Abstract
Purpose
We aimed to develop a hippocampal vascular injury surrogate marker for early prediction of late neurocognitive dysfunction in patients receiving brain radiotherapy (RT).
Methods and Materials
27 patients (17 males and 10 females, age 31–80 years) were enrolled in an IRB-approved prospective longitudinal study. Patients were diagnosed with a low-grade glioma or benign tumor and treated by 3-D conformal or intensity-modulated RT with a median dose of 54 Gy (50.4–59.4 Gy in 1.8-Gy fractions). Six dynamic-contrast enhanced MRI scans were performed from pre-RT to 18-month post-RT, and quantified for vascular parameters related to blood-brain barrier permeability, Ktrans, and the fraction of blood plasma volume, Vp. The temporal changes in the means of hippocampal Ktrans and Vp after starting RT were modeled by integrating the dose effects with age, sex, hippocampal laterality and presence of tumor/edema near a hippocampus. Finally, the early vascular dose-response in hippocampi was correlated with neurocognitive dysfunction 6 and 18-months post-RT.
Results
The Ktrans mean increased significantly from pre-RT to 1-month post-RT (p<0.0004), which significantly depended on sex (p<0.0007) and age (p<0.00004), with the dose-response more pronounced in older females. Also, the vascular dose-response in the left hippocampus of females correlated significantly with changes in memory function at 6 (r = −0.95, p<0.0006) and 18-months (r = −0.88, p<0.02) post-RT.
Conclusions
The early hippocampal vascular dose-response could be a predictor of late neurocognitive dysfunction. A personalized hippocampus sparing strategy may be considered in the future.
Keywords: Hippocampal Sparing, Radiation-Induced Neurocognitive Dysfunction, And Multivariate Interaction Model
Introduction
Radiation-induced injury to the central nervous system and neurocognitive dysfunction are severe complications in long-term brain tumor survivors receiving radiotherapy (RT). Clinical complications of RT include acute, delayed and late-delayed injury1. Acute injury, occurring hours to weeks after irradiation, may result from radiation-induced cerebral edema2, 3, while delayed injury, happening 1–6 months after irradiation, is thought partially due to transient interruption of myelin synthesis secondary to oligodendrocyte injury. Both acute and delayed injuries may be reversible and recover spontaneously5, 6. In contrast, the late-delayed effects, observed more than 6 months after irradiation, are thought to be irreversible and progressive. The most severe symptoms are formation of radiation-induced necrosis and progressive cognitive deterioration4, 7. The latter can occur without tissue necrosis or anatomic abnormality.
The exact mechanism of radiation-induced learning and memory declines in brain tumor survivors is unclear. However, it could relate to limbic system as well as hippocampus malfunctioning8. The hippocampus is believed to be responsible for the formation of verbal memory, so its dysfunction could decrease patient ability to consolidate short-term memory to long-term memory9. Radiation can induce vascular injury10 that may lead to hippocampus impairment. Considering limited treatment options for cognitive dysfunction, strategies for preventing detrimental cognitive effects after brain RT have been explored in RTOG clinical trials (0933 and 0614) of whole brain RT (WBRT) for brain metastases. One trial explores the hypothesis that sparing the hippocampus during WBRT preserves memory function11, and another tests a vascular dementia drug (memantine) for preventing cognitive declines after RT12. However, insufficient attention is given to the patients who are long-term survivors and received partial brain RT. Studying the hippocampal vascular response to radiation in this patient population and relating it to late-delayed memory function changes could provide critical dosimetric evidence to guide organ-at-risk sparing and/or drug intervention for preserving memory function in the patients. Also, the hippocampal dose-variation in the population provides an opportunity to evaluate dose effects, which is impossible in the patients receiving WBRT.
In this study, we investigated radiation-induced alterations in hippocampal vascular properties as a hippocampal injury surrogate marker for the delayed and late-delayed neurocognitive declines in patients treated by partial brain RT. We assessed radiation-induced longitudinal changes in hippocampal vasculature from pre-RT to 18 months post-RT, and the influence of clinical factors of sex, age, and edema on dose effects. To this end, we used the dynamic contrast enhanced (DCE)-MRI to characterize changes in vascular permeability and microvessel dilation or shrinkage as an indicator of vascular injury, and correlated their changes with delayed and late-delayed neurocognitive declines.
Materials and Methods
Patients
Between August 2004 and January 2012, 27 patients (17 men and 10 women, and the age range of 31–80 years) were enrolled in an institutional review board (IRB)-approved prospective longitudinal study (Table 1). The patients were treated by three-dimensional conformal or intensity-modulated RT, with a median dose of 54 Gy (range of 50.4–59.4 Gy in 1.8-Gy fractions), for low-grade glioma, grade 3 anaplastic ependymoma, meningioma, craniopharyngioma, or pituitary adenoma. None of these patients received chemotherapy during RT. Patients underwent MRI scans prior to RT (Pre-RT), 3 weeks after starting RT (Mid-RT), at the end of RT (End-RT), and 1 month (1M), 6 months (6M) and 18 months (18M) after completion of RT. Also, patients underwent a battery of standardized neurocognitive tests Pre-RT, and at 1M, 6M, and 18M post-RT. One patient (#13) continuously received hydrocortisone from Pre-RT up to 6M post-RT, and one other (#17) received dexamethasone during and until the end of RT. Of the 27 patients, one patient did not have the DCE-MRI data Mid-RT, 1 at End-RT, 4 at 1M post-RT, and 3 at 6M post-RT. Also, five patients did not have neurocognitive tests at 18M follow-up. So, the missing data were not available for analysis.
Table 1.
Patient characteristics
| Pt# | A/S | Tumor Type | Tumor Location | Total dose/Fx | Dmean(range) LH** | Dmean(range) RH** |
|---|---|---|---|---|---|---|
| 1* | 43/M | Grade 2 Gemistocytic Astrocytoma | Right Temporal | 59.4/1.8 | 15.2(11.7–24.4) | 53.8(49.5–54.9) |
| 2* | 64/M | Sphenoid Wing Meningioma | Left Temporal, Petrous ridge | 54/1.8 | 51.3(38.4–53.6) | 39.7(18.9–49.1) |
| 3 | 80/M | Pituitary Macroadenoma | Suprasellar | 50.4/1.8 | 23.4(7.6–42.8) | 28.2(8.8–44.7) |
| 4* | 47/M | Grade 2 mixed Oligoastrocytoma | Right Frontal, Temporal | 59.4/1.8 | 31.6(22.5–44.4) | 55.5(50.7–57.5) |
| 5 | 38/M | Craniopharyngioma | Suprasellar | 55.8/1.8 | 31.3(7.9–51.7) | 31.5(4.1–51.6) |
| 6* | 38/M | Grade 2 diffuse Astrocytoma | Left Frontal, Temporal | 54/1.8 | 51.4(50.8–52.2) | 46.2(35.9–50.3) |
| 7* | 43/M | Grade 3 Anaplastic Supratentorial Ependymoma | Left Frontal | 59.4/1.8 | 53.2(37.2–59.7) | 24.3(18.3–37.1) |
| 8 | 52/M | Pituitary Null-cell Adenoma | Suprasellar | 50.4/1.8 | 26.5(1.9–46.5) | 22.1(1.8–47.3) |
| 9 | 51/M | Pituitary Macroadenoma | Suprasellar | 50.4/1.8 | 12.3(1.05–43.6) | 23.7(1.57–47.4) |
| 10* | 32/F | Grade 2 Glioma | Left Frontal, Temporal | 54/1.8 | 55.2(49.7–59.3) | 14.4(8.97–20.9) |
| 11 | 70/M | Pituitary Macroadenoma | Suprasellar | 50.4/1.8 | 23.2(2.08–44.7) | 26.9(2.97–46.4) |
| 12 | 62/M | Grade 2 Astrocytoma | Left Frontal | 54/1.8 | 39.8(22.3–51.7) | 29.4(15.4–39.7) |
| 13 | 64/F | Pituitary Null-cell Adenoma | Suprasellar | 50.4/1.8 | 11.9(1.1–36.7) | 19.1(0.9–46.6) |
| 14 | 57/F | Meningioma | Left Temporal, Parasellar | 54/1.8 | 34.1(1.64–56) | 23.9(1.45–53.7) |
| 15* | 31/F | Low grade Glioma | Left Frontal | 54/1.8 | 54.3(45.4–56.3) | 20.1(18.1–24.5) |
| 16 | 60/M | Pituitary Null-cell Adenoma | Suprasellar | 50.4/1.8 | 19.3(0.8–49.7) | 11.7(0.7–46.8) |
| 17 | 48/F | Pituitary Null-cell Adenoma | Suprasellar | 50.4/1.8 | 4.4(0.9–29.6) | 7(1–42) |
| 18 | 48/M | Grade 2 diffuse Oligodendroglioma | Right Parietal | 54/1.8 | 3.6(1.6–15.1) | 23.6(9–53.2) |
| 19 | 50/F | Pituitary Macroadenoma | Suprasellar | 50.4/1.8 | 7.6(0.4–41.3) | 9.4(0.5–40.1) |
| 20 | 57/F | Meningioma | Left Parietal, Parasagittal | 54/1.8 | 2(1.1–7) | 4.2(1.4–11.4) |
| 21 | 58/F | Grade 2 Gemistocytic Astrocytoma | Left Frontal | 54/1.8 | 16.5(8.9–26.2) | 6.1(3.1–8.5) |
| 22 | 50/M | Pituitary Macroadenoma | Suprasellar | 50.4/1.8 | 8.6(1.3–20.5) | 12.2(2.7–30.9) |
| 23 | 40/F | Meningioma | Left Occipital, Tentorial and Posterior Fossa | 54/1.8 | 8.4(2.5–13.4) | 3.4(2.1–4.5) |
| 24 | 50/M | Meningioma | Right Cavernous sinus | 54/1.8 | 8.2(1.14–21.1) | 14.2(1.2–55.1) |
| 25* | 51/M | Grade 2 diffuse Astrocytoma | Left Temporal, Parietal | 59.4/1.8 | 56.1(55.2–57.5) | 16.8(15–18.8) |
| 26 | 48/M | Pituitary Null-cell Adenoma | Suprasellar | 54/1.8 | 17.5(1.2–49.3) | 38.6(1.6–55.2) |
| 27 | 32/F | Pituitary Craniopharyngioma | Suprasellar | 55.8/1.8 | 11.3(0.9–42.3) | 13.42(0.9–49.6) |
Abbreviations: Pt # = Patient number; A/S = Age/Sex; M = Male; F = Female; Dmean = mean radiation dose in the hippocampus; LH = left hippocampus; RH = right hippocampus; Fx = fraction size.
Hippocampus is affected by either tumor or edema.
2-Gy equivalent dose by the linear-quadratic equation with a/β = 2.5Gy.
MRI acquisition
MRI scans at each time included pre- and post-Gd-DTPA volumetric T1-weighted, T2-weighted fluid-attenuated inversion recovery (FLAIR), and 3-D volumetric DCE T1-weighted images. To eliminate the time-of-flight effect from in-flow blood proton spins, all DCE images were acquired in the sagittal plane and covered the whole brain. For the first 12 patients who were scanned on a 1.5-T system (General Electric HealthCare), the DCE images were acquired using a 3-D gradient echo sequence with a flip angle of 20°, TE/TR of 1.08/3.4 ms, a voxel size of 0.86 × 0.86 × 6 mm3 and a temporal resolution of approximately 6 seconds. Due to the system upgrade, the remaining 15 patients had scans on a 3-T scanner (Philips Healthcare), and had the DCE images acquired with TE/TR of 1.05/5.14 ms, a voxel size of 2 × 2 × 2 mm3. All patients had a single dose (0.1 mL/kg) of Gd-DTPA, injected at a rate of 2 mL/s. No patient was scanned on different scanners.
Neurocognitive tests
The neurocognitive tests included the Controlled Oral Word Association (COWA) test13, the revised Hopkins Verbal Learning Test (HVLT-R)14, and Trail Making Tests (TMT) A and B15. HVLT-R assesses verbal memory (recall and delayed recall) and learning component. The TMTA assesses attention and information processing efficiency. TMTB is used for mental flexibility and executive function. COWA assesses word fluency and executive function. These tests are well established in patients receiving RT16 and have been used in several RTOG trials, e.g., RTOG 0212, RTOG 0614 and RTOG 0933. The standardized Z-scores from all test scores were calculated to adjust age, sex, and education effects on raw scores accordingly14, 17, 18.
Image registration
All images, including anatomic MRI, DCE-MRI and treatment planning CT, were co-registered using a mutual information and simplex optimization algorithm from an in-house software package (FIAT) 19–20. Initially, the DCE images were co-registered within the series to correct any possible misalignment prior to kinetic variable estimation. At each time point, the high-resolution post-Gd T1-weighted images were registered to the DCE images, and other images were registered to the registered post-Gd T1-weighted images. Finally, the planned 3-D dose distribution maps were registered to the post-Gd T1-weighted images Pre-RT.
Hippocampus contouring
All hippocampi were manually contoured on the post-Gd-DTPA T1-weighted images according to the hippocampus contouring guidance developed for RTOG 0933. Both hippocampi of a patient were visually examined on T1-weighted and FLAIR MRI to make sure they are not adjacent to or affected by the tumor or edema. Nineteen of 27 patients (either benign or low-grade Glioma) had both intact hippocampi visually (Table 1), and were selected for assessment of radiation-induced neurocognitive dysfunction.
Estimation of kinetic variables from DCE-MRI
The modified Toft model21 was used to estimate the kinetic parameters including the transfer constant (Ktrans) of gadolinium influx from the intravascular space into the extravascular extracellular space and the fractional plasma volume (Vp) in tissue. An increase in Ktrans is an indicator of vascular leakiness (blood-brain barrier (BBB) permeability to gadolinium-DTPA), and changes in Vp could be related to vaso-constriction or dilation. The arterial input function was obtained from carotid arteries. Note that Vp obtained by this model was corrected for vascular leakage. For each hippocampal structure, we computed the mean (M) and standard deviation (STD) of Vp and Ktrans.
Statistical analysis
We focused on the dose-dependent changes in Vp and Ktrans of the hippocampal structures from pre-RT (baseline) to 1 month post-RT for prediction of the delayed (6M) and late-delayed (18M) neurocognitive dysfunction.
Dose effect on the temporal changes of vascular properties
The dose-effects on the mean Vp or Ktrans of a hippocampus were modeled by a linear regression. In the model, we considered dose interactions with co-variables, such as age, sex, left vs. right hippocampus, and edema as the disease effect as:
| (1) |
where τ represents the time after starting RT, Sex indicates male vs. female, Age is a continuous variable in year, LRH denotes left vs. right hippocampus, and Edema marks as 1 if a hippocampus is near edema and 0 otherwise. Also, Dose is the mean radiation dose in each hippocampus, in which the fraction-size variation was corrected to 2-Gy equivalent by the linear-quadratic equation with α/β = 2.5 Gy22. To control the false discovery rate in multiple regressions, a Bonfferoni-type sequential procedure23 was performed, in which a regression was considered statistically significant when pi < αi where pi is the ith smallest p-value from m regressions and αi = 0.05 * i/m. In our study, we had 2 kinetic variables (Vp and Ktrans) and 3 time points (Pre-RT to Mid-RT, End-RT, and 1 month post-RT), resulting in m = 6. We also tested whether the scanner type affected the results by including a regressor in Eq. [1], and then found the scanner type was not significant.
Correlation with delayed and late-delayed neurocognitive changes
We assessed whether the radiation-induced changes in vascular properties of either left or right hippocampus could predict the delayed and late-delayed neurocognitive decline. Hence, correlations between the early changes in the hippocampal means of Ktrans or Vp up to 1 month after RT and the delayed and late-delayed changes (6M and 18M after RT) in the standardized age- and education-adjusted Z-scores of neurocognitive functions were tested by Pearson correlation. To avoid the confounding effect of tumor/edema on analysis, we only considered the patients who had both hippocampi intact. Also, the patient with grade 3 Ependymoma was also excluded from the analysis and only benign tumors were considered.
Post-hoc analysis
For the significant clinical variables in Eq (1), we further investigated if there were any significant differences of the temporal changes in the mean hippocampal Vp or Ktrans between the subgroups using the paired t-test. Also, we performed the non-parametric Mann-Whitney U Test to investigate whether delayed and late-delayed neurocognitive declines were significantly different between males and females. The p values < 0.05 were considered significant.
Results
Clinical and radiological findings
Up to 18 months after the completion of RT, none of the patients showed tumor progression and radiation-induced lesions on T2-weighted FLAIR and post-Gd T1–weighted images. In all glioma patients, increased signal intensities on FLAIR images in the tumor and its vicinity prior to RT were stable or decreased during the 18-month follow-up. Also, no additional areas of radiation-induced abnormalities on the conventional MRI were present during follow-up. In patients with pituitary adenomas and craniopharyngioma, no signal abnormality outside the tumor was present pre-treatment and no interval change was seen during the 18-month follow-up. In the patient with meningioma, no changes on FLAIR signals beyond the surgical cavity were seen over follow-ups.
Dose effect on the temporal changes of vascular properties
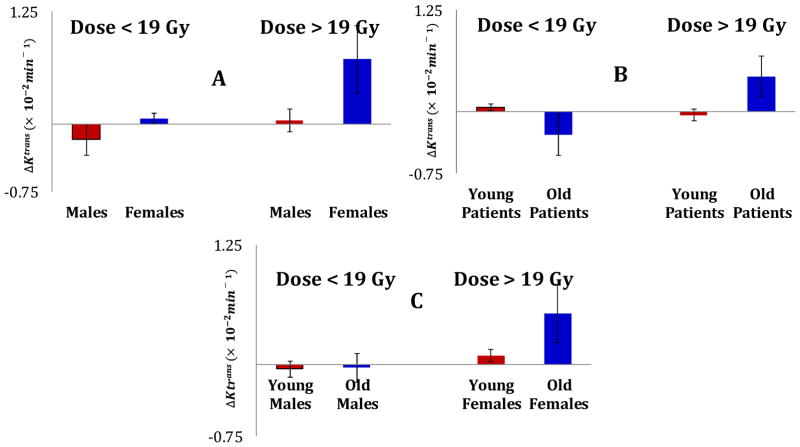
The multivariate linear regression model revealed that changes in the hippocampal Ktrans from pre-RT to 1M post-RT was significantly predicted (p < 0.0004) by the interactions of Dose and Sex (γ1 = 1.11 × 10−3, p = 0.0007), Dose and Age (γ2 = 0.005 × 10−3, p = 0.00004), and Dose and Edema near the hippocampus (γ4 = −1.1 × 10−3, p = 0.014). Post-hoc analysis further illustrated these effects, as (1) a higher radiation dose (> 19 Gy, the median hippocampal dose for all patients included in this study) induced a greater Ktrans change in female hippocampi (ΔKtrans = 0.007 ± 0.0038 min−1, p = 0.04) than in male hippocampi (Fig 1(A)); (2) a high dose affects older patients (>50 years, the median age of patients) (ΔKtrans = 0.004 ± 0.0024 min−1, p = 0.04) greater than younger patients (Fig 1(B)); and (3) the Ktrans change was more pronounced in old females (ΔKtrans = 0.005 ± 0.003 min−1, p = 0.05). Furthermore, the edema interaction with dose indicated that the edema-affected hippocampus had a lesser extent change in the Ktrans than the normal hippocampus receiving the same dose. However, there were no significant differences in radiation dose to the left and right hippocampi (p = 0.54) and the dose effect on the Ktrans changes between left and right hippocampi (p = 0.5).
Fig. 1.
Illustrations of dose effects interacting with Sex and Age on the mean Ktrans changes one month post-RT. The mean Ktrans changes are compared between males and females receiving greater or less than 19 Gy (A), between old and young (> or <50 years) patients receiving doses > and < 19 Gy (B), and between male and female and old and young patients receiving high and low doses (C). The dose effect was more pronounced in older females. The error bars represent Standard Error of Mean.
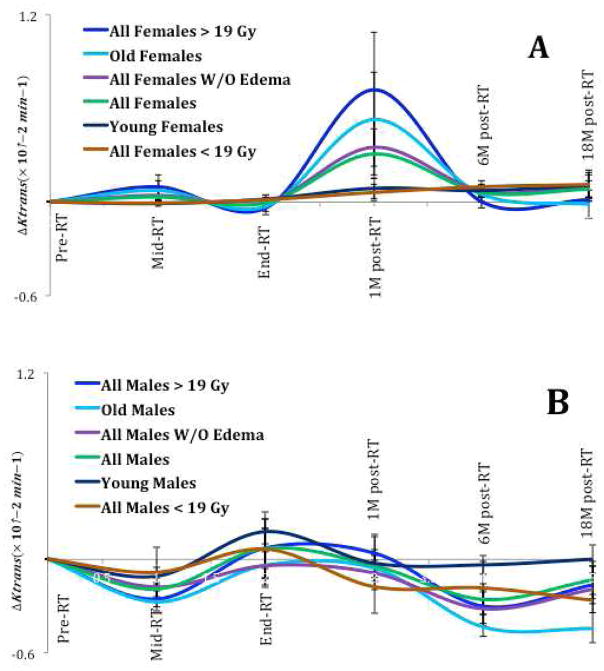
The time profile changes in the mean Ktrans of females and males are shown in Fig 2. Fig. 2(A) shows that the mean Ktrans in female hippocampi reached a maximum 1M post-RT and then were subsided 6M and 18M post-RT. In contrast, Fig 2(B) reveals that the mean Ktrans changes in male hippocampi are significantly less than in female, even though male hippocampi received significantly higher doses (27.3 ± 16.7 Gy) than female hippocampi (18.5 ± 16.3 Gy), p = 0.04.
Fig 2.
The radiation-induced time profile changes in the mean Ktrans of female (A) and male (B) hippocampi. The horizontal axes represent the time points: Pre-RT, week 3 during (Mid)-RT, End-RT, 1-month post-RT, 6-month post-RT and 18-month post-RT. The error bar represents the standard error of mean.
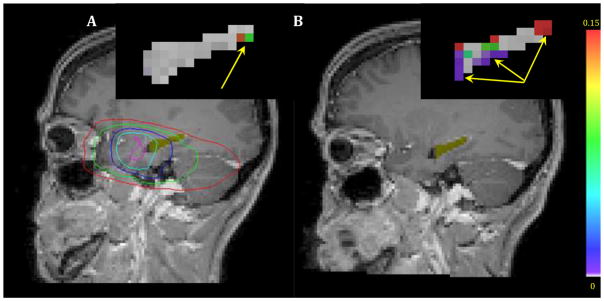
Finally, Fig. 3 shows an example of the Ktrans maps within the hippocampal contours (Yellow) pre-RT and 1M post-RT, and related planning isodose curves. Pre-RT, only a few voxels had a Ktrans value greater than zero (colored voxels). However, more voxels had non-zero Ktrans value 1M post-RT, indicating an increase in BBB permeability. Finally, we observed no significant dose effect on temporal changes in fractional plasma volume, Vp.
Fig. 3.
Hippocampi (yellow color) and zoomed and color-coded Ktrans maps overlaid on T1-weighted images pre-RT (A) and 1-month post-RT (B). The planned isodose curves, 5 (red), 10 (green), 20 (blue), 30 (cyan) and 40Gy (dark pink), are overlaid on the pre-RT image. Only a few voxels have Ktrans values greater than zero pre-RT (colored voxels pointed by yellow arrows) (A). The BBB permeability increased 1-month post-radiation (colored voxels pointed by yellow arrows) (B).
Correlation with delayed and late-delayed neurocognitive changes
The analysis of the neurocognitive test scores in patients with intact hippocampi (as described above) showed delayed-recall Z-scores of the HVLT-R test in females gradually decreased from Z = 0.31 ± 0.23 (SEM) pre-RT to Z = −0.66 ± 0.27 18M post-RT, which was significant with p < 0.05 (Fig 4). Previous data14, 17 have shown the average test-retest variability of Z-scores of the neurocognitive tests approximately ±0.25, and indicated a decline of >0.5 clinically significant. Hence, a Z-score decline of −0.97 (from 0.31 to −0.66) in the delayed-recall test of females indicated a clinically significant decline in memory function. No such trends were observed for men or other neurocognitive test scores.
Fig. 4.
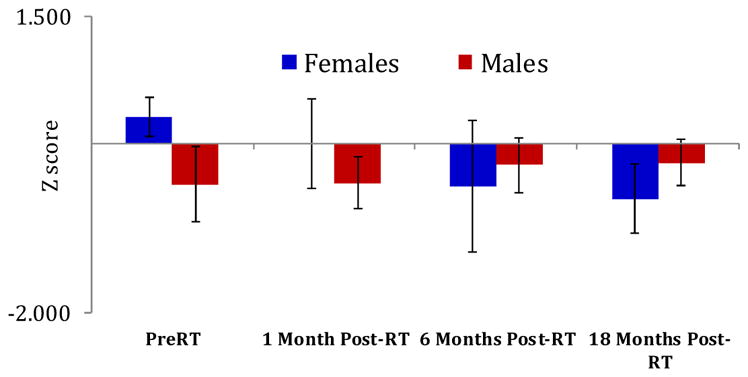
The temporal changes in the delayed recall Z-scores of the revised Hopkins Verbal Learning Test from pre-RT to post-RT in males and females. The negative Z-score mean a negative deviation from the age- and education-matched standard Z-score of the normal population. Each bar represents standard error of mean.
Most importantly, the changes in the mean Ktrans of the female left hippocampi from Pre-RT to 1M Post-RT were significantly correlated with changes in the delayed recall Z-scores of the HVLT-R 6M (r = −0.95, p = 0.0006) and 18M (r = −0.88, p = 0.019) post-RT. However, such correlations between the early Ktrans changes in the female right hippocampi and changes in the delayed-recall Z-scores 6M (r = −0.76, p = 0.04) and 18M (r = −0.37, p = 0.46) post-RT were much weaker and only significant at 6 month. However, we observed no such trend in males.
Discussion
In this paper, we developed a multivariate regression model to evaluate the hippocampal vascular dose-response as a potential surrogate marker for hippocampal dysfunction in the patients treated by partial brain RT. We investigated the dose effect on the blood-brain barrier permeability as well as the dose interactions with clinical factors, such as sex, age and edema. We observed that radiation induced BBB opening in hippocampi one month after completion of radiotherapy depending on the patient age and sex, with the dose-response more pronounced in older females. Most importantly, the early BBB opening in the female hippocampus was correlated with the delayed and late-delayed memory function declines. The greater sensitivity to radiation-induced neurocognitive declines in the elderly females is supported by the biological findings of radiation-caused aging-like pathology26.
Quantitative imaging allows us to study individual radiation sensitivity and analyze dose-responses in various subgroups systematically. In this study, we found that the radiation-induced hippocampal vascular injury and the subsequent neurocognitive decline are age- and sex- dependent. Although the exact mechanism of the age-dependent radiosensitivity remained unknown, previous studies24, 25 have shown that RT has more adverse effects on older patients. Moreover, the radiation-induced aging-like pathology in cerebrocortical cells has been suggested26, including shrinkage of cortical thickness, activation of cell-cycle arrest pathway and inhibition of DNA double strand break repair. Also, it has been shown that exposing cerebral cortex to radiation increases chronic oxidative stress, oxidant DNA damage, lipid peroxidation, and apoptosis26. In terms of sex-dependent radiation sensitivity, higher mortality and morbidity rates from radiation-induced injury in females have been reported27; also, it has been shown that girls could be more sensitive than boys in learning and memory decline following conformal radiotherapy28. However, the sex-dependency of the radiation-induced neurocognitive impairments has not been extensively studied, and needs to be interpreted cautiously. As noted, the patient number in this study is small. Most females underwent MR scans on the Philips scanner in our study. Whether the sex-dependency of the Ktrans change is due to variations in image acquisitions and magnet field strength was tested and found to be not significant. Also, no patient showed radiation-induced anatomic abnormality or tumor progression, the latter is unlikely a factor affecting our findings. Nonetheless, further verification of the results is needed using a larger dataset and standard imaging protocol. Also, to figure out why females are more sensitive to radiation than males could be the subject of radiobiology studies.
The findings of this study support personalized hippocampal sparing in patients receiving brain radiotherapy29 in a way that the patient’s sex and age may need to be considered in the treatment plan for preventing memory function. Indeed, due to short survival time, hippocampal sparing, although helpful, may be less significant in patients with brain metastases in contrast to mitigation of the disease symptoms. However, it would be extremely important in the patients who have low-grade glioma or benign brain tumor and are long-term survivors. In this study, we found that exposing hippocampus to a mean dose of 19-Gy (2-Gy equivalent) or less seemed to result in little or no memory function decline or vascular injury, which could be considered in the dosimetry guidance in a future study. Nonetheless, neurogenesis in the hippocampal subgranular zone has very high sensitivity to radiation doses30–32 where even a low dose at 2-Gy can decrease the production of new neurons and proliferating cells dramatically. Also, changes in neurogenesis have been associated with a significant inflammatory response as indicated by activation of microglia30–32. Based upon animal studies, it has been hypothesized that hippocampal neurogenesis damage is responsible for neurocognitive declines33. Hence, more studies are needed to further gain our understanding in the relationship between hippocampal dosimetry, neurogenesis, and neurocognitive dysfunction.
In this study, we focused on radiosensitivity of the hippocampal vasculature and its association with hippocampus dysfunction. Vasculatures of the hippocampus, an important structure for memory and learning function, are sensitive to radiation, and seem to be more for older females. Earlier vascular alterations are translated into late neurocognitive function declines. Considering brain functioning as a network, a future study that incorporates radiation effects on hippocampus with interconnecting white matter tracts, frontal lobe components, and clinical factors34–37 could lead to an even better prediction for late cognitive dysfunction.
Summary.
We studied the hippocampal vascular dose-response as an early indicator of neurocognitive dysfunction in patients receiving partial brain radiotherapy. Radiation-induced hippocampal vascular changes were pronounced one month after completion of radiotherapy and were significantly correlated with memory function decline. Clinical factors of sex, age and edema impacted on the hippocampal vascular dose-response, resulting in a large effect on the older females.
Acknowledgments
This work is supported in part by NIH grants RO1 NS064973.
Footnotes
None of the authors has any conflict of interest regarding this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fink J, Born D, Chamberlain MC. Radiation necrosis: relevance with respect to treatment of primary and secondary brain tumors. Curr Neurol Neurosci Rep. 2012;12(3):276–85. doi: 10.1007/s11910-012-0258-7. [DOI] [PubMed] [Google Scholar]
- 2.Young DF, Posner JB, Chu F, Nisce L. Rapid-course radiation therapy of cerebral metastases: results and complications. Cancer. 1974;34(4):1069–76. doi: 10.1002/1097-0142(197410)34:4<1069::aid-cncr2820340416>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Hindo WA, DeTrana FA, 3rd, Lee MS, Hendrickson FR. Large dose increment irradiation in treatment of cerebral metastases. Cancer. 1970;26(1):138–41. doi: 10.1002/1097-0142(197007)26:1<138::aid-cncr2820260117>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong CL, Shera DM, Lustig RA, Phillips PC. Phase measurement of cognitive impairment specific to radiotherapy. International journal of radiation oncology, biology, physics. 2012;83(3):e319–24. doi: 10.1016/j.ijrobp.2011.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rider WD. Radiation damage to the brain--a new syndrome. Journal of the Canadian Association of Radiologists. 1963;14:67–9. [PubMed] [Google Scholar]
- 6.Hoffman WF, Levin VA, Wilson CB. Evaluation of malignant glioma patients during the postirradiation period. J Neurosurg. 1979;50(5):624–8. doi: 10.3171/jns.1979.50.5.0624. [DOI] [PubMed] [Google Scholar]
- 7.Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217(2):377–84. doi: 10.1148/radiology.217.2.r00nv36377. [DOI] [PubMed] [Google Scholar]
- 8.Gondi V, Hermann BP, Mehta MP, Tome WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. International journal of radiation oncology, biology, physics. 2013;85(2):348–54. doi: 10.1016/j.ijrobp.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkey, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 10.Monje ML, Palmer T. Radiation injury and neurogenesis. Current opinion in neurology. 2003;16(2):129–34. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 11.Gondi V, Mehta MP, Pugh S, et al. Memory preservation with conformal avoidance of the hippocampus during whole-brain radiotherapy (WBRT) for patients with brain metastases: Primary endpoint results of RTOG 0933. ASTRO annual meeting; 2013. [Google Scholar]
- 12.Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro-oncology. 2013;15(10):1429–37. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benton AL, Hamsher Kd, Sivan AB. Multilingual Aphasia Examination. 3. Iowa City, IA: AJA Associates; 1994. [Google Scholar]
- 14.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – Revised: Normative Data and Analysis of Inter-Form and Test-Retest Reliability. The Clinical Neuropsychologist. 1998;12(1):43–55. [Google Scholar]
- 15.Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nature protocols. 2006;1(5):2277–81. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- 16.Mehta MP, Shapiro WR, Glantz MJ, et al. Lead-in phase to randomized trial of motexafin gadolinium and whole-brain radiation for patients with brain metastases: centralized assessment of magnetic resonance imaging, neurocognitive, and neurologic end points. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20(16):3445–53. doi: 10.1200/JCO.2002.07.500. [DOI] [PubMed] [Google Scholar]
- 17.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 1996;11(4):329–38. [PubMed] [Google Scholar]
- 18.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 2004;19(2):203–14. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 19.Meyer CR, Boes JL, Kim B, et al. Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Medical image analysis. 1997;1(3):195–206. doi: 10.1016/s1361-8415(97)85010-4. [DOI] [PubMed] [Google Scholar]
- 20.Cao Y. Development of Image Software Tools for Radiation Therapy Assessment. Medical Physics. 2005;32(6):2136. [Google Scholar]
- 21.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. Journal of magnetic resonance imaging: JMRI. 1999;10(3):223–32. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 22.Barton M. Tables of equivalent dose in 2 Gy fractions: a simple application of the linear quadratic formula. International journal of radiation oncology, biology, physics. 1995;31(2):371–8. doi: 10.1016/0360-3016(94)E0126-5. [DOI] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- 24.Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet neurology. 2009;8(9):810–8. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 25.Tabouret E, Tassy L, Chinot O, Cretel E, Retornaz F, Rousseau F. High-grade glioma in elderly patients: can the oncogeriatrician help? Clinical interventions in aging. 2013;8:1617–24. doi: 10.2147/CIA.S35941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suman S, Rodriguez OC, Winters TA, Fornace AJ, Jr, Albanese C, Datta K. Therapeutic and space radiation exposure of mouse brain causes impaired DNA repair response and premature senescence by chronic oxidant production. Aging. 2013;5(8):607–22. doi: 10.18632/aging.100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Research Council (U.S.) Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. Washington, D.C: National Academies Press; 2006. Committee to Assess Health Risks from Exposure to Low Level of Ionizing Radiation. [PubMed] [Google Scholar]
- 28.Di Pinto M, Conklin HM, Li C, Merchant TE. Learnin and memory following conformal radiation therapy for pediatric craniopharyngioma and low-grade glioma. International journal of radiation oncology, biology, physics. 2012;84(3):363–9. doi: 10.1016/j.ijrobp.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutierrez AN, Westerly DC, Tome WA, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: a planning study. International journal of radiation oncology, biology, physics. 2007;69(2):589–97. doi: 10.1016/j.ijrobp.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrer I, Serrano T, Alcantara S, Tortosa A, Graus F. X-ray-induced cell death in the developing hippocampal complex involves neurons and requires protein synthesis. Journal of neuropathology and experimental neurology. 1993;52(4):370–8. doi: 10.1097/00005072-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer research. 2003;63(14):4021–7. [PubMed] [Google Scholar]
- 32.Nagai R, Tsunoda S, Hori Y, Asada H. Selective vulnerability to radiation in the hippocampal dentate granule cells. Surgical neurology. 2000;53(5):503–6. doi: 10.1016/s0090-3019(00)00214-7. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 33.Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation-induced cognitive impairment. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19(9):2294–300. doi: 10.1158/1078-0432.CCR-11-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hau C, Merchant TE, Gajjar A, et al. Brain tumor therapy-induced changes in normal-appearing brainstem measured with longitudinal diffusion tensor imaging. International journal of radiation oncology, biology, physics. 2012;82(5):2047–54. doi: 10.1016/j.ijrobp.2011.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uh J, Merchant TE, Li Y, et al. Differences in brainstem fiber tract response to radiation: a longitudinal diffusion tensor imaging study. International journal of radiation oncology, biology, physics. 2012;86(2):292–7. doi: 10.1016/j.ijrobp.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapman CH, Nazem-Zadeh M, Lee OE, et al. Regional variation in brain white matter diffusion index changes following chemoradiotherapy: a prospective study using tract-based spatial statistics. Plus One. 2013;8(3):e57768. doi: 10.1371/journal.pone.0057768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hau C, Wu S, Chemaitilly W, et al. Predicting the probability of abnormal stimulated growth hormone response in children after radiotherapy for brain tumors. International journal of radiation oncology, biology, physics. 2012;84(4):990–5. doi: 10.1016/j.ijrobp.2012.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]