Abstract
Magnetic resonance imaging (MRI) at ultra-high field (UHF) strengths (7 T and above) offers unique opportunities for studying the human brain with increased spatial resolution, contrast and sensitivity. However, its reliability can be compromised by factors such as head motion, image distortion and non-neural fluctuations of the functional MRI signal. The objective of this review is to provide a critical discussion of the advantages and trade-offs associated with UHF imaging, focusing on the application to studying brain–heart interactions. We describe how UHF MRI may provide contrast and resolution benefits for measuring neural activity of regions involved in the control and mediation of autonomic processes, and in delineating such regions based on anatomical MRI contrast. Limitations arising from confounding signals are discussed, including challenges with distinguishing non-neural physiological effects from the neural signals of interest that reflect cardiorespiratory function. We also consider how recently developed data analysis techniques may be applied to high-field imaging data to uncover novel information about brain–heart interactions.
Keywords: functional magnetic resonance imaging, high-field imaging, high resolution, brain–heart
1. Introduction
Magnetic resonance imaging (MRI) has become the primary technique for investigating the macroscopic, systems-level structure and function of the living human brain. As the sensitivity of MRI to detecting neural activity improves with increasing field strength [1], there has been a continual movement towards higher-field imaging. Developments in ‘ultra-high-field’ MRI (UHF, referring to static magnetic fields of 7 T and above) have provided substantial gains in the spatial resolution and localization of neural activity, facilitating investigation of fine-scale cortical structure at the level of columns and layers, and of substructure in subcortical grey matter regions such as the basal ganglia and the thalamus (e.g. [2–4]). Throughout the past decade, high-field MRI has paved the way for novel discoveries and applications in multiple domains of human neuroscience (reviewed in [5–9]).
Interactions between the heart and the brain are vital for maintaining homeostasis and survival in an ever-changing environment. Moreover, abnormalities in cardiovascular function and other aspects of the autonomic nervous system are reciprocally linked with numerous brain disorders (e.g. [10–14]; see also [15]). While current knowledge about the pathways underpinning brain–heart interactions is based primarily on studies in the anaesthetized animal [16,17], much is unknown about its circuitry in the human. The interaction between cardiac activity and attentional, cognitive and emotional processes (e.g. [18–20]) is also rich ground for discovery, and there is growing recognition of the concept that the brain may best be studied in the context of its unification with the rest of the body (e.g. [20,21]; also [22]). Methodologically, the scope of research on brain–heart interactions in the human has to date been limited by the difficulty of non-invasively localizing and measuring the neural activity of key subcortical structures, and in modelling their temporal interactions with the rest of the brain. A number of key nuclei of the brainstem and forebrain cannot be adequately resolved with positron emission tomography (PET) or conventional functional magnetic resonance imaging (fMRI) protocols because of their limited spatial resolution [23].
However, recent advances in neuroimaging technology and data analysis are showing promise in surmounting these barriers. In particular, UHF MRI appears well suited to applications in autonomic neuroscience, including the investigation of brain–heart interactions. The improvement in spatial localization at UHF may better enable the study of small structures involved in the control and mediation of cardiac activity, along with their interactions within distributed brain networks that facilitate adaptive modulation to environmental and internal demands. While limitations of fMRI at presently ‘standard’ field strengths of 3 T and 1.5 T effectively restrict spatial resolution to above 2×2×2 mm3 (with voxel dimensions of 2.5–4 mm being typical of whole-brain acquisitions), field strengths of 7 T and above—in conjunction with progress in hardware and accelerated imaging methods—are permitting voxel sizes of 1 mm3 and below. Accordingly, as discussed below, data acquired at high field allows for differentiating between sub-regions of brainstem, amygdala, thalamus and other substrates of autonomic function. UHF MRI also has substantial advantages for the study of brain anatomy: large increases in contrast with susceptibility-weighted imaging at UHF have allowed structural resolution to approach 200 μm [4] which, in combination with fMRI, can help to better characterize the regions involved in brain–heart interactions. Yet, the increasing severity of spatial and temporal artefacts with increased field strengths (see below) necessitates careful treatment as well as continued methodological development in both data acquisition and post-processing aspects.
This article offers a review and perspective regarding the potential opportunities of high-field MRI (focusing on fMRI) in the study of brain–heart interactions, and outlines several major accompanying technological and physiological limitations. We also discuss recent and future directions for which high-field imaging data can be used in combination with novel data analysis techniques for probing reciprocal relationships between neural dynamics and cardiac activity. As our primary aim is to motivate the role of high-field imaging in studying brain–heart interactions, we refer the reader to review articles such as [5,6,24] for further information about high-field MRI physics and image acquisition.
2. Functional magnetic resonance imaging at ultra-high field
fMRI [25–27] is a method for non-invasively measuring changes in brain activity over time (see [28] for a recent review). A time series of images is acquired wherein the fluctuation in intensity at a voxel over time indirectly reflects the dynamics of the local neural activity. The vast majority of fMRI studies are based on the blood-oxygen-level-dependent (BOLD) effect [29], by which changes in neural activity are coupled with local changes in blood flow and blood oxygenation. As the magnetic properties of oxygenated versus deoxygenated blood differ, this haemodynamic response to neural activity is detectable by MRI. The spatial and temporal scales (millimetres and seconds, respectively) of BOLD fMRI are too coarse to fully capture the breadth of information contained in neuronal activity; rather, it is limited to detecting slow changes in activity of large neuronal assemblies, and hence sophisticated experimental design and data analysis are required to optimally study brain function with fMRI.
The functional involvement of a brain region can be examined by contrasting its time course of relative signal intensity in response to two or more well-controlled experimental conditions. More recently, spontaneous fluctuations in the fMRI signal, i.e. those that occur in the absence of (or seemingly independently of) explicitly presented stimuli and behaviour, have also been widely investigated and found to reveal topographies of distributed neurocognitive networks [30]. In both cases, the sensitivity of fMRI to detecting changes in neural activity depends on the ratio of contrast to noise (contrast-to-noise ratio, CNR). ‘Contrast’ reflects the magnitude of the fMRI signal change caused by neural activity, and ‘noise’ refers to temporal signal changes caused by other processes, often called ‘temporal noise’. Temporal noise contains contributions from the intrinsic MRI noise in each image (also called ‘thermal noise’) as well as structured fluctuations that are not related to neural activity, including head motion, instrumental instabilities and physiological (cardiac and respiratory) processes. In general, both BOLD contrast and physiological noise increase with the square of the field strength, while thermal noise increases linearly with field strength. Hence, CNR increases with field strength, though the gains are limited under conditions where there is a strong contribution from physiological noise [31]. The spatial specificity of BOLD contrast to neural activity also improves with increasing field strength, as the contribution from neural tissue increases at a faster rate than that of large vessels [1].
(a). Spatial resolution
Increases in CNR can be harnessed to image at higher spatial resolution, which is one of the main advantages of high-field MRI. The exact dependence of CNR on field strength (and on resolution) is complicated. For example, when neural activity produces BOLD contrast over a spatial extent greater than the voxel size, reducing the volume of the voxel will lead to a proportional decrease in contrast; however, when the spatial extent of contrast is smaller than the voxel size, reducing the voxel volume may not lead to appreciable reduction in contrast. In addition, while thermal noise is largely independent of voxel size, noise from physiological processes, subject motion and instrumental instabilities scale up with the signal level, and therefore will decrease when the voxel size is reduced (again, provided that the spatial extent of the noise source exceeds the voxel size) [32]. Thus, it is recommended that one decrease the voxel size until thermal noise is the dominant noise source. In practice, for voxel sizes of 1 mm3 and below, thermal noise will be the dominant noise source and the spatial extent of the functional contrast will often exceed the voxel size. Further reductions in voxel volume will lead to proportional (rapid) reductions in CNR.
In summary, the increased contrast at high field allows one to reduce the voxel size. At a given voxel size, the benefits of high field are limited by non-thermal noise that is due to sources including physiological processes and head motion. In addition to reducing the voxel size such that thermal noise dominates, isolating and modelling structured physiological noise may further improve the CNR and is an active area of research (see §4).
(b). Spatial specificity and BOLD contrast mechanism
Importantly, imaging at higher spatial resolution does not necessarily improve the spatial accuracy of measuring neural activity. In addition to head movement and within-brain pulsatility, which are of greater concern as one moves to higher resolution, BOLD contrast arises from vascular sources that may be somewhat distant from the active neural tissue. This is particularly the case for the larger (pial) veins, which may contain blood draining from an active site several millimetres away [33]. In this regard, a notable advantage of UHF fMRI is the inherent increase in the signal contribution of small vessels/capillaries close to the site of neural activity. Briefly, increasing the field strength changes the degree to which different vascular compartments contribute to BOLD contrast: the intravascular (blood) contribution decreases (due to the substantial reduction in the T2* of blood), but the extravascular contribution increases linearly near large vessels and quadratically near capillaries [34]. While the extravascular contribution near large vessels can still compromise spatial specificity, it is possible to suppress its contribution by performing a spin-echo acquisition (though at the expense of sensitivity and increased power deposition) [35] or by identifying them based on their appearance and location [36]. We note that, while BOLD fMRI contrast benefits substantially from UHF, this is not the case for all MRI contrasts. For example, the gains are much decreased with diffusion-weighted contrast due to the accelerated T2 relaxation at high field.
(c). Susceptibility artefacts and distortion
A potential drawback of UHF stems from magnetic field inhomogeneities caused by tissue interfaces with air and bone. Such inhomogeneities increase linearly with field strength and may cause image distortion as well as signal loss due to intra-voxel dephasing, also referred to as ‘susceptibility artefacts’. Improvements in accelerated imaging techniques and distortion-correction methods have been (and will continue to be) critical for minimizing these artefacts [5,7,37]. In typical fMRI studies, shimming cannot fully compensate for localized field inhomogeneities from susceptibility differences; this might only be possible for very small fields of view. Spin-echo methods, which suffer less from susceptibility-related signal loss compared to gradient-echo methods, benefit markedly at 7 T over 3 T due to the aforementioned changes in the BOLD contrast mechanism as well as from the increased CNR [35]. However, despite its gains at UHF, spin-echo techniques have reduced sensitivity and also result in greater RF power deposition and resulting tissue heating, limiting the extent of volume coverage ([38] and see [39] for a review).
(d). Applications of high-resolution functional magnetic resonance imaging at ultra-high field
Capitalizing on recent advances in UHF imaging, a number of studies have demonstrated the ability to map features of neural organization with exquisite spatial detail. fMRI studies with in-plane resolutions of 1 mm and below have revealed columnar structure [2,40] and layer-dependent specialization in cortex [3,41–43], tonotopic organization of the inferior colliculus [44] and benefits for characterizing networks at the whole-brain level [45].
3. Opportunities for ultra-high field functional magnetic resonance imaging in studying brain–heart interactions
Knowledge about the neurocircuitry underlying brain–heart interactions has primarily been established through invasive studies in anaesthetized animals [17,46,47]. More recently, non-invasive imaging techniques such as PET and fMRI have enabled further insight into central autonomic processing in the human, and in the context of higher-order emotional and cognitive responses [18,48,49]. The improved functional specificity of fMRI at higher field strengths is opening further opportunities for understanding the substrates and pathways of brain–heart interactions; many of the structures implicated in cardiovascular control and other autonomic functions consist of small-volume nuclei in the brainstem, as well as sub-regions of subcortical structures including hypothalamus, thalamus and amygdala, that cannot be adequately resolved at conventional field strengths and could benefit greatly from the increased resolution available at higher field (figure 1). Yet, critically, these regions are prone to susceptibility artefacts and physiological noise due to their locations near air–tissue boundaries and large vessels, limiting potential gains in functional contrast and requiring careful attention to acquisition and post-processing techniques. Below, we discuss findings from several domains of neuroscience that exemplify the promise of high-field fMRI in studying brain regions reported to be common to, or of similar size to and imaging difficulty as, structures likely implicated in human brain–heart interactions.
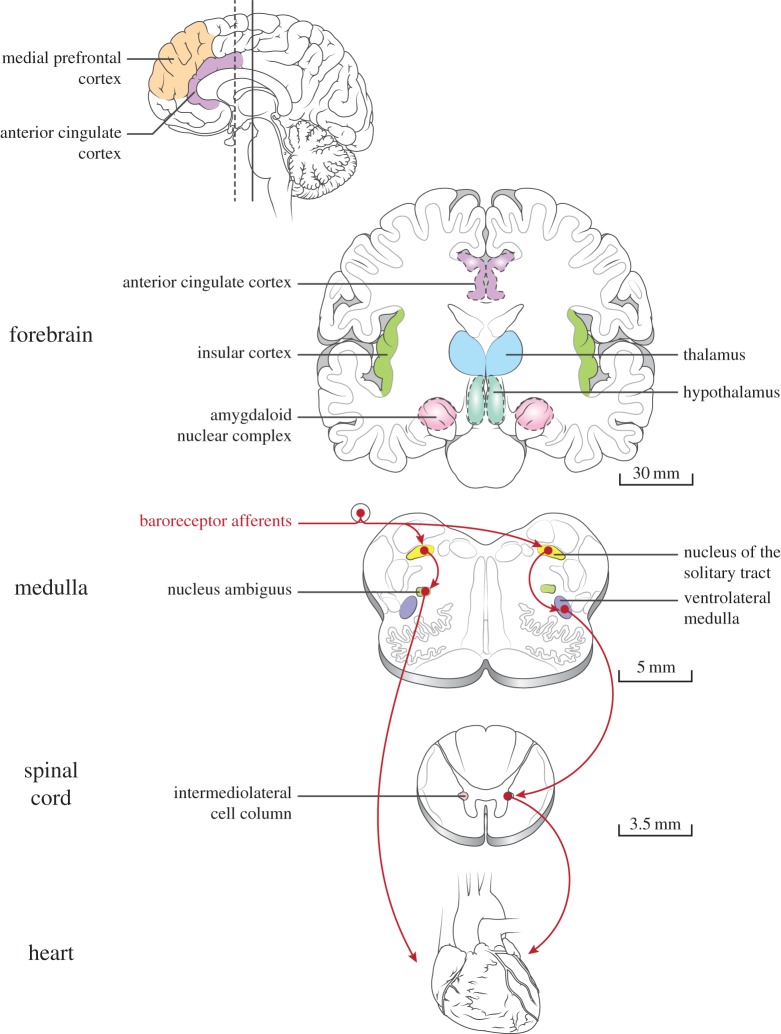
Figure 1.
Several key areas involved in brain–heart interactions. Selected areas of the forebrain, brainstem and spinal cord involved in human autonomic function are depicted. Arrows illustrate major pathways of the baroreceptor reflex, mediating the homeostatic control of blood pressure (left and right branches stemming from the baroreceptor afferents indicate parasympathetic and sympathetic outflow, respectively). Scale bars provide approximate dimensions in the human brain. For reviews, see [17,18,46,47]. Figure design inspired by Benarroch et al. [50]. (Online version in colour.)
(a). Functional magnetic resonance imaging of the brainstem
The brainstem houses small and interconnected nuclei which, via ascending and descending projections to cortex and spinal cord, play a vital role in maintaining homeostatic control and adaptively modulating physiological responses to environmental demands. The brainstem presents substantial challenges for neuroimaging and, as such, has remained one of the least-studied structures in the human brain in vivo. For instance, the brainstem is prone to high levels of cardiac and cerebrospinal fluid-induced pulsatile motion due to its proximity to large vessels and ventricles [51,52], and may also undergo bulk motion with the cardiac cycle [53,54]. In addition, prominent susceptibility artefacts may result from the bone and air-filled cavities such as the pontine cistern. Consequently, the BOLD signal quality in the brainstem is considerably lower than that of most other brain regions [55,56]. See [57] for a recent, detailed discussion focused on fMRI of the midbrain.
Nonetheless, with attention to acquisition and post-processing strategies (reviewed in [56] and see §4), several groups have reported success in resolving fMRI signals from functionally distinct brainstem areas. The central circuitry underlying the baroreflex, i.e. the homeostatic regulation of blood pressure, was examined at 3 T with a concurrently recorded index of sympathetic output [58,59]. Using an interleaved acquisition scheme and optimized coverage of brainstem areas, increases in muscle sympathetic activity were associated with fMRI signal increases in the rostral ventrolateral medulla along with decreases in its caudal aspect and in the nucleus of the solitary tract, consistent with known baroreflex pathways (figure 2). At 7 T, the internal organization of the periaqueductal gray (PAG) rostrocaudally and into columns was shown to be functionally differentiated in emotional and volitional respiration tasks, using spatial resolutions of 0.75 mm and 1 mm, respectively [60,61].
Figure 2.
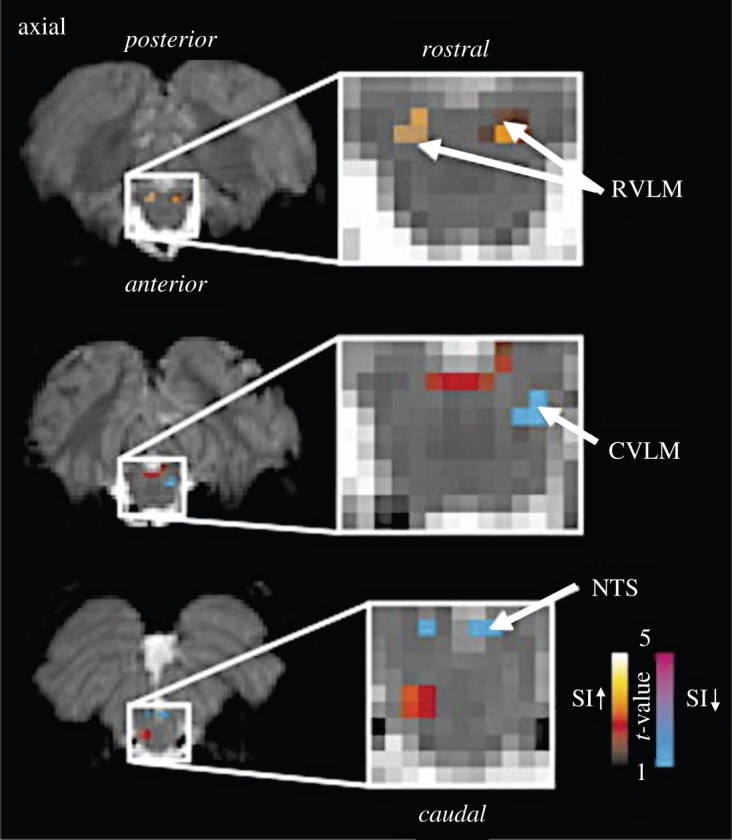
fMRI signal intensity changes correlated with sympathetic outflow. fMRI signal intensity changes correlated with spontaneous fluctuations in muscle sympathetic nerve activity (MSNA) in eight subjects. Increases (hot colour scale) and decreases(cool colour scale) in fMRI signal intensity with increases in MSNA are overlaid onto a series of axial fMRI slices from an individual subject. RVLM, rostral ventrolateral medulla; NTS, nucleus tractus solitarius; CVLM, caudal ventrolateral medulla; SI, signal intensity. Adapted from [58]. (Online version in colour.)
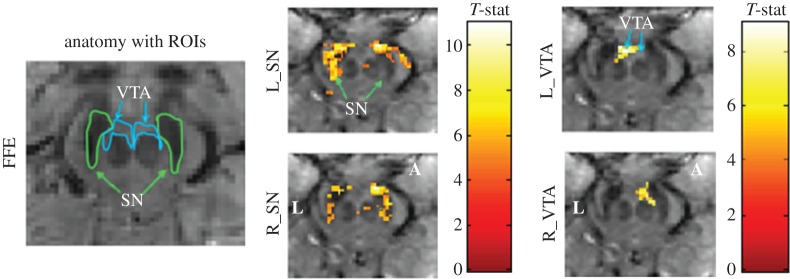
In addition to studying the activation of individual brainstem nuclei, exploring their functional connectivity may also inform us about the mechanisms involved in brain–heart interactions. However, brainstem functional connectivity in the human is largely uncharted territory, and this endeavour may also be advanced as the possible gains of UHF fMRI are realized. Recently, Beissner et al. [23] demonstrated patterns of cortical connectivity with distinct brainstem sub-regions in human resting-state fMRI at 3 T, which was achieved by masking areas containing high physiological noise prior to assessing functional connectivity (a technique also suggested in [62]). A subsequent analysis of 7 T data yielded robust detection of brainstem nuclei in single subjects, whereas this detection required multi-subject averaging at 3 T [63]. Functional connectivity of the ventral tegmental area and substantia nigra, structures in the midbrain, displayed resting-state functional connectivity patterns within the brainstem (figure 3) and to various cortical regions at 7 T [55].
Figure 3.
Functional connectivity in the midbrain at 7 T. Functional connectivity maps at 7 T in a representative participant, obtained with a three-dimensional fast field echo (FFE) sequence. An uncorrected voxel level threshold of p<10−6 (cluster size = 30 voxels) was used. Seed regions ofinterest in the midbrain include left substantia nigra (L_SN), right substantia nigra (R_SN), left ventral tegmental area (L_VTA) and right ventral tegmental area (R_VTA). Colour bar represents T-statistic. FFE voxel size = 1.33 mm3. Axial midbrain slice sections are displayed at the level of the superior colliculus for N=6 participants. L denotes the left side of the brain and A denotes the anterior portion of the brain. Data underwent motion correction, RETROICOR, band-pass filtering between 0.01 and 0.1 Hz. Robust bilateral spatial distribution of functional connectivity was observed in most ROIs. Adapted from [55]. (Online version in colour.)
(b). Functional magnetic resonance imaging of forebrain regions
Autonomic pathways of the brainstem and spinal cord are integrated with, and mediated by, higher centres in cortex as well as subcortical and limbic regions including hypothalamus, thalamus and amygdala. These structures adaptively modulate cardiovascular activity in response to changing emotional, cognitive and physical demands [50]. Like the brainstem, these regions have characteristics that present formidable challenges for neuroimaging. For one, they are small and themselves contain heterogeneous subdivisions, though the majority of fMRI studies effectively treat them as uniform areas, as it is difficult to resolve their finer structure. This may give rise to apparent variability across the literature in their reported functions and may vastly oversimplify any resulting inferences. Further, ventral brain areas (e.g. amygdala, hypothalamus, nucleus accumbens) suffer from susceptibility artefacts as well as elevated amounts of physiological fluctuation.
Advances in UHF fMRI are showing promise in obtaining sensitive measures of neural activity from forebrain areas and in revealing their fine internal organization. Considerable improvement in the BOLD CNR of the amygdala was observed at 7 T compared to 3 T in an emotion discrimination task [64], and the use of smaller voxels has been shown to mitigate susceptibility-induced signal dropout in the amygdala and orbitofrontal cortex [65]. Interestingly, high-resolution imaging at 3 T has also indicated the possible involvement of the amygdala in the default-mode network, an observation not typically made at lower resolutions [66], and has revealed valence-dependent responses to emotional stimuli in the hypothalamus, suggesting a broader role for the latter in emotional processing than is typically recognized [67]. Data-driven clustering methods at conventional image resolutions (3 mm isotropic) at 3 T have suggested that three primary amygdalar subdivisions can be identified on the basis of distinct resting-state connectivity patterns [68], and there is evidence that the performance of connectivity-based parcellation techniques will improve with even higher field [63]. Very recently, resting-state functional connectivity of the bed nucleus of the stria terminalis, a portion of the ‘extended amygdala’ that is implicated in autonomic arousal and anxiety, was mapped in humans at 7 T at 1.3 mm isotropic resolution [69]. In contrast with a similar analysis conducted at 3 T [70], greater specificity was reported in identifying functional connectivity to small structures (e.g. sublenticular extended amygdala, PAG, hypothalamus and habenula) and sub-regions of hippocampus and thalamus. Collectively, these studies suggest that improvements in resolving and measuring neural activity in ventral brain areas may enable novel studies of their involvement in emotional and stress-induced cardiac responses, as well as their integration with other cortical and subcortical regions.
(c). Studying large-scale interactions with high-resolution functional magnetic resonance imaging
Cortical areas, prominently the insular, cingulate and prefrontal cortices, also have a well-established involvement in cardiovascular modulation (reviewed in [18,71,72]). Non-invasive imaging is making exciting headway in extending findings from the animal to the human and in shedding light on the integration between the autonomic nervous system, cognition and emotion. At present, an array of findings point to the complexity of cortical autonomic processing, suggesting that different pathways may be recruited depending on the particular task employed (e.g. affective, cognitive, motor [73]) and also perhaps as a function of individual experience [48]. Meta-analyses are beginning to elucidate the segregation and integration between parasympathetic and sympathetic divisions [73,74] and to interrogate their relationship with large-scale networks such as default-mode and salience networks (discussed in [18,73]; see also [19] with regard to attentional and affective networks). Of particular relevance to UHF fMRI, regions attributed to parasympathetic and sympathetic involvement described in [73] were situated in closely overlapping clusters of anterior insula, angular gyrus and amygdala that may be more precisely delineated with high spatial resolution.
fMRI at UHF may convey unique benefits in studying interactions between cortical and subcortical areas. For instance, the thalamus—containing nuclei through which information related to brain–heart interactions is relayed between the cortex and subcortical/spinal cord regions [14,17]—has a well-described functional parcellation in animals that is also supported by non-invasive human studies (e.g. [75,76]), though its nuclei are not commonly differentiated in human fMRI reports. Functional integration of different thalamic sub-regions with distinct cortical and basal ganglia circuits may be studied with greater precision with UHF fMRI [77]. In one study at 7 T, distinct activation of mediodorsal versus centromedian/parafascicular thalamic nuclei was observed in emotional versus anticipatory attention phases of a task; further, these nuclei exhibited distinct co-activation with affective and cognitive divisions, respectively, of the anterior cingulate cortex (ACC) and insula [78]. Although much of the neocortex can be imaged with adequate sensitivity at 3 T and below, examination of cortical subdivisions having differential autonomic involvement, e.g. within the insula [79], may also be better accomplished with UHF fMRI.
(d). Outlook and clinical potential
UHF MRI is a burgeoning component of large multi-site neuroimaging projects, including the Human Connectome Project ([80], which includes a 7 T protocol). Progress towards optimizing imaging hardware, acquisition and data analysis in association with these projects [81] is likely to continue advancing the use of UHF and its role in understanding brain–heart interactions. The growing number of open data repositories are also broadening the accessibility of UHF data ([82,83] and see [5] for further discussion of practical issues concerning UHF scanners).
While UHF is not presently established in clinical practice, its advantages over lower field strengths for visualizing certain pathological features offers clear promise for the diagnosis and understanding of disease processes [5,84,85]. As one possible future direction for fMRI, the activity of deep-brain and brainstem nuclei—along with their functional interactions with the rest of the brain—may be contrasted between normal and pathological conditions for insight into how human brainstem abnormalities may underlie autonomic impairments. Preliminary research at 3 T provides evidence that in major depression, a condition marked by reduced heart rate (HR) variability [86,87], autonomic dysregulation may be linked with deficient functional connectivity between autonomic brainstem nuclei and the rostral anterior cingulate [88].
4. Challenges in ultra-high-field imaging of brain–heart interactions
While the enhanced temporal contrast and spatial fidelity of UHF MRI make it an appealing tool for the study of brain–heart interactions, there are a number of counteracting technical and analytic hurdles. Several issues pertaining to the study of brain–heart interactions are discussed below.
(a). Balancing regional sensitivity with wide spatial coverage
One challenge in studying large-scale functional interactions with small nuclei involves balancing optimized acquisition of specific brain areas with the desire for broad spatial coverage. Owing to limitations in MRI acquisition speed, high-resolution fMRI of specific brain regions has typically been achieved by restricting the field of view such that it covers only a fraction of the brain. Fortunately, continued progress in hardware and accelerated imaging techniques is enabling the acquisition of high-resolution images with extensive spatial coverage [89,90]. Leveraging advances in gradient and RF hardware along with parallel imaging, De Martino et al. [45] examined resting-state networks as a function of resolution (1–2 mm isotropic) in 7 T data with whole-brain coverage [45]. Beyond demonstrating the detection of canonical resting-state networks at high resolution (with 1.5 mm reported to be the best compromise in their comparisons), higher spatial resolutions were found to yield significantly reduced partial volume effects and display tighter correspondence with the subject-specific anatomic images. However, default-mode network connectivity in frontal brain areas (ACC) was reduced compared to that seen in lower-field data acquired at lower resolution, an effect suggested to result from reduced SNR in the high-resolution data. Indeed, optimal spatial resolutions may depend on the brain region and particular neuroscience question of interest [6]. Also, consistent with observations in the visual cortex by Bianciardi et al. [91], BOLD signals of opposite temporal phase near large draining vessels were identified in the precuneus, suggesting that large-vessel effects are still present at high field and may interfere with interpretation of resting-state fMRI.
(b). Combining anatomy and function
Localizing activation observed with BOLD contrast fMRI, particularly within zones of small, densely situated nuclei, can be exceedingly difficult and requires precise alignment with an anatomical reference image. Fortunately, structural MRI also benefits from increased resolution and contrast available at UHF (figure 4). Susceptibility-weighted imaging at field strengths of 7 T and above has improved the visualization of structures, including white matter fibres and cortical layers, of the order of hundreds of micrometres [5]. Anatomic parcellation of amygdalar and hypothalamic subdivisons, and delineation of midbrain dopaminergic structures, has also seen improvement at 7 T compared to lower field strengths [93–96]. Efforts to evaluate different anatomic contrasts (and combinations thereof) for in vivo visualization of brainstem nuclei are under way [97,98], as are assessments of optimal processing and alignment strategies for both functional and structural data [99–101]. Recent studies have taken initial steps towards mapping human brainstem nuclei into a standard-space atlas (at 7 T [97]) and towards visualizing neuroanatomic connections between autonomic nuclei in the brainstem and limbic forebrain sites using high-resolution diffusion spectrum imaging tractography (at 3 T [102]).
Figure 4.
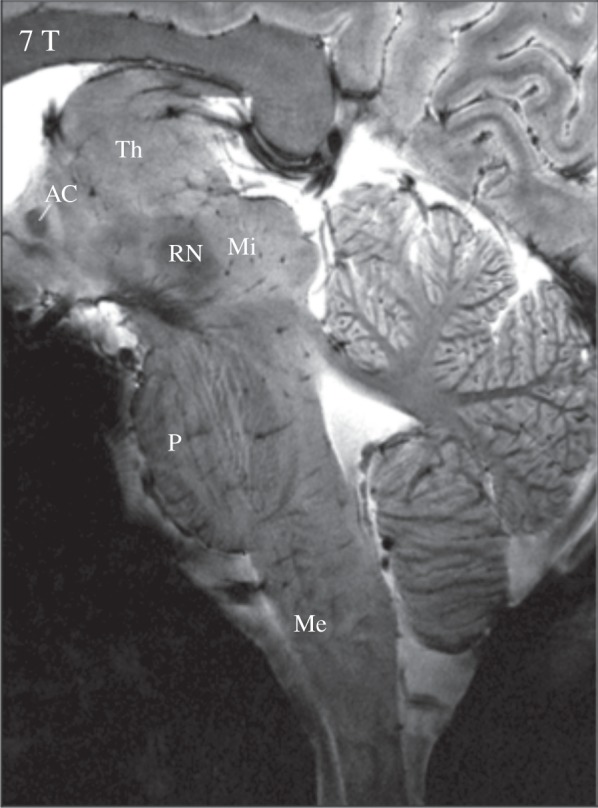
High-resolution anatomical contrast at 7 T. This sagittal image was acquired at 7 T using a two-dimensional gradient-echo sequence and elongated head coil for full brainstem coverage.Spatial resolution is 0.25 mm in-plane (slice thickness = 2 mm), allowing for detailed discrimination of brainstem structures and nuclei. Th, thalamus; AC, anterior commissure; Mi, midbrain; RN, red nucleus; P, pons; Me, medulla. Adapted from [92].
Importantly, the increased severity of distortions at UHF poses a major challenge for aligning functional and anatomic data. Tools for distortion correction are available and under development (e.g. [103,104]). Acquiring T1-contrast echo-planar images with matched distortion can also facilitate registration to anatomical landmarks or serve as an intermediate step between aligning distorted functional images with a non-distorted T1-contrast anatomic reference [105]. The quality of T1-weighted images may also be compromised at high field, due to the increased propensity for B1 field inhomogeneities, though recent improvements in RF coil technology and pulse sequences (e.g. [106]) can greatly ameliorate these issues. Finally, it is possible that standard tools for whole-brain spatial registration may not perform well at high (less than 1 mm) spatial resolution; beyond the lower tolerance of high-resolution data to head motion, true differences in fine-scale functional localization across individuals introduce challenges in assessing results at the group level [107].
(c). Temporal noise
Non-neural sources of temporal fluctuation have been estimated to constitute more than half of the total fMRI signal variability at 7 T [108]; also, with BOLD contrast comprising only a few per cent of the raw signal, effect sizes in cognitive neuroscience studies tend to be small, and accounting for non-neural influences can critically influence the outcome of analysis. Below, we discuss three major classes of temporal noise.
(i). Physiological noise: non-BOLD
Physiological noise refers to temporal noise that arises from systemic physiology, primarily from cardiac and respiratory processes (see [56,109] for reviews). Physiological noise is amplified at high magnetic fields and influences fMRI time series through multiple distinct mechanisms. One class of physiological noise results in non-BOLD fMRI signal deflections that are synchronized with the respiratory and cardiac cycles [51,110,111]. Static magnetic field modulations caused by the motion of the chest during breathing will result in voxel shifts or blurring, causing the greatest deflections at boundaries between tissue compartments and around the edges of the brain [112]; the cardiac cycle can induce cerebral pulsation and inflow (T1) effects [51]. As noted above, these artefacts unfortunately affect the brainstem and other regions that reside near vessels and fluid-filled cavities. Retrospective filtering methods [113,114], including RETROICOR [115] and independent component analysis (ICA [116,117]) have shown success in reducing these cyclic, non-BOLD artefacts, even in the brainstem [118,119]. On the acquisition side, artefacts from respiratory motion can be additionally reduced by the use of navigator methods to demodulate dynamic phase shifts [120,121]. Cardiac gating (synchronizing the fMRI image acquisition with the cardiac cycle) is often used to minimize pulsation artefacts, and undesired fluctuations introduced by the (heart-dependent) variability in TR can be normalized by collecting images at multiple echo times per TR (‘multi-echo imaging’) [100,122].
(ii). Physiological noise: BOLD
A second form of physiological noise acts directly on BOLD contrast by dynamically altering cerebral blood flow and volume. In other words, BOLD signal changes that are not coupled to the local neural activity (and thereby regarded as ‘noise’) can be induced through systemic respiratory and cardiac modulation of variables such as arterial CO2 and blood pressure. An extreme example is breath holding, which is known to trigger a spatially extensive, large-amplitude BOLD signal change [123] through physiological mechanisms postulated in, for example, [124,125]. The more subtle, inevitable changes in respiration volume and rate (RV [126,127]) (as well as HR [128,129]) across a typical fMRI are also found to induce considerable fluctuation in widespread, grey matter BOLD signal, compromising the ability to detect resting-state networks. These haemodynamic RV and HR effects are, compared to the non-BOLD artefacts described above, more difficult to disentangle from neurally driven BOLD due to their shared spatial and temporal characteristics; linear transfer function models have been found to provide effective nuisance regressors [129–131], though further investigation is needed.
The mechanism by which HR correlates with BOLD is not as well understood as that of respiratory modulation. One possibility is that correlations between HR and the BOLD signal arise indirectly from correlations between respiration and HR (e.g. respiratory sinus arrhythmia [132]); it is also plausible that HR acts independently from respiration to some degree, separately modulating blood pressure and haemodynamics. While correlations between HR and BOLD are observed to be quite global in nature [128,129], suggestive of systemic modulation rather than particular neural substrates of cardiac regulation, it is difficult to rule out (or disentangle) the contribution of neural activity. Another possibility is that changes in vigilance and arousal state, which are associated with changes in both HR and global neural activity [133,134], mediate the observed correlation between HR and BOLD.
Indeed, physiological noise (particularly that of the BOLD variety) poses an obvious confound for studies investigating neural correlates of autonomic processing. Such studies have employed tasks that actively modulate physiology (e.g. Valsalva manoeuvre [135], volitional breath holding [136] and hand-grip effort [137,138]), and/or they assess relationships between fMRI signals and simultaneously acquired peripheral autonomic measures (e.g. HR variability (HRV, [74,139]), skin conductance [140,141] and microneurographic recordings of nerve activity [59]). Respiratory manoeuvres and physical tasks can elicit large changes in systemic physiology and subject motion that can obscure specific neural responses. Passive monitoring of autonomic variables may reduce such artefacts, but also elicits less autonomic variation and still does not circumvent the question of disambiguating autonomic neural responses from physiological noise (and from activity underlying cognitive/mental effort or skeletal motor activity in performing a task). How can one disentangle non-neural physiological influences on fMRI signals from those implicated in cardio-respiratory control [142]?
One possibility for dissociating BOLD ‘noise’ from neural signals arises when these components possess spatial and/or temporal separability. Birn et al. [143] suggested that the temporal dynamics of the artefactual, respiratory-driven BOLD signal modulation may differ enough from the haemodynamic response to neural activity such that they can be disentangled to some extent using linear modelling [143]. The transfer function between RV and BOLD signal time series indeed exhibits longer latency (and primarily of opposite polarity) compared to the haemodynamic response of BOLD to neural activity [129,144]. Dissociation between vascular and neural signals in respiratory paradigms may also be augmented by experimentally manipulating the time course of the former. As two examples: (i) a cued-breathing task was performed in addition to a respiratory challenge at 7 T in an effort to discern brainstem areas related to volitional control of breathing [60]; and (ii) BOLD signal changes from external CO2 administration were contrasted with those correlated with natural fluctuations in end-tidal CO2, with the hypothesis that the coupling between PaCO2 and BOLD would become stronger in respiratory control centres [145]. Spatial ICA appears to capture well the cyclic cardiac pulsation and respiratory artefact into a collection of ‘noise’ components, though is less effective for BOLD respiratory volume/rate artefacts [146], perhaps due to the prevalence of the latter in grey matter and its strong spatial overlap with task- and resting-state networks. ICA in conjunction with a multi-echo pulse sequence can offer a principled way of sorting BOLD and non-BOLD components based on their echo-time dependence (see below), though again demonstrating better performance in dissociating cyclic, non-BOLD physiological noise [118,147] compared to BOLD physiological noise.
(iii). Non-physiological sources of noise
Examples of non-physiological noise sources include head motion and instrumental drift. Some of these noise sources are very slow and may be suppressed by filtering out very low frequencies or regressing out low-order polynomials [148]; however, the challenge is to remove such nuisance fluctuations without removing BOLD signal of interest. One alternative is to use regressors based on motion parameters or reference regions within the brain that are known to be neurally inactive [149,150]. Another approach is to examine the dependence on echo time: non-BOLD noise should scale with MRI signal strength and decay approximately exponentially with echo time, whereas BOLD signals show a characteristic gamma-variate dependence on echo time [151]. Thus, by performing a multi-echo acquisition, it may be possible to isolate non-BOLD noise sources [152,153]. Recent work has demonstrated that ICA of multi-echo data can offer a practical approach for mitigating non-BOLD sources of fluctuation [118,147,154,155]. It will be interesting to see whether this, and other filtering approaches, will enable the study of autonomic processes that vary too slowly to be resolved with conventional BOLD fMRI, such as tonically active sites of autonomic control. One drawback of the multi-echo approach is the high data acquisition rate, which complicates the acquisition of fMRI scans with high spatial and temporal resolution; the use of simultaneous multi-slice excitation may facilitate this (discussed in [156]).
5. New directions in analysis of brain–heart interactions
Recent developments in high-dimensional data analysis may, in conjunction with UHF fMRI, uncover novel information about brain–heart interactions and their deviation in pathological states. We briefly discuss two relevant directions.
(a). Dynamic measures of functional interaction
Methods for studying the interactions between brain regions typically assume that temporal covariation is fixed over time, particularly in resting-state fMRI. While static analysis frameworks have yielded tremendous insight into large-scale functional architecture, considerable variation over time can be observed in the properties of resting-state fMRI time series from individual brain regions [157,158], and in metrics of inter-regional coupling [159,160]. Variability in intrinsic activity has been suggested to arise in part from changes in arousal and autonomic processes, underscoring the state dependence of spontaneous brain activity and connectivity [140,161–163]. A better understanding of autonomic influences on intrinsic fluctuations may shed light on previously unexplained patterns of structured brain activity, as well as provide a new window into the neurobiology of brain–body interactions.
In addition to mapping regions whose fMRI activity tracks fluctuations in peripheral autonomic indices such as HRV, one may also ask whether time-varying autonomic states are linked with time-varying associations between brain regions. In an exploratory study of this nature [162], spontaneous fluctuations in HRV—whose power in high frequencies (0.15–0.4 Hz) is regarded as an indirect measure of parasympathetic activity—were accompanied by changes in functional connectivity with regard to the dorsal anterior cingulate cortex (dACC) and amygdala. A cluster in the brainstem whose correlation with dACC and amygdala specifically tracked high-frequency HRV was identified; while the low spatial resolution of the data precluded localization to specific nuclei, the analysis framework could certainly be extended to resolve autonomic nuclei in the brainstem and elsewhere with higher spatial resolution. The extent to which time-averaged functional connectivity differences between clinical populations are mediated by varying autonomic states (and corresponding functional connectivity differences) in neurological and psychiatric disorders is also an open question.
A number of approaches for analysing time-varying functional interactions have been proposed. Recently, a multivariate technique was described by Liu et al. wherein each BOLD fMRI image (volume) is regarded as a snapshot of co-activation, and representative co-activation patterns (CAPs) are then derived by clustering a set of volumes based on their spatial similarity [164]. CAPs derived from resting-state data revealed transient associations between brain regions that are not apparent from typical, longer time averages; the default-mode network, for instance, appears to co-activate with different areas and sub-networks within salience and executive control networks at different points in time. We may speculate that greater spatial detail revealed by analysing CAPs (and extensions thereof [165]) at higher resolution, and in conjunction with cardiac measurements, may provide a new technique for investigating the network behaviour underlying brain–heart interactions.
(b). Multivariate pattern analysis
Machine learning methods are having a rapidly growing impact on the neuroimaging field (reviewed in [166,167]). Distinct from traditional univariate approaches, which query the activity of individual voxels, multivariate methods capitalize on the joint activity expressed over collections of voxels to understand differences in brain states, differences in structure or function between clinical populations, and neural representations of stimuli, mental imagery and dream content. A possible future direction may use multivariate pattern analysis in the study of brain–heart interactions. For instance, within small-volume regions of highly heterogeneous structure, the pattern of activity across a neighbourhood of voxels (i.e. ‘searchlight’ [168]) spanning functional subdivisions may reveal complementary information about how they jointly encode or underlie changes in autonomic state.
We may speculate that UHF imaging and increased spatial resolution enhance the information that can be mapped at fine scales with multivariate methods (an idea also suggested and further described in [6,24]). The benefit of higher resolution may depend on the particular brain regions studied as well as dimensionality reduction trade-offs (e.g. using more, lower-resolution brain areas in a classifier compared to fewer high-resolution areas) and the particular classification methods applied. In addition, there are several examples in which discriminative information is shown to persist at coarse spatial scales, the underlying mechanisms of which are under discussion (e.g. [169–172]). Presently, whether high-resolution fMRI can offer advantages for multivariate pattern analysis in assessing brain–heart interactions has yet to be determined; the potential benefits may be countered by significant trade-offs (see [173] and those discussed above), and is a topic currently under investigation (e.g. [174]).
6. Summary
High-resolution fMRI at magnetic fields at and above 7 T has yielded remarkably fine-scale measurements of neural activity and structure, and is currently well poised for novel discoveries about interactions between the heart and the brain. The ability to achieve sub-millimetre functional and anatomic resolution in vivo permits the study of small nuclei in areas such as brainstem and forebrain that serve as key integration and relay nodes for bidirectional brain–heart modulation. Advances in accelerated imaging also permit studying their large-scale functional connectivity, which may be further facilitated by drawing upon recent concepts in spatio-temporal data analysis. However, successfully isolating functional signals from small regions, especially in areas strongly contaminated by physiological and susceptibility artefacts, requires considerable effort in acquisition and post-processing. Subject motion can obscure signals from smaller, densely interwoven nuclei (even if the imaging resolution can be further increased), and disentangling temporal noise from autonomic neural activity remains an outstanding challenge. Nonetheless, evidence suggests that UHF MRI will continue to open unique opportunities for investigating brain–heart interactions in the human.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke. This material is based in part upon work supported by the National Science Foundation Graduate Research Fellowship under grant no. DGE-1444316.
References
- 1.Uludag K, Muller-Bierl B, Ugurbil K. 2009. An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage 48, 150–165. ( 10.1016/j.neuroimage.2009.05.051) [DOI] [PubMed] [Google Scholar]
- 2.Yacoub E, Harel N, Ugurbil K. 2008. High-field fMRI unveils orientation columns in humans. Proc. Natl Acad. Sci. USA 105, 10 607–10 612. ( 10.1073/pnas.0804110105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polimeni JR, Fischl B, Greve DN, Wald LL. 2010. Laminar analysis of 7 T BOLD using an imposed spatial activation pattern in human V1. Neuroimage 52, 1334–1346. ( 10.1016/j.neuroimage.2010.05.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duyn JH, van Gelderen P, Li TQ, de Zwart JA, Koretsky AP, Fukunaga M. 2007. High-field MRI of brain cortical substructure based on signal phase. Proc. Natl Acad. Sci. USA 104, 11 796–11 801. ( 10.1073/pnas.0610821104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duyn JH. 2012. The future of ultra-high field MRI and fMRI for study of the human brain. Neuroimage 62, 1241–1248. ( 10.1016/j.neuroimage.2011.10.065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olman CA, Yacoub E. 2011. High-field FMRI for human applications: an overview of spatial resolution and signal specificity. Open Neuroimag. J. 5, 74–89. ( 10.2174/1874440001105010074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ugurbil K. 2014. Magnetic resonance imaging at ultrahigh fields. IEEE Trans. Biomed. Eng. 61, 1364–1379. ( 10.1109/TBME.2014.2313619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Kolk AG, Hendrikse J, Zwanenburg JJ, Visser F, Luijten PR. 2013. Clinical applications of 7 T MRI in the brain. Eur. J. Radiol. 82, 708–718. ( 10.1016/j.ejrad.2011.07.007) [DOI] [PubMed] [Google Scholar]
- 9.Turner R. 2012. Neuroscientific applications of high-field MRI in humans. In High-field MR imaging. Medical radiology (eds Hennig J, Speck O), pp. 137–149. Berlin, Germany: Springer. [Google Scholar]
- 10.Oppenheimer S. 2006. Cerebrogenic cardiac arrhythmias: cortical lateralization and clinical significance. Clin. Auton. Res. 16, 6–11. ( 10.1007/s10286-006-0276-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortelli P, Lombardi C, Montagna P, Parati G. 2012. Baroreflex modulation during sleep and in obstructive sleep apnea syndrome. Auton. Neurosci. 169, 7–11. ( 10.1016/j.autneu.2012.02.005) [DOI] [PubMed] [Google Scholar]
- 12.Kemp AH, Quintana DS. 2013. The relationship between mental and physical health: insights from the study of heart rate variability. Int. J. Psychophysiol. 89, 288–296. ( 10.1016/j.ijpsycho.2013.06.018) [DOI] [PubMed] [Google Scholar]
- 13.Koenig J, Kemp AH, Feeling NR, Thayer JF, Kaess M. 2016. Resting state vagal tone in borderline personality disorder: a meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 18–26. ( 10.1016/j.pnpbp.2015.07.002) [DOI] [PubMed] [Google Scholar]
- 14.Palma JA, Benarroch EE. 2014. Neural control of the heart: recent concepts and clinical correlations. Neurology 83, 261–271. ( 10.1212/WNL.0000000000000605) [DOI] [PubMed] [Google Scholar]
- 15.Silvani A, Calandra-Buonaura G, Dampney RAL, Cortelli P. 2016. Brain–heart interactions: physiology and clinical implications. Phil. Trans. R. Soc. A 374, 20150181 ( 10.1098/rsta.2015.0181) [DOI] [PubMed] [Google Scholar]
- 16.Dampney RA. 1981. Functional organization of central cardiovascular pathways. Clin. Exp. Pharmacol. Physiol. 8, 241–259. ( 10.1111/j.1440-1681.1981.tb00156.x) [DOI] [PubMed] [Google Scholar]
- 17.Saper CB, Stornetta RL. 1995. Central autonomic system. In The rat nervous system, 4th edn (ed. G Paxinos), pp. 155–185. San Diego, CA: Academic Press. [Google Scholar]
- 18.Critchley HD, Eccles J, Garfinkel SN. 2013. Interaction between cognition, emotion, and the autonomic nervous system. Handb. Clin. Neurol. 117, 59–77. ( 10.1016/B978-0-444-53491-0.00006-7) [DOI] [PubMed] [Google Scholar]
- 19.Thayer JF, Lane RD. 2000. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 61, 201–216. ( 10.1016/S0165-0327(00)00338-4) [DOI] [PubMed] [Google Scholar]
- 20.Park HD, Correia S, Ducorps A, Tallon-Baudry C. 2014. Spontaneous fluctuations in neural responses to heartbeats predict visual detection. Nat. Neurosci. 17, 612–618. ( 10.1038/nn.3671) [DOI] [PubMed] [Google Scholar]
- 21.Critchley HD, Harrison NA. 2013. Visceral influences on brain and behavior. Neuron 77, 624–638. ( 10.1016/j.neuron.2013.02.008) [DOI] [PubMed] [Google Scholar]
- 22.Lin A, Liu KKL, Bartsch RP, Ivanov PCh. 2016. Delay-correlation landscape reveals characteristic time delays of brain rhythms and heart interactions. Phil. Trans. R. Soc. A 374, 20150182 ( 10.1098/rsta.2015.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beissner F, Schumann A, Brunn F, Eisentrager D, Bar KJ. 2014. Advances in functional magnetic resonance imaging of the human brainstem. Neuroimage 86, 91–98. ( 10.1016/j.neuroimage.2013.07.081) [DOI] [PubMed] [Google Scholar]
- 24.Francis S, Panchuelo RS. 2014. Physiological measurements using ultra-high field fMRI: a review. Physiol. Meas. 35, R167–R185. ( 10.1088/0967-3334/35/9/R167) [DOI] [PubMed] [Google Scholar]
- 25.Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. 1992. Time course EPI of human brain function during task activation. Magn. Reson. Med. 25, 390–397. ( 10.1002/mrm.1910250220) [DOI] [PubMed] [Google Scholar]
- 26.Kwong KK. et al. 1992. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl Acad. Sci. USA 89, 5675–5679. ( 10.1073/pnas.89.12.5675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. 1992. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc. Natl Acad. Sci. USA 89, 5951–5955. ( 10.1073/pnas.89.13.5951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JE, Glover GH. 2015. Functional magnetic resonance imaging methods. Neuropsychol. Rev. 25, 289–313. ( 10.1007/s11065-015-9294-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa S, Lee TM, Kay AR, Tank DW. 1990. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl Acad. Sci. USA 87, 9868–9872. ( 10.1073/pnas.87.24.9868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711. ( 10.1038/nrn2201) [DOI] [PubMed] [Google Scholar]
- 31.Hutton C, Josephs O, Stadler J, Featherstone E, Reid A, Speck O, Bernarding J, Weiskopf N. 2011. The impact of physiological noise correction on fMRI at 7 T. Neuroimage 57, 101–112. ( 10.1016/j.neuroimage.2011.04.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyde JS, Biswal BB, Jesmanowicz A. 2001. High-resolution fMRI using multislice partial k-space GR-EPI with cubic voxels. Magn. Reson. Med. 46, 114–125. ( 10.1002/mrm.1166) [DOI] [PubMed] [Google Scholar]
- 33.Turner R. 2002. How much cortex can a vein drain? Downstream dilution of activation-related cerebral blood oxygenation changes. Neuroimage 16, 1062–1067. ( 10.1006/nimg.2002.1082) [DOI] [PubMed] [Google Scholar]
- 34.Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. 1995. MR contrast due to intravascular magnetic susceptibility perturbations. Magn. Reson. Med. 34, 555–566. ( 10.1002/mrm.1910340412) [DOI] [PubMed] [Google Scholar]
- 35.Yacoub E, Duong TQ, Van de Moortele PF, Lindquist M, Adriany G, Kim SG, Hu X. 2003. Spin-echo fMRI in humans using high spatial resolutions and high magnetic fields. Magn. Reson. Med. 49, 655–664. ( 10.1002/mrm.10433) [DOI] [PubMed] [Google Scholar]
- 36.Menon RS. 2002. Postacquisition suppression of large-vessel BOLD signals in high-resolution fMRI. Magn. Reson. Med. 47, 1–9. ( 10.1002/mrm.10041) [DOI] [PubMed] [Google Scholar]
- 37.Wald LL. 2012. The future of acquisition speed, coverage, sensitivity, and resolution. Neuroimage 62, 1221–1229. ( 10.1016/j.neuroimage.2012.02.077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyacioglu R, Schulz J, Muller NC, Koopmans PJ, Barth M, Norris DG. 2014. Whole brain, high resolution multiband spin-echo EPI fMRI at 7 T: a comparison with gradient-echo EPI using a color–word Stroop task. Neuroimage 97, 142–150. ( 10.1016/j.neuroimage.2014.04.011) [DOI] [PubMed] [Google Scholar]
- 39.Norris DG. 2012. Spin-echo fMRI: the poor relation? Neuroimage 62, 1109–1115. ( 10.1016/j.neuroimage.2012.01.003) [DOI] [PubMed] [Google Scholar]
- 40.Yacoub E, Shmuel A, Logothetis N, Ugurbil K. 2007. Robust detection of ocular dominance columns in humans using Hahn spin echo BOLD functional MRI at 7 tesla. Neuroimage 37, 1161–1177. ( 10.1016/j.neuroimage.2007.05.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goense J, Merkle H, Logothetis NK. 2012. High-resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative BOLD responses. Neuron 76, 629–639. ( 10.1016/j.neuron.2012.09.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olman CA, Harel N, Feinberg DA, He S, Zhang P, Ugurbil K, Yacoub E. 2012. Layer-specific fMRI reflects different neuronal computations at different depths in human V1. PLoS ONE 7, e32536 ( 10.1371/journal.pone.0032536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmermann J. et al. 2011. Mapping the organization of axis of motion selective features in human area MT using high-field fMRI. PLoS ONE 6, e28716 ( 10.1371/journal.pone.0028716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Martino F, Moerel M, Van de Moortele PF, Ugurbil K, Goebel R, Yacoub E, Formisano E. 2013. Spatial organization of frequency preference and selectivity in the human inferior colliculus. Nat. Commun. 4, 1386 ( 10.1038/ncomms2379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Martino F, Esposito F, Van de Moortele PF, Harel N, Formisano E, Goebel R, Ugurbil K, Yacoub E. 2011. Whole brain high-resolution functional imaging at ultra high magnetic fields: an application to the analysis of resting state networks. Neuroimage 57, 1031–1044. ( 10.1016/j.neuroimage.2011.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benarroch EE. 1993. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin. Proc. 68, 988–1001. ( 10.1016/S0025-6196(12)62272-1) [DOI] [PubMed] [Google Scholar]
- 47.Spyer KM. 1994. Annual review prize lecture. Central nervous mechanisms contributing to cardiovascular control. J. Physiol. 474, 1–19. ( 10.1113/jphysiol.1994.sp019997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cechetto DF. 2014. Cortical control of the autonomic nervous system. Exp. Physiol. 99, 326–331. ( 10.1113/expphysiol.2013.075192) [DOI] [PubMed] [Google Scholar]
- 49.Park G, Thayer JF. 2014. From the heart to the mind: cardiac vagal tone modulates top-down and bottom-up visual perception and attention to emotional stimuli. Front. Psychol. 5, 278 ( 10.3389/fpsyg.2014.00278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benarroch EE. 2012. Central autonomic control. In Primer on the autonomic nervous system, 3rd edn (eds D Robertson, I Biaggioni, G Burnstock, PA Low, JFR Paton), ch. 2, pp. 9–12. Waltham, MA: Academic Press ( 10.1016/B978-0-12-386525-0.00002-0) [DOI] [Google Scholar]
- 51.Dagli MS, Ingeholm JE, Haxby JV. 1999. Localization of cardiac-induced signal change in fMRI. Neuroimage 9, 407–415. ( 10.1006/nimg.1998.0424) [DOI] [PubMed] [Google Scholar]
- 52.Klose U, Strik C, Kiefer C, Grodd W. 2000. Detection of a relation between respiration and CSF pulsation with an echoplanar technique. J. Magn. Reson. Imaging 11, 438–444. () [DOI] [PubMed] [Google Scholar]
- 53.Soellinger M, Ryf S, Boesiger P, Kozerke S. 2007. Assessment of human brain motion using CSPAMM. J. Magn. Reson. Imaging 25, 709–714. ( 10.1002/jmri.20882) [DOI] [PubMed] [Google Scholar]
- 54.Enzmann DR, Pelc NJ. 1992. Brain motion: measurement with phase-contrast MR imaging. Radiology 185, 653–660. ( 10.1148/radiology.185.3.1438741) [DOI] [PubMed] [Google Scholar]
- 55.Barry RL, Coaster M, Rogers BP, Newton AT, Moore J, Anderson AW, Zald DH, Gore JC. 2013. On the origins of signal variance in FMRI of the human midbrain at high field. PLoS ONE 8, e62708 ( 10.1371/journal.pone.0062708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brooks JC, Faull OK, Pattinson KT, Jenkinson M. 2013. Physiological noise in brainstem FMRI. Front. Hum. Neurosci. 7, 623 ( 10.3389/fnhum.2013.00623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Düzel E, Guitart-Masip M, Maass A, Hämmerer D, Betts MJ, Speck O, Weiskopf N, Kanowski M. 2015. Midbrain fMRI: applications, limitations and challenges. In fMRI: from nuclear spins to brain functions (eds Uludag K, Ugurbil K, Berliner L), Biological Magnetic Resonance, vol. 30, pp. 581–609. New York, NY: Springer; ( 10.1007/978-1-4899-7591-1.20) [DOI] [Google Scholar]
- 58.Macefield VG, Henderson LA. 2010. Real-time imaging of the medullary circuitry involved in the generation of spontaneous muscle sympathetic nerve activity in awake subjects. Hum. Brain Mapp. 31, 539–549. ( 10.1002/hbm.20885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henderson LA, James C, Macefield VG. 2012. Identification of sites of sympathetic outflow during concurrent recordings of sympathetic nerve activity and fMRI. Anat. Rec. (Hoboken) 295, 1396–1403. ( 10.1002/ar.22513) [DOI] [PubMed] [Google Scholar]
- 60.Faull OK, Jenkinson M, Clare S, Pattinson KT. 2015. Functional subdivision of the human periaqueductal grey in respiratory control using 7 tesla fMRI. Neuroimage 113, 356–364. ( 10.1016/j.neuroimage.2015.02.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Satpute AB, Wager TD, Cohen-Adad J, Bianciardi M, Choi JK, Buhle JT, Wald LL, Barrett LF. 2013. Identification of discrete functional subregions of the human periaqueductal gray. Proc. Natl Acad. Sci. USA 110, 17 101–17 106. ( 10.1073/pnas.1306095110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Formisano E, Esposito F, Di Salle F, Goebel R. 2004. Cortex-based independent component analysis of fMRI time series. Magn. Reson. Imaging 22, 1493–1504. ( 10.1016/j.mri.2004.10.020) [DOI] [PubMed] [Google Scholar]
- 63.Beissner F. et al. (eds). 2014. Ultra-high field fMRI of pain-related brainstem nuclei in single subjects. Annual Meeting of the Organization for Human Brain Mapping. [Google Scholar]
- 64.Sladky R, Baldinger P, Kranz GS, Trostl J, Hoflich A, Lanzenberger R, Moser E, Windischberger C. 2013. High-resolution functional MRI of the human amygdala at 7 T. Eur. J. Radiol. 82, 728–733. ( 10.1016/j.ejrad.2011.09.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson S, Windischberger C, Rauscher A, Moser E. 2004. Optimized 3 T EPI of the amygdalae. Neuroimage 22, 203–210. ( 10.1016/j.neuroimage.2003.12.048) [DOI] [PubMed] [Google Scholar]
- 66.Robinson SD, Pripfl J, Bauer H, Moser E. 2008. The impact of EPI voxel size on SNR and BOLD sensitivity in the anterior medio-temporal lobe: a comparative group study of deactivation of the default mode. Magn. Reson. Mater. Phys., Biol. Med. (MAGMA) 21, 279–290. ( 10.1007/s10334-008-0128-0) [DOI] [PubMed] [Google Scholar]
- 67.Karlsson KA, Windischberger C, Gerstl F, Mayr W, Siegel JM, Moser E. 2010. Modulation of hypothalamus and amygdalar activation levels with stimulus valence. Neuroimage 51, 324–328. ( 10.1016/j.neuroimage.2010.02.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mishra A, Rogers BP, Chen LM, Gore JC. 2014. Functional connectivity-based parcellation of amygdala using self-organized mapping: a data driven approach. Hum. Brain Mapp. 35, 1247–1260. ( 10.1002/hbm.22249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Torrisi S, O'Connell K, Davis A, Reynolds R, Balderston N, Fudge JL, Grillon C, Ernst M. 2015. Resting state connectivity of the bed nucleus of the stria terminalis at ultra-high field. Hum. Brain Mapp. 36, 4076–4088. ( 10.1002/hbm.22899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU. 2014. BNST neurocircuitry in humans. Neuroimage 91, 311–323. ( 10.1016/j.neuroimage.2014.01.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shoemaker JK, Goswami R. 2015. Forebrain neurocircuitry associated with human reflex cardiovascular control. Front. Physiol. 6, 240 ( 10.3389/fphys.2015.00240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saper CB. 2002. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 25, 433–469. ( 10.1146/annurev.neuro.25.032502.111311) [DOI] [PubMed] [Google Scholar]
- 73.Beissner F, Meissner K, Bar KJ, Napadow V. 2013. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 33, 10 503–10 511. ( 10.1523/JNEUROSCI.1103-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thayer JF, Ahs F, Fredrikson M, Sollers JJ 3rd , Wager TD. 2012. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 36, 747–756. ( 10.1016/j.neubiorev.2011.11.009) [DOI] [PubMed] [Google Scholar]
- 75.Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. 2010. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb. Cortex 20, 1187–1194. ( 10.1093/cercor/bhp182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Behrens TE. et al. 2003. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 6, 750–757. ( 10.1038/nn1075) [DOI] [PubMed] [Google Scholar]
- 77.Metzger CD, van der Werf YD, Walter M. 2013. Functional mapping of thalamic nuclei and their integration into cortico-striatal-thalamo-cortical loops via ultra-high resolution imaging—from animal anatomy to in vivo imaging in humans. Front. Neurosci. 7, 24 ( 10.3389/fnins.2013.00024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Metzger CD. et al. 2010. High field fMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Front. Neuroanat. 4, 138 ( 10.3389/fnana.2010.00138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macey PM, Wu P, Kumar R, Ogren JA, Richardson HL, Woo MA, Harper RM. 2012. Differential responses of the insular cortex gyri to autonomic challenges. Auton. Neurosci. 168, 72–81. ( 10.1016/j.autneu.2012.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K. 2013. The WU-Minn Human Connectome Project: an overview. Neuroimage 80, 62–79. ( 10.1016/j.neuroimage.2013.05.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ugurbil K. et al. 2013. Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. Neuroimage 80, 80–104. ( 10.1016/j.neuroimage.2013.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanke M, Baumgartner FJ, Ibe P, Kaule FR, Pollmann S, Speck O, Zinke W, Stadler J. 2014. A high-resolution 7-tesla fMRI dataset from complex natural stimulation with an audio movie. Sci. Data 1, 140003 ( 10.1038/sdata.2014.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gorgolewski KJ. et al. 2015. A high resolution 7-tesla resting-state fMRI test–retest dataset with cognitive and physiological measures. Sci. Data 2, 140054 ( 10.1038/sdata.2014.54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van der Kolk AG, Hendrikse J, Luijten PR. 2012. Ultrahigh-field magnetic resonance imaging: the clinical potential for anatomy, pathogenesis, diagnosis, and treatment planning in brain disease. Neuroimaging Clin. N. Am. 22, 343–362. ( 10.1016/j.nic.2012.02.004) [DOI] [PubMed] [Google Scholar]
- 85.Moser E, Stahlberg F, Ladd ME, Trattnig S. 2012. 7-T MR—from research to clinical applications? NMR Biomed. 25, 695–716. ( 10.1002/nbm.1794) [DOI] [PubMed] [Google Scholar]
- 86.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. 2010. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol. Psychiatry 67, 1067–1074. ( 10.1016/j.biopsych.2009.12.012) [DOI] [PubMed] [Google Scholar]
- 87.Licht CM, de Geus EJ, Zitman FG, Hoogendijk WJ, van Dyck R, Penninx BW. 2008. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA). Arch. Gen. Psychiatry 65, 1358–1367. ( 10.1001/archpsyc.65.12.1358) [DOI] [PubMed] [Google Scholar]
- 88.Smith R, Allen JJ, Thayer JF, Lane RD. 2015. Altered functional connectivity between medial prefrontal cortex and the inferior brainstem in major depression during appraisal of subjective emotional responses: a preliminary study. Biol. Psychol. 108, 13–24. ( 10.1016/j.biopsycho.2015.03.007) [DOI] [PubMed] [Google Scholar]
- 89.Feinberg DA, Setsompop K. 2013. Ultra-fast MRI of the human brain with simultaneous multi-slice imaging. J. Magn. Reson. 229, 90–100. ( 10.1016/j.jmr.2013.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Uğurbil K. 2010. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 63, 1144–1153. ( 10.1002/mrm.22361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bianciardi M, Fukunaga M, van Gelderen P, de Zwart JA, Duyn JH. 2011. Negative BOLD-fMRI signals in large cerebral veins. J. Cereb. Blood Flow Metab. 31, 401–412. ( 10.1038/jcbfm.2010.164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cho Z-H, Kim Y-B, Han J-Y, Min H-K, Kim K-N, Choi S-H, Veklerov E, Shepp LA. 2008. New brain atlas—mapping the human brain in vivo with 7.0 T MRI and comparison with postmortem histology: will these images change modern medicine? Int. J. Imaging Syst. Technol. 18, 2–8. ( 10.1002/ima.20143) [DOI] [Google Scholar]
- 93.Solano-Castiella E, Schafer A, Reimer E, Turke E, Proger T, Lohmann G, Trampel R, Turner R. 2011. Parcellation of human amygdala in vivo using ultra high field structural MRI. Neuroimage 58, 741–748. ( 10.1016/j.neuroimage.2011.06.047) [DOI] [PubMed] [Google Scholar]
- 94.Makris N, Swaab DF, van der Kouwe A, Abbs B, Boriel D, Handa RJ, Tobet S, Goldstein JM. 2013. Volumetric parcellation methodology of the human hypothalamus in neuroimaging: normative data and sex differences. Neuroimage 69, 1–10. ( 10.1016/j.neuroimage.2012.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schindler S. et al. 2013. Development and evaluation of an algorithm for the computer-assisted segmentation of the human hypothalamus on 7-tesla magnetic resonance images. PLoS ONE 8, e66394 ( 10.1371/journal.pone.0066394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eapen M, Zald DH, Gatenby JC, Ding Z, Gore JC. 2011. Using high-resolution MR imaging at 7 T to evaluate the anatomy of the midbrain dopaminergic system. Am. J. Neuroradiol. 32, 688–694. ( 10.3174/ajnr.A2355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bianciardi M. et al. 2015. Toward an in vivo neuroimaging template of human brainstem nuclei of the ascending arousal, autonomic, and motor systems. Brain Connect. 5, 597–607. ( 10.1089/brain.2015.0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deistung A, Schafer A, Schweser F, Biedermann U, Gullmar D, Trampel R, Reichenbach JR. 2013. High-resolution MR imaging of the human brainstem in vivo at 7 tesla. Front. Hum. Neurosci. 7, 710 ( 10.3389/fnhum.2013.00710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Napadow V, Dhond R, Kennedy D, Hui KK, Makris N. 2006. Automated brainstem co-registration (ABC) for MRI. Neuroimage 32, 1113–1119. ( 10.1016/j.neuroimage.2006.05.050) [DOI] [PubMed] [Google Scholar]
- 100.Beissner F, Deichmann R, Baudrexel S. 2011. fMRI of the brainstem using dual-echo EPI. Neuroimage 55, 1593–1599. ( 10.1016/j.neuroimage.2011.01.042) [DOI] [PubMed] [Google Scholar]
- 101.Limbrick-Oldfield EH, Brooks JC, Wise RJ, Padormo F, Hajnal JV, Beckmann CF, Ungless MA. 2012. Identification and characterisation of midbrain nuclei using optimised functional magnetic resonance imaging. Neuroimage 59, 1230–1238. ( 10.1016/j.neuroimage.2011.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Edlow BL, McNab JA, Witzel T, Kinney HC. In press The structural connectome of the human central homeostatic network. Brain Connect. ( 10.1089/brain.2015.0378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.In MH, Posnansky O, Beall EB, Lowe MJ, Speck O. 2015. Distortion correction in EPI using an extended PSF method with a reversed phase gradient approach. PLoS ONE 10, e0116320 ( 10.1371/journal.pone.0116320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andersson JL, Skare S, Ashburner J. 2003. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870–888. ( 10.1016/S1053-8119(03)00336-7) [DOI] [PubMed] [Google Scholar]
- 105.Renvall V, Witzel T, Wald LL, Polimeni JR. 2014. Fast variable inversion-recovery time EPI for anatomical reference and quantitative T1 mapping. In Proc. 22nd Joint Annual Meeting of ISMRM-ESMRMB, Milan, Italy, 10–16 May. [Google Scholar]
- 106.O'Brien KR, Kober T, Hagmann P, Maeder P, Marques J, Lazeyras F, Krueger G, Roche A. 2014. Robust T1-weighted structural brain imaging and morphometry at 7 T using MP2RAGE. PLoS ONE 9, e99676 ( 10.1371/journal.pone.0099676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khan R, Zhang Q, Darayan S, Dhandapani S, Katyal S, Greene C, Bajaj C, Ress D. 2011. Surface-based analysis methods for high-resolution functional magnetic resonance imaging. Graph. Models 73, 313–322. ( 10.1016/j.gmod.2010.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Shmueli K, Duyn JH. 2009. Sources of functional magnetic resonance imaging signal fluctuations in the human brain at rest: a 7 T study. Magn. Reson. Imaging 27, 1019–1029. ( 10.1016/j.mri.2009.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Murphy K, Birn RM, Bandettini PA. 2013. Resting-state fMRI confounds and cleanup. Neuroimage 80, 349–359. ( 10.1016/j.neuroimage.2013.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raj D, Anderson AW, Gore JC. 2001. Respiratory effects in human functional magnetic resonance imaging due to bulk susceptibility changes. Phys. Med. Biol. 46, 3331–3340. ( 10.1088/0031-9155/46/12/318) [DOI] [PubMed] [Google Scholar]
- 111.Windischberger C, Langenberger H, Sycha T, Tschernko EM, Fuchsjager-Mayerl G, Schmetterer L, Moser E. 2002. On the origin of respiratory artifacts in BOLD-EPI of the human brain. Magn. Reson. Imaging 20, 575–582. ( 10.1016/S0730-725X(02)00563-5) [DOI] [PubMed] [Google Scholar]
- 112.Van de Moortele PF, Pfeuffer J, Glover GH, Ugurbil K, Hu X. 2002. Respiration-induced B0 fluctuations and their spatial distribution in the human brain at 7 tesla. Magn. Reson. Med. 47, 888–895. ( 10.1002/mrm.10145) [DOI] [PubMed] [Google Scholar]
- 113.Deckers RH, van Gelderen P, Ries M, Barret O, Duyn JH, Ikonomidou VN, Fukunaga M, Glover GH, de Zwart JA. 2006. An adaptive filter for suppression of cardiac and respiratory noise in MRI time series data. Neuroimage 33, 1072–1081. ( 10.1016/j.neuroimage.2006.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu X, Le TH, Parrish T, Erhard P. 1995. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magn. Reson. Med. 34, 201–212. ( 10.1002/mrm.1910340211) [DOI] [PubMed] [Google Scholar]
- 115.Glover GH, Li TQ, Ress D. 2000. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 44, 162–167. () [DOI] [PubMed] [Google Scholar]
- 116.Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. 2014. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 90, 449–468. ( 10.1016/j.neuroimage.2013.11.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thomas CG, Harshman RA, Menon RS. 2002. Noise reduction in BOLD-based fMRI using component analysis. Neuroimage 17, 1521–1537. ( 10.1006/nimg.2002.1200) [DOI] [PubMed] [Google Scholar]
- 118.Kundu P, Inati SJ, Evans JW, Luh WM, Bandettini PA. 2012. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage 60, 1759–1770. ( 10.1016/j.neuroimage.2011.12.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harvey AK, Pattinson KT, Brooks JC, Mayhew SD, Jenkinson M, Wise RG. 2008. Brainstem functional magnetic resonance imaging: disentangling signal from physiological noise. J. Magn. Reson. Imaging 28, 1337–1344. ( 10.1002/jmri.21623) [DOI] [PubMed] [Google Scholar]
- 120.Pfeuffer J, Van de Moortele PF, Ugurbil K, Hu X, Glover GH. 2002. Correction of physiologically induced global off-resonance effects in dynamic echo-planar and spiral functional imaging. Magn. Reson. Med. 47, 344–353. ( 10.1002/mrm.10065) [DOI] [PubMed] [Google Scholar]
- 121.Hu X, Kim SG. 1994. Reduction of signal fluctuation in functional MRI using navigator echoes. Magn. Reson. Med. 31, 495–503. ( 10.1002/mrm.1910310505) [DOI] [PubMed] [Google Scholar]
- 122.Zhang WT, Mainero C, Kumar A, Wiggins CJ, Benner T, Purdon PL, Bolar DS, Kwong KK, Sorensen AG. 2006. Strategies for improving the detection of fMRI activation in trigeminal pathways with cardiac gating. Neuroimage 31, 1506–1512. ( 10.1016/j.neuroimage.2006.02.033) [DOI] [PubMed] [Google Scholar]
- 123.Kastrup A, Li TQ, Takahashi A, Glover GH, Moseley ME. 1998. Functional magnetic resonance imaging of regional cerebral blood oxygenation changes during breath holding. Stroke 29, 2641–2645. ( 10.1161/01.STR.29.12.2641) [DOI] [PubMed] [Google Scholar]
- 124.Nakada K, Yoshida D, Fukumoto M, Yoshida S. 2001. Chronological analysis of physiological T2* signal change in the cerebrum during breath holding. J. Magn. Reson. Imaging 13, 344–351. ( 10.1002/jmri.1049) [DOI] [PubMed] [Google Scholar]
- 125.Thomason ME, Burrows BE, Gabrieli JD, Glover GH. 2005. Breath holding reveals differences in fMRI BOLD signal in children and adults. Neuroimage 25, 824–837. ( 10.1016/j.neuroimage.2004.12.026) [DOI] [PubMed] [Google Scholar]
- 126.Birn RM, Diamond JB, Smith MA, Bandettini PA. 2006. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage 31, 1536–1548. ( 10.1016/j.neuroimage.2006.02.048) [DOI] [PubMed] [Google Scholar]
- 127.Wise RG, Ide K, Poulin MJ, Tracey I. 2004. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage 21, 1652–1664. ( 10.1016/j.neuroimage.2003.11.025) [DOI] [PubMed] [Google Scholar]
- 128.Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH. 2007. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage 38, 306–320. ( 10.1016/j.neuroimage.2007.07.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chang C, Cunningham JP, Glover GH. 2009. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage 44, 857–869. ( 10.1016/j.neuroimage.2008.09.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chang C, Glover GH. 2009. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage 47, 1448–1459. ( 10.1016/j.neuroimage.2009.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Falahpour M, Refai H, Bodurka J. 2013. Subject specific BOLD fMRI respiratory and cardiac response functions obtained from global signal. Neuroimage 72, 252–264. ( 10.1016/j.neuroimage.2013.01.050) [DOI] [PubMed] [Google Scholar]
- 132.Berntson GG, Cacioppo JT, Quigley KS. 1993. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology 30, 183–196. ( 10.1111/j.1469-8986.1993.tb01731.x) [DOI] [PubMed] [Google Scholar]
- 133.de Munck JC, Goncalves SI, Faes TJ, Kuijer JP, Pouwels PJ, Heethaar RM, Lopes da Silva FH. 2008. A study of the brain's resting state based on alpha band power, heart rate and fMRI. Neuroimage 42, 112–121. ( 10.1016/j.neuroimage.2008.04.244) [DOI] [PubMed] [Google Scholar]
- 134.Olbrich S, Mulert C, Karch S, Trenner M, Leicht G, Pogarell O, Hegerl U. 2009. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. Neuroimage 45, 319–332. ( 10.1016/j.neuroimage.2008.11.014) [DOI] [PubMed] [Google Scholar]
- 135.Henderson LA. et al. 2002. Brain responses associated with the Valsalva maneuver revealed by functional magnetic resonance imaging. J. Neurophysiol. 88, 3477–3486. ( 10.1152/jn.00107.2002) [DOI] [PubMed] [Google Scholar]
- 136.McKay LC, Adams L, Frackowiak RS, Corfield DR. 2008. A bilateral cortico-bulbar network associated with breath holding in humans, determined by functional magnetic resonance imaging. Neuroimage 40, 1824–1832. ( 10.1016/j.neuroimage.2008.01.058) [DOI] [PubMed] [Google Scholar]
- 137.Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. 2000. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J. Physiol. 523, 259–270. ( 10.1111/j.1469-7793.2000.t01-1-00259.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sander M, Macefield VG, Henderson LA. 2010. Cortical and brain stem changes in neural activity during static handgrip and postexercise ischemia in humans. J. Appl. Physiol. 108, 1691–1700. ( 10.1152/japplphysiol.91539.2008) [DOI] [PubMed] [Google Scholar]
- 139.Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. 2008. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage 42, 169–177. ( 10.1016/j.neuroimage.2008.04.238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fan J, Xu P, Van Dam NT, Eilam-Stock T, Gu X, Luo YJ, Hof PR. 2012. Spontaneous brain activity relates to autonomic arousal. J. Neurosci. 32, 11 176–11 186. ( 10.1523/JNEUROSCI.1172-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Critchley HD. 2002. Electrodermal responses: what happens in the brain. Neuroscientist 8, 132–142. ( 10.1177/107385840200800209) [DOI] [PubMed] [Google Scholar]
- 142.Iacovella V, Hasson U. 2011. The relationship between BOLD signal and autonomic nervous system functions: implications for processing of ‘physiological noise’. Magn. Reson. Imaging 29, 1338–1345. ( 10.1016/j.mri.2011.03.006) [DOI] [PubMed] [Google Scholar]
- 143.Birn RM, Murphy K, Handwerker DA, Bandettini PA. 2009. fMRI in the presence of task-correlated breathing variations. Neuroimage 47, 1092–1104. ( 10.1016/j.neuroimage.2009.05.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Birn RM, Smith MA, Jones TB, Bandettini PA. 2008. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage 40, 644–654. ( 10.1016/j.neuroimage.2007.11.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pattinson KT, Mitsis GD, Harvey AK, Jbabdi S, Dirckx S, Mayhew SD, Rogers R, Tracey I, Wise R. 2009. Determination of the human brainstem respiratory control network and its cortical connections in vivo using functional and structural imaging. Neuroimage 44, 295–305. ( 10.1016/j.neuroimage.2008.09.007) [DOI] [PubMed] [Google Scholar]
- 146.Birn RM, Murphy K, Bandettini PA. 2008. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Hum. Brain. Mapp. 29, 740–750. ( 10.1002/hbm.20577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kundu P. et al. 2013. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc. Natl Acad. Sci. USA 110, 16 187–16 192. ( 10.1073/pnas.1301725110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tanabe J, Miller D, Tregellas J, Freedman R, Meyer FG. 2002. Comparison of detrending methods for optimal fMRI preprocessing. Neuroimage 15, 902–907. ( 10.1006/nimg.2002.1053) [DOI] [PubMed] [Google Scholar]
- 149.Lund TE, Madsen KH, Sidaros K, Luo WL, Nichols TE. 2006. Non-white noise in fMRI: does modelling have an impact? Neuroimage 29, 54–66. ( 10.1016/j.neuroimage.2005.07.005) [DOI] [PubMed] [Google Scholar]
- 150.Behzadi Y, Restom K, Liau J, Liu TT. 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. ( 10.1016/j.neuroimage.2007.04.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Posse S. et al. 1999. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magn. Reson. Med. 42, 87–97. () [DOI] [PubMed] [Google Scholar]
- 152.Buur PF, Poser BA, Norris DG. 2009. A dual echo approach to removing motion artefacts in fMRI time series. NMR Biomed. 22, 551–560. ( 10.1002/nbm.1371) [DOI] [PubMed] [Google Scholar]
- 153.Speck O, Hennig J. 1998. Functional imaging by I0- and T2∗-parameter mapping using multi-image EPI. Magn. Reson. Med. 40, 243–248. ( 10.1002/mrm.1910400210) [DOI] [PubMed] [Google Scholar]
- 154.Evans JW, Kundu P, Horovitz SG, Bandettini PA. 2015. Separating slow BOLD from non-BOLD baseline drifts using multi-echo fMRI. Neuroimage 105, 189–197. ( 10.1016/j.neuroimage.2014.10.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Boyacioglu R, Schulz J, Koopmans PJ, Barth M, Norris DG. 2015. Improved sensitivity and specificity for resting state and task fMRI with multiband multi-echo EPI compared to multi-echo EPI at 7 T. Neuroimage 119, 352–361. ( 10.1016/j.neuroimage.2015.06.089) [DOI] [PubMed] [Google Scholar]
- 156.Olafsson V, Kundu P, Wong EC, Bandettini PA, Liu TT. 2015. Enhanced identification of BOLD-like components with multi-echo simultaneous multi-slice (MESMS) fMRI and multi-echo ICA. Neuroimage 112, 43–51. ( 10.1016/j.neuroimage.2015.02.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Barnes A, Bullmore ET, Suckling J. 2009. Endogenous human brain dynamics recover slowly following cognitive effort. PLoS ONE 4, e6626 ( 10.1371/journal.pone.0006626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fukunaga M. et al. 2006. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn. Reson. Imaging 24, 979–992. ( 10.1016/j.mri.2006.04.018) [DOI] [PubMed] [Google Scholar]
- 159.Hutchison RM. et al. 2013. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80, 360–378. ( 10.1016/j.neuroimage.2013.05.079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Calhoun VD, Miller R, Pearlson G, Adali T. 2014. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron 84, 262–274. ( 10.1016/j.neuron.2014.10.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chang C, Liu Z, Chen MC, Liu X, Duyn JH. 2013. EEG correlates of time-varying BOLD functional connectivity. Neuroimage 72, 227–236. ( 10.1016/j.neuroimage.2013.01.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. 2013. Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage 68, 93–104. ( 10.1016/j.neuroimage.2012.11.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tagliazucchi E, von Wegner F, Morzelewski A, Borisov S, Jahnke K, Laufs H. 2012. Automatic sleep staging using fMRI functional connectivity data. Neuroimage 63, 63–72. ( 10.1016/j.neuroimage.2012.06.036) [DOI] [PubMed] [Google Scholar]
- 164.Liu X, Chang C, Duyn JH. 2013. Decomposition of spontaneous brain activity into distinct fMRI co-activation patterns. Front. Syst. Neurosci. 7, 101 ( 10.3389/fnsys.2013.00101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Karahanoglu FI, Van De Ville D. 2015. Transient brain activity disentangles fMRI resting-state dynamics in terms of spatially and temporally overlapping networks. Nat. Commun. 6, 7751 ( 10.1038/ncomms8751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tong F, Pratte MS. 2012. Decoding patterns of human brain activity. Annu. Rev. Psychol. 63, 483–509. ( 10.1146/annurev-psych-120710-100412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pereira F, Mitchell T, Botvinick M. 2009. Machine learning classifiers and fMRI: a tutorial overview. Neuroimage 45 (Suppl. 1), S199–S209. ( 10.1016/j.neuroimage.2008.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kriegeskorte N, Goebel R, Bandettini P. 2006. Information-based functional brain mapping. Proc. Natl Acad. Sci. USA 103, 3863–3868. ( 10.1073/pnas.0600244103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kriegeskorte N, Cusack R, Bandettini P. 2010. How does an fMRI voxel sample the neuronal activity pattern: compact-kernel or complex spatiotemporal filter? Neuroimage 49, 1965–1976. ( 10.1016/j.neuroimage.2009.09.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Op de Beeck HP. 2010. Against hyperacuity in brain reading: spatial smoothing does not hurt multivariate fMRI analyses? Neuroimage 49, 1943–1948. ( 10.1016/j.neuroimage.2009.02.047) [DOI] [PubMed] [Google Scholar]
- 171.Shmuel A, Chaimow D, Raddatz G, Ugurbil K, Yacoub E. 2010. Mechanisms underlying decoding at 7 T: ocular dominance columns, broad structures, and macroscopic blood vessels in V1 convey information on the stimulated eye. Neuroimage 49, 1957–1964. ( 10.1016/j.neuroimage.2009.08.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Formisano E, Kriegeskorte N. 2012. Seeing patterns through the hemodynamic veil—the future of pattern-information fMRI. Neuroimage 62, 1249–1256. ( 10.1016/j.neuroimage.2012.02.078) [DOI] [PubMed] [Google Scholar]
- 173.Kriegeskorte N, Bandettini P. 2007. Analyzing for information, not activation, to exploit high-resolution fMRI. Neuroimage 38, 649–662. ( 10.1016/j.neuroimage.2007.02.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mandelkow H, de Zwart JA, Duyn JH. 2014. Does multivariate pattern analysis (MVPA) of BOLD fMRI data benefit from higher resolution at 7T? In Proc. 22nd Joint Annual Meeting of ISMRM–ESMRMB, Milan, Italy, 10–16 May. [Google Scholar]