Abstract
Background & objectives:
Methicillin resistant Staphylococcus aureus (MRSA) are the commonest cause of osteomyelitis. The aim of this study was to evaluate the role of an alternative therapy i.e. application of S. aureus specific bacteriophages in cases of osteomyelitis caused by MRSA in animal model.
Methods:
Twenty two rabbits were included in this study. The first two rabbits were used to test the safety of phage cocktail while the remaining 20 rabbits were divided into three groups; group A (n=4) to assess the establishment of osteomyelitis; group B (n=4) osteomyelitis developed but therapy started only after six weeks; and group C (n=12) osteomyelitis developed and therapy started after three weeks. Groups B and C rabbits were treated with four doses of cocktail of seven virulent bacteriophages at the interval of 48 h. Comparison between three groups was made on the basis of observation of clinical, radiological, microbiological, and histopathological examinations.
Results:
Experimental group rabbits recovered from the illness in the subsequent two weeks of the therapy. Appetite and activity of the rabbits improved, local oedema, erythema and induration subsided. There were minimal changes associated with osteomyelitis in X-ray and histopathology also showed no signs of infection with new bone formation. Control B group rabbits also recovered well from the infection.
Interpretation & conclusions:
The present study shows a potential of phage therapy to treat difficult infections caused by multidrug resistant bacteria.
Keywords: MRSA, osteomyelitis, phage therapy, Staphylococcus aureus
Chronic osteomyelitis is defined as suppurative/non-supurative bone inflammation established for ≥ 6 wk. This chronic infection may arise either as a complication of acute osteomyelitis or as a result of infection by indolent pathogen. It has been observed that appropriate antibiotics if started in cases of stage-1 osteomyelitis (early acute), may cure the infections1,2. However, it becomes difficult to cure higher stage acute and chronic osteomyelitis specially when caused by multidrug resistant bacteria. In addition, biofilm formation and accumulation of dead tissues, e.g. necrosed soft tissues and bone (sequestrum) at the site of infection further aggravates the problem3. If the causative bacterium is sensitive to a particular antibiotic and proper surgical debridement is ensured, it often fails because of several predictable reasons such as lower concentrations (10-20%) of antibiotics achieved in bone tissue due to poor vascular perfusion, variation in tissue pH and oxidative microenvironment interfering with the activity of antibiotics in bone4,5,6.
Staphylococcus aureus is the commonest aetiological agent implicated in acute and chronic osteomyelitis7. S. aureus has an extraordinary capacity to adapt the hostile environment with a proven ability to develop resistance against antibiotics. Non-antimicrobial approaches to prevent and treat the infection have also been tried. Use of lysostaphin8, antimicrobial peptides9, some natural products (tea, tree, oil)10, anti-staphylococcal vaccines11 and bacteriophages12,13 are some of the alternative modalities to deal with S. aureus including methicillin resistant (MRSA) and vancomycin resistant (VRSA). The present study was planned to see the effect of S. aureus specific bacteriophages on osteomyelitis especially the chronic infection caused by MRSA strain in experimental animal model (rabbit).
Material & Methods
The study protocol was approved by the Animal Ethics Committee of Banaras Hindu University, Varanasi, India. The study was carried out during January 2011 to March 2012 in the Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi in collaboration with departments of Orthopaedics, Pathology and Radiodiagnosis and Imaging. The study was divided into two parts: The in vitro study included isolation of bacteria, isolation and purification of phages and assessment of their anti-staphylococcal activity; whereas in vivo study included creation of osteomyelitis in distal end of femur of rabbits, confirmation of infection, phage therapy and assessment of result on the basis of clinical, microbiological, radiological and histopathological examinations.
Bacterial isolation: A total of 100 S. aureus isolates were obtained from pus, blood, urine, CSF, wound swab specimens of the patients admitted in the university hospital. The pure isolated colonies were identified by Gram's staining, slide coagulase, catalase tests and mannitol fermentation while confirmation was done by amplification with S. aureus specific coagulase gene14. MRSA were identified on the basis of antibiotic susceptibility using modified Kirby-Bauer disk diffusion method15 and minimum inhibitory concentration (MIC) determination by the agar dilution method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines16. Antibiotic discs (Hi-Media, Mumbai, India) were used for susceptibility testing. Basic set included penicillin (10 Unit), oxacillin (1μg), gentamicin (10 μg), ciprofloxacin and ofloxacin (30 μg each), erythromycin, (15 μg), trimethoprim (1.25μg) + sulphamethoxazole (23.75μg), cefoxitin (30 μg), ceftriaxone (30 μg), tobramycin (10 μg), amikacin (30 μg), vancomycin (30 μg), linezolid (30 μg), piperacillin (100 μg) + tazobactam (10 μg), and teicoplanin (30 μg). A zone of inhibition less than 10 mm or any discernible growth within zone of inhibition around oxacillin disc was taken as methicillin resistance. A total of 17 MRSA isolates could be identified. One of the MRSA isolated from a patient suffering from chronic osteomyelitis for >10 yr was used for further experimentation. For further experiments the bacterial isolates were grown in Luria-Bertani (L-B) broth at 37°C and harvested while in exponential phase of the growth cycle. The harvested bacteria were washed with normal saline (0.85% NaCl in distilled water) and re-suspended in saline to achieve 106 cfu/ml.
Phage isolation and purification: Isolation of bacteriophages was done from different water sources (river, ponds and sewer) by using double agar overlay method with slight modification as described earlier17. In brief, for isolation of bacteriophages, the host bacteria were plated as lawn culture (108 cfu/ml) on Muller-Hinton agar (MHA). Water specimens from different water bodies were treated with 1 per cent chloroform (v/v) for 20 min and centrifuged for 15 min at 10778×g. The supernatant in the volume of one ml was flooded on the 5 h old lawn culture growth (log phage) of the S. aureus on 90 mm nutrient agar plate and incubated overnight at 37 °C. Next day the lawn was washed with 3 ml TMG (tris HCl, magnesium sulphate, gelatin pH 7.4) buffer and centrifuged at 10778×g for 15 min. The supernatant (1 ml) was transferred to a 1.5 ml microcentrifuge tube. One drop of chloroform was added and mixed well by vortexing or by inversion. Centrifugation was done at 10778×g for 10 min. The lawn culture in log phase of the host was again prepared and the supernatant collected as mentioned above was inoculated in the volume of 100μl at 8-10 places to screen for lysis. The surface with clear plaque was swabbed and collected in 1ml of the TMG buffer and propagated further for plaque counting by soft agar overlay method18. Centrifugation was done and supernatant was transferred into a fresh microcentrifuge tube. To pellet the bacteria and cell debris centrifugation was done at 10778×g for 10 min. Transfer of the supernatant of second spin was done to another fresh microfuge tube. The clarified supernatant was preserved at 4°C for further use.
For purification and concentration of phages the harvested fluid was subjected to formalin (1%) for 20 min and then centrifuged at 10778×g for 10 min and supernatant was mixed with polyethylene glycol (PEG) (20% in 2.5M NaCl) at the final concentration of 5 per cent (7.5ml PEG + 30ml washings) for overnight at 4°C. Spinning of the tube was done for 30 min at 21124×g and milky pellet was collected. Re-spinning was done three times to remove all of PEG solution. Phages were resuspended in sodium chloride-Tris-EDTA (STE) (1000 μl). The phage suspension was made free from DNAse by adding protienase K enzyme which was inactivated later by heating at 50° C.
Assessment of antistaphylococcal activity: A total of 100 clinical isolates of S. aureus were subjected to all the 47 isolated phages for the assessment of their antibacterial activity. The lawn culture of S. aureus (108 cfu/ml) was made on MHA. Each of the phages having concentration of 1012 plaque forming unit (pfu)/ ml was spotted on the plate in the volume of 10 μl. The plates were observed for the clear zone after overnight incubation at 37°C.
Characterization of phages: The seven most virulent phages (SA-BHU1, SA-BHU2, SA-BHU8, SA-BHU15 and SA-BHU21, SA-BHU37, SA-BHU47) were characterized by using FEI tecnai™ transmission electron microscope at 200 KV (FEI Company, NE, USA). Briefly, bacteriophage lysate was centrifuged at 20,000×g for 60 min using an ultracentrifuge machine (Hitachi- HIMAC CR21, HITACH KOKI India Ltd. Bengaluru). The supernatant was discarded and pellet was washed twice in 0.1 M ammonium acetate (pH 7.0). A drop of the above solute was deposited on copper grid provided with carbon coated Formvar films Ted Pella Inc, CA, USA), stained with 2 per cent w/v potassium phosphotungstate. Further, the mapping of the genome was done by using molecular methods (RFLP, RAPD and ERIC PCR). The viruses with stable genomic structure as repeated analysis showed almost similar banding pattern, were used for the phage therapy.
Dose standardization of phage cocktail and phage therapy: The most virulent seven phages (SA-BHU1, SA-BHU2, SA-BHU8, SA-BHU15 and SA-BHU21, SA-BHU37, SA-BHU47) were selected to prepare the cocktail. Each of the phages (5x1012pfu/ml) in 15 μl volume was mixed (105μl) for therapy.
Safety of bacteriophage cocktail: Two adult rabbits about 6-8 months old were taken and 100 μl of phage cocktail (~2x1012pfu/ml) was given through intraperitoneal (i.p.) injection. These rabbits were observed for one month and no sickness was observed in them. Therefore, it was decided to inject the cocktail of phages at a concentration of 5x1012pfu/ml which was found safe and most likely able to lyse the S. aureus bacteria from outside.
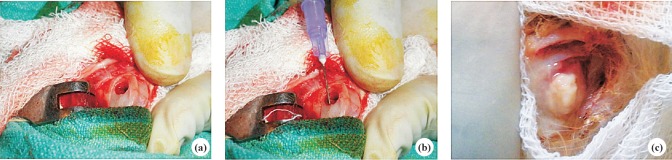
Development of osteomyelitis on distal end of femur of rabbit: Rabbits were anaesthetized using 2 mg/kg of ketamine hydrochloride and 2 mg/kg of midazolam (Sedoz, Claris Life Sciences Ltd, Ahmedabad, India). After part preparation using alcohol (70%) and iodine (2%), a 2 cm long incision was made at the lateral aspect of distal end of femur and metaphysial area was exposed. With the help of a hand drill, a 5 mm diameter unicortical defect was created19 (Fig. 1 a & b). Methicillin resistant S. aureus (5 x 106 cfu/ml diluted by 10μl in 1ml) were inoculated by the intramedullary injection. The incised area was covered by sterile bandages with one stitch at middle of open area. The establishment of infection of S. aureus was confirmed by repeated isolation of the bacteria form wound site. The phage cocktail was injected in soft tissues of infected area in the volume 15μl.
Fig. 1.
Exposure and inoculation of S. aureus through drill hole (a) and (b) in distal femur and frank pus discharge (c).
A total of 20 rabbits of approximately 2.5-3.0 kg weight were taken and divided into three groups (Table).
Table.
Bacteriophage therapy of acute and chronic osteomyelitis in rabbit model
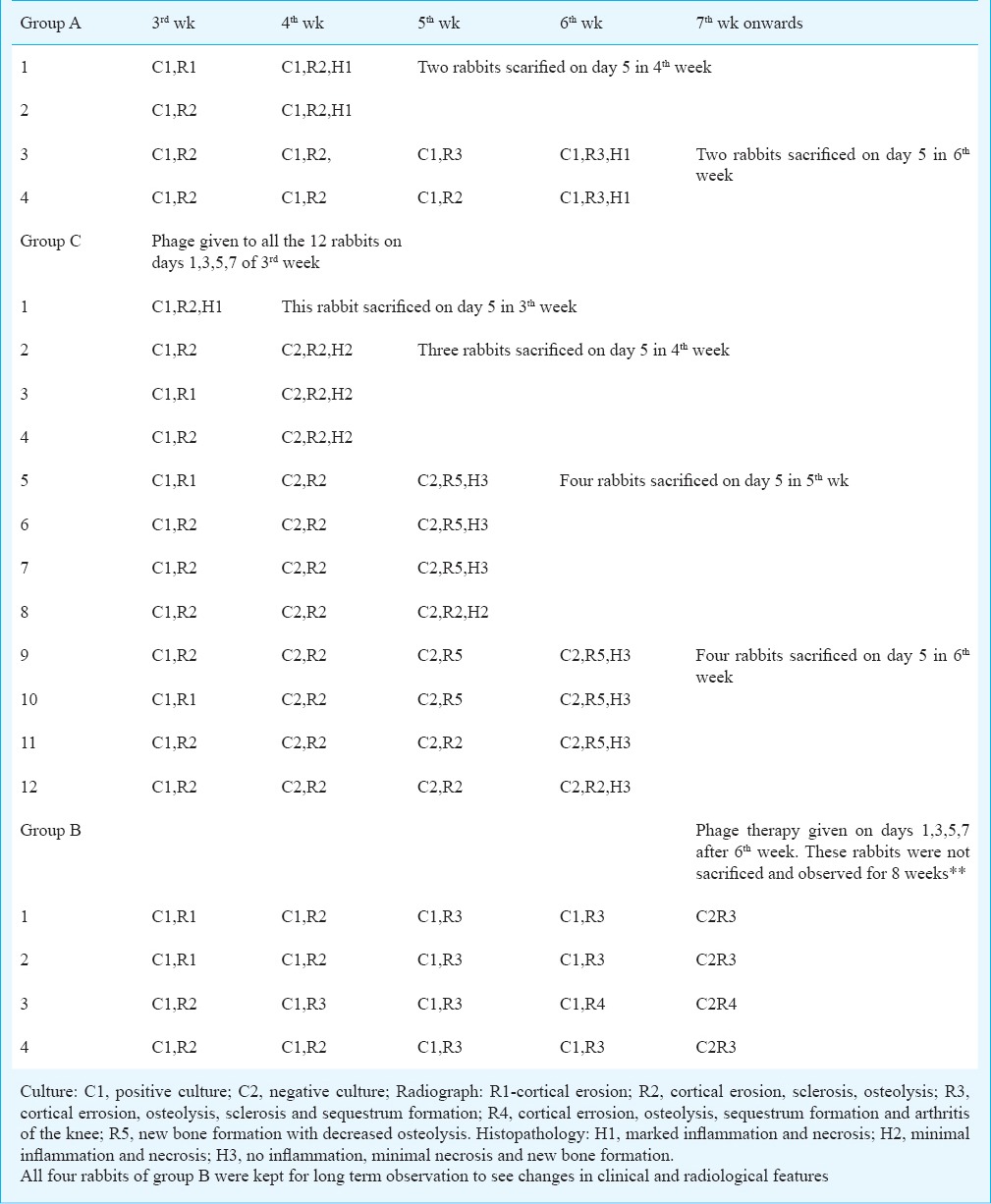
Group A (n=4) rabbits were used to see the progression of osteomyelitis without any treatment. They were observed for clinical, microbiological and radiological signs of infection at regular intervals and sacrificed at 4th week (N=2) and 6th week (n=2) for gross and microscopic evaluation. In group C (n=12), phage therapy was started in 3rd week on 16th days and a total four doses were given at the interval 48h (on days 1,3,5,7). One rabbit was sacrificed after 2nd dose in the 3rd week, three rabbits in 4th week, four rabbits in 5th week and four in 6th week to compare the gross and microscopic changes. In group B (n=4), phage therapy was started after 6th wk of the infection, i.e. after the development of chronic osteomyelitic changes. They were not sacrificed to observe long term effect of phage therapy.
Clinical assessment: Rabbits were observed for local soft tissue oedema, induration, pus discharge and constitutional symptoms like general condition, loss of appetite and activity.
Bacterial isolation from wound: Serial swabs were taken from the operated site on every 3rd day. The swabs were inoculated on blood agar plate and incubated at 370 C for overnight.
Radiographic assessment of osteomyelitis: Bilateral hind limbs of rabbits of all three groups were processed for digital X -ray radiography specially focusing to diaphyseal and distal metaphyseal to examine osteomyelitic changes.
Radiographic changes i.e. cortical errosion, osteolysis, sclerosis, soft tissue swelling, periosteal reaction, and sequestra formation were assessed.
Histopathological evaluation: Rabbits of groups A and C were scarified to confirm osteomyelitic changes and therapeutic effect of phage therapy on femur. The operated femurs from rabbis in both A and C groups (n=16 total) were used for histopathological evaluation. After removal of soft tissues, the intact femur was fixed in 10 per cent neutral buffered formalin for 48 h, after which it was transferred to 70 per cent ethanol. Decalcification was done in 10 per cent EDTA. The bisected femur was processed for histology and embedded, longitudinally, with the cut surface down, in paraffin. Two, 5.0 μm thick sections were obtained from one block from each femur and were stained with hematoxylin and eosin. The sections were evaluated histopathologically20.
Results
Group A rabbits were observed with local oedema, erythema and induration in 2nd week that increased gradually in 3rd, 4th, and 5th weeks. Frank pus discharge was present along with increase in severity of clinical signs of infection (Fig. 1c). In group C, phage therapy was started after 2nd post operative week on day 16; and four doses of phage cocktail were given in the next seven days at an interval of 48 h. In the 4th wk onwards, local oedema, erythema and induration started decreasing with the improvement in general condition, appetite and activity of rabbits. Findings in group B rabbits were same as in group A before phage therapy but after phage therapy rabbits improved clinically with one rabbit observed with clinical signs of arthritis.
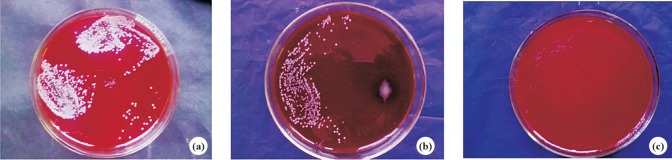
Culture and colony count: S. aureus could be isolated throughout the whole duration of observation i.e. upto 4th week in group A rabbits. Culture became negative after four doses of phage cocktail given in the 3rd week in group C rabbits (Fig. 2 a, b & c) and colony count also decreased after 3rd dose. The wound site was observed in all the remaining four rabbits and swab collected up to six week and it remained negative for the isolation of S. aureus.
Fig. 2.
Pus culture showing positive result in 2nd (a) and 5th (b) week, negative result in 7th week (c) in group B rabbits.
Four rabbits in group B were kept as control to have continued infection up to six weeks without phage therapy. They continued to have pus discharges which were positive for isolation of S. aureus. After the four doses of phage cocktail administration at the wound site all four rabbits improved clinically, wound healed and culture became sterile in 8th wk. These rabbits were not sacrificed and watched for next two months.
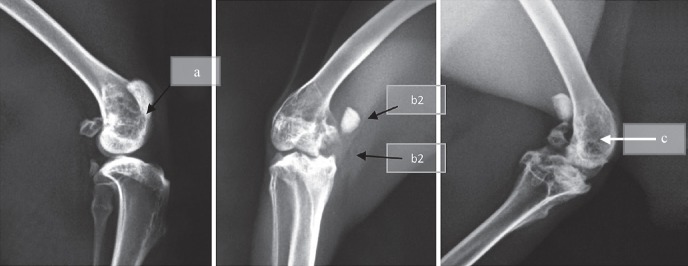
Radiological evaluation: Radiological examination of group A rabbits showed periosteal reaction, increased sclerosis and osteolysis, sequestrum formation and other sequelae such as arthritis of the knee. In group C, there was minimal periosteal reaction with no or minimal osteolysis, sclerosis and no sequestrum formation. Group B rabbits were observed with severe osteomyelitis radiologically in the 6th week (Fig. 3a,b,c). After phage therapy, radiological features of osteomyelitis persisted with one rabbit having developed arthritis of the knee.
Fig. 3.
X-ray of group C rabbits showing sclerosis and osteolysis at 3rd week (a), periosteal reaction with sequestrum formation (b1) and arthritis (b2) at 5th week, similar features but decrease in sclerosis and ostelysis at 7th week (c).
Histopathological evaluation: In group A, there was marked inflammation and tissue necrosis in 4th and 6th weeks. There was no sign of new bone formation. In group C, after phage therapy there was marked reduction in inflammation and necrosis in the 4th week (Fig. 4a) and inflammation was minimal with good amount of new bone formation in 5th and 6th weeks (Fig. 4b). No histological examination was done in case of group B rabbits as they were not sacrificed.
Fig. 4.
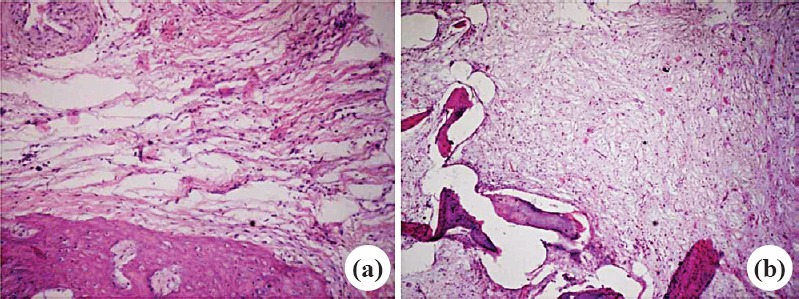
Histopathological slides of group C rabbits showing minimal inflammation at 3rd week (a) and no inflammation with new formation at 6th week (b). Stain H & E, magnification 10x.
Outcome of the therapy: All 12 rabbits in group C initially subjected to therapy at different intervals starting from day 16 could be cured which was evident by microbiological, radiological and histopathological examinations. However, the group A rabbits were observed with continued discharge positive for S. aureus from the wound site with radiological features of progression of osteomyelitis and inflammation on histopathologic examinations. In group B rabbits, after giving four doses, some of the radiologic features of chronic osteomyelitis like periosteal reactions, arthritis persisted but wound healed and the site became sterile. Thus, all the 16 rabbits subjected to therapy before (acute osteomyelitis) and after six weeks (chronic osteomyelitis) were cured of infection but one of the rabbits receiving therapy after six weeks had some residual effect of destruction (features of arthritis) at the site of infection despite bacteriological cure.
Discussion
In this preliminary study successful eradication of S. aureus was observed from acute and chronic osteomyelitis in rabbits. A cocktail of seven different virulent phage was injected intralesionally in the infected soft tissues. In studies carried out on mice, rabbits and dairy animals protective role of S. aureus specific phages has been reported in septicaemia, abscess and mastitis2,21,22.
In the beginning of antibiotic era when antibiotic resistance was not reported, osteomyelitis caused by S. aureus could not be cured in all the (100%) cases. The possible reasons for the failure may be biofilm formation; low antibiotic levels achieved in bone tissue and reduced activity of antibiotics in bone and its marrow4,5,6. In contrast to antibiotics, phages are known to penetrate the biofilm as they propagate in their bacterial host. Many phages produce depolymerases that hydrolyze biofilm extracellular polymers and they can penetrate the inner layers of the biofilm by degrading components of the biofilm exopolymeric matrix6. There is a report suggesting that the phages can enter the macrophages to kill the intracellular form23. It is known that there is a decrease in lysis of the bacterial cells when they are in dormant state22,24,25,26. This might be the reason for isolation of S. aureus till the third dose of the phage, i.e. upto 6th day of the therapy. However, single dose of the phage cocktail has been found to be effective in acute bacterial infection, e.g. septicaemia in mouse burn model caused by Pseudomonas aeruginosa (our unpublished data) as bacteria actively multiply in blood circulation. Contrary to this, in chronic infection where along with slow multiplication, biofilm formation is quite likely; multiple doses seem to be mandatory2. Systemic infections are an example of active in vivo phage multiplication and lysis of the bacteria occurring from inside while osteomyelitis like local chronic infection (abscess) is an example of passive phage therapy where no or little phage multiplication occurs inside the bacteria. In such cases, high number of phages has to be given to achieve lysis of bacteria from outside25,26. In our study we gave high dosage (1012pfu/ml) of phage cocktail.
In conclusion, it may be suggested that given the cost and long term sufferings of the osteomyelitis caused by either methicillin sensitive S. aureus (MSSA) or MRSA, the phage therapy will be beneficial to treat the infection. The phage therapy could be an alternative to antibiotics for the treatment of chronic and acute infections caused by MRSA or VRSA. The low costs, high specificity to bacterial hosts and easy administration of phage therapy advocate its consideration for replacing antibiotic usage to treat difficult infections caused by multidrug resistant bacteria.
Acknowledgment
The second author (RRM) acknowledges the Council of Scientific and Industrial Research, New Delhi, for granting Research Associateship in 2010.
Footnotes
Conflicts of Interest: None.
References
- 1.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–79. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 2.Wills QF, Kerrigan C, Soothill JS. Experimental bacteriophage protection against Staphylococcus aureus abscesses in a rabbit model. Antimicrob Agents Chemother. 2005;49:1220–1. doi: 10.1128/AAC.49.3.1220-1221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol. 2008;52:13–22. doi: 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 4.Lazzarini L, de Lalla F, Mader JT. Long bone osteomyelitis. Curr Infect Dis Rep. 2002;4:439–45. doi: 10.1007/s11908-002-0012-4. [DOI] [PubMed] [Google Scholar]
- 5.Lazzarini L, Lipsky BA, Mader JT. Antibiotic treatment of osteomyelitis: what have we learned from 30 years of clinical trials? Int J Infect Dis. 2005;9:127–38. doi: 10.1016/j.ijid.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Stengel D, Bauwens K, Sehouli J, Ekkernkamp A, Porzsolt F. Systematic review and meta-analysis of antibiotic therapy for bone and joint infections. Lancet Infect Dis. 2001;1:175–88. doi: 10.1016/S1473-3099(01)00094-9. [DOI] [PubMed] [Google Scholar]
- 7.Krogstad P. Osteomyelitis. In: Feigin RD, Cherry JD, Demmler-Harrison GJ, Kaplan SL, editors. Textbook of pediatric infectious diseases. 6th ed. PA, USA: Saunders Elsevier; 2009. pp. 725–42. [Google Scholar]
- 8.Dajcs JJ, Thibodeaux BA, Hume EB, Zheng X, Sloop GD, O’Callaghan RJ. Lysostaphin is effective in treating methicillin-resistant Staphylococcus aureus endophthalmitis in the rabbit. Curr Eye Res. 2001;22:451–7. doi: 10.1076/ceyr.22.6.451.5486. [DOI] [PubMed] [Google Scholar]
- 9.Lawton EM, Ross RP, Hill C, Cotter PD. Two-peptide lantibiotics: a medical perspective. Mini Rev Med Chem. 2007;7:1236–47. doi: 10.2174/138955707782795638. [DOI] [PubMed] [Google Scholar]
- 10.Stapleton PD, Shah S, Ehlert K, Hara Y, Taylor PW. The beta-lactam-resistance modifier (-)- epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology. 2007;153:2093–103. doi: 10.1099/mic.0.2007/007807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–94. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Górski A, Borysowski J, Miedzybrodzki R, Weber-Dabrowska B. Bacteriophages in medicine. In: McGrath S, van Sinderen D, editors. Bacteriophage: Genetics and molecular biology. Norfolk, UK: Caister Academic Press; 2007. pp. 126–58. [Google Scholar]
- 13.Sulakvelidze A, Kutter E. Bacteriophage therapy in humans. In: Kutter E, Sulakvelidze A, editors. Bacteriophages: Biology and Application. Boca Raton, FL: CRC Press; 2005. pp. 381–436. [Google Scholar]
- 14.Gharib AA, Adel Attia MA, Bendary MM. Detection of the Coa gene in Staphylococcus aureus from different sources by polymerase chain reaction. Int J Microbiol Res. 2013;4:37–42. [Google Scholar]
- 15.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 16.Wayne, PA: 2008. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 15th informational supplement, M100-S15. [Google Scholar]
- 17.Ellis EL, Delbruck M. The growth of bacteriophages. J Gen Physiol. 1939;22:365–84. doi: 10.1085/jgp.22.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams M, editor. Bacteriophages. London, United Kingdom: Interscience Publishers; 1959. [Google Scholar]
- 19.Saraf SK, Yadav A, Nagwani S, Sen M. Decal bone matrix as a local antibiotic delivery vehicle in a MRSA-infected bone model: an experimental study. Indian J Orthop. 2010;44:246–51. doi: 10.4103/0019-5413.65140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smeltzer MS, Thomas JR, Hickmon SG, Skinner RA, Nelson CL, Griffith D, et al. Characterization of a rabbit model of staphylococcal osteomyelitis. J Orthop Res. 1997;15:414–21. doi: 10.1002/jor.1100150314. [DOI] [PubMed] [Google Scholar]
- 21.Capparelli R, Parlato M, Borriello G, Salvatore P, Iannelli D. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob Agents Chemother. 2007;51:2765–73. doi: 10.1128/AAC.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuzaki S, Rashel M, Uchiyama J, Sakurai S, Ujihara T, Kuroda M, et al. Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. J Infect Chemother. 2005;11:211–9. doi: 10.1007/s10156-005-0408-9. [DOI] [PubMed] [Google Scholar]
- 23.Broxmeyer L, Sosnowska D, Miltner E, Chacón O, Wagner D, McGarvey J, et al. Killing of Mycobacterium avium and Mycobacterium tuberculosis by a mycobacteriophage delivered by a nonvirulent Mycobacterium: a model for phage therapy of intracellular bacterial pathogens. J Infect Dis. 2002;186:1155–60. doi: 10.1086/343812. [DOI] [PubMed] [Google Scholar]
- 24.Sillankorva S, Oliveira R, Vieira MJ, Sutherland IW, Azeredo J. Bacteriophage Φ S1 infection of Pseudomonas fluorescens planktonic cells versus biofilms. Biofouling. 2004;20:133–8. doi: 10.1080/08927010410001723834. [DOI] [PubMed] [Google Scholar]
- 25.Payne RJ, Phil D, Jansen VA. Phage therapy: the peculiar kinetics of self-replicating pharmaceuticals. Clin Pharmacol Ther. 2000;68:225–30. doi: 10.1067/mcp.2000.109520. [DOI] [PubMed] [Google Scholar]
- 26.Heilmann S, Sneppen K, Krishna S. Coexistence of phage and bacteria on the boundary of self-organized refuges. Proc Natl Acad Sci USA. 2012;109:12828–33. doi: 10.1073/pnas.1200771109. [DOI] [PMC free article] [PubMed] [Google Scholar]