Abstract
Age-related fragility fractures are an enormous public health problem. Both acquisition of bone mass during growth and bone loss associated with ageing affect fracture risk late in life. The development of high-resolution peripheral quantitative CT (HRpQCT) has enabled in vivo assessment of changes in the microarchitecture of trabecular and cortical bone throughout life. Studies using HRpQCT have demonstrated that the transient increase in distal forearm fractures during adolescent growth is associated with alterations in cortical bone, which include cortical thinning and increased porosity. Children with distal forearm fractures in the setting of mild, but not moderate, trauma also have increased deficits in cortical bone at the distal radius and in bone mass systemically. Moreover, these children transition into young adulthood with reduced peak bone mass. Elderly men, but not elderly women, with a history of childhood forearm fractures have an increased risk of osteoporotic fractures. With ageing, men lose trabecular bone primarily by thinning of trabeculae, whereas the number of trabeculae is reduced in women, which is much more destabilizing from a biomechanical perspective. However, age-related losses of cortical bone and increases in cortical porosity seem to have a much larger role than previously recognized, and increased cortical porosity might characterize patients at increased risk of fragility fractures.
Introduction
The population of the West is ageing at an unprecedented rate.1 For example, in the USA, the number of individuals aged ≥65 years is expected to nearly double from ~39 million (corresponding to ~13% of the population) in 2009 to ~72 million in 2030 (corresponding to one in five of the population). An overwhelming number of these individuals will develop age-related degenerative dis orders, such as osteoporosis and its precursor, osteopenia (analogous to diabetes mellitus and prediabetes), which increase fracture susceptibility.1 As noted in the Surgeon General’s Report on bone health and osteoporosis,2 10 million individuals in the USA >50 years of age already have osteoporosis, and an additional 34 million individuals have osteopenia. Indeed, the total number of people in the USA with low bone mass could reach 61 million by 2020.3 Moreover, the 2 million osteoporosis-related fractures reported in 2005 could increase to 3 million by 2025, with annual costs increasing from US$16.9 billion to US$25.3 billion.4
Given the growing prevalence and costs of fractures associated with age-related bone loss and osteoporosis, increased understanding of the structural changes that occur in the ageing skeleton are crucial for the development of improved, more-targeted therapies to prevent bone loss and fracture. Moreover, considerable evidence now exists that peak bone mass attained during childhood and adolescent growth is a major determinant of bone mass and fracture risk later in life.5 Indeed, on the basis of computer simulations of bone loss over life, the age at which an individual crosses the threshold for a diagnosis of osteoporosis is predicted to be delayed by 13 years if young adult BMD is 10% higher than the mean.6 Beyond bone mass and BMD, however, the structure of bone might also make important contributions to its strength and thus its resistance to fracture.
In this Review, we address key aspects of our understanding of the structural changes that occur in the skeleton during growth as well as during senescence. We focus specifically on studies in humans, with an emphasis on skeletal changes at the distal radius, a clinically relevant site of forearm fractures. The advent of new imaging modalities, such as high-resolution peripheral quantitative CT (HRpQCT),7,8 now provide the opportunity to address this issue not only at the macrostructural level of changes in bone mass, but also at the level of changes in bone microarchitecture. Thus, although fractures at other sites (for example, the spine and hip) are also clearly of great public health importance,4 the inability of conventional imaging to characterize bone microarchitecture at these central sites (owing to concerns regarding radiation exposure) limits our ability to directly address this issue at these sites. Nonetheless, we discuss available data regarding bone macrostructural changes at these sites with ageing and the limited bone biopsy sample and cadaveric data examining bone microarchitecture in patients with and without hip fractures.
Skeletal changes during growth
Acquisition of bone mass
Bone mass increases steadily during childhood, but markedly so during adolescent growth. Indeed, >95% of the adult skeleton is formed by the end of adolescence.9 Most studies evaluating skeletal growth have used dual-energy X-ray absorptiometry (DXA), which can measure bone mineral content (BMC) as well as areal BMD (aBMD)—defined as BMC divided by the projected bone area. Consequently, as aBMD is influenced by bone size, DXA measurements of aBMD overestimate the true volumetric BMD (vBMD) in large bones and underestimate vBMD in small bones.10 In addition, DXA cannot discriminate between changes in trabecular versus cortical bone. Despite these limitations, DXA has nonetheless proven to be quite useful not only for assessment of bone mass in adult individuals, but also in children and adolescents during growth.
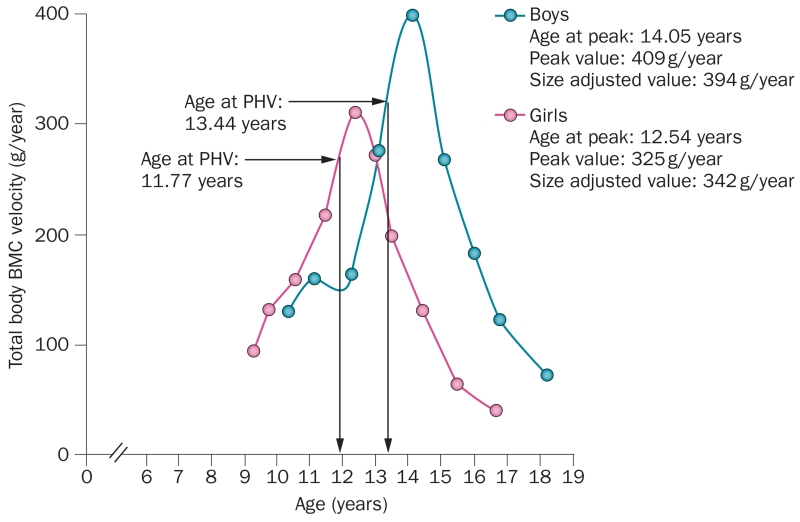
Bone mineral accrual during adolescence was investigated using annual DXA measurements in a prospective study of 53 girls and 60 boys aged 8–14 years over 6 years.9 The mean age for peak bone mineral accrual (peak BMC velocity) occurred ~2 years earlier in girls (12.5 ± 0.90 years) than in boys (14.1 ± 0.95 years; Figure 1), and in the girls coincided closely with the mean age at menarche (12.5 ± 0.98 years). In both girls and boys, peak height velocity occurred ~0.7 years before peak BMC velocity. In addition, peak bone mass is greater in boys than in girls,11 which might have considerable implications for the onset and diagnosis of osteoporosis later in life. However, as most studies conducted to date have included predominantly white individuals, the extent to which peak bone mass differs among ethnic groups is an important, but unresolved, question.
Figure 1.
Bone mineral accrual during adolescence. Total body peak BMC velocity curve is shown, which illustrates velocity at peak BMC, age at peak BMC and PHV by chronological age in boys and girls. Abbreviations: BMC, bone mineral content; PHV, peak height velocity. Reproduced with permission from Bailey, D. A. et al. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the University of Saskatchewan bone mineral accrual study. J. Bone Miner. Res. 14(10), 1672–1679 © (1999) John Wiley & Sons, Inc.
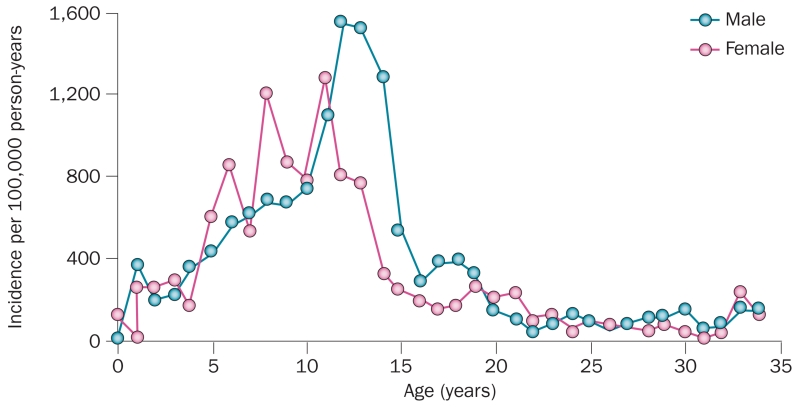
The timing of peak BMC acquisition in girls and boys is relevant to understanding distal forearm fractures, which are common during adolescence. In a population-based study conducted over 30 years, the age-specific and sex-specific incidence of distal forearm fractures in children from Olmsted County, MN, USA12 (Figure 2) were consistent with several other studies.13–15 The incidence of these fractures tended to peak around the time of the adolescent growth spurt, which is earlier in girls than in boys. By late puberty, the incidence of these fractures fell to the low levels observed in young adults, and then increased again late in life in women to a much greater extent than in men.16 Of concern, the incidence of distal forearm fractures seemed to increase substantially between 1969–1971 and 1999–2001; age-adjusted incidence rates per 100,000 were 32% higher in boys and 56% higher in girls in 1999–2001 than in 1969–1971 in Olmsted County, MN, USA.12 Whether these increases were due to changing patterns of physical activity, decreased bone acquisition due to poor nutrition (for example, reduced calcium intake), or both is unclear but warrants further investigation.
Figure 2.
Incidence of distal forearm fractures in residents of Olmsted County, MN, USA. Data shown for male and female residents for the period 1999–2001. Reproduced with permission from Khosla, S. et al. Incidence of childhood distal forearm fractures over 30 years: a population-based study. JAMA 290(11), 1479–1485 © (2003) American Medical Association. All rights reserved.
Bone microarchitecture
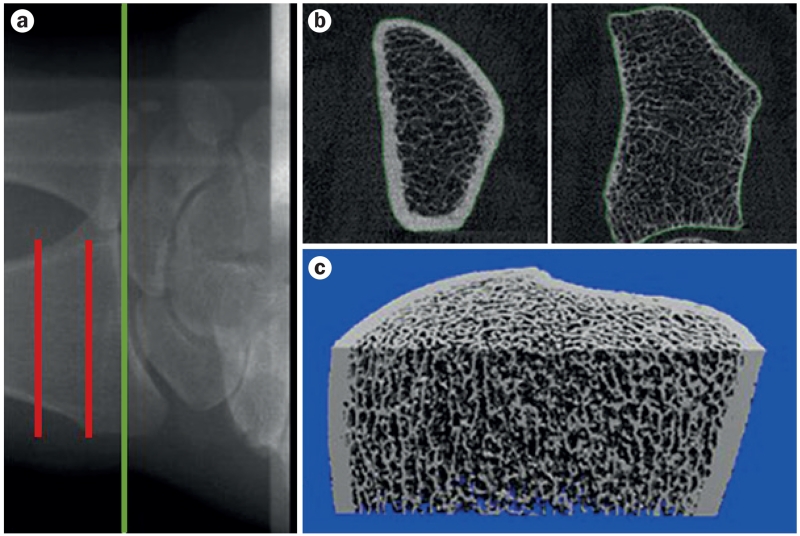
The availability of HRpQCT imaging which, with a resolution of 82 μm (and lately, of 61 μm), provides a noninvasive, in vivo ‘bone biopsy’,7,8 has led to a number of studies examining changes in bone microarchitecture during growth. This technology can define trabecular and cortical microstructural parameters that correlate well with the ‘gold standard’ of ex vivo μCT (resolution <10 μm).7,8,17 In addition, HRpQCT scans can also be used to construct micro-finite element (μFE) models of bone strength, which correlate well with biomechanically measured bone strength.18 Representative images from the distal and proximal scanning sites of the distal radius using the newest generation HRpQCT scanner demonstrate the remarkable resolution of bone microarchitecture possible with this technology (Figure 3). However, a limitation of this technique is that due to the radiation doses needed to achieve this resolution, scanning is only possible at peripheral skeletal sites (such as the radius and tibia). Nonetheless, when peripheral sites are scanned, the total body effective radiation dose is extremely low (<0.005 mSv, compared with 14.2 mSv for a routine abdomen and/or pelvic CT scan).
Figure 3.
HRpQCT-resolved bone microarchitecture of the distal radius.
a | Radiograph showing the HRpQCT site of imaging at the distal radius. Green line indicates the beginning of the joint space and red lines indicate the section of bone over which images were acquired. b | Representative cross-sectional images from the stack of CT slices from the most proximal slice (left) to the distal slice (right). c | Representative 3D reconstruction of the distal radius from the HRpQCT scan slices. Abbreviation: HRpQCT, high-resolution peripheral quantitative CT.
Although HRpQCT has the potential to provide important insights into skeletal changes throughout the lifespan and the implications of such changes in the pathogenesis of fragility fractures, this approach does have limitations. For example, although new HRpQCT imaging permits much higher resolution than conventional CT, a concern is that measures of bone microarchitecture (for example, cortical porosity) are, in fact, only estimates of their true values. Nonetheless, this technique represents the current limit of feasibility for assessing bone microarchitecture in humans in vivo, and such measurements discriminate between patients with and without fractures.19 Moreover, studies utilizing XtremeCT II (SCANCO Medical, Switzerland), which has a resolution of 61 μm, are on the horizon.
A better understanding of changes in bone microarchitecture during growth was provided by a cross-sectional study of 66 girls and 61 boys aged 6–21 years that utilized HRpQCT scanning of the distal radius.20 Trabecular parameters (bone volume:tissue volume [BV:TV], trabecular number [TbN] or trabecular thickness [TbTh]) did not change through puberty in girls, but increased in boys from late puberty. Cortical thickness (CtTh) and vBMD decreased markedly from prepuberty to midpuberty in girls, with a similar trend in boys, before increasing markedly towards late puberty in both sexes. Total bone strength, calculated using μFE models, increased with age in both sexes; bone strength was higher in boys than in girls by late puberty, which was due mainly to the larger bone size in boys than in girls. Interestingly, cortical porosity peaked at midpuberty in both sexes, around the time of maximal longitudinal growth, and coinciding with the timing of adolescent forearm fractures in previous studies.12–15 These findings suggest that transient, regional deficits in cortical bone, particularly cortical thinning and increased cortical porosity during the time of rapid longitudinal growth, result in marked increases in the incidence of forearm fractures during adolescence.
Overall, similar findings were subsequently reported in a HRpQCT study involving 69 boys and 60 girls aged 5–18 years.21 Although cortical porosity was not assessed in this study, the investigators reported notable decreases in CtTh and in cortical vBMD in midpuberty, particularly in boys. Another study reported that through all stages of puberty, boys had higher cortical porosity than girls,22 perhaps explaining, at least in part, the higher incidence of distal forearm fractures in boys than in girls during adolescence.12–15
Data from these studies using HRpQCT have essentially validated the hypothesis proposed by Parfitt23 many years ago regarding the pathogenesis of adolescent forearm fractures. On the basis of clinical and epidemiological data, which demonstrate a marked increase in distal forearm fractures following the menopause in women,16 Parfitt postulated that high bone turnover, whether following the menopause or during rapid adolescent growth, leads to an increase in cortical porosity.23 This increase is clearly transient during growth,20 but is permanent and progressive in ageing women (and ageing men).24 According to Parfitt,23 the increased demand for calcium associated with the adolescent growth spurt is probably met, at least in part, by increased calcium mobilization from cortical bone, which leads to the observed increases in cortical porosity.23 This increased calcium mobilization might be driven by transient increases in secretion of parathyroid hormone, as this factor is the primary regulator of calcium homeostasis and is known to increase cortical porosity.25–27 The distal radius might be particularly vulnerable to the generalized increase in cortical porosity, as the cortex is extremely thin at this site. Thus, according to the Parfitt hypothesis,23 distal forearm fractures during adolescence are an inevitable consequence of the growth spurt associated with puberty and the systemic need for calcium required to sustain longitudinal growth.
Distal forearm fractures during growth
Implications
The high incidence of distal forearm fractures during growth12–15 and the observation that these fractures might be increasing over time12 has led to three important questions. Firstly, do adolescents with distal forearm fractures have skeletal deficits? Secondly, do these skeletal deficits persist into young adulthood? Thirdly, do children who sustain distal forearm fractures have an increased risk of fragility fractures later in life?
Skeletal deficits
A number of studies, summarized in a meta-analysis,28 used DXA to address the question of whether adolescents with distal forearm fractures have skeletal deficits. Using data from the eight studies included in the meta-analysis, the standardized mean difference (the difference in means divided by the pooled SD of the participants) in bone mass between children with fractures and control children without fractures was −0.32 (95% CI −0.43 to −0.21, P <0.001).28 Consistent with this analysis, the only prospective cohort study available to date, which included 6,213 children (mean age 9.9 years) who were followed up for 24 months, found an 89% increased risk of fractures per SD decrease in size-adjusted BMC.29
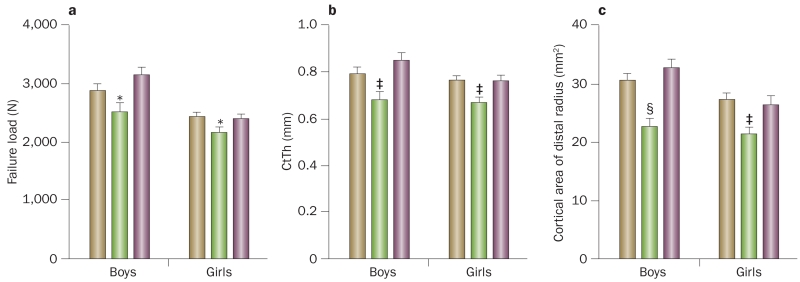
To better understand possible bone microstructural changes in adolescents with distal forearm fractures and to address the role of the level of trauma leading to these fractures, a 2014 study30 used HRpQCT and a careful definition of trauma levels (mild versus moderate), which were based on a validated trauma classification scheme (Box 1).31 Study participants included 115 boys and girls who had sustained a distal forearm fracture within the past year and 108 control individuals aged 8–15 years without a fracture. The key findings of this study30 were that compared with controls, boys and girls with a mild trauma distal forearm fracture (for example, resulting from a fall from standing height) had 13% and 11% reductions in bone strength (assessed by μFE models, P <0.05), respectively (Figure 4a), as well as statistically significant reductions in CtTh of 14% and 13% (P <0.01), respectively, (Figure 4b) and in cortical area of 26% and 23% (P <0.01), respectively (Figure 4c). Similar changes were evident at the distal tibia, which suggests that these deficits were generalized. By contrast, both boys and girls with a moderate trauma distal forearm fracture (for example, resulting from a fall from a bicycle) had similar values to control individuals for all of the measured skeletal parameters, including bone strength, CtTh and cortical area (Figure 4). In addition, even though cortical porosity had previously been shown to increase during rapid longitudinal growth,20 this parameter was not different between individuals with a distal forearm fracture and control individuals, including those in the subset of individuals with fractures caused by mild trauma. However, by use of a somewhat different method for assessing cortical porosity, another study reported that girls with distal forearm fractures had greater cortical porosity at the distal radius than their peers without a fracture;32 boys with distal forearm fractures were not evaluated.
Box 1. Categories of Landin’s modified trauma levels.
Events classified as mild trauma
-
■
Falling onto the ground from standing height or less (<0.5 m)
-
■
Falling onto a resilient surface (for example, rubber, grass or sand) from 0.5–3.0 m
-
■
Falling from a bed or couch
-
■
Playing injuries, including playground scuffles
-
■
Falling while moving at slow speed on a scooter, skateboard, skis, rollerblades or skates
-
■
Low-energy collisions with an object moving at slow speed
-
■
Low-energy sport injuries (for example, attained during basketball and soccer)
Events classified as moderate trauma
-
■
Falling onto concrete or other nonresilient surface from 0.5–3.0 m
-
■
Falling onto another person, which results in a moderate-energy collision
-
■
Falling from a bunk bed
-
■
Falling down stairs
-
■
Falling from a bicycle or horse
-
■
Falling from a swing, slide or similar playground equipment
-
■
Falling while moving at fast speed on a scooter, skateboard, skis, rollerblades or skates
-
■
Moderate-energy collisions with an object moving at fast speed
-
■
Moderate-energy collisions between two moving objects (for example, during football and hockey)
Events classified as severe trauma
-
■
Falling from a height >3.0 m
-
■
Traffic accidents
-
■
Being hit by a moving heavy object
Adapted with permission from Farr, J. N. et al. Bone strength and structural deficits in children and adolescents with a distal forearm fracture resulting from mild trauma. J. Bone Miner. Res. 29(3), 590–599 © (2014) John Wiley & Sons, Inc.
Figure 4.
Bone microstructural changes in adolescents with distal forearm fractures. a | Bone strength (failure load, [N, newtons]), b | CtTh and c | cortical area of the distal radius in healthy children without a fracture (brown bars), children with mild trauma distal forearm fractures (green bars) and children with moderate trauma distal forearm fractures (purple bars). Values presented as mean ± SEM and adjusted for bone age. *P <0.05, ‡P <0.01 and §P <0.001 compared with the respective sex-matched control group without a fracture, using the Dunnett adjustment for multiple comparisons. Abbreviation: CtTh, cortical thickness. Adapted with permission from Farr, J. N. et al. Bone strength and structural deficits in children and adolescents with a distal forearm fracture resulting from mild trauma. J. Bone Miner. Res. 29(3), 590–599 © (2014) John Wiley & Sons, Inc.
Collectively, DXA28,29 and HRpQCT30,32 data demonstrate that adolescents who sustain distal forearm fractures have systemic skeletal deficits, but only if they attained a fracture in the setting of mild trauma. Additionally, adolescents with distal forearm fractures following mild trauma have more severe cortical thinning at the distal radius and tibia (leading to reduced bone strength) during the pubertal growth spurt than those without a fracture.30 Whether these systemic skeletal deficits or cortical thinning are due to genetic predisposition or nutritional and/or environmental factors remains unclear at this point and further study is justified.
Persistence of deficits into young adulthood
Several studies have attempted to address the question of whether skeletal deficits persist into young adulthood.33,34 One study used a fairly old technique, single-photon absorptiometry, at the distal forearm to measure aBMD in 47 boys and 26 girls (mean age 10 years, range 3–16 years) with an index fracture (defined as any fracture except hand, finger, skull, tooth and rib) and a similar number of age-matched and sex-matched control children at baseline and again 27 years later.33 Boys with an index fracture had very similar deficits in aBMD at baseline (Z-score, −0.4, 95% CI −0.7 to −0.1) and 27 years later (Z-score −0.4, 95% CI −0.7 to 0.1), which suggests that the skeletal deficits in these children ‘tracked’ into young adulthood. Similar findings were also present in the girls in the study. In the boys, the deficit in bone mass was driven by mild, rather than moderate or severe trauma fractures. However, to our knowledge, no study has examined the relationship between reduced peak bone mass and fracture risk in late decades of life.
To examine possible alterations in bone microarchitecture in young adults with a history of childhood distal forearm fractures, a second study used HRpQCT to study 75 women and 75 men (age range 20–40 years) with a history of childhood distal forearm fractures and an equal number of age-matched and sex-matched control individuals without a history of fracture.34 Similar to the findings in children,30 young women and men with a history of childhood distal forearm fractures due to mild, but not moderate, trauma had statistically significant reductions in bone strength as well as in cortical area at the radius; bone strength and cortical area were similarly reduced at the tibia.34 Of note, these skeletal deficits in young adults with a history of distal forearm fractures were of a similar magnitude to those present in adolescents with these fractures, which suggests that the deficits present in adolescence ‘track’ into young adult life. Individuals with a history of mild trauma distal forearm fractures also had considerable deficits in aBMD, which were assessed by DXA at the radius, hip and total body. Thus, a mild trauma distal forearm fracture might predict the attainment of suboptimal peak bone density, structure and strength in young adulthood.
Risk of fragility fractures later in life
Owing to the potential length of follow-up required, the question of whether children who sustain distal forearm fractures have an increased risk of fragility fractures later in life has been difficult to address. From participants of the European Prospective Osteoporosis Study,35 6,451 men (mean age 63.8 years) and 6,936 women (mean age 63.1 years) were recruited and queried about their history of childhood fractures by recall.36 8.9% of the men and 4.5% of the women reported a first fracture (at any site) between the ages of 8 years and 18 years.36 However, as the prevalence of fractures in childhood is estimated at 42% in girls and 54% in boys,37 the results of this study could have been affected by ascertainment bias. This caveat notwithstanding, a history of any childhood fracture or forearm fracture (by recall) was not associated with an increased risk of future fractures in either sex.36
To further address the risk of fragility fractures later in life, a population-based cohort of 1,776 children on the Olmsted County database aged <18 years who had a distal forearm fracture in 1935–1992 (confirmed by medical records) were studied and incident fractures occurring after the age of 50 years identified.38 In this study, men who sustained a distal forearm fracture in childhood were at a significantly increased risk (OR ~2–3) of any fragility fracture, a major osteoporotic fracture (hip, spine, distal forearm or proximal humerus) or other fragility fractures.38 By contrast, women who sustained a childhood forearm fracture did not have an increased risk of fragility fractures later in life. This sex difference could be due to a number of factors: for example, any ‘tracking’ of skeletal fragility from childhood into old age might be masked in women by the major skeletal insult of the menopause, which causes marked bone loss.39 In addition, if the relationship between childhood distal forearm fractures and a fragility fracture later in life is weaker in women than in men, this study might have missed a possible association due to the inability to distinguish between mild versus moderate trauma fractures that occurred many years previously in childhood.
Age-related bone loss and fractures
Patterns of bone loss in women and men
In addition to optimal bone mass acquisition, the subsequent bone loss associated with ageing clearly has a major role in determining fracture risk late in life. Given the limitations of DXA noted previously, particularly the inability to distinguish between trabecular and cortical bone, recent studies have used quantitative CT (QCT) to assess changes in trabecular and cortical vBMD, as well as bone size at various skeletal sites, including the spine, hip, distal radius and distal tibia.
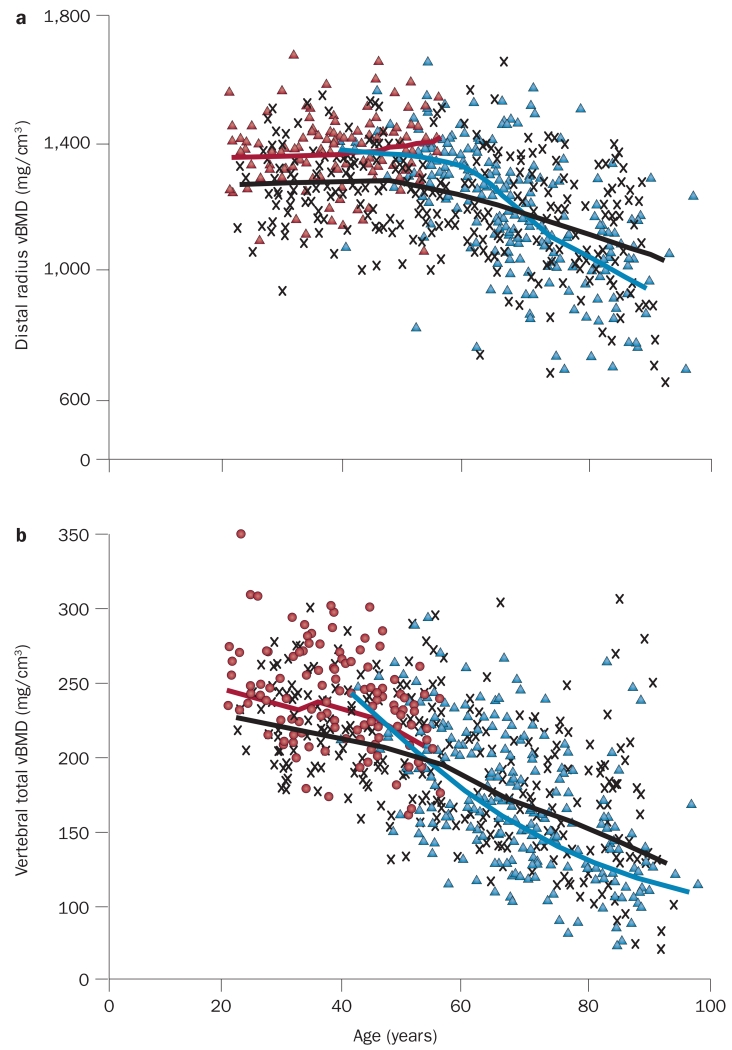
In a cross-sectional study involving 373 men and 323 women aged 20–97 years, men had 35–42% larger bone areas (depending on the specific skeletal site examined) than women in young adulthood (age range 20–29 years), which is consistent with their larger body size.40 In both sexes, bone area increased over life by ~15%, which indicates ongoing periosteal bone growth is occurring during adult life. This biomechanically beneficial change in bone size was, however, offset by marked decreases in vBMD. Specifically, although cortical vBMD at multiple sites was unchanged until ~50 years of age and then decreased in both sexes (Figure 5a), trabecular vBMD declined even in young adulthood, with ongoing trabecular bone loss evident throughout life (Figure 5b). Decreases in vBMD in both compartments (cortical and trabecular) were greater in women than in men, which is consistent with the added effects of the menopause and subsequent almost complete estrogen deficiency in women.
Figure 5.
Age-related changes in cortical and trabecular vBMD. a | Values for vBMD (mg/cm3) of cortical bone at the distal radius (>95% cortical bone) in a population sample of women and men from Rochester, MN, USA, aged 20–97 years. Individual values and smoothed lines are shown for women who have not undergone the menopause (red), women after the menopause (blue) and men (black). b | Values for vBMD of the total vertebral body (>80% trabecular bone) in the same cohort (colour code is as in part a). All changes with age were significant (P <0.05). Abbreviation: vBMD, volumetric BMD. Adapted with permission from Riggs, B. L. et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J. Bone Miner. Res. 19(12), 1945–1954 © (2004) John Wiley & Sons, Inc.
To further validate these cross-sectional data, a longitudinal analysis of the same cohort was performed, which produced similar findings.41 These data thus indicate that cortical bone remains fairly stable in both sexes until mid-life, when estrogen deficiency in women39 and gradual sex steroid deficiency in ageing men42 might begin to drive cortical bone loss. By contrast, the early onset (in young adult life) of substantial trabecular bone loss in both sexes during sex steroid sufficiency remains largely unexplained. Nevertheless, these findings suggest that interventions that optimize peak bone mass early in life and prevent trabecular bone loss in young adulthood have important implications for the prevention of osteoporosis and fractures in elderly people.
Bone microarchitectural changes over life were assessed by use of HRpQCT in a population-based, cross-sectional study involving 324 women and 278 men aged 21–97 years.43 Young men (aged 20–29 years) had greater BV/TV and TbTh at the distal radius (by 26% and 28%, respectively,) than young women, but similar values for TbN and trabecular separation. Over life, decreases in BV/TV were similar in women and men, but the under-lying structural basis for this decrease differed between the sexes: women underwent bone loss principally due to loss of trabeculae (decreases in TbN), whereas men primarily had trabecular thinning (decreases in TbTh). This difference has important biomechanical consequences, as decreases in TbN lead to considerably larger deficits in bone strength than comparable decreases in TbTh.44 These findings might, in part, explain the lower risk of fragility fractures in ageing men than in ageing women.
An emerging role for increased cortical porosity
The ability to noninvasively assess bone microarchitecture using HRpQCT has revealed an important role for increased cortical porosity in age-related bone loss and fractures. By use of HRpQCT and a novel analysis of cortical porosity along with scanning electron microscopy, ageing was demonstrated to be associated with greater loss of cortical bone than of trabecular bone, as well as with marked increases in cortical porosity.24 This study also highlighted the importance of bone resorption on endocortical surfaces (so-called ‘trabecularization’ of cortical bone) in the progressive cortical porosity as sociated with ageing.
Another study took a somewhat different approach to evaluate age-related changes in bone structure but arrived at a similar conclusion regarding the importance of cortical porosity as a key microarchitectural change in bone with ageing.45 Although previous studies have shown that age is a key predictor of fracture risk independent of aBMD,46 the possible effect of age on bone ‘quality’ has not been well defined. To address this issue, 44 women aged <50 years (mean age 41.0 years) were matched to 44 women aged ≥50 years (mean age 62.7 years) by aBMD measurements at the ultradistal radius using DXA; 57 men aged <50 years (mean age 41.3 years) were also similarly matched to 57 men aged ≥50 years (mean age 68.1 years).45 In individuals matched for aBMD, none of the trabecular microstructural parameters (for example, BV/TV, TbN and TbTh) differed between young and old participants. By contrast, women and men aged ≥50 years had markedly increased cortical porosity (by 91% and 56%, respectively) that was not captured by DXA. Thus, the combined data from these studies24,45 demonstrate that cortical porosity increases markedly with age and this increase is not captured by DXA.
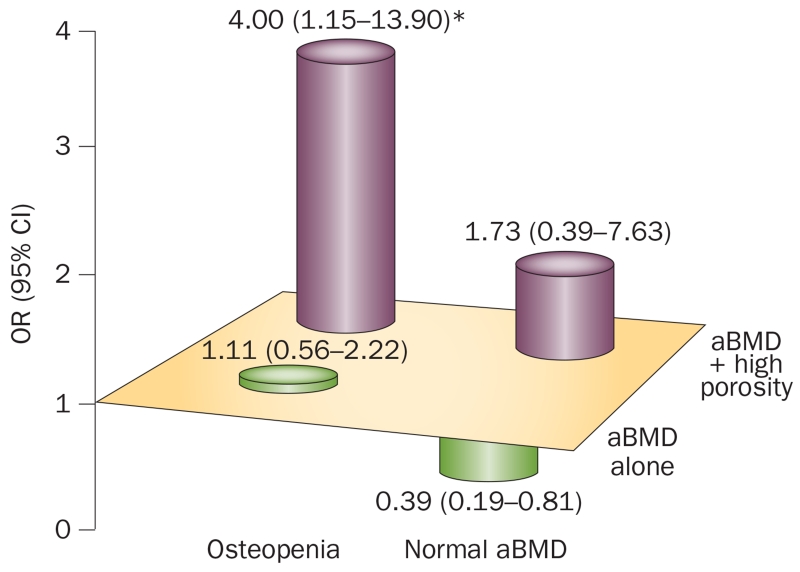
The potential importance of cortical porosity in determining risk of distal forearm fractures was highlighted in a study of 68 women who had been through the menopause and 70 age-matched control individuals using HRpQCT.19 In women who already had osteoporosis diagnosed by DXA (ultradistal aBMD T-score ≤−2.5), cortical porosity was not useful in differentiating between individuals with or without fractures as high porosity (defined as >90th percentile of young adult women) was present in 92% and 86% of each group, respectively. By contrast, in women with osteopenia (aBMD T-score between −1.0 and −2.5), increased cortical porosity was associated with a fourfold (P <0.05) increased odds ratio for a distal forearm fracture (Figure 6). Of interest, previous work, albeit performed on small numbers of biopsy samples from patients with a hip fracture and cadaveric control samples matched for age and sex, quantified circumferential variations in the characteristics of cortical bone osteons using segmental analysis and demonstrated regional differences in cortical porosity47 and merging of adjacent, clustered remodelling osteons in the fractured femoral neck.48,49 These findings suggest that composite osteons lead to increased cortical porosity and trabecularization of the hip cortex at regions commonly loaded on fall impact, which might, in part, explain increased susceptibility to hip fracture. Although larger studies examining not only distal forearm fractures, but also other fractures, are clearly needed to further validate these findings, the available data suggest that assessment of cortical porosity enhances the identification of individuals at increased risk of fracture among the large group of patients with osteopenia, in whom fracture risk assessment remains most ambiguous.50
Figure 6.
Cortical porosity and risk of distal forearm fractures. OR (95% CI) for distal forearm fracture associated with aBMD alone and in combination with high cortical porosity in women with osteopenia at the ultradistal radius. *P <0.05. Abbreviation: aBMD, areal BMD. Reproduced with permission from Bala, Y. et al. Cortical porosity identifies women with osteopenia at increased risk of forearm fractures. J. Bone Miner. Res. 29(6), 1356–1362 © (2014) John Wiley & Sons, Inc.
Alterations in bone material properties
Although the previous discussion has focused on changes in bone microarchitecture associated with ageing or predisposition to fracture, the material composition of bone is another important component of bone quality.51 However, assessment of how the properties of bone material might be altered with ageing or in patients with fragility fractures has been difficult, owing to the invasive nature of the procedures previously needed to quantify these properties in vivo. The availability of a novel microindentation device might now enable the safe measurement of properties of bone material (bone material strength index) in humans.52,53 Using a prototype device (called a reference point indenter), several small studies found that indices of properties of bone material might be reduced in patients with hip fractures54 and atypical femoral fractures.55 The potential utility of this approach for assessing properties of bone material in humans was demonstrated in a proof-of-concept study.56 Women with type 2 diabetes mellitus who had undergone the menopause had a statistically significantly lower bone material strength index than matched controls without type 2 diabetes mellitus, despite no significant differences in aBMD (assessed by DXA) between the two groups.56 This finding suggests that impaired properties of bone material are a cause of the increased fracture risk observed in these patients.57,58 Thus, the availability of this tool should now make possible the assessment of age-related changes in properties of bone material in humans as well as addressing whether patients with fragility fractures have alterations not only in bone microarchitecture, but also in properties of bone material. Combined with HRpQCT, assessment of properties of bone material might improve understanding of age-related changes in bone quality and how these changes might be further impaired in patients with fragility fractures.
Conclusions
Although DXA has been an extremely important tool for the clinical assessment of fracture risk, new approaches to assess bone microarchitecture in humans have provided novel insights into the structural changes in bone that occur during growth and senescence. These studies have highlighted the importance of changes in cortical bone, particularly cortical porosity, in predisposition to increased fracture risk both during adolescent growth and with ageing. Nonetheless, a number of important questions remain to be answered by future research. Firstly, do changes in bone microarchitecture predict fractures at clinically relevant skeletal sites other than the forearm (for example, the hip and spine)? Secondly, to what extent do changes in bone microarchitecture at the distal radius or tibia relate to changes at the hip and spine? Thirdly, does measurement of bone microarchitecture enhance identification of individual patients at risk of rapid bone loss and/or fragility fractures? In addition, the availability of new tools to assess properties of bone material, used in combination with innovative approaches to assess bone microarchitecture in humans, might further increase our understanding of the changes in bone that occur with ageing and perhaps refine our ability to identify patients at highest risk of fracture and thereby target these patients for treatment.
Key points.
-
■
Both peak bone mass attained during childhood and adolescent growth, and bone loss associated with senescence are major determinants of bone mass and fracture risk late in life
-
■
Incidence of distal forearm fractures increases markedly around the time of puberty and children who sustain these fractures in the setting of mild, but not moderate, trauma have systemic skeletal deficits that persist into young adult life
-
■
With ageing, cortical bone at multiple skeletal sites remains fairly stable in both sexes until mid-life, when estrogen deficiency in women and gradual sex steroid deficiency in men begins to drive cortical bone loss
-
■
By contrast, substantial trabecular bone loss occurs in both sexes during young adult life, in conditions of sex steroid sufficiency
-
■
Cortical porosity is increasingly recognized as an important component of bone ‘quality’ that increases markedly with age; changes in cortical porosity are not captured by dual-energy X-ray absorptiometry
-
■
Assessment of cortical porosity might help identify individuals at increased risk of fracture from the large group of patients with osteopenia, in whom assessment of fracture risk remains most ambiguous
Acknowledgements
The authors acknowledge support from the NIH (grants AG004875 and AR027065 to S.K.) and a Mayo Clinical and Translational Science Award (UL1 TR000135 to the Mayo Foundation).
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
J.N.F. and S.K. researched data for the article, provided substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
References
- 1.Centers for Disease Control and Prevention The state of aging and health in America 2013. 2013 [online], http://www.cdc.gov/features/agingandhealth/state_of_aging_and_health_in_america_2013.pdf.
- 2.US Departments of Health and Human Services Bone health and osteoporosis: a report of the surgeon general. 2004:187–217. [online], http://www.dsls.usra.edu/meetings/bonehealth_2005/SG_full_report.pdf.
- 3.National Osteoporosis Foundation . America’s bone health: the state of osteoporosis and low bone mass in our nation. The Foundation; 2002. [Google Scholar]
- 4.Burge R, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 5.Rizzoli R, Bianchi ML, Garabedian M, McKay EA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez CJ, Beaupré GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos. Int. 2003;14:843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]
- 7.Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol. Health Care. 1998;6:329–337. [PubMed] [Google Scholar]
- 8.Laib A, Ruegsegger P. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-μm-resolution microcomputed tomography. Bone. 1999;24:35–39. doi: 10.1016/s8756-3282(98)00159-8. [DOI] [PubMed] [Google Scholar]
- 9.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J. Bone Miner. Res. 1999;14:1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 10.Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J. Bone Miner. Res. 1999;14:1796–1802. doi: 10.1359/jbmr.1999.14.10.1796. [DOI] [PubMed] [Google Scholar]
- 11.Heaney RP, et al. Peak bone mass. Osteoporos. Int. 2000;11:985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 12.Khosla S, et al. Incidence of childhood distal forearm fractures over 30 years: A population-based study. JAMA. 2003;290:1479–1485. doi: 10.1001/jama.290.11.1479. [DOI] [PubMed] [Google Scholar]
- 13.Landin LA. Fracture patterns in children. Analysis of 8,682 fractures with special reference to incidence, etiology and secular changes in a Swedish urban population 1950–1979. Acta Orthop. Scand. Suppl. 1983;202:1–109. [PubMed] [Google Scholar]
- 14.Kramhøft M, Bødtker S. Epidemiology of distal forearm fractures in Danish children. Acta Orthop. Scand. 1988;59:557–559. doi: 10.3109/17453678809148784. [DOI] [PubMed] [Google Scholar]
- 15.Bailey DA, Wedge JH, McCulloch RG, Martin AD, Bernhardson SC. Epidemiology of fractures of the distal end of the radius in children as associated with growth. J. Bone Joint Surg. Am. 1989;71:1225–1231. [PubMed] [Google Scholar]
- 16.Cooper C, Melton LJ., 3rd Epidemiology of osteoporosis. Trends Endocrinol. Metab. 1992;3:224–229. doi: 10.1016/1043-2760(92)90032-v. [DOI] [PubMed] [Google Scholar]
- 17.MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med. Eng. Phys. 2007;29:1096–1105. doi: 10.1016/j.medengphy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Pistoia W, et al. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone. 2002;30:842–848. doi: 10.1016/s8756-3282(02)00736-6. [DOI] [PubMed] [Google Scholar]
- 19.Bala Y, et al. Cortical porosity identifies women with osteopenia at increased risk of forearm fractures. J. Bone Miner. Res. 2014;29:1356–1362. doi: 10.1002/jbmr.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirmani S, et al. Bone structure at the distal radius during adolescent growth. J. Bone Miner. Res. 2009;24:1033–1042. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, et al. Rapid growth produces transient cortical weakness: a risk factor for metaphyseal fractures during puberty. J. Bone Miner. Res. 2010;25:1521–1526. doi: 10.1002/jbmr.46. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama KK, et al. Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during puberatal growth: an HR-pQCT study. J. Bone Miner. Res. 2012;27:273–282. doi: 10.1002/jbmr.552. [DOI] [PubMed] [Google Scholar]
- 23.Parfitt AM. The two faces of growth: benefits and risks to bone integrity. Osteoporos. Int. 1994;4:382–398. doi: 10.1007/BF01622201. [DOI] [PubMed] [Google Scholar]
- 24.Zebaze RM, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 25.Fox J, et al. Effects of daily treatment with parathyroid hormone 1–84 for 16 months on density, architecture and biomechanical properties of cortical bone in adult ovariectomized rhesus monkeys. Bone. 2007;41:321–330. doi: 10.1016/j.bone.2007.04.197. [DOI] [PubMed] [Google Scholar]
- 26.Recker RR, et al. Cancellous and cortical bone architecture and turnover at the iliac crest of postmenopausal osteoporotic women treated with parathyroid hormone 1–84. Bone. 2009;44:113–119. doi: 10.1016/j.bone.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Gafni RI, et al. Daily parathyroid hormone 1–34 replacement therapy for hypoparathyroidism induces marked changes in bone turnover and structure. J. Bone Miner. Res. 2012;27:1811–1820. doi: 10.1002/jbmr.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark EM, Tobias JH, Ness AR. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics. 2006;117:291–297. doi: 10.1542/peds.2005-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: a prospective cohort study. J. Bone Miner. Res. 2006;21:1489–1495. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farr JN, et al. Bone strength and structural deficits in children and adolescents with a distal forearm fracture resulting from mild trauma. J. Bone Miner. Res. 2014;29:590–599. doi: 10.1002/jbmr.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark EM, Ness AR, Tobias JH. Bone fragility contributes to the risk of fracture in children, even after moderate and severe trauma. J. Bone Miner. Res. 2008;23:173–179. doi: 10.1359/jbmr.071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bala Y, et al. Trabecular and cortical microstructure and fragility of the distal radius in women. J. Bone Miner. Res. 2015;30:621–629. doi: 10.1002/jbmr.2388. [DOI] [PubMed] [Google Scholar]
- 33.Buttazzoni C, et al. Does a childhood fracture predict low bone mass in young adulthood? A 27-year prospective controlled study. J. Bone Miner. Res. 2013;28:351–359. doi: 10.1002/jbmr.1743. [DOI] [PubMed] [Google Scholar]
- 34.Farr JN, et al. Diminished bone strength is observed in adult women and men who sustained a mild trauma distal forearm fracture during childhood. J. Bone Miner. Res. 2014;29:2193–2202. doi: 10.1002/jbmr.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Neill TW, et al. The prevalence of vertebral deformity in European men and women: the European Vertebral Osteoporosis Study. J. Bone Miner. Res. 1996;11:1010–1018. doi: 10.1002/jbmr.5650110719. [DOI] [PubMed] [Google Scholar]
- 36.Pye SR, et al. Childhood fractures do not predict future fractures: results from the European prospective osteoporosis study. J. Bone Miner. Res. 2009;24:1314–1318. doi: 10.1359/jbmr.090220. [DOI] [PubMed] [Google Scholar]
- 37.Jones IE, Williams SM, Dow N, Goulding A. How many children remain fracture-free during growth? A longitudinal study of children and adolescents participating in the Dunedin Multidisciplinary Health and Development Study. Osteoporos. Int. 2002;13:990–995. doi: 10.1007/s001980200137. [DOI] [PubMed] [Google Scholar]
- 38.Amin S, et al. A distal forearm fracture in childhood is associated with an increased risk for future fragility fractures in adult men, not women. J. Bone Miner. Res. 2013;28:1751–1759. doi: 10.1002/jbmr.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 40.Riggs BL, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J. Bone Miner. Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 41.Riggs BL, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J. Bone Miner. Res. 2008;23:205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr. Rev. 2008;29:441–464. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khosla S, et al. Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J. Bone Miner. Res. 2006;21:124–131. doi: 10.1359/JBMR.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva MJ, Gibson LJ. Modeling the mechanical behavior of vertebral trabecular bone: effects of age-related changes in microstructure. Bone. 1997;21:191–199. doi: 10.1016/s8756-3282(97)00100-2. [DOI] [PubMed] [Google Scholar]
- 45.Nicks KM, et al. Relationship of age to bone microstructure independent of areal bone mineral density. J. Bone Miner. Res. 2012;27:637–644. doi: 10.1002/jbmr.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hui SL, Slemenda W, Johnston CC., Jr. Age and bone mass as predictors of fracture in a prospective study. J. Clin. Invest. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bell KL, et al. Regional differences in cortical porosity in the fractured femoral neck. Bone. 1999;24:57–64. doi: 10.1016/s8756-3282(98)00143-4. [DOI] [PubMed] [Google Scholar]
- 48.Jordan GR, et al. Spatial clustering of remodeling osteons in the femoral neck cortex: a cause of weakness in hip fracture? Bone. 2000;26:305–313. doi: 10.1016/s8756-3282(99)00272-0. [DOI] [PubMed] [Google Scholar]
- 49.Bell KL, et al. A novel mechanism for induction of increased cortical porosity in cases of intracapsular hip fracture. Bone. 2000;27:297–304. doi: 10.1016/s8756-3282(00)00318-5. [DOI] [PubMed] [Google Scholar]
- 50.Khosla S, Melton LJ., 3rd Osteopenia. N. Engl. J. Med. 2007;356:2293–3000. doi: 10.1056/NEJMcp070341. [DOI] [PubMed] [Google Scholar]
- 51.Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 52.Bridges D, Randall C, Hansma PK. A new device for performing reference point indentation without a reference probe. Rev. Sci. Instrum. 2012;83:044301. doi: 10.1063/1.3693085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Randall C, et al. Applications of a new handheld reference point indentation instrument measuring bone material strength. J. Med. Device. 2013;7:410051–410056. doi: 10.1115/1.4024829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diez-Perez A, et al. Microindentation for in vivo measurement of bone tissue mechanical properties in humans. J. Bone Miner. Res. 2010;25:1877–1885. doi: 10.1002/jbmr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guerri-Fernandez RC, et al. Microindentation for in vivo measurement of bone tissue material properties in atypical femoral fracture patients and controls. J. Bone Miner. Res. 2013;28:162–168. doi: 10.1002/jbmr.1731. [DOI] [PubMed] [Google Scholar]
- 56.Farr JN, et al. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J. Bone Miner. Res. 2014;29:787–795. doi: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leslie WD, Rubin MR, Schwartz AV, Kanis JA. Type 2 diabetes and bone. J. Bone Miner. Res. 2012;27:2231–2237. doi: 10.1002/jbmr.1759. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz AV, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305:2184–2192. doi: 10.1001/jama.2011.715. [DOI] [PMC free article] [PubMed] [Google Scholar]