (Prague, Czech Republic), which had a measurement range of 0.4-180 IU/l with an inter-assay variation of 6.7 per cent and an intra-assay variation of 3.7 per cent. FSH was also measured by IRMA using kits from Beckman Coulter (IM2125-IM3301), which had a measurement range of 0.5-180 IU/l and an intra- and inter-assay variations of 2.6 and 6.3 per cent, respectively. Free thyroxin was estimated by commercial radioimmunoassay (RIA) kits (SA1535S by Diasorin Inc., USA) which had a range of measurement between 0.07 - 4.3 ng/dl and inter- and intra-assay variations ranging from 2.52-9.62 and 6.09-16.28 per cent, respectively at different concentrations of fT4. Cortisol was also estimated by an RIA kit from Diasorin which had a measurement range of 0.21-60 μg/dl and intra- and inter-assay variations ranging from 6.6-7.7 and 8.8-9.8 per cent, respectively at different concentrations of cortisol. SHBG was also estimated by an RIA (kit IM3532, Beckman Coulter, Czech Republic) with a measurement range of 0.22-300 nmol/l and an intra- and inter-assay variations of 6.1 and 8.6 per cent, respectively. TSH was measured by IRMA using kits (IRMAK-9) from the Board of Radiation Technology (BRIT), Mumbai, having a measurement range of 0.025-100 μIU/ml. Androstenedione was estimated by RIA kit (DSL3800) manufactured by Beckman Coulter which had a range of measurement of 0.03-10 ng/ml. All measurements were made on an I125 Gamma Counter (IC 4702A, ECIL, Hyderabad). Prolactin, fT3 and E2 were estimated on a fully automated chemiluminesence platform (Access 2 Immunoassay System by Beckman Coulter, USA). For prolactin, the range of measurement was 0.25-200 ng/ml, for estradiol, 20-4800 pg/ml and for fT3 it was 1.4-47.7 pmol/l. DHEA was measured by enzyme linked immunosorbent assay (ELISA) using the DHEA kits manufactured by Diagnostics Biochem Canada Inc., Ontario, Canada. The measurement range was from 0.082 to 40 ng/ml. The intra-assay coefficients of variation ranged from 5.9 to 13.4 per cent, while the inter-assay coefficients of variation ranged from 9.2-13.5 per cent.
Statistical analysis: Continuous variables were represented either as mean ± standard deviation (SD) when these were normally distributed. Else these were represented as median (inter-quartile range - IQR). Where means of continuous variables in patients were compared with those in healthy controls, the differences were tested for significance by the Student t test. However, when the medians of continuous variables in patients were compared with that in healthy controls, the differences were tested for significance by the non-parameteric Mann Whitney U test. Correlations between continuous variables were determined using the Pearson's coefficient of correlation.
Results
Thirty five critically ill postmenopausal patients older than 60 yr of age (66.6 ± 6.5 yr) and 35 apparently healthy postmenopausal controls aged more than 60 yr (64.6 ± 4.2 yr) were studied. In patients, the mean SAPS II score was 43.9 ± 9.1 and the mean predicted in-hospital mortality was 34.1±18.1 per cent. Ten of 35 patients died in hospital during the study period giving an observed in-hospital mortality of 28.6 per cent.
Comparison of hormone levels between patients and controls: Hormonal characteristics of patients and healthy controls are shown in Tables I and II. Levels of LH and FSH were significantly lower among sick patients in comparison to healthy controls (P<0.001). However, oestradiol values were significantly higher among patients than in controls (P<0.001) even though the sex hormone binding globulin (SHBG) levels were similar. Likewise, cortisol and DHEA but not androstenedione were elevated in patients compared to controls, while the fT3, fT4 and TSH all were significantly lower. Prolactin levels were similar between patients and controls.
Table I.
Comparison of hormones between sick postmenopausal women versus that in healthy postmenopausal controls
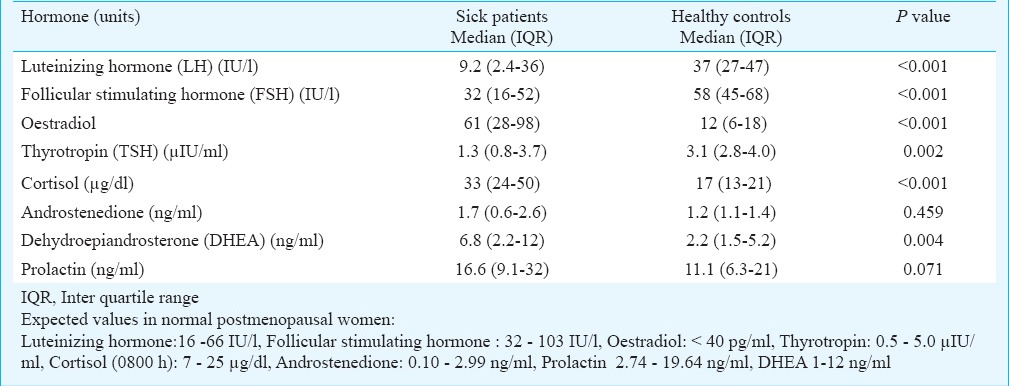
Table II.
Comparison of hormones between sick postmenopausal women versus that in healthy postmenopausal controls
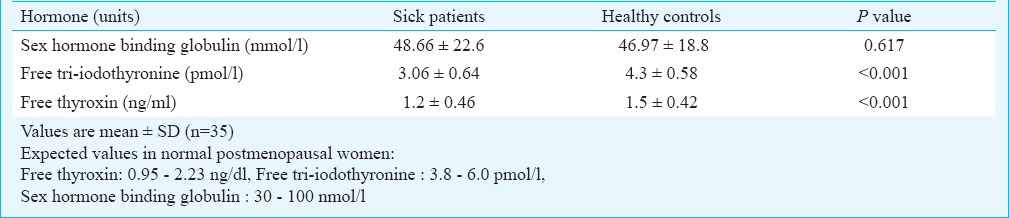
Correlations between hormones and SAPS II score in sick patients group: Among sick patients both FSH and fT4 showed a significant negative correlation with the SAPS II score (r = -0.35; P=0.04 for FSH and r = -0.42; P=0.012 for fT4). No correlations with SAPS II score were obtained for any of other hormones estimated.
Correlations between pairs of hormones in sick patients group: FSH and fT4 in patients showed a significant positive correlation with each other (r=0.471; P=0.004). The two gonadotropins LH and FSH also showed a strong positive correlation with each other (r=0.601; P<0.001). The adrenocortical steroid hormones, cortisol and androstenedione were also significantly and positively correlated with each other (r=0.356; P=0.036). No other significant correlations were observed among the various hormones in the group of sick patients.
Discussion
In our study the SAPSII scoring system was used to quantify severity of the underlying illness and to predict the likelihood of mortality. We chose to include only those patients who had a predicted in-hospital mortality greater than or equal to 10 per cent to ensure that only genuinely critically ill patients were included as cases. A significant negative correlation was observed between severity of illness as quantified by the SAPSII score and serum FSH but not with LH. Spratt et al12 have used the APACHE (Acute Physiological and Chronic Health Evaluation II) scoring system to divide their patients into mild, moderate and severe illness. They reported that in postmenopausal women nadir serum FSH but not LH levels during hospitalization were lower in patients with APACHE II scores > 15 than in patients with APACHE scores of < 15. Thus, while both gonadotropins are suppressed in acute illness, the FSH level of suppression correlates with the severity of illness.
A significant correlation was observed between the FSH and LH levels in the critically ill patients. Both these hormones are produced by the same cells and are released in response to the same hypothalamic trophic hormone namely gonadodotropin releasing hormone (GnRH). Cytokines are the probable mediators of the gonadotropin suppression in the sick patients via their negative impact on GnRH pulses. Injection of interleukin (IL)-1α in primates caused suppression of gonadotropin secretion that was reversed by the administration of corticotrophin releasing hormone (CRH) antagonist, implying the role of CRH in reducing GnRH pulses in cytokine induced gonadotropin suppression15.
Cytokines can also activate the HPA axis via their effects on hypothalamic CRH and arginine vasopressin (AVP) secretion. In our study, levels of cortisol were significantly higher in patients than in the healthy controls. This has been observed in several other studies as well6,7. Even though there is a suppressive effect of cortisol on gonadotropins16, no correlation was observed between cortisol levels with either of the two gonadotropins in our study. Thus cortisol was not probably responsible for the gonadotropin suppression. Samuels et al17 found no effect of 100 and 300 mg hydrocortisone (HC) infusion over 24 h on pulsatile LH, FSH and alpha-subunit secretion in ten healthy young subjects on three occasions. They have concluded that the effects of stress and/or hypercortisolism on the gonadal axis may require higher cortisol levels, more prolonged exposure, or other mediators of the stress response.
Parallel with the rise in cortisol in our critically ill patient group, higher levels of DHEA in comparison to healthy controls were also observed. Both cortisol and androstenedione showed a significant positive correlation among patients, as reported earlier13. This may be explained by the fact that ultimately both androgens and cortisol are secreted by the adrenal cortex under control of the same trophic hormone namely adrenocorticotropic hormone (ACTH).
An interesting observation in our study was higher levels of oestradiol in critically ill postmenopausal women when compared to healthy postmenopausal controls despite the lower levels of gonadotropins in them. This suggests that the oestrogen elevation is not under gonadotropin stimulation and is thus not of ovarian origin. The only other source of oestrogen is that derived from the peripheral aromatization of adrenal androgens to oestrogen. Moreover, the adrenal specific androgen DHEA, like oestrogen was elevated in the critically ill postmenopausal women in comparison to controls. This could have been the source for the increased production of oestrogen in sick postmenopausal women, via aromatization in the periphery.
Though no correlation was observed between DHEA or androstenedione with oestradiol, it may be noted that DHEA has to be initially converted to androstenedione which on aromatization directly forms oestrone rather than oestradiol. Thus oestradiol, being not a direct product of DHEA or androstenedione may lack correlation with it.
The clinical significance of oestrogen elevation is not clear. Oestrogen has been shown to have many beneficial effects during acute severe illness. Physiologic levels of oestrogen, like those seen during the oestrus/menstrual cycle, stimulate the immune response, whereas high levels such as those found during pregnancy are suggested to downregulate cell-mediated immune responses18. Estrogen receptors are found in reproductive tissue, as well as certain immune cells including T cells, monocytes, and macrophages. Oestrogens have an anti-apoptotic effect on cultured human macrophage cell lines19, and has also shown a protective effect in pneumonia as it enhances the transport of IgA into the respiratory mucosal cells by increasing toll like receptor 4 (TLR 4) expression20. Oestrogens also have prothrombotic effects21 which may be of help with haemostasis in acute trauma patients.
In our study there was no significant correlation between the SAPS II score and levels of oestrogen. However, Dosselt et al22 showed that oestrogen levels could be a predictor of mortality in critically ill and injured patients in both sexes as the level of oestrogen elevation correlated with the severity of illness22,23,24. Oestradiol levels were higher in non-survivors than survivors22,23. A serum oestradiol cut-point of 50 pg/ml was 48 per cent sensitive and 80 per cent specific in predicting death and classified the outcome of 76 per cent of patients correctly22. Analysis by quartiles of oestradiol among critically ill trauma and surgery patients demonstrated a greater than three-fold increase in the mortality rate for the highest vs. the lowest estradiol quartiles24. Further research is required to determine whether oestrogen is merely a marker of severity of illness and consequently mortality or whether it contributes to deterioration and death of the patient.
In sick postmenopausal women, as expected fT3 and fT4 as well as TSH levels were lower than in control, which was consistent with what is expected in the sick euthyroid syndrome8,9,10. For fT4 we were also able to demonstrate a significant negative correlation with the severity of illness as quantified by the SAPS II.
Prolactin is an important stress induced hormone and is known to rise in acute critically ill patients25,26,27. However, in our study, which focused exclusively on postmenopausal women, there was no difference between the prolactin levels in patients and controls. Levels of prolactin in critically ill patients also did not correlate with the SAPS II. Spratt et al12 also showed no correlation between prolactin and the APACHE II score in sick men and postmenopausal women. This lack of prolactin response to the stress of critical illness could be due the atrophy of lactotrophs in postmenopausal women due to the prolonged lack of oestrogen. Oestrogen is trophic to the lactotrophs and maintains the mass and function of these cells. Prolactin has important immunomodulatory roles28,29. It is not clear whether the absence of elevated prolactin and consequently of its immunomodulatory effect could have any effect on the outcome in critically ill postmenopausal women.
To conclude, critically ill postmenopausal women showed an elevated oestradiol despite suppression of gonadotropins. This oestradiol is likely to be due to aromatization of elevated andrenal androgens, found in these patients, to oestrogens. The suppression of FSH correlated with severity of the illness. Prolactin levels were not altered in postmenopausal critically ill women. The impact of these hormonal alterations on outcome of the underlying disease needs more study.
Acknowledgment
This research work was funded by a research grant from the Indian Council of Medical Research, New Delhi.
Footnotes
Conflicts of Interest: None.
References
- 1.Kalyani RR, Gavini S, Dobs AS. Male hypogonadism in systemic disease. Endocrinol Metab Clin North Am. 2007;36:333–48. doi: 10.1016/j.ecl.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Baker HW. Reproductive effects of nontesticular illness. Endocrinol Metab Clin North Am. 1998;27:831–50. doi: 10.1016/s0889-8529(05)70043-8. [DOI] [PubMed] [Google Scholar]
- 3.Woolf PD, Hamill RW, McDonald JV, Lee LA, Kelly M. Transient hypogonadotropic hypogonadism caused by critical illness. J Clin Endocrinol Metab. 1985;60:444–50. doi: 10.1210/jcem-60-3-444. [DOI] [PubMed] [Google Scholar]
- 4.Bing-You RG, Spratt DI. Serum estradiol but not gonadotropin levels decrease acutely after insulin-induced hypoglycemia in cycling women. J Clin Endocrinol Metab. 1992;75:1054–9. doi: 10.1210/jcem.75.4.1400870. [DOI] [PubMed] [Google Scholar]
- 5.van Steenbergen W, Naert J, Lambrecht S, Scheys I, Lesaffre E, Pelemans W. Suppression of gonadotropin secretion in the hospitalized postmenopausal female as an effect of acute critical illness. Neuroendocrinology. 1994;60:165–72. doi: 10.1159/000126747. [DOI] [PubMed] [Google Scholar]
- 6.Drucker D, Shandling M. Variable adrenocortical function in acute medical illness. Crit Care Med. 1985;13:477–9. doi: 10.1097/00003246-198506000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Chernow B, Alexander HR, Smallridge RC, Thompson WR, Cook D, Beardsley D, et al. Hormonal responses to graded surgical stress. Arch Intern Med. 1987;147:1273–8. [PubMed] [Google Scholar]
- 8.Docter R, Krenning EP, DeJong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol. 1993;39:499–518. doi: 10.1111/j.1365-2265.1993.tb02401.x. [DOI] [PubMed] [Google Scholar]
- 9.McIver B, Gorman CA. Euthyroid sick syndrome: an overview. Thyroid. 1997;7:125–32. doi: 10.1089/thy.1997.7.125. [DOI] [PubMed] [Google Scholar]
- 10.DeGroot LJ. Non-thyroidal illness syndrome is functional central hypothyroidism, and if severe, hormone replacement is appropriate in light of present knowledge. J Endocrinol Invest. 2003;26:1163–70. doi: 10.1007/BF03349151. [DOI] [PubMed] [Google Scholar]
- 11.Quint AR, Kaiser FE. Gonadotropin determinations and thyrotropin-releasing hormone and luteinizing hormone-releasing hormone testing in critically ill postmenopausal women with hypothyroxinemia. J Clin Endocrinol Metab. 1985;60:464–71. doi: 10.1210/jcem-60-3-464. [DOI] [PubMed] [Google Scholar]
- 12.Spratt DI, Cox P, Orav J, Moloney J, Bigos T. Reproductive axis suppression in acute illness is related to disease severity. J Clin Endocrinol Metab. 1993;76:1548–54. doi: 10.1210/jcem.76.6.8501163. [DOI] [PubMed] [Google Scholar]
- 13.Spratt DI, Longcope C, Cox PM, Bigos ST, Wilbur-Welling C. Differential changes in serum concentrations of androgens and estrogens (in relation with cortisol) in postmenopausal women with acute illness. J Clin Endocrinol Metab. 1993;76:1542–7. doi: 10.1210/jcem.76.6.8501162. [DOI] [PubMed] [Google Scholar]
- 14.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 15.Feng YJ, Shalts E, Xia LN, Rivier J, Rivier C, Vale W, et al. An inhibitory effect of interferon 1a on basal gonadotropin release in the ovariectomized rhesus monkey: reversal by a corticotrophin - releasing factor antagonist. Endocrinology. 1991;128:2077–82. doi: 10.1210/endo-128-4-2077. [DOI] [PubMed] [Google Scholar]
- 16.Saketos M, Sharma N, Santoro NE. Suppression of the hypothalamo-pituitary-ovarian axis in normal women by glucocorticoids. Biol Reprod. 1993;49:1270–6. doi: 10.1095/biolreprod49.6.1270. [DOI] [PubMed] [Google Scholar]
- 17.Samuels MH, Luther M, Henry P, Ridgway EC. Effects of hydrocortisone on pulsatile pituitary glycoprotein secretion. J Clin Endocrinol Metab. 1994;78:211–5. doi: 10.1210/jcem.78.1.8288706. [DOI] [PubMed] [Google Scholar]
- 18.Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–84. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- 19.Cutolo M, Capellino S, Montagna P, Ghiorzo P, Sulli A, Villaggio B. Sex hormone modulation of cell growth and apoptosis of the human monocytic/macrophage cell line. Arthritis Res Ther. 2005;7:R1124–32. doi: 10.1186/ar1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali AA, Diebel LN, Liberati DM. Estrogen modulation of pneumonia?. An immunoglobulin A effect. Trauma Acute Care Surg. 2012;72:908–15. doi: 10.1097/TA.0b013e3182468989. [DOI] [PubMed] [Google Scholar]
- 21.DeLoughery TG. Estrogen and thrombosis: controversies and common sense. Rev Endocr Metab Disord. 2011;12:77–84. doi: 10.1007/s11154-011-9178-0. [DOI] [PubMed] [Google Scholar]
- 22.Dossett LA, Swenson BR, Evans HL, Bonatti H, Sawyer RG, May AK. Serum estradiol concentration as a predictor of death in critically ill and injured adults. Surg Infect (Larchmt) 2008;9:41–8. doi: 10.1089/sur.2007.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dossett LA, Swenson BR, Heffernan D, Bonatti H, Metzger R, Sawyer RG, et al. High levels of endogenous estrogens are associated with death in the critically injured adult. J Trauma. 2008;64:580–5. doi: 10.1097/TA.0b013e31816543dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.May AK, Dossett LA, Norris PR, Hansen EN, Dorsett RC, Popovsky KA, et al. Estradiol is associated with mortality in critically ill trauma and surgical patients. Crit Care Med. 2008;36:62–8. doi: 10.1097/01.CCM.0000292015.16171.6D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akbaş T, Karakurt S, Unlügüzel G, Celikel T, Akalin S. The endocrinologic changes in critically ill chronic obstructive pulmonary disease patients. COPD. 2010;7:240–7. doi: 10.3109/15412555.2010.496815. [DOI] [PubMed] [Google Scholar]
- 26.Sahana PK, Ghosh A, Mukhopadhyay P, Pandit K, Chowdhury BR, Chowdhury S. A study on endocrine changes in patients in intensive care unit. J Indian Med Assoc. 2008;106:362–4. [PubMed] [Google Scholar]
- 27.Güven M, Bayram F, Unlühizarci K, Keleştimur F. Endocrine changes in patients with acute organophosphate poisoning. Hum Exp Toxicol. 1999;18:598–601. doi: 10.1191/096032799678839419. [DOI] [PubMed] [Google Scholar]
- 28.Buckley AR. Prolactin, a lymphocyte growth and survival factor. Lupus. 2001;10:684–90. doi: 10.1191/096120301717164912. [DOI] [PubMed] [Google Scholar]
- 29.De Bellis A, Bizzarro A, Pivonello R, Lombardi G, Bellastella A. Prolactin and autoimmunity. Pituitary. 2005;8:25–30. doi: 10.1007/s11102-005-5082-5. [DOI] [PubMed] [Google Scholar]


