Abstract
Aggregation behaviour is the tendency for animals to group together, which may have important consequences on individual fitness. We used a combination of experimental and simulation approaches to study how genetic variation and social environment interact to influence aggregation dynamics in Drosophila. To do this, we used two different natural lines of Drosophila that arise from a polymorphism in the foraging gene (rovers and sitters). We placed groups of flies in a heated arena. Flies could freely move towards one of two small, cooler refuge areas. In groups of the same strain, sitters had a greater tendency to aggregate. The observed behavioural variation was based on only two parameters: the probability of entering a refuge and the likelihood of choosing a refuge based on the number of individuals present. We then directly addressed how different strains interact by mixing rovers and sitters within a group. Aggregation behaviour of each line was strongly affected by the presence of the other strain, without changing the decision rules used by each. Individuals obeying local rules shaped complex group dynamics via a constant feedback loop between the individual and the group. This study could help to identify the circumstances under which particular group compositions may improve individual fitness through underlying aggregation mechanisms under specific environmental conditions.
Keywords: aggregation behaviour, Drosophila melanogaster, social interaction, foraging gene, interacting phenotypes
1. Introduction
Many animals aggregate—which means they are socially attracted by others and group together—and receive in return benefits such as reduced predation risk or better access to resources or mates. When individuals aggregate, they interact with other group members, which can change the behaviour of the group as a whole.
Adult fruit flies (Drosophila melanogaster) frequently aggregate on rotten fruit. The size and structure of these aggregates change over space and time [1,2]. Group formation presents an opportunity for various types of intra- and inter-sexual social interactions. When an individual encounters a group on a substrate, it may signal potential for sexual partners, oviposition sites or food resources. But it also means there will be competition for these resources. Deciding whether or not to join a group may therefore impact individual fitness [3].
Recent laboratory studies have investigated how group composition may modify individual phenotypes, and have detected strong impacts on traits including the circadian clock, sleep duration, pheromone synthesis and mating behaviour [4,5]. The nature of the effect on phenotype depends on the size and genetic diversity of the group. For example, increased group size can lead to increased sleep duration [6]. Mating behaviours increase in more genetically heterogeneous groups [7]. Behavioural modifications may then, in turn, affect group dynamics. In social spiders, groups composed of both social and asocial individuals forage more efficiently than groups of only one phenotype [8]—the two phenotypes interact [9], combining the individual decision rules used by each genetic variant.
In Drosophila, preference for different sizes of social aggregates appears to have a strong genetic basis, with some lines preferring to join small aggregates and others preferring to join larger ones [3]. In the common lizard Lacerta vivipara, less social individuals tend to leave larger groups to join smaller ones, while more social individuals do the reverse [10]. However, in previous studies, individuals chose between already formed, unbalanced groups—an experimental design that does not reveal the impact of initial conditions and subsequent dynamics of the aggregation process [11]. When given the choice between two equally rewarding, empty refuges, groups of cockroaches Blattella germanica tend to aggregate on a single refuge [12]. Small differences in initial stochastic individual distribution are amplified as subsequent cockroaches evaluate the groups, and as individuals stay longer within larger groups [13]. The individual decision rules that lead to this positive feedback cycle and ultimate asymmetrical distribution among groups depend primarily on two simple parameters: the probability of choosing a refuge depending on the relative number of individuals already present and the probability of leaving a refuge depending on the absolute number already present [14,15].
Few experiments have investigated how interactions among individuals with different decision rules will affect aggregation dynamics. The outcome of a group could reflect its composition in an exclusively additive way. In the nematode Caenorhabditis elegans there is a natural variant, linked to the expression of the npr-1 gene, that exhibits either solitary or gregarious patterns of foraging [16]. Solitary strains tend to forage evenly throughout the resource whereas gregarious strains tend to forage on the periphery of the resource [17]. When the two phenotypes are mixed, each keeps its own foraging pattern without being affected by interactions with the other [17]. In other situations, phenotypes can interact, with the behaviour of an individual influenced both by genetics and by the phenotype of other individuals in the population. Simple interaction among individuals that use different decision rules may lead to complex collective behaviours [18]. For instance, a very opinionated minority can dictate group choice in animals. However, if there are many uninformed individuals, their behaviour can counter the choice of the opinionated few [19].
Here, we investigated the impact of social environment, measured as group composition and group size, on aggregation tendency and decision rules. Like C. elegans, D. melanogaster exhibits a foraging polymorphism that affects both larvae and adults [20,21]. It has now been described in many other species and is known to have pleiotropic effects [22–24]. The foraging gene encodes for a c-GMP-dependent protein kinase (PKG) and presents two behavioural variants associated with allelic variation at the for locus: rover (forR) and sitter (forS) [25]. In natural populations, rovers and sitters coexist within the same populations [26], and laboratory experiments suggest that they could be maintained by frequency-dependent selection [27]. When on food, rovers tend to be very active foragers as larvae; as adults they disperse farther and more frequently than do sitters [20,21]. Larval and adult rovers leave food patches readily and explore more food patches, whereas sitters tend to remain on food patches [28]. There seems to be a strong connection between PKG activity and olfactory response to food, which in turn may lead to variation in aggregation patterns [29]. Variation in the tendency to aggregate may impact group dynamics and population structure, even in the absence of food, though this has not been studied.
Here, we used a combination of experimental and simulation approaches to ask the following questions. When facing several options, how does the composition of a group influence whether an individual will join it? How do these choices affect the dynamics of the overall group structure? Groups of adult flies were placed in a moderate aversive heated arena where they could freely move toward one of two small, cooler refuge areas. Our aim was to determine whether rovers and sitters differed in their tendency to aggregate, or group together in a single refuge zone, and how the social context affects aggregation. We first compared groups of rovers and sitters for their tendency to aggregate, and analysed how group size impacted this tendency. We then directly addressed how different strains interact by mixing rover and sitter flies within the same group and analysing the aggregation behaviour of each. We also used simulations to extract the decision rules at the level of the individual and to compare the behaviours that arise from these decision rules under different group sizes and compositions.
2. Material and methods
(a). Flies
We used two D. melanogaster lines, rover (forR) and sitter (forS), which differ as adults in their foraging activity and dispersal [20]. Rover and sitter individuals were initially selected from a natural population maintained by Marla B. Sokolowski at the University of Toronto [26]. The foraging (for) gene maps to the second chromosome. To partially control for common genetic background, genetic manipulation was done so that both lines share common forR-derived third chromosomes [30].
Flies were reared in a controlled temperature room (21°C) in tubes containing 40–60 individuals on standard medium (yeast–cornmeal agar). Only 7-day-old adult females were tested.
To prevent flies from escaping from the arena during the experiment, they were anaesthetized on ice and their wings were cut 24 h before the test. Then they were maintained in groups of 10 flies on standard medium.
(b). Heat maze apparatus
We used a heat maze apparatus that was originally developed to study spatial learning and search strategies in D. melanogaster [31]. This device uses the thermo-sensitivity of flies as a negative reinforcement. It is composed of an arena of 18 cm diameter surrounded by a metal ring 5 cm high that was heated by a wire at 60°C to prevent flies from escaping. Above this metal ring, there is a wall of white paper 20 cm high that hides any spatial cues that flies could use to orient themselves.
To control the ground temperature, the arena was composed of 31 Peltier elements (organized in a 5 × 5 grid; 23 measured 4 × 4 cm and 8 measured 2 × 2 cm). Twenty-nine Peltier elements were maintained at 30°C, which is a high but non-lethal temperature for flies, and serves as weak negative reinforcement. The last two Peltier elements were maintained at 25°C and served as refuge zones. The two refuge zones were diametrically separated by 8.5 cm. Each refuge zone can easily support 20 individuals, but could be limiting with 30 individuals (A. S. Philippe, personal communication). On top of this grid of Peltier elements, we put a thermally conductive plastic sheet. We were able to wash this floor surface to remove potential odour cues and the surface-masked visual cues that might have allowed flies to distinguish between the safe and unsafe zones. The heat maze apparatus was placed in a wooden box to limit distant visual cues and maintain a constant temperature of 28 ± 2°C.
For each experiment, groups of flies were simultaneously placed in one of the two quadrants that did not contain a refuge zone. A webcam was coupled to a computer to record the arena during the 20 min trials. We analysed all videos, noting the time and the number of individuals on the safe zones every time an individual left or entered a refuge zone. At the end of an experiment, we removed the flies and washed the floor of the arena.
We performed three experiments simultaneously. In the first, there was only one refuge zone (the other refuge was set to 30°C). We used this set-up to test the aggregation behaviour of flies in groups of one to six individuals (18 replicates each). In the second and the third experiments, there were two refuges. In the second experiment, groups of flies included 1, 2, 6, 10, 20 or 30 individuals from a single strain. In the third experiment, groups of flies were composed of 20 rovers, 15 rovers and 5 sitters, 10 rovers and 10 sitters, 5 rovers and 15 sitters, or 20 sitters. To distinguish individual rovers and sitters during the third experiment, we randomly marked individuals from one strain with a coloured dot on the thorax. Marking the flies did not affect their aggregation tendency.
(c). Model description
We developed a simple numerical model (Monte Carlo simulations) to extract the behavioural rules from homogeneous groups of the two strains and predict how mixed groups of rovers and sitters should distribute themselves between the two refuge zones if there is no behavioural interaction genotypic changes. The Monte Carlo method allowed us to obtain simulated results that could be compared with empirical data. Thus, the model used only parameters derived from experimental data. A complete description of the model can be found in the electronic supplementary material. Briefly, in our model, we considered three compartments: the two refuge zones and the rest of the arena. At the beginning of a simulation run, flies were in the arena outside of the refuge zones. At each time step, flies could either join or leave a refuge zone with certain probabilities, depending on the number of other individuals in the zone. The probability of leaving a refuge was calculated from experimental data of homogeneous groups of one to six flies and in the presence of a single refuge zone. The same probability of leaving a refuge was used for groups of more than six individuals.
To assess the probability that an individual will leave a refuge zone, we measured both how long a single individual stays in a refuge zone and how long aggregates of different sizes remain within the refuge zone, or the ‘lifetime’ of the aggregate. We defined the lifetime of an aggregate as the time between the arrival of one fly and the spontaneous departure of any individual from the refuge zone (e.g. we used groups of four individuals to assess the lifetime of aggregates of four on the refuge zone).
Using the data from experiments with two refuge zones, we calculated the probability of joining a specific refuge based on the relative number of individuals already present on that refuge.
Simulations were first done to determine the distribution of groups of 10, 20 and 30 rovers or sitters between the two refuge zones. We then performed simulations to predict the distribution of individuals in mixed groups of rovers and sitters. Note that all behavioural rules implemented in the simulations were derived from empirical data collected exclusively in homogeneous groups of rovers or sitters (see details in the electronic supplementary material).
(d). Data analysis
All experimental data analyses were done using SPSS statistics v. 20. For each experiment, we measured the number of individuals on the two refuge zones at the end of the experiment (1200 s) and calculated the proportion of individuals on the most crowded refuge zone.
For the experimental portion of our study, we used a generalized linear model for proportional data, fixing the over-dispersion of the data using quasi-binomial error distribution and ‘Probit’ link function to determine whether the social context (group size variation) and the genetic variation (rover and sitter lines) affected aggregation behaviour.
3. Results
(a). A single choice experiment of aggregation behaviour in homogeneous groups
We placed between one and six flies in an arena with only one refuge zone for 20 min. The distribution of stay time (as survival analysis) of single individuals and of the lifetime of aggregates was characterized by a fast decay followed by a slow decay (electronic supplementary material, figure S1). This suggested that the probability of leaving an aggregate decreased with the time spent in the aggregate.
For both rovers and sitters, the individual tendency to leave a refuge zone decreased with the number of conspecifics on the refuge (figure 1). When alone, rover individuals had a higher probability of leaving than sitters. However, the probability of leaving decreased dramatically for both as soon as two individuals were in the refuge zone.
Figure 1.
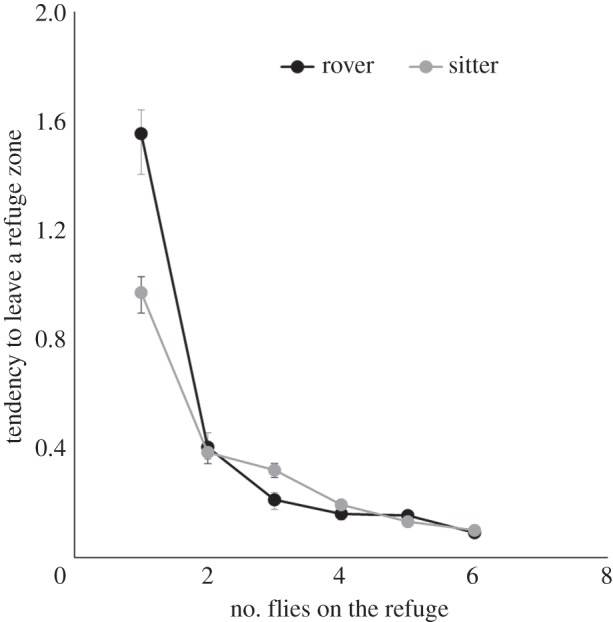
Variation in the estimated tendency to leave a refuge zone as a function of group size on the refuge in a single refuge zone experiment (error bars: ±95% CI).
(b). Aggregation behaviour of homogeneous groups in a dual choice experiment
In a second experiment, 1, 2, 6, 10, 20 or 30 rover or sitter flies were placed in an arena with two refuge zones for 20 min.
(i). Probability of joining a refuge zone when alone
We assessed the individual probability of joining a refuge zone by introducing a single fly into an arena with two refuge zones. The survival curves of the time spent in the arena between two successive visits of each refuge also showed a bilinear pattern (electronic supplementary material, figure S2). The number of observations where a fly was in the arena decreased slowly at first, meaning that few flies initially went on refuge zones. After 50 s, the proportion of lone flies moving to one of the refuge zones increased dramatically. Rovers tended to join refuge zones faster than did sitters.
(ii). Probability of joining a refuge zone when in groups
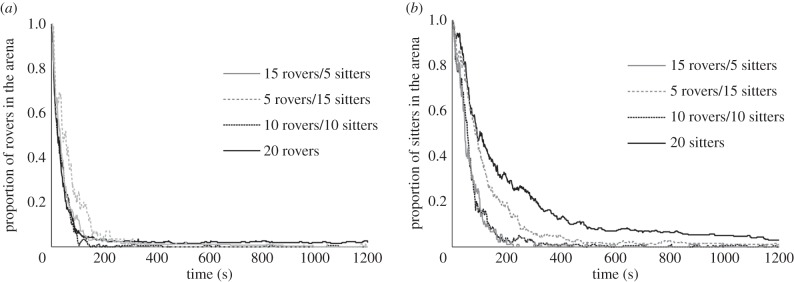
Interestingly, rovers and sitters in groups showed different aggregation dynamics. Rovers entered the refuge zones much faster than did sitters (comparison of half-life with group size > 6: F1,86 = 21.43; p < 10−3; figure 2 for groups of 20 individuals). Group sizes of 10, 20 or 30 individuals did not differ in their dynamics for either rovers or sitters (comparison of half-life: F1,86 = 0.02; p = 0.87).
Figure 2.
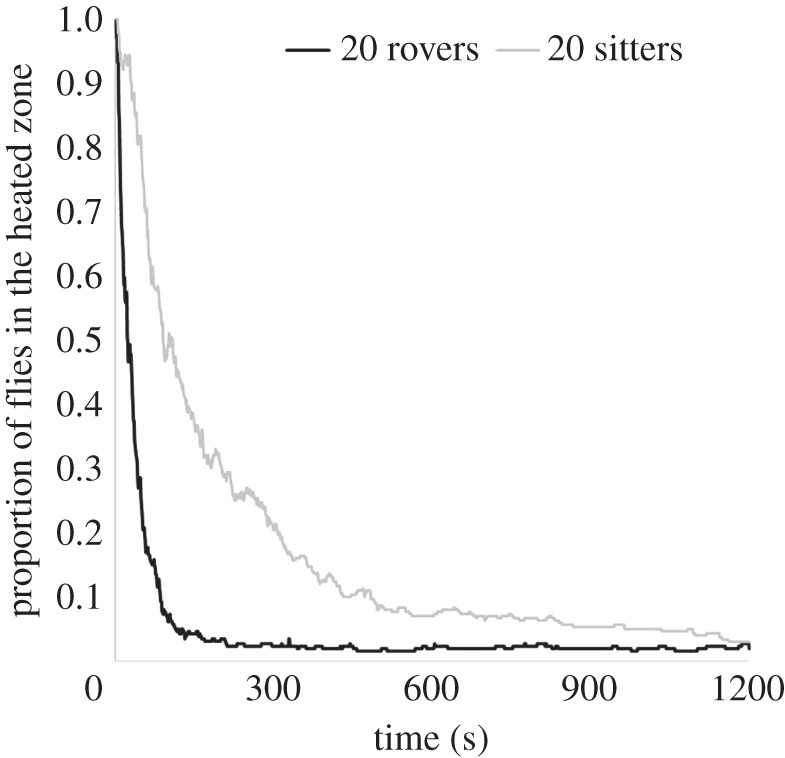
Proportion of flies that remained in the heated zone as a function of time for homogeneous groups of 20 individuals (n = 18).
(iii). Probability of choosing a refuge zone
The proportion of individuals on the most crowded zone at the end of each experiment was negatively related to group size (group size effect: t = −5.902; p < 10−3). For small groups (two to six), most rover or sitter individuals aggregated on the same refuge zone (figure 3). However, for bigger groups, rover individuals tended to distribute themselves more evenly on the two refuge zones, while sitters tended to aggregate together on a single refuge zone (figure 3; line effect: t = 3.769; p < 10−3). There was a quantitative agreement in the experimental and simulated scores of aggregation for groups of 10, 20 and 30 rovers and sitters (electronic supplementary material, figure S4).
Figure 3.
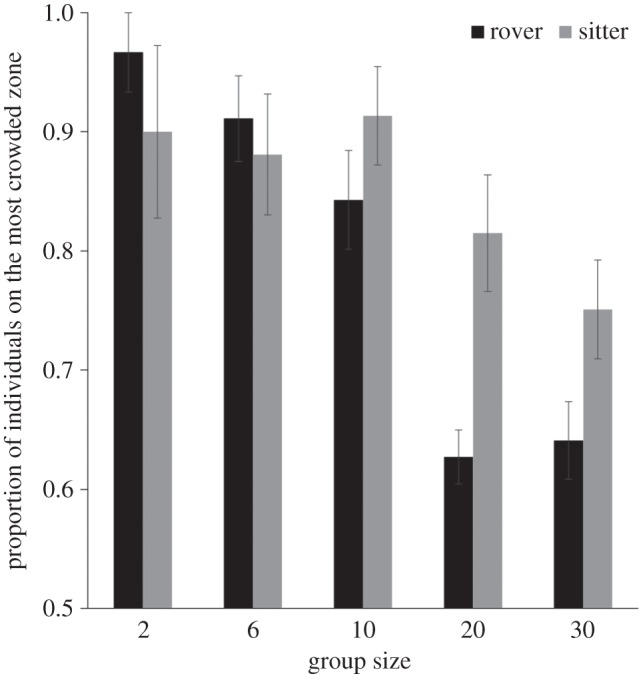
Proportion of individuals on the most crowded refuge zone as a function of group size in the dual choice experiment (n = 18; mean ± s.e.m.).
To assess the probability that an individual fly would choose a refuge zone as a function of the number of individuals already present, we used the data from the experiments with two refuge zones and groups of 10, 20 and 30 individuals for each strain. For each combination of individuals on each refuge zone (for example, two flies on one refuge and four on the other one), we determined the proportion of individuals that joined each refuge zone. In sitters, the probability of joining the more crowded refuge zone increased linearly as the difference between the number of individuals in the refuge zones increased (figure 4; linear regression: F1,8 = 26.48, p < 10−3, R2 = 0.74). By contrast, rovers were not more likely to join the more crowded refuge zone (linear regression: F1,8 = 0.35, p = 0.57, R2 = −0.08). This shows that sitters have a stronger tendency to aggregate than do rovers.
Figure 4.
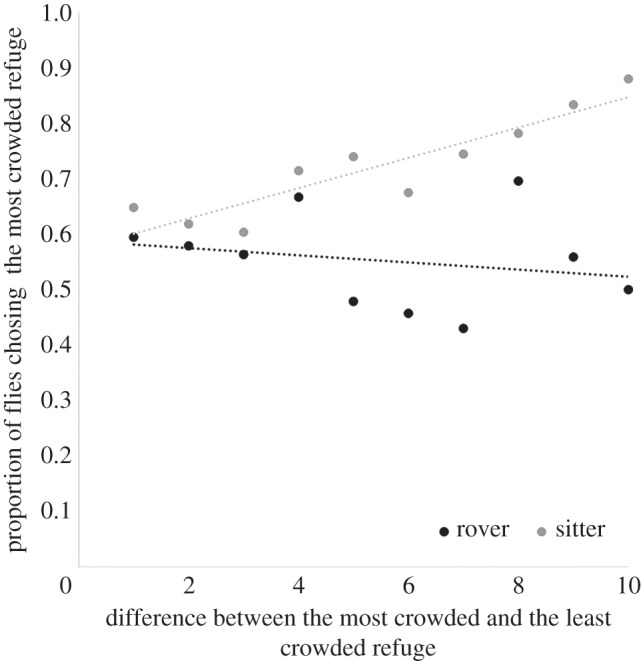
Proportion of flies that chose the most crowded refuge zone as a function of the difference in the number of individuals already present in the two zones (n = 18).
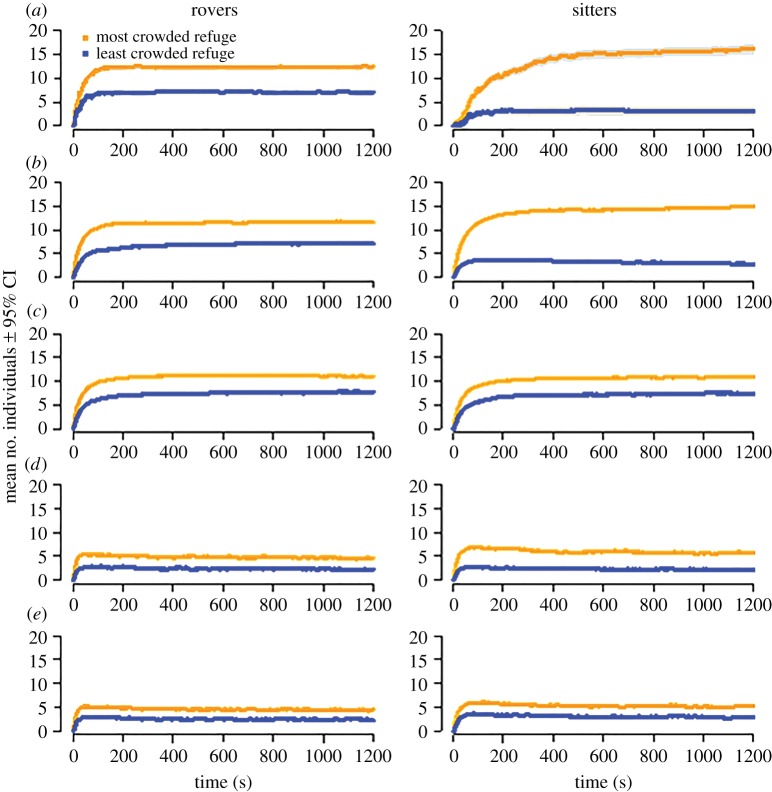
Overall, the model revealed that the behaviour of rovers and sitters can be explained by two necessary and sufficient parameters: (i) aggregation requires modulation of resting time as a function of the number of conspecifics present on a refuge and (ii) the difference between rovers and sitters in how asymmetrically they distribute themselves between refuges relies on the ability of sitters to select the more crowded refuge (figure 5).
Figure 5.
Experimental and theoretical dynamics of aggregation in groups of 20 flies in the presence of two refuges. (a) Experimental data. (b–e) Theoretical data (n = 200 simulations for each condition). (b) Influence of group size on the probabilities of leaving and of choosing a refuge. (c) Influence of group size on the probability of leaving a refuge, not on the probability of choosing a refuge. (d) Influence of group size on the probability of choosing a refuge not on the probability of leaving a refuge. (e) No influence of group size on the probabilities of leaving or choosing a refuge (mean ± 95% CI). (Online version in colour.)
(c). Aggregation behaviour in mixed groups of rovers and sitters
Aggregation behaviour depended on the proportion of rovers and sitters in a group: the more rover phenotypes present within a group, the faster flies tended to join a refuge, but the fewer flies tended to group on a single refuge (z = 4.3; p = 0.03; figure 6). The simulated and experimental distributions of individuals in mixed groups in the two refuge zones clearly agreed quantitatively (electronic supplementary material, figure S5).
Figure 6.
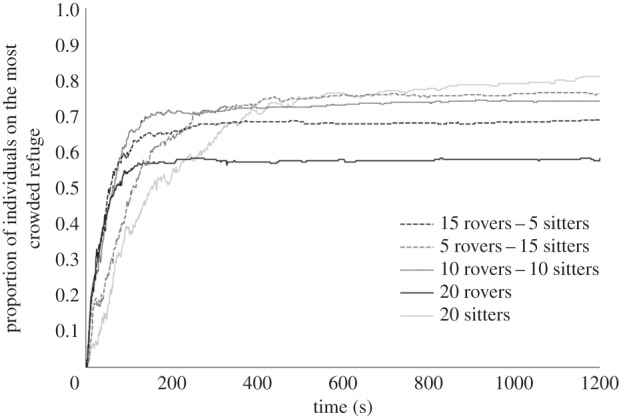
Number of flies on the most crowded refuge zone as a function of time and group composition (n = 18).
Interestingly, when analysed separately, we observed that group composition had a different effect on the aggregation profile of rovers than it did on sitters. First, rovers tended to take more time to join a refuge zone when they were in a group that had a strong excess of sitters (figure 7a; half-life comparison: F1,66 = 11.73; p = 0.001). However, sitters tended to join a refuge zone much faster when they were in a group that also had rovers, even in a small proportion (figure 7b; half-life comparison: F1,66 = 9.57; p = 0.003). Second, the number of rovers within a group affected the proportion of individuals of each phenotype within the most crowded group. This effect was stronger for rovers (number of rovers: z = 82.9; p < 10−3; line: z = 28.3; p < 10−3; line × number of rovers: z = 58.5; p < 10−3; figure 8). Again, experimental and simulation results strongly converged (electronic supplementary material, figure S6). This suggests that, despite the observed variation in aggregation tendency for rovers and sitters in mixed groups compared with homogeneous groups, the behavioural rules used by individual rovers and sitters were not changed.
Figure 7.
Proportion of flies (number of rovers or sitters present in the arena/total number of rovers or sitters) that are still present in the arena as a function of time and group composition for (a) rovers and (b) sitters (n = 18).
Figure 8.
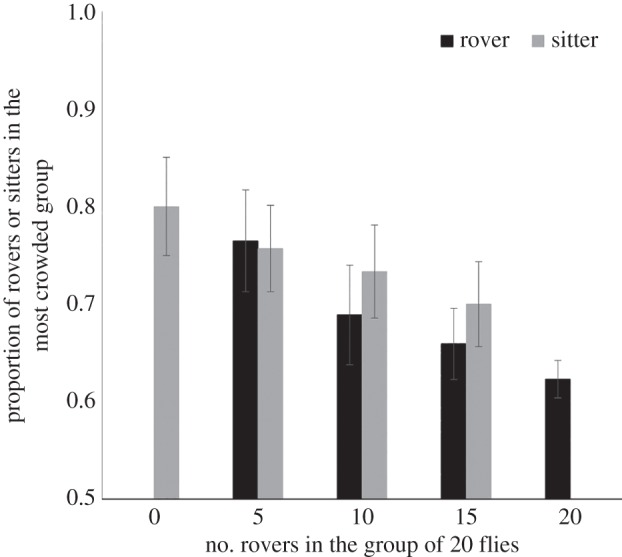
Aggregation level of rovers or sitters (number of rovers or sitters present in the most crowded group/total number of rovers or sitters) as a function of their proportion in a group of 20 individuals (n = 18, mean ± s.e.m.).
4. Discussion
Very few studies have focused on social dynamics within the framework of collective animal behaviour [11,32]. Our study aimed to investigate how social environment may affect decision rules and aggregation patterns using D. melanogaster as a model species. Our experimental and simulation data agreed strongly, and revealed strong differences in aggregation behaviour between rovers and sitters that can be explained by few simple parameters. Two different strategies with different dynamics were driven by the following two parameters: the probability of entering a refuge and the choice of the refuge based on the number of individuals present.
When tested in homogeneous groups with a single refuge, the presence of other individuals in a refuge induced newcomers to increase their resting time in that refuge. This led to a positive feedback cycle: the more individuals in a refuge, the more each of them tended to stay in the refuge. Our results are consistent with previous studies on cockroaches [13] and ants [33], and the mechanism is typical of self-organized behaviours such as an ‘all or nothing’ or snowball effect [11], where we do not observe a normal (uniform or Gaussian) distribution between two alternatives (here the two refuges) but a U-shaped distribution (all individuals choosing one alternative and none the other). Interestingly, this social effect was stronger for rovers than sitters: when tested alone rovers tended to leave the refuge much more often than sitters, but when tested in groups there was no difference between rovers and sitters.
When given the choice between two refuge zones separated by only 8.5 cm, we observed non-random distribution of the flies and variation in aggregation dynamics that depended on social parameters. Sitters tended to choose the more crowded refuge. Interestingly, this choice depended on the difference in density of individuals on the two refuges, and it therefore reinforced the tendency to aggregate. Rovers, on the other hand, always had a slight tendency to choose the more crowded refuge (approx. 60% of the time), but this did not depend on the difference in density of individuals on the two refuges. Sitters chose to aggregate in a progressive way, leading to an unbalanced distribution of flies on the two refuges. Rovers rapidly chose a refuge, leading to a more even distribution of individuals between the two refuges. This was particularly pronounced in groups of 20 or more individuals. Further studies should investigate how differences in the genetic background of the two variants impact the sensorial modalities of each line and determine their sensitivity to conspecifics. Interestingly, the majority of the for-expressing nerves terminate in the antennal mechanosensory brain region of the fly [34]. Recent work [32] showed a link between the formation of collective behaviour in Drosophila and activity of these mechanosensory sensilla neurons. Whether the observed variation in aggregation dynamics is restricted to variation in the activity of these neurons between rovers and sitters remains to be investigated.
When groups of flies were composed of both rover and sitter individuals (as seen in natural populations [35]), aggregation behaviour was strongly affected by the proportion of each strain, and reflected their respective behaviour that was observed when they were tested in homogeneous groups. This is reminiscent of the quorum sensing or majority rule process underlying collective decision-making during group movement [19]. However, we observed interactions between phenotypes at the individual level. When rovers were in the minority, they tended to respond like sitters (they were more likely to aggregate on the more crowded refuge). When sitters were in the minority, they tended to respond like rovers (they joined a refuge more quickly and were less likely to aggregate on the more crowded refuge). We propose the following scenario based on a two-sided interaction. (i) When sitters are in the minority, rovers distribute between refuges more evenly. This more even distribution prevents sitters from disproportionately choosing the more crowded refuge, which would also lead to a lower aggregation profile. (ii) When rovers are in the minority, sitters are progressively more likely to aggregate on the more crowded refuge. Rovers have a weak tendency to aggregate on the more crowded refuge, independent of how evenly other individuals are distributed between refuges. The distribution of rovers between refuges becomes more uneven when they are in a mixed group than when they are in a homogeneous group.
This double-sided interaction does not require any particular modulation of individual decision rules in a mixed group, consistent with the principle of self-organized processes [11]: individuals do not need to recognize the presence of another phenotype and adjust their behaviour accordingly. The observed differences in behaviour are based on the differential dynamics of aggregation, which interact with and affect the behaviour of others, leading to a separate group behaviour. This suggests that interacting phenotypes—or the modification of individual behaviour as a function of group composition—may emerge even without any modification of individual decision rules and inter-individual recognition. Individuals follow the same rules whatever the group composition, but the pattern emerging from social interactions is more complex than the sum of individual behaviours. Interestingly, a similar pattern was found in anti-predator behaviour in guppies Poecilia reticulata [36]. When two guppy strains were mixed, the observed group behaviour directly reflected the additive behaviour of each strain; however, at the individual level each individual responded to the specific social environment.
Interaction between rovers and sitters led to a decrease in the aggregation level of sitters but an increase in the aggregation level of rovers, resulting in additive global aggregation level. These results suggest a mechanism by which interactions, even between different phenotypes, can even out the differences observed at the individual level and induce groups to behave homogeneously [37]. One should note the dissimilarity between the homogeneous behaviour observed here and the behaviour observed previously in C. elegans [16], for example, where the interaction of two similar behavioural gene backgrounds with different aggregation behaviour did not lead to collective behaviour—each strain kept its own aggregation tendency. The conditions under which homogeneous versus non-homogeneous behaviour is induced require further investigation.
This study revealed an interaction between genetic variation of a group and individual behaviour, which in turn affects global collective decision-making. We show that line-dependent individual aggregation rules shape complex group dynamics via a constant feedback loop between the individual and the group. Our approach contributes to a proximal understanding of the impact of genetic variation on the emergence of collective behaviours, and highlights potential mechanisms that might have supported evolutionary processes and the diversification of behavioural phenotypes. The strong impact of individual response to social environment may raise questions about the process and trajectory of behavioural evolution [38]. When individuals behave the same way independent of group composition, evolution of the individual behaviour can directly predict the evolution of the group behaviour. However, the presence of interacting phenotypes may decouple these two levels of selection and, depending on the relative strength of selection on each level, induce different evolutionary outcomes. In a study a social spiders [8], one mechanism by which behavioural variation could be maintained is by complementary phenotypes, where the strategies of some individuals are facilitated by the presence of others. How interaction between genotypes and group phenotypic composition underlies selection and evolution of species needs further consideration.
Supplementary Material
Acknowledgements
We thank S. Wardrop for useful corrections on the manuscript. We thank Dr V. Orgogozo and two anonymous reviewers for their very constructive comments on previous version of the manuscript.
Data accessibility
All data are deposited in the Dryad repository: http://dx.doi.org/10.5061/dryad.n9m02.
Authors' contributions
A.-S.P. and F.M. designed the experiments; A.-S.P. conducted the experiments; R.J. and F.R. conducted the simulations; A.-S.P., C.P. and F.M. analysed the data; A.-S.P., R.J., C.P., F.R., C.S. and F.M. wrote the manuscript. All the authors gave final approval for publication.
Competing interests
Authors declare no competing interests.
Funding
This project is funded by an ANR programme blanc (ANR 12 BSV7 0013 02) to F.M. and C.S. C.S is funded by the University of Strasbourg Institute for Advanced Study (USIAS) and the Fyssen Foundation.
References
- 1.Stamps J, Buechner M, Alexander K, Davis J, Zuniga N. 2005. Genotypic differences in space use and movement patterns in Drosophila melanogaster. Anim. Behav. 70, 609–618. ( 10.1016/j.anbehav.2004.11.018) [DOI] [Google Scholar]
- 2.Wertheim B, Dicke M, Vet L. 2002. Behavioural plasticity in support of a benefit for aggregation pheromone use in Drosophila melanogaster. Entomol. Exp. Appl. 103, 61–71. ( 10.1046/j.1570-7458.2002.00954.x) [DOI] [Google Scholar]
- 3.Saltz JB. 2011. Natural genetic variation in social environment choice: context-dependent gene–environment correlation in Drosophila melanogaster. Evolution 65, 2325–2334. ( 10.1111/j.1558-5646.2011.01295.x) [DOI] [PubMed] [Google Scholar]
- 4.Kent C, Azanchi R, Smith B, Formosa A, Levine JD. 2008. Social context influences chemical communication in D. melanogaster males. Curr. Biol. 18, 1384–1389. ( 10.1016/j.cub.2008.07.088) [DOI] [PubMed] [Google Scholar]
- 5.Levine JD. 2002. Resetting the circadian clock by social experience in Drosophila melanogaster. Science 298, 2010–2012. ( 10.1126/science.1076008) [DOI] [PubMed] [Google Scholar]
- 6.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. 2006. Waking experience affects sleep need in Drosophila. Science 313, 1775–1781. ( 10.1126/science.1130408) [DOI] [PubMed] [Google Scholar]
- 7.Krupp JJ, Kent C, Billeter J-C, Azanchi R, So AKC, Schonfeld JA, Smith BP, Lucas C, Levine JD. 2008. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr. Biol. 18, 1373–1383. ( 10.1016/j.cub.2008.07.089) [DOI] [PubMed] [Google Scholar]
- 8.Pruitt JN, Riechert SE. 2011. How within-group behavioural variation and task efficiency enhance fitness in a social group. Proc. R. Soc. B 278, 1209–1215. ( 10.1098/rspb.2010.1700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore AJ, Brodie ED III, Wolf JB. 1997. Interacting phenotypes and the evolutionary process. I. Direct and indirect genetic effects of social interactions. Evolution 51, 1352–1362. ( 10.2307/2411187) [DOI] [PubMed] [Google Scholar]
- 10.Cote J, Clobert J. 2007. Social personalities influence natal dispersal in a lizard. Proc. R. Soc. B 274, 383–390. ( 10.1098/rspb.2006.3734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumpter DJT. 2006. The principles of collective animal behaviour. Phil. Trans. R. Soc. B 361, 5–22. ( 10.1098/rstb.2005.1733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ame J, Rivault C, Deneubourg J. 2004. Cockroach aggregation based on strain odour recognition. Anim. Behav. 68, 793–801. ( 10.1016/j.anbehav.2004.01.009) [DOI] [Google Scholar]
- 13.Jeanson R, Rivault C, Deneubourg J-L, Blanco S, Fournier R, Jost C, Theraulaz G. 2005. Self-organized aggregation in cockroaches. Anim. Behav. 69, 169–180. ( 10.1016/j.anbehav.2004.02.009) [DOI] [Google Scholar]
- 14.Jeanson R, Deneubourg JL. 2007. Conspecific attraction and shelter selection in gregarious insects. Am. Nat. 170, 47–58. ( 10.1086/518570) [DOI] [PubMed] [Google Scholar]
- 15.Halloy J, et al. 2007. Social integration of robots into groups of cockroaches to control self-organized choices. Science 318, 1155–1158. ( 10.1126/science.1144259) [DOI] [PubMed] [Google Scholar]
- 16.de Bono M, Bargmann C. 1998. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell 94, 679–689. ( 10.1016/S0092-8674(00)81609-8) [DOI] [PubMed] [Google Scholar]
- 17.Gloria-Soria A, Azevedo RB. 2008. npr-1 Regulates foraging and dispersal strategies in Caenorhabditis elegans. Curr. Biol. 18, 1694–1699. ( 10.1016/j.cub.2008.09.043) [DOI] [PubMed] [Google Scholar]
- 18.Farine DR, Montiglio P-O, Spiegel O. 2015. From individuals to groups and back: the evolutionary implications of group phenotypic composition. Trends Ecol. Evol. 30, 609–621. ( 10.1016/j.tree.2015.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couzin ID, Ioannou CC, Demirel G, Gross T, Torney CJ, Hartnett A, Conradt L, Levin SA, Leonard NE. 2011. Uninformed individuals promote democratic consensus in animal groups. Science 334, 1578–1580. ( 10.1126/science.1210280) [DOI] [PubMed] [Google Scholar]
- 20.Edelsparre AH, Vesterberg A, Lim JH, Anwari M, Fitzpatrick MJ. 2014. Alleles underlying larval foraging behaviour influence adult dispersal in nature. Ecol. Lett. 17, 333–339. ( 10.1111/ele.12234) [DOI] [PubMed] [Google Scholar]
- 21.Pereira HS, Sokolowski MB. 1993. Mutations in the larval foraging gene affect adult locomotory behavior after feeding in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 90, 5044–5046. ( 10.1073/pnas.90.11.5044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben-Shahar Y. 2005. The foraging gene, behavioral plasticity, and honeybee division of labor. J. Comp. Physiol. A 191, 987–994. ( 10.1007/s00359-005-0025-1) [DOI] [PubMed] [Google Scholar]
- 23.Ingram KK, Oefner P, Gordon DM. 2005. Task-specific expression of the foraging gene in harvester ants. Mol. Ecol. 14, 813–818. ( 10.1111/j.1365-294X.2005.02450.x) [DOI] [PubMed] [Google Scholar]
- 24.Sokolowski MB. 1998. Genes for normal behavioral variation: recent clues from flies and worms. Neuron 21, 463–466. ( 10.1016/s0896-6273(00)80556-5) [DOI] [PubMed] [Google Scholar]
- 25.Osborne KA, Robichon A, Burgess E, Butland S, Shaw RA, Coulthard A, Pereira HS, Greenspan RJ, Sokolowski MB. 1997. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science 277, 834–836. ( 10.1126/science.277.5327.834) [DOI] [PubMed] [Google Scholar]
- 26.Sokolowski MB. 1980. Foraging strategies of Drosophila melanogaster—a chromosomal analysis. Behav. Genet. 10, 291–302. ( 10.1007/bf01067774) [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick MJ, Feder E, Rowe L, Sokolowski MB. 2007. Maintaining a behaviour polymorphism by frequency-dependent selection on a single gene. Nature 447, U210–U215. ( 10.1038/nature05764) [DOI] [PubMed] [Google Scholar]
- 28.Tortorici C, Bell W. 1988. Search orientation in adult Drosophila melanogaster: responses of rovers and sitters to resource dispersion in a food patch. J. Insect Behav. 1, 209–223. ( 10.1007/bf01052239) [DOI] [Google Scholar]
- 29.Shaver S, Varnam C, Hilliker A, Sokolowski M. 1998. The foraging gene affects adult but not larval olfactory-related behavior in Drosophila melanogaster. Behav. Brain Res. 95, 23–29. ( 10.1016/S0166-4328(97)00206-4) [DOI] [PubMed] [Google Scholar]
- 30.Debelle JS, Hilliker AJ, Sokolowski MB. 1987. Genetic localization of the rover sitter larval foraging polymorphism in Drosophila melanogaster. Behav. Genet. 17, 620. [Google Scholar]
- 31.Foucaud J, Burns JG, Mery F. 2010. Use of spatial information and search strategies in a water maze analog in Drosophila melanogaster. PLoS ONE 5, e0015231 ( 10.1371/journal.pone.0015231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramdya P, Lichocki P, Cruchet S, Frisch L, Tse W, Floreano D, Benton R. 2015. Mechanosensory interactions drive collective behaviour in Drosophila. Nature 519, 233–236. ( 10.1038/nature14024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sumpter DJ, Beekman M. 2003. From nonlinearity to optimality: pheromone trail foraging by ants. Anim. Behav. 66, 273–280. ( 10.1006/anbe.2003.2224) [DOI] [Google Scholar]
- 34.Belay AT, Scheiner R, So AKC, Douglas SJ, Chakaborty-Chatterjee M, Levine JD, Sokolowski MB. 2007. The foraging gene of Drosophila melanogaster: spatial-expression analysis and sucrose responsiveness. J. Comp. Neurol. 504, 570–582. ( 10.1002/cne.21466) [DOI] [PubMed] [Google Scholar]
- 35.Sokolowski MB, Pereira HS, Hughes K. 1997. Evolution of foraging behavior in Drosophila by density-dependent selection. Proc. Natl Acad. Sci. USA 94, 7373–7377. ( 10.1073/pnas.94.14.7373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bleakley B, Parker D, Brodie E. 2007. Nonadditive effects of group membership can lead to additive group phenotypes for anti-predator behaviour of guppies, Poecilia reticulata. J. Evol. Biol. 20, 1375–1384. ( 10.1111/j.1420-9101.2007.01342.x) [DOI] [PubMed] [Google Scholar]
- 37.Dussutour A, Nicolis SC, Despland E, Simpson SJ. 2008. Individual differences influence collective behaviour in social caterpillars. Anim. Behav. 76, 5–16. ( 10.1016/j.anbehav.2007.12.009) [DOI] [Google Scholar]
- 38.Agrawal AF, Brodie ED III, Wade MJ. 2001. On indirect genetic effects in structured populations. Am. Nat. 158, 308–323. ( 10.1086/321324) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are deposited in the Dryad repository: http://dx.doi.org/10.5061/dryad.n9m02.