Abstract
Emotion perception, occurring in brain areas such as the prefrontal cortex and amygdala, involves autonomic responses affecting cardiovascular dynamics. However, how such brain–heart dynamics is further modulated by emotional valence (pleasantness/unpleasantness), also considering different arousing levels (the intensity of the emotional stimuli), is still unknown. To this extent, we combined electroencephalographic (EEG) dynamics and instantaneous heart rate estimates to study emotional processing in healthy subjects. Twenty-two healthy volunteers were elicited through affective pictures gathered from the International Affective Picture System. The experimental protocol foresaw 110 pictures, each of which lasted 10 s, associated to 25 different combinations of arousal and valence levels, including neutral elicitations. EEG data were processed using short-time Fourier transforms to obtain time-varying maps of cortical activation, whereas the associated instantaneous cardiovascular dynamics was estimated in the time and frequency domains through inhomogeneous point-process models. Brain–heart linear and nonlinear coupling was estimated through the maximal information coefficient (MIC). Considering EEG oscillations in the θ band (4–8 Hz), MIC highlighted significant arousal-dependent changes between positive and negative stimuli, especially occurring at intermediate arousing levels through the prefrontal cortex interplay. Moreover, high arousing elicitations seem to mitigate changes in brain–heart dynamics in response to pleasant/unpleasant visual elicitation.
Keywords: electroencephalographic, heart rate variability, maximal information coefficient, brain–heart dynamics, emotions, International Affective Picture System
1. Introduction
The autonomic nervous system (ANS) and the central nervous system (CNS) are strictly interconnected through anatomical and functional links, and influence each other continuously [1–4]. As an example, cortical and subcortical brain areas including the amygdala, insular cortex and pregenual anterior cingulate cortex play crucial homeostatic–interoceptive functions involving ANS dynamics [1,5]. Moreover, such cingulate cortex and amygdala activities are involved in regulating the sympathovagal balance [3,4]. On the other hand, changes in ANS signalling relevantly affect the CNS, both in physiological and in pathological conditions [2,4,6–9]. Dysfunctions of the ANS were found in acute and chronic stressful conditions [10–12], insomnia [13,14], epilepsy [15,16], parkinsonisms [17,18], psychosomatic disorders [19] and schizophrenia, anxiety and mood disorders [6,20–24], which are typically considered CNS-related conditions. Moreover, vagal nerve stimulation has been shown as an effective treatment for major depression [25,26], while relaxation techniques based on cardio-feedback are used for managing negative emotions and psychological symptoms [27,28].
A typical and paradigmatic brain–heart interaction occurs during an emotional experience. Human emotions involve several areas for their perception and processing. The prefrontal cortex and amygdala specifically represent the essence of two specific pathways. The prefrontal cortex encodes the affective elicitations longer than 6 s transmitting the related information to other areas of the Central Autonomic Network [29], whereas the amygdala encodes the briefly presented stimuli. Indeed, previous studies on emotions were mainly carried out investigating brain and neurovegetative activities [30–33]. As an example, estimates of vagal activity alone predicted neural responses during subjective rating of fearful faces [33], whereas heart rate (HR) increases were predicted by the level of activity of interconnected brain regions, including the amygdala, insula, anterior cingulate and brainstem during visual perception of emotional facial expression [34]. Concerning pathological mental states, mood disorders were linked to Takotsubo cardiomyopathy [35], which is one of the brain–heart disorders, whereas depressive states were associated with a functional disconnection between rostral anterior cingulate cortex and autonomic brainstem nuclei [36].
The scientific debate on the physiological origin of emotions is still open: whether they originate from the peripheral reactivity of the ANS, or from specific areas of the brain, or from both. As Damasio stated, ‘emotions are the most complex expression of homeostatic regulatory systems’. He hypothesized that emotions (or emotional memories) can modify our behaviour through conscious or unconscious signals [37], p. 86. Note that the latter belongs to ANS signalling whose role is to generate re-entry vegetative information to pre-existing cortical maps [37,30]. Although it is reasonable to hypothesize that emotions and emotional reactivity strongly affect brain–heart coupling, how such a brain–heart dynamics is further modulated by the specific kind of an emotional stimulus is still unknown.
Considering emotions as continuous traits, each state can be described and mapped in a multidimensional space, portraying different psychophysiological and neurobiological underpinnings [30,38,39]. According to the circumplex model of affect (CMA) [40], emotions can be mapped in two dimensions through a combination of arousal and valence levels [30]. Valence refers to the pleasantness or unpleasantness of an emotion, whereas arousal refers to the intensity of the emotional stimuli, expressed in terms of degree of activation from low to high. Importantly, CMA assumes that these dimensions are orthogonal, thus with no mutual influence (or interaction) among them.
At a peripheral level, the study of emotional responses is especially related to the analysis of heart rate variability (HRV) [30–32,41,42]. This is justified by the fact that oscillations of HRV above 0.15 Hz (i.e. the high-frequency band) are exclusively mediated by vagal activity [41,42], and oscillations below 0.15 Hz (i.e. low-frequency band) are mediated by both vagal and sympathetic activities [43]. At a central level, emotions have mainly been studied through functional magnetic resonance imaging, and continuous electroencephalographic (EEG) and evoked related potentials recordings [31,44,45].
Previous studies investigated the coupled brain–heart dynamics during healthy and pathological emotional responses (see reviews in [46,47]), highlighting connections in the vagally mediated regulation of physiological, affective and cognitive processes. As a general approach, previous studies have tried to link the EEG power in specific bands to HRV measures. Although significant correlations were found for the α (8–12 Hz) [48–52], β (13–30 Hz) [49,50,53] and γ (>30 Hz) bands [53,54], the psychophysiological meaning of such associations is still ill-defined. For instance, complexity of HRV series was used to predict changes in the EEG α band after stress [48]. However, physiological correlates of HRV complexity are still unknown.
On the other hand, the link between the EEG θ band (4–8 Hz) and ANS activity is quite consistent. Specifically, in healthy controls, both sympathetic- and parasympathetic-related parameters were correlated with EEG θ power in temporal areas [55]. Moreover, the frontal θ power has been demonstrated to be sensitive to emotions [56–60]. Unpleasant music evoked a significant decrease of HR associated with an increase of frontal midline θ power [61], whereas θ event-related synchronization were found to occur in frontal regions of the brain during the earliest phases of affective auditory stimuli processing [58]. In response to negative emotional patterns, EEG activity in the θ band was associated with the right prefrontal cortex activity, following an increase in the sympathetic response [62]. Furthermore, a positive correlation between HRV high-frequency power and EEG frontal midline θ power was found during meditation [63]. The same kind of correlation was found between HRV low-frequency power and EEG posterior θ power during biofeedback task [64]. More in general, the EEG θ power was associated with a general emotional response and states of relaxation and internal attention [63], whereas alterations of EEG dynamics in the θ band were found in case of ANS dysfunctions [55]. Cardiac and brain dynamics were also quantitatively assessed during sleep in the frame of dynamical information theory [65], highlighting the role of EEG low-frequency bands in the ‘from-brain-to-heart’ information transfer.
Limitations of the above-mentioned literature can be summarized into two categories: (i) correlation measures quantifying brain–heart coupling were performed at a groupwise level, considering few sample measures for each subject and, thus, totally disregarding intra-recording time-varying brain–heart dynamics; (ii) previously proposed correlation measures have considered only linear couplings, thus disregarding intrinsic nonlinear brain–heart interactions.
To overcome these limitations, in this study we provide a unique insight into the brain–heart dynamics during emotion perception in healthy subjects, showing experimental results using high-resolution EEG signals (128 channels) and instantaneous HR estimates. We present here a novel approach to study brain–heart interactions, quantifying the linear and nonlinear brain–heart coupling mechanisms through the calculation of the maximal information coefficient (MIC) index [66], a statistical method for detecting associations between pairs of variables. Importantly, MIC calculations were performed at a single-subject level, between time-varying estimates of high-resolution EEG power spectra and instantaneous HR, during visual emotional elicitation. Twenty-two healthy volunteers were emotionally elicited through passive viewing of pictures taken from the International Affective Picture System (IAPS) [67], associated with 25 different combinations of arousal and valence levels, including neutral elicitations.
More in detail, EEG data were processed using short-time Fourier transform representations in order to obtain time-varying maps of cortical activation, whereas the associated instantaneous cardiovascular dynamics was estimated through inhomogeneous point-process models of RR interval series [68], which were gathered from the electrocardiogram (ECG). The use of inhomogeneous point-process on heartbeat dynamics allows to obtain instantaneous time domain and spectral estimates, which can be considered as covariate measures of brain–heart interaction during emotional processing. Details on the inhomogeneous point-process modelling can be found in [68–70]. Briefly, we model the probability function of the next heartbeat given the past R-events. The probability function is fully parametrized to model its first-order moment. Importantly, as the probability function is defined at each moment in time, the parameter estimation is performed instantaneously. In particular, the linear terms allow for instantaneous time domain and spectral estimation. Recently, using point-process modelling, we reported on how to recognize emotional valence swings (positive or negative), as well as two levels of arousal (low-medium and medium-high), using heartbeat data only, being also able to instantaneously assess the subject's state even in short-time events (less than 10 s) [70]. In other words, emotional stimuli with high/low arousing and high/low valence levels produce changes in ANS dynamics, through both sympathetic and parasympathetic pathways, that can be tracked by a multidimensional representation estimated in continuous time by the proposed point-process model [70].
In the rest of the paper, as a proof of concept of the proposed methodology, we particularly focus on the experimental results gathered analysing EEG oscillations in the θ band, and its coupling with instantaneous heartbeat measures. Although we are aware that oscillations in other EEG frequency bands may be coupled with heartbeat dynamics, we focused on these low-frequency oscillations because the emotional processes consistently elicit changes in the θ band, together with the ANS responses [55–65]. However, for the sake of completeness, experimental results related to all of the EEG frequency bands, including α, β and γ bands, are reported in the electronic supplementary material.
2. Material and methods
(a). Experimental set-up
The recording paradigm related to this work has been previously described in [70]. As mentioned in the Introduction, we adopted a common dimensional model which uses multiple dimensions to categorize the emotions, the CMAs [40]. The CMA used in our experiment takes into account two main dimensions conceptualized by the terms of valence and arousal. Accordingly, we employed visual stimuli belonging to an international standardized database (IAPS) [67] having a specific emotional rating expressed in terms of valence and arousal. The IAPS is one of the most frequently cited tools in the area of affective stimulation and consists of a set of 944 images with emotional ratings obtained using the self-assessment manikin (the arousal scales ranging from 0 to 10, and valence scales ranging from −2 to 2).
An homogeneous population of 22 healthy subjects (aged from 21 to 24), not suffering from cardiovascular, neurological and mental disorders, was recruited to participate in the experiment. All subjects were naive to the purpose of the experiment. The experimental protocol for this study was approved by the ethics committee of the University of Pisa and informed consent was obtained from all participants involved in the experiment. All participants were screened using the Patient Health QuestionnaireTM, and only the ones with a score lower than 5 were included in this study [71].
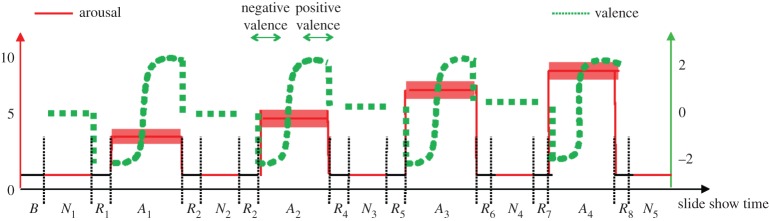
A general overview of the experimental protocol and analysis is shown in figure 1. The affective elicitation was performed by visualizing the IAPS pictures onto a PC monitor. Experimental protocol began with a resting session of 5 min with the eyes closed (session B). Then, the slideshow started, comprising nine sessions, alternating neutral sessions (from N1 to N5) and arousal sessions (from A1 to A4) (figure 1). One-minute resting-state sessions (from R1 to R8) were in between each neutral/arousal session. Neutral sessions consisted of six images having valence range (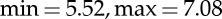 ) and arousal range (
) and arousal range (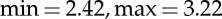 ). The arousal sessions included 20 images eliciting an increasing level of valence (from unpleasant to pleasant). Arousal sessions had a valence range (
). The arousal sessions included 20 images eliciting an increasing level of valence (from unpleasant to pleasant). Arousal sessions had a valence range (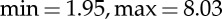 ) and an arousal range (
) and an arousal range (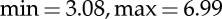 ). The overall protocol used 110 images. Each image was presented for 10 s for a whole duration of the experiment of 18 min and 20 s. In order to check for attention lapses throughout the protocol, we used a home-made eye-tracking system [72] that was precise enough to check the sight orientation to the computer monitor during each picture viewing. However, it did not allow to get information about gaze path in exploring pictures. Subjects that did not pay attention to the pictures were excluded from further statistical analyses.
). The overall protocol used 110 images. Each image was presented for 10 s for a whole duration of the experiment of 18 min and 20 s. In order to check for attention lapses throughout the protocol, we used a home-made eye-tracking system [72] that was precise enough to check the sight orientation to the computer monitor during each picture viewing. However, it did not allow to get information about gaze path in exploring pictures. Subjects that did not pay attention to the pictures were excluded from further statistical analyses.
Figure 1.
Sequence scheme over time of image presentation in terms of arousal and valence levels. The y-axis relates to the official IAPS score, whereas the x-axis relates to the time. After the first 5 min baseline (b) acquisition, the neutral sessions (Nx) alternate with resting-state sessions Rx and the arousal ones Ax. Along the time, the red line followsthe four arousal sessions having increasing intensity of activation. The dotted green line indicates the valence levels, distinguishing negative and positive levels within an arousing session. (Online version in colour.)
For the whole experimental session, high-resolution 128 channels EEG and ECG signals were acquired through the Geodesic EEG Systems 300 from Electrical Geodesics, Inc. The sampling frequency was set at 1 kHz. The average of mastoid signals was used as reference. This monitoring device allowed for a rapid application and comfortable fit of the cuff. All available EEG signals were taken into account for MIC analysis between every HRV time-varying feature. However, for the sake of conciseness, signals exclusively gathered from electrodes placed on the scalp at standardized positions Fp1, Fp2, F7, F3, Fz, F4, F8, T7, C3, C4, Cz, T8, P7, P3, Pz, P4, P8, O1, O2, according to the International Standard System 10–20, were considered for post hoc statistical analyses.
(b). Electroencephalographic signal processing
EEG signals were filtered by a sixth-order infinite impulse response bandpass filter with cut-off frequencies of 1–45 Hz. The Matlab toolbox EEGLAB [73] was used for the entire processing of the EEG data. EEG spectral analysis was performed using discrete fast Fourier transform to estimate the power spectra in 4 s moving windows, with 75% overlapping, within the classical frequency bandwidths: θ (4–8 Hz), α (8–14 Hz), β (14–30 Hz), γ (30–40 Hz). Frequency band δ (less than 4 Hz) was not taken into account in this study because its related to deeper stages of sleep.
EEG signal pre-processing consisted in three main steps: removal of head/body movement-related artefacts; removal of eye movement artefacts; and ‘bad’ channels identification. Concerning head/body and eye artefacts, we have simply excluded all contaminated epochs from further analyses. This choice was a cautious approach aimed to avoid any effects of artefact-related residuals following the artefact-removal procedures, which could alter true EEG power and synchronization estimates. Looking for head/body movement-related artefacts, all EEG channels were analysed in order to find synchronous, sudden increases in signal amplitude. We classified EEG epochs with amplitude exceeding the threshold of the 95th percentile of the signal amplitude distribution as epochs likely to contain movement or muscular artefacts. After confirmatory visual inspection, such EEG epochs were discarded. Eye movement artefacts were detected by computing a moving-window cross-correlation between the frontal EEG channels and electro-oculogram: high values of cross-correlation were marked as ocular artefacts. We considered cross-correlation values as significantly high if greater than a specific threshold value. Such a value was derived by computing the same moving-window cross-correlation between phase-randomized surrogated [74] frontal EEG channels and the electro-oculogram. Furthermore, we considered only artefacts producing fluctuations greater than 50 μV on frontal EEG channels, lasting at least 70 ms. Epochs marked as artefact-corrupted were tagged and, after visual inspection, definitively discarded [75]. ‘Bad’ channels identification refers to the detection of low-quality EEG signal, frequent unexpected events and presence of high-frequency noise [73]. To this aim, for each channel, we calculated the second-, third- and fourth-central moments and identified the ‘bad’ channels as the outliers present in such a three-dimensional space. Good channels, in fact, usually cluster together, whereas the bad ones drift apart in different directions according to their artefactual nature (for example, channels highly contaminated by the power-line have lower kurtosis than other channels). For each dimension of this space, channels distant more than twice the interquartile range from the cluster centroid were classified as artefactual and, after visual inspection, were discarded.
(c). Instantaneous heart rate variability analysis
The ECG signal was analysed off-line to extract the RR intervals [41]. Firstly, ECG was pre-filtered through a moving average filter in order to extract and subtract the baseline. Then, a QRS complex detection algorithm was used. We adopted the automatic algorithm developed by Pan–Tompkins [76]. This algorithm allowed us to extract each QRS complex and to detect the corresponding R-peak. Erroneous and ectopic beats were corrected by a previously developed algorithm, based on the point-process modelling [77].
Starting from the RR interval series, instantaneous time and frequency domain features were estimated through point-process modelling [68,69,78,79]. The point-process framework primarily defines the probability of having a heartbeat event at each moment in time. A parametric formulation of the probability function allows for a systematic, parsimonious estimation of the parameter vector in a recursive way and at any desired time resolution. Instantaneous cardiovascular indices can then be derived from the parameters in order to quantify important features as related to cardiovascular control dynamics.
Mathematically, let (0,T] denote the observation interval and 0≤u1<⋯<uk<uk+1<⋯<uK≤T the times of the events. For t∈(0,T], let 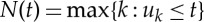 be the sample path of the associated counting process. Its differential, dN(t), denotes a continuous-time indicator function, where dN(t)=1, when there is an event (such as the ventricular contraction) or dN(t)=0, otherwise. Let define also a left continuous function
be the sample path of the associated counting process. Its differential, dN(t), denotes a continuous-time indicator function, where dN(t)=1, when there is an event (such as the ventricular contraction) or dN(t)=0, otherwise. Let define also a left continuous function 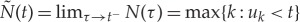 which will be useful in the following definitions.
which will be useful in the following definitions.
Given a set of R-wave events 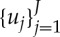 detected from the ECG, let RRj=uj−uj−1>0 denote the jth R−R interval, or, equivalently, the waiting time until the next R-wave event. Assuming history dependence, the probability density of the waiting time t−uj until the next R-wave event follows an inverse Gaussian model:
detected from the ECG, let RRj=uj−uj−1>0 denote the jth R−R interval, or, equivalently, the waiting time until the next R-wave event. Assuming history dependence, the probability density of the waiting time t−uj until the next R-wave event follows an inverse Gaussian model:
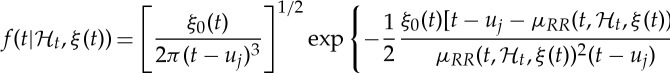 |
2.1 |
where 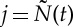 denotes the index of the previous R-wave event occurred before time t,
denotes the index of the previous R-wave event occurred before time t, 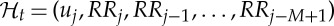 , ξ(t) is the vector of the time-varying parameters,
, ξ(t) is the vector of the time-varying parameters, 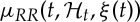 represents the first-moment statistic (mean) of the distribution, and ξ0(t)=θ>0 denotes the shape parameter of the inverse Gaussian distribution. The function
represents the first-moment statistic (mean) of the distribution, and ξ0(t)=θ>0 denotes the shape parameter of the inverse Gaussian distribution. The function 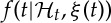 indicates the probability of having a beat at time t given that a previous beat has occurred at uj and
indicates the probability of having a beat at time t given that a previous beat has occurred at uj and 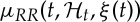 can be interpreted as signifying the prediction of the time when the next beat is expected to occur. Of note, the use of an inverse-Gaussian distribution to characterize the R–R intervals occurrences is motivated by both algorithmic and physiological reasons [68,69].
can be interpreted as signifying the prediction of the time when the next beat is expected to occur. Of note, the use of an inverse-Gaussian distribution to characterize the R–R intervals occurrences is motivated by both algorithmic and physiological reasons [68,69].
Here, we model 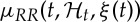 as
as
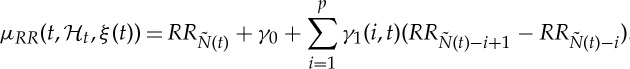 |
2.2 |
Since 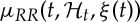 is defined in a continuous-time fashion, we can obtain an instantaneous R–R mean estimate at a very fine timescale (with an arbitrarily small bin size Δ), which requires no interpolation between the arrival times of two beats. Given the proposed parametric model, the indexes of the HR and HRV will be defined as a time-varying function of the parameters ξ(t)=[θ(t),g0(t),g1(1,t),…,g1(p,t)]. A local maximum-likelihood method [68,69] was used to estimate the unknown time-varying parameter set ξ(t) within a sliding window of W=90 s. We used a Newton–Raphson procedure to maximize the local log-likelihood and compute the local maximum-likelihood estimate of ξ(t) [68,69] within W. Because there is significant overlap between adjacent local likelihood intervals, we started the Newton–Raphson procedure at t with the previous local maximum-likelihood estimate at time t−Δ in which Δ define how much the local likelihood time interval is shifted to compute the next parameter update.
is defined in a continuous-time fashion, we can obtain an instantaneous R–R mean estimate at a very fine timescale (with an arbitrarily small bin size Δ), which requires no interpolation between the arrival times of two beats. Given the proposed parametric model, the indexes of the HR and HRV will be defined as a time-varying function of the parameters ξ(t)=[θ(t),g0(t),g1(1,t),…,g1(p,t)]. A local maximum-likelihood method [68,69] was used to estimate the unknown time-varying parameter set ξ(t) within a sliding window of W=90 s. We used a Newton–Raphson procedure to maximize the local log-likelihood and compute the local maximum-likelihood estimate of ξ(t) [68,69] within W. Because there is significant overlap between adjacent local likelihood intervals, we started the Newton–Raphson procedure at t with the previous local maximum-likelihood estimate at time t−Δ in which Δ define how much the local likelihood time interval is shifted to compute the next parameter update.
The model goodness-of-fit is based on the Kolmogorov–Smirnov (KS) test and associated KS statistics (see details in [68,69]). Autocorrelation plots were considered to test the independence of the model-transformed intervals [68,69]. Once the order p is determined, the initial model coefficients were estimated by the method of least squares [68,69].
(i). Instantaneous indices of heart rate and heart rate variability
Our framework allows for a quantitative characterization of heartbeat dynamics based on instantaneous time- and frequency-domain estimations. The time-domain characterization is based on the first- and the second-order moments of the underlying probability structure. Namely, given the time-varying parameter set ξ(t), the instantaneous estimates of mean 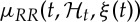 , R–R interval standard deviation
, R–R interval standard deviation 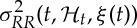 , mean HR
, mean HR 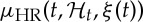 and HR standard deviation
and HR standard deviation 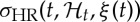 can be derived at each moment in time as follows [68,69]:
can be derived at each moment in time as follows [68,69]:
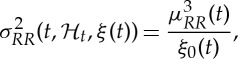 |
2.3 |
| 2.4 |
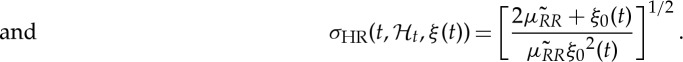 |
2.5 |
The linear power spectrum estimation reveals the linear mechanisms governing the heartbeat dynamics in the frequency domain. In particular, given the model of 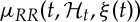 , we can compute the time-varying parametric (linear) autospectrum [68,69] as follows:
, we can compute the time-varying parametric (linear) autospectrum [68,69] as follows:
| 2.6 |
where H1 represents the Fourier transform of the γ1 terms. By integrating equation (2.6) in each frequency band, we compute the indices within the low frequency (LF = 0.04–0.15 Hz) and high frequency (HF = 0.14–0.45 Hz) ranges, along with their ratio (LF/HF).
(d). Maximal information coefficient
In order to quantify the coupling between two variables, we calculated the MIC [66]. This index, in fact, is able to quantify linear and nonlinear couplings occurring between two variables over time, x and y [66]. MIC relies on the fact that, if two variables are somehow coupled, then a grid can be drawn on the scatterplot of the two variables.
Formally, let Gx×y indicate all the possible partitions with x rows and y columns of the scatterplot for the ordered pairs of two vectors, and Ig the mutual information for one specific partition with x×y grids that are applied to the ordered samples of the two vectors.
MIC is defined as the maximal value of mx×y over the ordered pairs (x,y), with x≤n and y≤n, where n is the length of the vectors:
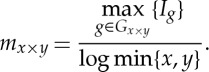 |
2.7 |
In practice, it is possible to compute the estimation as 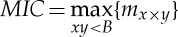 with B empirically defined as B=n0.6 [66].
with B empirically defined as B=n0.6 [66].
(e). Statistical analysis
Statistical analyses were performed on MIC values, considering data gathered from all of the subjects, in order to perform (i) comparison between resting state sessions; (ii) comparison between neutral sessions; (iii) comparison between (whole) arousal sessions; (iv) comparison between pleasant and unpleasant stimuli of each arousal sessions; and (v) comparison between pleasant/unpleasant stimuli among different arousal levels. These statistical comparisons were performed, for each EEG signal, between each pair of time-varying EEG-PSD, calculated in the θ, α, β and γ frequency bands, and each of the time-varying HRV features. All HRV features were instantaneously calculated with a δ=5 ms temporal resolution, and then averaged each within a 1 s sliding time window, in order to achieve temporal correspondence with the time-varying EEG-PSD series.
Before performing the statistical analysis, we implemented the Lilliefors (a KS-based approach) test to check whether the data were normally distributed. As most of the samples taken into account did not show a normal distribution, non-parametric rank-based statistical analyses were carried out. Moreover, central tendency and dispersion of samples were expressed in terms of median and median absolute deviation, respectively. In particular, according to the number of sample groups under comparison, non-parametric Wilcoxon and Friedman tests for paired data were applied, considering the null hypothesis of equal medians among samples. Statistical significance was set considering p-values <0.05. Results from the statistical analysis on MIC values are shown as topographic colourmaps.
3. Results
After applying the EEG preprocessing steps (see §2b), all recordings showed more than 90% of artefact-free epochs. Moreover, among all subjects, up to eight EEG channels were discarded after the ‘bad’ channels identification procedure (on average, five channels were removed).
Concerning the application of the point-process modelling on RR series, excellent results were achieved in terms of goodness-of-fit. To this extent, optimal model order was found to be p=7. KS distances were as low as 0.0328±0.0052 and were never above 0.051. Moreover, the independence test performed through autocorrelation plots was verified for all subjects [69], showing that in 21 out of 22 recordings, more than 98% of the autocorrelation samples fell within 95% CIs. A total of 16 out of 22 recordings showed KS plots within 95% CIs.
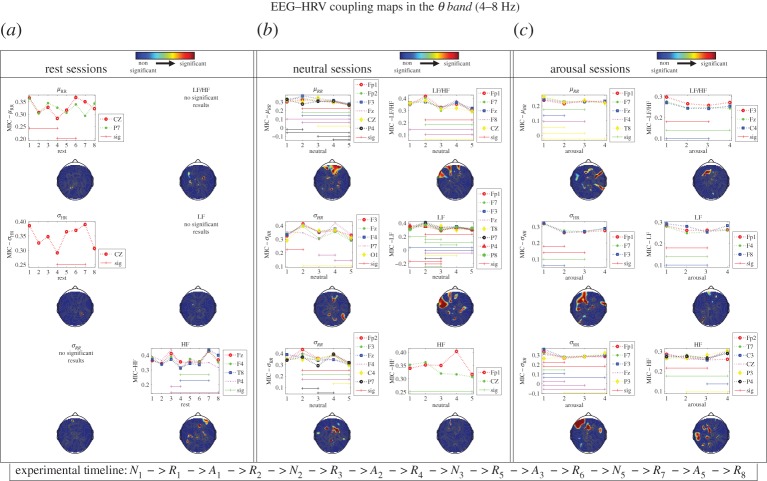
Figure 2 shows p-value topographic maps resulting from the statistical comparison of MIC values between resting-state sessions, neutral sessions and arousal sessions, considering each pair of time-varying HRV features and time-varying EEG-PSD calculated in the θ band. Differences between resting-state sessions were found in coupling with μRR, σHR and HF only. Differences in all of the couplings between EEG θ band and HRV features, instead, were found between all neutral and between all arousing sessions. In particular, major differences between neutral sessions occurred in the prefrontal cortex, mainly due to significant changes occurring in the neutral session 5, whereas major differences between arousal sessions occurred in the prefrontal cortex and parietal lobes, mainly due to significant changes occurring in the intermediate arousal session 3. Note that, in the experimental protocol timeline, arousal level 3 follows neutral session 5. Similar EEG–HRV coupling maps, calculated for the EEG α, β and γ bands, are reported in electronic supplementary material, figures S1–S3. All MIC values, expressed as median and its absolute deviation, gathered during the first 5 min baselines, are reported in electronic supplementary material, tables S1–S4 , for each EEG frequency band. Likewise, MIC values of resting-state sessions are in electronic supplementary material, tables S5–S8, MIC values of arousal sessions in electronic supplementary material, tables S9–S12, and MIC values of neutral sessions in electronic supplementary material, tables S12–S16.
Figure 2.
p-Value topographic maps resulting from the statistical comparison (Friedman non-parametric tests) of MIC values between resting-state sessions (a), neutral sessions (b) and arousal sessions (c), considering each pair of time-varying HRV features and time-varying EEG–PSD calculated in the θ band. Blue regions are associated with no significant difference between sessions, whereas green/yellow/red activations are associated with significant differencesof the brain–heart coupling in at least two of the considered sessions. Each p-value topographic map is reported along plots of significantly different MIC values, and significant differences among sessions. Such significant differences (sig.) result from multiple comparison analysis considering a Bonferroni correction.
(a). Positive versus negative emotion elicitation
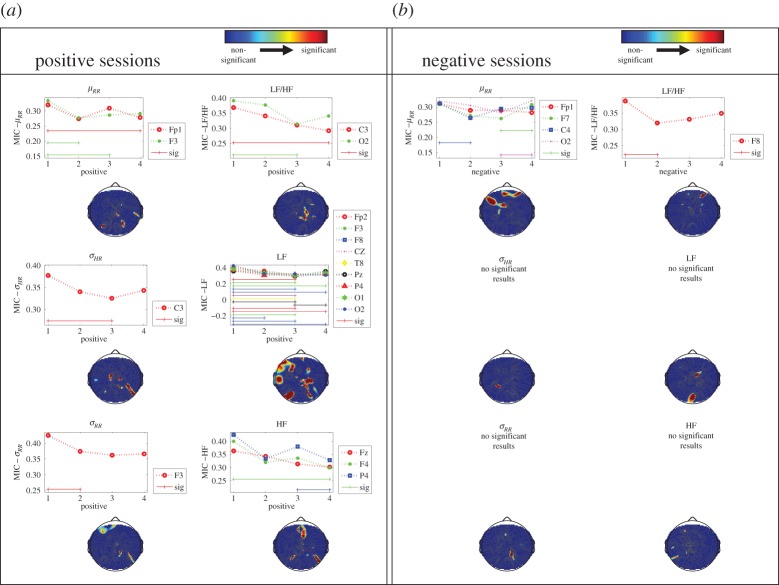
Figure 3 shows p-value topographic maps resulting from the statistical comparison (Friedman non-parametric tests) of MIC values between all of the positive, and all of the negative elicitation sessions, considering each pair of time-varying HRV features and time-varying EEG-PSD calculated in the θ band. Significant arousal-dependent differences were found between positive elicitation sessions, especially affecting the EEG θ–HRV-LF coupling in the left temporal region. This was mainly due to significant changes occurring in the intermediate arousal session 3. Negative elicitation sessions showed arousal-dependent differences, occurring exclusively on the coupling between EEG θ–μRR and EEG θ–HRV LF/HF ratio in the prefrontal cortex region. Similar EEG–HRV coupling maps, calculated for the EEG α, β and γ bands, are reported in the electronic supplementary material, figures S10–S12.
Figure 3.
p-Value topographic maps resulting from the statistical comparison (Friedman non-parametric test) of MIC values between all the positive elicitation sessions (a), and all the negative elicitation sessions (b). Results are shown for each HRV feature and the EEG θ frequency band, considering each arousal level. Blue regions are associated with no significant difference between sessions,whereas green/yellow/red activations are associated with significant differences of the brain–heart coupling in at least one of the considered sessions.
The electronic supplementary material, figures S4–S5, shows p-value topographic maps of MIC values between positive/negative elicitation sessions, across each arousal level, whereas electronic supplementary material, figures S6–S9, show actual MIC values calculated on each pair of time-varying HRV features and time-varying EEG-PSD, calculated in all of the EEG frequency bands, for positive and negative elicitation sessions, among all of the arousal levels
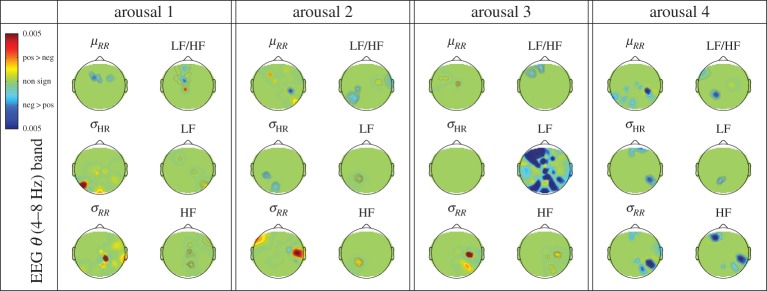
Figure 4 shows, for each arousal level, p-value topographic maps resulting from the statistical comparison (Wilcoxon non-parametric test) of MIC values between positive and negative elicitation. Results show that, at arousal levels 1, 2 and 3, positive emotional pictures increased the brain–heart coupling with respect to the negative ones, as estimated through MIC values between EEG θ power and time-domain features μRR, σRR and σHR, and the parasympathetic component (HF) of HRV. At arousal level 4, brain–heart dynamics switches to opposite MIC trends, i.e. negative emotional pictures increased the brain–heart coupling with respect to the positive ones, as estimated through MIC values between EEG θ power and HRV time-domain features. Moreover, at arousal levels 1, 2 and 3, negative emotional pictures significantly increased the brain–heart coupling with respect to the positive ones as estimated through MIC values between EEG θ power and HRV frequency-domain features. It is noteworthy that the strong effect of negative pictures at arousal level 3 on of MIC values calculated between EEG θ power and HRV-LF. Interestingly, at arousal level 4, such a EEG θ power and HRV–LF coupling seems to switch to EEG θ power and frequency-domain feature HF, though with less spatial activation. Similar EEG–HRV coupling maps, calculated for the EEG α, β and γ bands, are reported in electronic supplementary material, figures S13–S14.
Figure 4.
p-Value topographic maps resulting from the statistical comparison (Wilcoxon non-parametric test) of MIC values between positive and negative elicitation. Results are shown for each HRV feature and the EEG θ frequency band, considering each arousal level. Green regions are associated with no significant difference between positiveand negative elicitations, whereas red/blue activations are associated with significant increase of the brain–heart coupling during the positive/negative elicitation sessions.
4. Discussion and conclusion
We studied brain–heart dynamics using MIC values calculated between time-varying series of EEG power spectra and instantaneous heartbeat dynamics during visual emotional elicitation. Twenty-two healthy volunteers were emotionally elicited through passive viewing of IAPS pictures, covering 25 different combinations of arousal and valence levels. The proposed methodological approach and experimental protocol are, to our knowledge, of great novelty in the current literature because (i) we considered linear and nonlinear brain–heart couplings, (ii) we considered all possible arousal-, valence-dependent brain–heart couplings, and (iii) such coupling measures were calculated with subject-specific, time-varying features. Furthermore, experimental results were performed using high-resolution EEG signals (128 channels). At a group level, results were shown as p-value topographic maps and related multiple comparisons, highlighting brain regions whose activity significantly correlated with heartbeat dynamics.
Importantly, from a methodological point of view, the study of brain–heart dynamics performed on uneven heartbeat samples, taking into account short-time emotional elicitation (less than 10 s), was possible thanks to the use of the point-process paradigm [68]. Through this approach, we obtained instantaneous time domain and spectral cardiovascular estimates, which are known to track ANS changes due to emotional elicitation and mood changes [70,80]. Accordingly, we hypothesized that different emotional stimuli would differently affect ANS–CNS signalling. As a proof of concept of the proposed methodology, we focused on EEG oscillations in the θ band as emotional processes consistently elicit changes in the EEG θ power, regulating ANS responses accordingly [55–65].
As expected, we found that the prefrontal cortex plays a crucial role in brain–heart coupling modulation during visual emotional elicitation. This cortex was involved in the switching mechanisms between neutral and arousing elicitation, especially during negative elicitation sessions. In particular, a strong coupling between prefrontal cortex activity and heartbeat dynamics was found at intermediate arousal (arousal level 3). In such an arousing elicitation, we also found a significant EEG θ–HRV-LF coupling in the left temporal region, especially due to images with negative valence.
Furthermore, MIC differences between negative and positive valence (figure 4), which are visible at the lowest level of arousal (i.e. arousal level 1), changed both in sign and in spatial location at higher levels of arousal (level 2 and 3) and disappear at the highest level of arousal (level 4). Note that this behaviour was consistently found across all EEG frequency bands (see figure 4 and electronic supplementary material, figures S4–S14). This suggests the following conclusion: (i) the assumption of having orthogonal dimensions in the CMA model, as associated with arousal and valence dimensions, has to be reconsidered. This is also in agreement with previous findings suggesting that the effect of emotional valence on affective picture perception is modulated by levels of arousal at both early and late stages of brain processing [81]; (ii) increasing arousing elicitations seems to mitigate, or even to washout, the impact of valence on brain–heart dynamics in response to visual elicitation.
Positive emotions elicited greater MIC than negative ones for the total RR variability (σRR and σHR) and for the HRV parasympathetic component HF across arousal levels 1, 2 and 3. For σRR despite this behaviour (higher brain–heart coupling for positive emotions) is diffuse over the scalp, the topology of significance involves central regions of the right hemisphere. Concerning HRV–HF, MIC significance shifts from left (arousal 2) to right (arousal 3) hemisphere.
Negative emotions elicited a significantly higher brain–heart reaction with respect to the positive ones, as indicated by a higher MIC over large cortical areas for HRV–LF power. This was especially evident between EEG oscillations in θ band, at arousal 3, in the areas related to visuospatial attention. Interestingly, such a strong interaction vanishes at the highest arousal level (arousal 4), being replaced by a stronger (parasympathetic) HF activity associated with EEG θ power increase in left frontal regions and in right parietal regions. Importantly, this patterns of activations seem in line with a classical attentional-bradicardic reaction to hyper-aroused images with negative emotional content [82]. We believe that the vanishing effect hereby observed at high arousing elicitation has to be related to the processing of emotions at a CNS level exclusively. At a ANS level, in fact, through the same experimental protocol, our previous findings confirm arousal-specific patterns of skin conductance [83,84], and HRV and respiratory dynamics [85], allowing for a four-class discrimination of all of the arousing visual elicitations.
Statistically significant differences between sessions of the same type should be linked to the quantification of the effect of (pleasant/unpleasant) arousing stimuli to subsequent neutral elicitations and resting-state sessions. Interestingly, while we found no variations in brain–heart dynamics between resting-state sessions, we found significant differences between neutral sessions. In particular, major differences between neutral sessions occurred in the prefrontal cortex, mainly due to significant changes occurring in the neutral session 5. Note that, in the experimental protocol timeline, arousal level 3 (i.e. the elicitation with higher changes in brain–heart dynamics) follows neutral session 5. In this view, an early/late effect can be associated to an arousing visual elicitation. We also remark that brain–heart coupling, as estimated through our MIC-based method, is significantly lower during non-elicitation states than the ones with elicitations. This can be easily seen from electronic supplementary material, tables S1–S4, showing MIC values calculated during the first 5 min baseline acquisition of the experimental protocol.
It is worthwhile noting that stronger associations of parasympathetic activity with frontal and parietal cortices could be related to the attentional noradrenergic network, which was previously identified in fMRI studies [86]. This network extends to both hemispheres, whose stronger connectivity is derived from the right posterior parietal cortices [86] as indicated in our results. More in general, at a speculation level, we believe that part of our results may be explained by both noradrenergic and dopaminergic signalling modulating the brain–heart coupling, along with their interaction. Indeed, for the dopaminergic system, the nucleus accumbens integrates affective inputs from the amygdala and the prefrontal cortex, and its role in salience processing, signals novelty and contextual deviance has been previously identified [87]. Moreover, prefrontal feedbacks to limbic structures are able to modulate subcortical excitability and, thus, change the brain–heart coupling in different regions. On the other hand, the noradrenergic modulation on prefrontal feedbacks to the limbic output could explain the observed different levels of brain–heart coupling as a function of arousal [88].
Our findings are consistent with Scherer's theory, which argues that synchronization of oscillatory physiological systems is fundamental to emotion [89], and with Porges's polivagal theory [90], which points out how afferent feedback from the heart to the brain through the vagus nerve and nucleus tractus solitarius could play a regulatory role in emotional response.
As this study should be intended is a proof-of-concept of a novel methodology to investigate brain–heart coupling, limitations should also be mentioned. First, although we did check that subjects were actually looking at pictures throughout the experimental protocol, we could not control for attention and habituation effects since the task was a complete passive one. However, although we had a repetition of five neutral sessions throughout the elicitation, we did include different images in such sessions, minimizing the repetition effects on emotional processing. Note that our previous endeavours demonstrate that no significant changes occur in ANS dynamics among neutral IAPS elicitation sessions [91,72]. Moreover, self-assessment scores of elicited IAPS images after the experiment were not taken into account in this study. We relied on the standardization of the IAPS images, which had been performed on a large number of healthy subjects [67], ensuring highly consistent results in terms of valence and arousal ratings. However, we cannot exclude that individual differences in valence and arousal perceptions, with respect to the elicited ones, occurred. Furthermore, we are aware that brain–heart dynamics might be further mediated by psychological factors like mood, anxiety or personality traits that we did not take into account in this study. Future works should account for all these aspects in order to achieve a more detailed and precise brain–heart model, also accounting for the evaluation of brain–heart coupling directionality, as well as for the brain–brain, and ANS–ANS interactions [65]. MIC, in fact, is unable to assess how much the past samples in one series affect its future values or the future of the other time series (like in Granger causality measures). However, is important to point out that these issues has a limited impact on our results. We were interested in assessing the coupling between EEG oscillations and heartbeat dynamics, tracking their changes despite external elicitation. In other words, despite the individual emotional processing of each subject, we were able to identify significant changes between θ oscillations and HRV metrics. The effect of slight difference between the window duration for the estimation of EEG (4 s) and HRV parameters (about 7 s due to the point-process model order p=7) on experimental results should also be investigated. Finally, although in this study we performed a high-resolution EEG recording with 128 channels, our conclusion involving the prefrontal cortex activity have limited physiological interpretation due to limits in the spatial resolution. In future studies, precise electrode localization, paired with individual morphological magnetic resonance imaging, would be acquired in order to have a reliable increase of spatial resolution and thus to perform analysis at a cortical surface levels such as Loreta-based approaches.
To conclude, we demonstrated that a point-to-point linear and nonlinear correlation measure between instantaneous heartbeat dynamics and EEG time-varying spectra may be a feasible method to understand the coupling between ANS and CNS. We suggest that EEG oscillations in the θ band are the most promising metrics to be used in such an evaluation, especially because this band have a significant role in monitoring the attentional significance of emotions [92]. However, it is possible to hypothesize that different EEG bands couple with different aspects of ANS dynamics. As a matter of fact, in the electronic supplementary material, we were able to show distinct pattern of coupling between different EEG bands and heartbeat dynamics, although they were less supported by psychological and neurobiological bases. To this extent, further studies should effectively focus on other specific bands than θ, also including a larger number of subjects in order to take into account other covariates such as personality traits and subjective IAPS rating, which may influence central and peripheral signalling. Moreover, quantification of the exact amount of nonlinear coupling occurring in brain–heart dynamics should also be performed. Further studies should also address whether any early/late phenomena as a response of affective stimuli can be identified in brain–heart dynamics. Moreover, it is important to investigate this coupling in psychiatric as well as neurological disorders which have been shown to involve both cognition and ANS dysfunction [93], and whether its alterations could be a specific marker of mental disorders. According to our findings, we believe that the analysis of EEG θ rhythms will provide a significant decision support tool for managing mental health, also considering the role of insular and pregenual cingulate cortices in psychiatric disorders including mood disorders, panic disorders, obsessive-compulsive disorders, eating disorders and schizophrenia [1,5,46,47,90,94].
Supplementary Material
Supplementary Material
Ethics
The experimental protocol for this study was approved by the ethics committee of the University of Pisa and informed consent was obtained from all participants involved in the experiment.
Competing interests
We declare we have no competing interests.
Funding
The research leading to these results has received funding from the European Commission Horizon 2020 Programme under grant agreement no. 689691 “NEVERMIND”.
References
- 1.Craig A. 2003. Pain mechanisms: labeled lines versus convergence in central processing. Annu. Rev. Neurosci. 26, 1–30. ( 10.1146/annurev.neuro.26.041002.131022) [DOI] [PubMed] [Google Scholar]
- 2.Beissner F, Meissner K, Bär K-J, Napadow V. 2013. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 33, 10 503–10 511. ( 10.1523/JNEUROSCI.1103-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang C, Metzger CD, Glover GH, Duyn JH, Heinze H-J, Walter M. 2013. Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage 68, 93–104. ( 10.1016/j.neuroimage.2012.11.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. 2012. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 36, 747–756. ( 10.1016/j.neubiorev.2011.11.009) [DOI] [PubMed] [Google Scholar]
- 5.Nagai M, Hoshide S, Kario K. 2010. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J. Am. Soc. Hypertens. 4, 174–182. ( 10.1016/j.jash.2010.05.001) [DOI] [PubMed] [Google Scholar]
- 6.Lanata A, Valenza G, Nardelli M, Gentili C, Scilingo EP. 2015. Complexity index from a personalized wearable monitoring system for assessing remission in mental health. IEEE J. Biomed. Health Inform. 19, 132–139. ( 10.1109/JBHI.2014.2360711) [DOI] [PubMed] [Google Scholar]
- 7.Basile B, Bassi A, Calcagnini G, Strano S, Caltagirone C, Macaluso E, Cortelli P, Bozzali M. 2013. Direct stimulation of the autonomic nervous system modulates activity of the brain at rest and when engaged in a cognitive task. Hum. Brain Mapp. 34, 1605–1614. ( 10.1002/hbm.22013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray MA, Minati L, Paoletti G, Critchley HD. 2010. Baroreceptor activation attenuates attentional effects on pain-evoked potentials. Pain 151, 853–861. ( 10.1016/j.pain.2010.09.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riganello F, Garbarino S, Sannita WG. 2015. Heart rate variability, homeostasis, and brain function. J. Psychophysiol. 26, 178–203. ( 10.1027/0269-8803/a000080) [DOI] [Google Scholar]
- 10.Jones K, Amawi F, Bhalla A, Peacock O, Williams J, Lund J. 2015. Assessing surgeon stress when operating using heart rate variability and the state trait anxiety inventory: will surgery be the death of us? Colorectal Dis. 17, 335–341. ( 10.1111/codi.12844) [DOI] [PubMed] [Google Scholar]
- 11.Parker SL, Laurie KR, Newton CJ, Jimmieson NL. 2014. Regulatory focus moderates the relationship between task control and physiological and psychological markers of stress: a work simulation study. Int. J. Psychophysiol. 94, 390–398. ( 10.1016/j.ijpsycho.2014.10.009) [DOI] [PubMed] [Google Scholar]
- 12.Taggart P, Boyett MR, Logantha SJR, Lambiase PD. 2011. Anger, emotion, and arrhythmias: from brain to heart. Front. Physiol. 2, 67 ( 10.3389/fphys.2011.00067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X-L, Zhang Z-G, Ye C-P, Lei Y, Wu L, Zhang Y, Chen Y-Y, Xiao Z-J. 2015. Attenuated or absent hrv response to postural change in subjects with primary insomnia. Physiol. Behav. 140, 127–131. ( 10.1016/j.physbeh.2014.12.018) [DOI] [PubMed] [Google Scholar]
- 14.Maes J. et al. 2014. Sleep misperception, EEG characteristics and autonomic nervous system activity in primary insomnia: a retrospective study on polysomnographic data. Int. J. Psychophysiol. 91, 163–171. ( 10.1016/j.ijpsycho.2013.10.012) [DOI] [PubMed] [Google Scholar]
- 15.Calandra-Buonaura G. et al. 2012. Physiologic autonomic arousal heralds motor manifestations of seizures in nocturnal frontal lobe epilepsy: implications for pathophysiology. Sleep Med. 13, 252–262. ( 10.1016/j.sleep.2011.11.007) [DOI] [PubMed] [Google Scholar]
- 16.Romigi A. et al. In press Heart rate variability in untreated newly diagnosed temporal lobe epilepsy: evidence for ictal sympathetic dysregulation. Epilepsia. ( 10.1111/epi.13309) [DOI] [PubMed] [Google Scholar]
- 17.Valenza G. et al. 2016. Assessment of spontaneous cardiovascular oscillations in parkinson's disease. Biomed. Signal Process. Control 26, 80–89. ( 10.1016/j.bspc.2015.12.001) [DOI] [Google Scholar]
- 18.Tessa C. et al. 2014. Progression of brain atrophy in the early stages of parkinson's disease: a longitudinal tensor-based morphometry study in de novo patients without cognitive impairment. Hum. Brain Mapp. 35, 3932–3944. ( 10.1002/hbm.22449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvioli B, Pellegatta G, Malacarne M, Pace F, Malesci A, Pagani M, Lucini D. 2015. Autonomic nervous system dysregulation in irritable bowel syndrome. Neurogastroenterol. Motil. 27, 423–430. ( 10.1111/nmo.12512) [DOI] [PubMed] [Google Scholar]
- 20.Akar SA, Kara S, Latifoğlu F, Bilgiç V. 2015. Analysis of heart rate variability during auditory stimulation periods in patients with schizophrenia. J. Clin. Monit. Comput. 29, 153–162. ( 10.1007/s10877-014-9580-8) [DOI] [PubMed] [Google Scholar]
- 21.Moon E, Lee S-H, Kim D-H, Hwang B. 2013. Comparative study of heart rate variability in patients with schizophrenia, bipolar disorder, post-traumatic stress disorder, or major depressive disorder. Clin. Psychopharmacol. Neurosci. 11, 137–143. ( 10.9758/cpn.2013.11.3.137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clamor A, Hartmann MM, Köther U, Otte C, Moritz S, Lincoln TM. 2014. Altered autonomic arousal in psychosis: an analysis of vulnerability and specificity. Schizophr. Res. 154, 73–78. ( 10.1016/j.schres.2014.02.006) [DOI] [PubMed] [Google Scholar]
- 23.Kemp AH, Quintana DS, Quinn CR, Hopkinson P, Harris AW. 2014. Major depressive disorder with melancholia displays robust alterations in resting state heart rate and its variability: implications for future morbidity and mortality. Front. Psychol. 5, 1387 ( 10.3389/fpsyg.2014.01387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Gonzalez MA, Guzik P, May RW, Koutnik AP, Hughes R, Muniz S, Kabbaj M, Fincham FD. 2014. Trait anxiety mimics age-related cardiovascular autonomic modulation in young adults. J. Hum. Hypertens 29, 274–280. ( 10.1038/jhh.2014.72) [DOI] [PubMed] [Google Scholar]
- 25.Berry SM, Broglio K, Bunker M, Jayewardene A, Olin B, Rush AJ. 2013. A patient-level meta-analysis of studies evaluating vagus nerve stimulation therapy for treatment-resistant depression. Med. Dev. 6, 17–35. ( 10.2147/MDER.S41017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin J, Martin-Sanchez E. 2012. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur. Psychiatry 27, 147–155. ( 10.1016/j.eurpsy.2011.07.006) [DOI] [PubMed] [Google Scholar]
- 27.Lehrer PM, Gevirtz R. 2014. Heart rate variability biofeedback: how and why does it work? Front. Psychol. 5, 756 ( 10.3389/fpsyg.2014.00756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karavidas MK, Lehrer PM, Vaschillo E, Vaschillo B, Marin H, Buyske S, Malinovsky I, Radvanski D, Hassett A. 2007. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl. Psychophysiol. Biofeedback 32, 19–30. ( 10.1007/s10484-006-9029-z) [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Padial E, Vila J, Thayer JF. 2011. The effect of conscious and non-conscious presentation of biologically relevant emotion pictures on emotion modulated startle and phasic heart rate. Int. J. Psychophysiol. 79, 341–346. ( 10.1016/j.ijpsycho.2010.12.001) [DOI] [PubMed] [Google Scholar]
- 30.Coan JA, Allen JJ. 2007. Handbook of emotion elicitation and assessment. Oxford, UK: Oxford University Press. [Google Scholar]
- 31.Calvo R, D'Mello S. 2010. Affect detection: an interdisciplinary review of models, methods, and their applications. IEEE Trans. Affect. Comput. 1, 18–37. ( 10.1109/T-AFFC.2010.1) [DOI] [Google Scholar]
- 32.Kreibig SD. 2010. Autonomic nervous system activity in emotion: a review. Biol. Psychol. 84, 394–421. ( 10.1016/j.biopsycho.2010.03.010) [DOI] [PubMed] [Google Scholar]
- 33.Makovac E. et al. 2015. Effect of parasympathetic stimulation on brain activity during appraisal of fearful expressions. Neuropsychopharmacology 40, 1649–1658. ( 10.1038/npp.2015.10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Critchley HD, Rotshtein P, Nagai Y, O'Doherty J, Mathias CJ, Dolan RJ. 2005. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage 24, 751–762. ( 10.1016/j.neuroimage.2004.10.013) [DOI] [PubMed] [Google Scholar]
- 35.Goldfinger JZ, Nair A, Sealove BA. 2013. Brain-heart interaction in takotsubo cardiomyopathy. Heart Failure Clin. 9, 217–223. ( 10.1016/j.hfc.2012.12.013) [DOI] [PubMed] [Google Scholar]
- 36.Smith R, Allen JJ, Thayer JF, Lane RD. 2015. Altered functional connectivity between medial prefrontal cortex and the inferior brainstem in major depression during appraisal of subjective emotional responses: a preliminary study. Biol. Psychol. 108, 13–24. ( 10.1016/j.biopsycho.2015.03.007) [DOI] [PubMed] [Google Scholar]
- 37.Damasio AR. 1998. Emotion in the perspective of an integrated nervous system. Brain Res. Rev. 26, 83–86. ( 10.1016/S0165-0173(97)00064-7) [DOI] [PubMed] [Google Scholar]
- 38.Panksepp J. 1998. Affective neuroscience: the foundations of human and animal emotions. Oxford, UK: Oxford University Press. [Google Scholar]
- 39.Lang PJ, Bradley MM, Cuthbert BN. 1998. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol. Psychiatry 44, 1248–1263. ( 10.1016/S0006-3223(98)00275-3) [DOI] [PubMed] [Google Scholar]
- 40.Posner J, Russell JA, Peterson BS. 2005. The circumplex model of affect: an integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev. Psychopathol. 17, 715–734. ( 10.1017/S0954579405050340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim C, Suri J. 2006. Heart rate variability: a review. Med. Biol. Eng. Comput. 44, 1031–1051. ( 10.1007/s11517-006-0119-0) [DOI] [PubMed] [Google Scholar]
- 42.Henry BL, Minassian A, Paulus MP, Geyer MA, Perry W. 2010. Heart rate variability in bipolar mania and schizophrenia. J. Psychiatr. Res. 44, 168–176. ( 10.1016/j.jpsychires.2009.07.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saul J, Berger R, Albrecht P, Stein S, Chen M, Cohen R. 1991. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am. J. Physiol. Heart Circ. Physiol. 261, H1231. [DOI] [PubMed] [Google Scholar]
- 44.Sebastiani L, Simoni A, Gemignani A, Ghelarducci B, Santarcangelo E. 2003. Autonomic and eeg correlates of emotional imagery in subjects with different hypnotic susceptibility. Brain Res. Bull. 60, 151–160. ( 10.1016/S0361-9230(03)00025-X) [DOI] [PubMed] [Google Scholar]
- 45.Martini N, Menicucci D, Sebastiani L, Bedini R, Pingitore A, Vanello N, Milanesi M, Landini L, Gemignani A. 2012. The dynamics of eeg gamma responses to unpleasant visual stimuli: from local activity to functional connectivity. NeuroImage 60, 922–932. ( 10.1016/j.neuroimage.2012.01.060) [DOI] [PubMed] [Google Scholar]
- 46.Thayer JF, Lane RD. 2009. Claude bernard and the heart–brain connection: further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 33, 81–88. ( 10.1016/j.neubiorev.2008.08.004) [DOI] [PubMed] [Google Scholar]
- 47.Hagemann D, Waldstein SR, Thayer JF. 2003. Central and autonomic nervous system integration in emotion. Brain Cogn. 52, 79–87. ( 10.1016/S0278-2626(03)00011-3) [DOI] [PubMed] [Google Scholar]
- 48.Chiu H-C, Lin Y-H, Lo M-T, Tang S-C, Wang T-D, Lu H-C, Ho Y-L, Ma H-P, Peng C-K. 2015. Complexity of cardiac signals for predicting changes in alpha-waves after stress in patients undergoing cardiac catheterization. Sci. Rep. 5, 13315 ( 10.1038/srep13315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagendra H, Kumar V, Mukherjee S. 2015. Cognitive behavior evaluation based on physiological parameters among young healthy subjects with yoga as intervention. Comput. Math. Methods Med. 2015, 821061 ( 10.1155/2015/821061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Triggiani AI. et al. In press Resting state rolandic mu rhythms are related to activity of sympathetic component of autonomic nervous system in healthy humans. Int. J. Psychophysiol. ( 10.1016/j.ijpsycho.2015.02.009) [DOI] [PubMed] [Google Scholar]
- 51.Simor P, Körmendi J, Horváth K, Gombos F, Ujma PP, Bódizs R. 2014. Electroencephalographic and autonomic alterations in subjects with frequent nightmares during pre-and post-rem periods. Brain Cogn. 91, 62–70. ( 10.1016/j.bandc.2014.08.004) [DOI] [PubMed] [Google Scholar]
- 52.Papousek I, Weiss EM, Schulter G, Fink A, Reiser EM, Lackner HK. 2014. Prefrontal eeg alpha asymmetry changes while observing disaster happening to other people: cardiac correlates and prediction of emotional impact. Biol. Psychol. 103, 184–194. ( 10.1016/j.biopsycho.2014.09.001) [DOI] [PubMed] [Google Scholar]
- 53.Amin HU, Malik AS, Subhani AR, Badruddin N, Chooi W-T. 2013. Dynamics of scalp potential and autonomic nerve activity during intelligence test. In Neural information processing (eds M Lee, A Hirose, Z Hou, RM Kil), pp. 9–16. Berlin, Germany: Springer.
- 54.Vecchiato G, Astolfi L, Fallani FDV, Cincotti F, Mattia D, Salinari S, Soranzo R, Babiloni F. 2010. Changes in brain activity during the observation of tv commercials by using eeg, gsr and hr measurements. Brain Topogr. 23, 165–179. ( 10.1007/s10548-009-0127-0) [DOI] [PubMed] [Google Scholar]
- 55.Liou L-M, Ruge D, Kuo M-C, Tsai J-C, Lin C-W, Wu M-N, Hsu C-Y, Lai C-L. 2014. Functional connectivity between parietal cortex and the cardiac autonomic system in uremics. Kaohsiung J. Med. Sci. 30, 125–132. ( 10.1016/j.kjms.2013.11.001) [DOI] [PubMed] [Google Scholar]
- 56.Aftanas L, Golosheykin S. 2005. Impact of regular meditation practice on eeg activity at rest and during evoked negative emotions. Int. J. Neurosci. 115, 893–909. ( 10.1080/00207450590897969) [DOI] [PubMed] [Google Scholar]
- 57.Aftanas L, Varlamov A, Pavlov S, Makhnev V, Reva N. 2001. Affective picture processing: event-related synchronization within individually defined human theta band is modulated by valence dimension. Neurosci. Lett. 303, 115–118. ( 10.1016/S0304-3940(01)01703-7) [DOI] [PubMed] [Google Scholar]
- 58.Bekkedal MY, Rossi J, Panksepp J. 2011. Human brain eeg indices of emotions: delineating responses to affective vocalizations by measuring frontal theta event-related synchronization. Neurosci. Biobehav. Rev. 35, 1959–1970. ( 10.1016/j.neubiorev.2011.05.001) [DOI] [PubMed] [Google Scholar]
- 59.Craig A, Tran Y, Wijesuriya N, Nguyen H. 2012. Regional brain wave activity changes associated with fatigue. Psychophysiology 49, 574–582. ( 10.1111/j.1469-8986.2011.01329.x) [DOI] [PubMed] [Google Scholar]
- 60.Knyazev GG, Barchard KA, Razumnikova OM, Mitrofanova LG. 2012. The relationship of positive and negative expressiveness to the processing of emotion information. Scand. J. Psychol. 53, 206–215. ( 10.1111/j.1467-9450.2012.00941.x) [DOI] [PubMed] [Google Scholar]
- 61.Sammler D, Grigutsch M, Fritz T, Koelsch S. 2007. Music and emotion: electrophysiological correlates of the processing of pleasant and unpleasant music. Psychophysiology 44, 293–304. ( 10.1111/j.1469-8986.2007.00497.x) [DOI] [PubMed] [Google Scholar]
- 62.Balconi M, Grippa E, Vanutelli ME. 2015. What hemodynamic (fnirs), electrophysiological (eeg) and autonomic integrated measures can tell us about emotional processing. Brain Cogn. 95, 67–76. ( 10.1016/j.bandc.2015.02.001) [DOI] [PubMed] [Google Scholar]
- 63.Tang Y-Y. et al. 2009. Central and autonomic nervous system interaction is altered by short-term meditation. Proc. Natl Acad. Sci. USA 106, 8865–8870. ( 10.1073/pnas.0904031106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prinsloo GE, Rauch HL, Karpul D, Derman WE. 2013. The effect of a single session of short duration heart rate variability biofeedback on eeg: a pilot study. Appl. Psychophysiol. Biofeedback 38, 45–56. ( 10.1007/s10484-012-9207-0) [DOI] [PubMed] [Google Scholar]
- 65.Faes L, Marinazzo D, Jurysta F, Nollo G. 2015. Linear and non-linear brain–heart and brain–brain interactions during sleep. Physiol. Measure. 36, 683–698. ( 10.1088/0967-3334/36/4/683) [DOI] [PubMed] [Google Scholar]
- 66.Reshef DN, Reshef YA, Finucane HK, Grossman SR, McVean G, Turnbaugh PJ, Lander ES, Mitzenmacher M, Sabeti PC. 2011. Detecting novel associations in large data sets. Science 334, 1518–1524. ( 10.1126/science.1205438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lang P, Bradley MM. 2007. The International Affective Picture System (IAPS) in the study of emotion and attention. Handb. Emotion Elicitation Assess. 29, 29–46. [Google Scholar]
- 68.Barbieri R, Matten E, Alabi A, Brown E. 2005. A point-process model of human heartbeat intervals: new definitions of heart rate and heart rate variability. Am. J. Physiol. Heart Circ. Physiol. 288, H424. [DOI] [PubMed] [Google Scholar]
- 69.Valenza G, Citi L, Scilingo EP, Barbieri R. 2013. Point-process nonlinear models with laguerre and volterra expansions: instantaneous assessment of heartbeat dynamics. IEEE Trans. Signal Process. 61, 2914–2926. ( 10.1109/TSP.2013.2253775) [DOI] [Google Scholar]
- 70.Valenza G, Citi L, Lanatá A, Scilingo EP, Barbieri R. 2014. Revealing real-time emotional responses: a personalized assessment based on heartbeat dynamics. Sci. Rep. 4, 4998 ( 10.1038/srep04998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kroenke K, Spitzer RL, Williams JB. 2001. The phq-9. J. Gen. Intern. Med. 16, 606–613. ( 10.1046/j.1525-1497.2001.016009606.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lanata A, Valenza G, Scilingo EP. 2013. Eye gaze patterns in emotional pictures. J. Ambient Intell. Humanized Comput. 4, 705–715. ( 10.1007/s12652-012-0147-6) [DOI] [Google Scholar]
- 73.Delorme A, Makeig S. 2004. Eeglab: an open source toolbox for analysis of single-trial eeg dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. ( 10.1016/j.jneumeth.2003.10.009) [DOI] [PubMed] [Google Scholar]
- 74.Theiler J. 1994. Two tools to test time series data for evidence of chaos and/or nonlinearity. Integr. Physiol. Behav. Sci. 29, 211–216. ( 10.1007/BF02691326) [DOI] [PubMed] [Google Scholar]
- 75.Artoni F, Chisari C, Menicucci D, Fanciullacci C, Micera S. 2012. Remov: Eeg artifacts removal methods during lokomat lower-limb rehabilitation. In Proc. (BioRob), 4th IEEE RAS and EMBS Int. Conf. on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June, pp. 992–997. IEEE.
- 76.Pan J, Tompkins WJ. 1985. A real-time qrs detection algorithm. IEEE Trans. Biomed. Eng. 3, 230–236. ( 10.1109/TBME.1985.325532) [DOI] [PubMed] [Google Scholar]
- 77.Citi L, Brown EN, Barbieri R. 2012. A real-time automated point-process method for the detection and correction of erroneous and ectopic heartbeats. IEEE Trans. Biomed. Eng. 59, 2828–2837. ( 10.1109/TBME.2012.2211356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valenza G, Citi L, Scilingo EP, Barbieri R. 2014. Inhomogeneous point-process entropy: an instantaneous measure of complexity in discrete systems. Phys. Rev. E 89, 052803 ( 10.1103/PhysRevE.89.052803) [DOI] [PubMed] [Google Scholar]
- 79.Valenza G, Citi L, Barbieri R. 2014. Estimation of instantaneous complex dynamics through lyapunov exponents: a study on heartbeat dynamics. PLoS ONE 9, e105622 ( 10.1371/journal.pone.0105622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valenza G, Citi L, Gentili C, Lanata A, Scilingo EP, Barbieri R. 2015. Characterization of depressive states in bipolar patients using wearable textile technology and instantaneous heart rate variability assessment. IEEE J. Biomed. Health Inform. 19, 263–274. ( 10.1109/JBHI.2014.2307584) [DOI] [PubMed] [Google Scholar]
- 81.Feng C, Li W, Tian T, Luo Y, Gu R, Zhou C, Luo Y-J. 2014. Arousal modulates valence effects on both early and late stages of affective picture processing in a passive viewing task. Soc. Neurosci. 9, 364–377. ( 10.1080/17470919.2014.896827) [DOI] [PubMed] [Google Scholar]
- 82.Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. 2001. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion 1, 276–298. ( 10.1037/1528-3542.1.3.276) [DOI] [PubMed] [Google Scholar]
- 83.Greco A, Valenza G, Lanata A, Scilingo EP, Citi L. In press cvxEDA: a convex optimization approach to electrodermal activity processing. IEEE Trans. Biomed. Eng. ( 10.1109/TBME.2015.2474131) [DOI] [PubMed] [Google Scholar]
- 84.Greco A, Valenza G, Lanata A, Rota G, Scilingo EP. 2014. Electrodermal activity in bipolar patients during affective elicitation. IEEE J. Biomed. Health Inform. 18, 1865–1873. ( 10.1109/JBHI.2014.2300940) [DOI] [PubMed] [Google Scholar]
- 85.Valenza G, Lanatá A, Scilingo EP. 2013. Improving emotion recognition systems by embedding cardiorespiratory coupling. Physiol. Meas. 34, 449–464. ( 10.1088/0967-3334/34/4/449) [DOI] [PubMed] [Google Scholar]
- 86.Büchel C, Friston K. 1997. Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modelling and fMRI. Cereb. Cortex 7, 768–778. ( 10.1093/cercor/7.8.768) [DOI] [PubMed] [Google Scholar]
- 87.Zaehle T, Bauch EM, Hinrichs H, Schmitt FC, Voges J, Heinze H-J, Bunzeck N. 2013. Nucleus accumbens activity dissociates different forms of salience: evidence from human intracranial recordings. J. Neurosci. 33, 8764–8771. ( 10.1523/JNEUROSCI.5276-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coull J, Büchel C, Friston K, Frith C. 1999. Noradrenergically mediated plasticity in a human attentional neuronal network. Neuroimage 10, 705–715. ( 10.1006/nimg.1999.0513) [DOI] [PubMed] [Google Scholar]
- 89.Grandjean D, Sander D, Scherer KR. 2008. Conscious emotional experience emerges as a function of multilevel, appraisal-driven response synchronization. Conscious. Cogn. 17, 484–495. ( 10.1016/j.concog.2008.03.019) [DOI] [PubMed] [Google Scholar]
- 90.Porges SW. 2007. The polyvagal perspective. Biol. Psychol. 74, 116–143. ( 10.1016/j.biopsycho.2006.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Valenza G, Citi L, Gentili C, Lanatá A, Scilingo E, Barbieri R. 2014. Point-process nonlinear autonomic assessment of depressive states in bipolar patients. Methods Inform. Med. 53, 296–302. ( 10.3414/ME13-02-0036) [DOI] [PubMed] [Google Scholar]
- 92.Balconi M, Brambilla E, Falbo L. 2009. Bis/bas, cortical oscillations and coherence in response to emotional cues. Brain Res. Bull. 80, 151–157. ( 10.1016/j.brainresbull.2009.07.001) [DOI] [PubMed] [Google Scholar]
- 93.Passamonti L, Salsone M, Toschi N, Cerasa A, Giannelli M, Chiriaco C, Cascini GL, Fera F, Quattrone A. 2013. Dopamine-transporter levels drive striatal responses to apomorphine in parkinson's disease. Brain Behav. 3, 249–262. ( 10.1002/brb3.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valenza G, Nardelli M, Bertschy G, Lanata A, Scilingo E. 2014. Mood states modulate complexity in heartbeat dynamics: a multiscale entropy analysis. Europhys. Lett. 107, 18003 ( 10.1209/0295-5075/107/18003) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.