Abstract
In this study, we investigated two potentially important intersexual postcopulatory gametic interactions in a population of chinook salmon (Oncorhynchus tshawytscha): (i) the effect of female ovarian fluid (OF) on the behaviour of spermatozoa during fertilization and (ii) the effects of multilocus heterozygosity (MLH) (as an index of male quality) and female–male genetic relatedness on sperm behaviour and male fertilization success when there is sperm competition in the presence of that OF. To do this, we conducted a series of in vitro competitive fertilization experiments and found that, when ejaculates from two males are competing for access to a single female's unfertilized eggs, fertilization success was significantly biased towards the male whose sperm swam fastest in the female's OF. Embryo survival—a measure of fitness—was also positively correlated with both sperm swimming speed in OF and male MLH, providing novel evidence that cryptic female choice is adaptive for the female, enhancing the early survival of her offspring and potentially influencing her fitness.
Keywords: cryptic female choice, sperm competition, ovarian fluid, sexual selection, embryo survival, salmon
1. Introduction
Two mechanisms of postcopulatory sexual selection—sperm competition and cryptic female choice (CFC)—are major determinants of fertilization success in many taxa [1–4]. The gametic interactions between two (or more) males and a given female, for example, has led to adaptations that enhance a male's ability to compete with a rival [2,5–10]. Females are also able to bias the outcome of sperm competition by differentially enhancing the fertilization success of ‘chosen’ males, a process known as CFC [1]. There is now convincing evidence to suggest that, in species with either internal or external fertilization, male–female compatibility at the gamete-level biases fertilization towards those males most likely to maximize offspring fitness [11–13]. Thus, for example, when there is a risk of inbreeding by close genetic relatives, there is often gamete incompatibility [14–17].
Despite the recognized importance of CFC on fertilization success under sperm competition, relatively little is known about how CFC is mediated, nor is there clear evidence that such choice enhances female fitness. The identification of mechanisms that allow females to promote fertilization after copulation or spawning have only been identified in a handful of taxa [14,18–20], in part because it is notoriously difficult to establish that CFC has in fact occurred. Particularly in internal fertilizers, for example, it is near impossible to visualize and measure egg and sperm interactions under natural conditions [21,22]. Moreover, variation in the quality of a male's sperm may confound the process of CFC as sperm traits alone can also influence a male's fertilization success [2,5–10].
Some of the clearest examples of CFC have been observed in broadcast spawning species, where females differentially favour fertilization by sperm from certain males. For example, in a hermaphroditic ascidian, Diplosoma listerianum, self-fertilization of ova is avoided by discrimination against sperm that are genetically similar to the individual being fertilized [23]. Likewise, the fertilization of sea urchin (Echinometra sp.) eggs can only be achieved by sperm containing a bindin genotype compatible with their own receptor, which acts to limit hybridization and enhance local adaptation [24–26].
In this study, we build upon our previous work [27] using an externally fertilizing fish species, chinook salmon, Oncorhynchus tshawytscha, to explore the mechanism of CFC at the gamete level, and to determine whether CFC has any clear fitness consequences for the female. In this system, males often mate in a competitive environment during spawning [28], such that postcopulatory selection can act on both males and females.
In salmonids, a viscous ovarian fluid (OF) surrounds the unfertilized ova and is released with those ova into fresh or salt water during spawning. This OF comprises 10–30% of the volume of the spawned egg mass [29]. Spermatozoa likely encounter increasing concentrations of OF as they approach an unfertilized ovum, with the highest concentration on the ovum's porous outer shell and inside its micropyle [27].
In chinook salmon (and other fishes), sperm swimming speed is an important predictor of sperm competitiveness, and thus paternity success, under conditions similar to those in the natural spawning environment [5–7]. When the spermatozoa come into contact with OF, sperm performance is enhanced: sperm swim faster, survive longer, and swim in a more linear trajectory compared with sperm behaviour measured in water alone [30]. The positive effect of OF on sperm behaviour is now well studied in a number of fish species [20,31–35] but, in chinook salmon at least, sperm swimming speed in OF differentially depends upon female and male identities, with certain sperm–OF combinations resulting in faster sperm velocity than others [27]. This finding leads us to suggest that the OF may in fact bias the success of spermatozoa carrying superior genotypes to be sire's for the female's offspring [27,36–38].
Similar effects of OF on sperm performance have been reported in guppies, Poecilia reticulata [20], lake trout, Salvelinus namaycush [33], and Arctic charr, Salvelinus alpinus [35]. In the internally fertilizing guppy [20], sperm–OF interactions biased fertilization success towards males that were not related to the female—the sperm of those males generally swam faster in the focal female's OF than did the sperm of their competitors that were more closely related to the female [20]. In addition, recent work on Atlantic salmon, Salmo salar, and brown trout, Salmo trutta, indicates that OF plays a key role in promoting the fertilization of ova by conspecific sperm in preference to genetically incompatible heterospecific sperm, and that OF is likely more important than interactions at the ovum's porous outer shell in promoting reproductive isolation between these sympatric species [18].
Here we use a combination of paired-male, in vitro, competitive fertilization trials in chinook salmon, coupled with microsatellite-based analyses of paternity and multilocus heterozygosity (MLH), to investigate (i) the influence of OF on sperm performance and male fertilization success when there is sperm competition and (ii) the early life stage survival and development of embryos resulting from fertilization by ‘preferred’ versus ‘non-preferred’ males, where ‘preferred’ males are defined as those that had the higher fertilization success under sperm competition. We also examined whether any bias in fertilization outcomes was related to (iii) the genetic relatedness between each of the competing males and the female whose ova they were competing to fertilize [18,33,39] and (iv) the quality of competing males as estimated by their MLH determined with microsatellite markers [40,41].
2. Material and methods
(a). Study species and maintenance
Chinook salmon were caught during their annual spawning run (April–May) in a trap located on the Kaiapoi River, a tributary of the Waimakariri River system, Canterbury, New Zealand [42]. We studied sexually mature, 3-year-old, ‘hooknose’ males and 3-year-old females captured in 2010 (n = 13 males, 4 females) and 2011 (n = 15 males, 6 females), taken from a sample of fish (25 and 37 males in 2010 and 2011, respectively, and about 100 females in each year) captured and individually marked for gamete harvesting at a hatchery.
Fish were maintained in a natural river-water raceway (12.5–13°C) at a hatchery (Salmon Smolt NZ, Canterbury, New Zealand) using standard husbandry procedures. A small fin clip was taken from each fish and stored in 95% ethanol for later DNA extraction and analysis.
(b). Competitive fertilization trials
To assess whether differences between males in sperm velocity (measured in a female's OF) influenced a male's fertilization success, we set up competitive fertilization trials between two males (dyads) at a time. This allowed us to directly compare the relative fertilization success of each male (difference between the number of eggs fertilized) that was competing to fertilize the same clutch of ova, in an experimental design that mimicked the natural spawning environment. We tested two different concentrations of OF (50% OF in 2010, 100% OF in 2011) as spermatozoa are expected to be in contact with an increasing concentration of OF as they approach the unfertilized ova.
We conducted a total of 90 replicated competitive fertilization trials (35 in 50% OF, 55 in 100% OF) involving a total of 28 individual males (13 in 2010, 15 in 2011) and 10 individual females (4 in 2010, 6 in 2011). For each of the two replicates per trial, one female and two males were haphazardly chosen, so individuals were sometimes used in more than one replicated trial, but each trial involved a unique triad.
Milt was obtained from males, and OF from females, as described previously [7,27,30]. All unfertilized ova, OF and, milt samples were held at 4°C for up to 5 h until the trial was replicated. Sperm density was determined using an improved Neubauer haemocytometer prior to each fertilization trial so that approximately the same number of sperm per male (1 × 108 spermatozoa) could be used in each trial.
For each in vitro competitive fertilization trial, we placed a batch of about 100 unfertilized ova from the focal female in a dry 2 l plastic beaker, then added milt samples from each male simultaneously by injecting them separately into a steady stream of raceway water (250 ml at 12.5°–13°C) being poured into the beaker. This technique ensured a rapid and heterogeneous mixing of gametes. We added the milt samples separately into the water to ensure that the spermatozoa were activated before the milt samples came into contact, to minimize any effects of each male's seminal fluid on the other male's sperm function. For each trial, the two beakers containing fertilized eggs, milt, and water were left undisturbed in the dark for 5 min to ensure that sperm were no longer motile and eggs had begun to harden. Then, each batch of fertilized eggs was gently transferred into its own compartment in a vertical incubation stack. Clutches of fertilized eggs were allowed to develop undisturbed in a constant water flow at natural spawning-water temperatures for 28 days (350 accumulated thermal units, ATUs) approximately 4–5 days before hatching (which typically occurs after 480 ATUs). At 28 days after fertilization, an average of 116 eggs (range 68–204) were examined in each of those 180 batches to determine the proportion of eggs that had viable embryos. Viable embryos with vascularized yolk sacs were clearly visible inside the egg at that stage [43,44].
To determine the paternity of offspring resulting from these trials, we conducted microsatellite genotyping of 4 320 unhatched embryos at day 28. DNA was extracted from preserved tissue samples using a standard Chelex procedure [45]. For each of the 180 (i.e. 90 replicated) trials, the two potential sires, the dam, and a haphazard sample of 24 progeny were genetically typed by Genomnz (AgResearch Ltd, Mosgiel, New Zealand) using a multiplex of nine highly polymorphic microsatellite markers (Ocl-1 [46], Omy-325 [47], Ots-101 [47], Ots-104 [47], Ots-107 [47], Ots-2 [47], Ots-3 [47], Ssa-197 [48], Ssa-85 [49]). We assigned paternity using a maximum-likelihood approach in CERVUS (v. 3.0) [50].
The results from these trials were not significantly different between the two replicates with each triad (see the electronic supplementary material, statistical appendix for details), and we pooled the replicates for further analysis. We designated the male that sired the majority of offspring in each triad as the ‘winner’, and estimated his fertilization advantage as the number of offspring that he sired in excess of those sired by the loser.
To determine the most accurate measure of genetic relatedness (rMF) between each male and the female in a trial, we used a simulation program in COANCESTRY v. 1.0.1.0 [51] as outlined in Taylor [52]. Based on the nine microsatellite markers that we genotyped, these simulations showed that the triadic likelihood (TrioML) estimator [51] produced pairwise relatedness estimates most highly correlated with true values (Pearson's r = 0.82, n = 600 simulated dyad populations).
Using GenAIEx [53], we also calculated each individual's MLH from the same nine microsatellite loci as the proportion of loci that are heterozygous. Calculating MLH using neutral loci such as microsatellites provides a good estimate of heterozygosity and is an index of genome-wide genetic diversity [40,41] that is commonly used to measure heterozygosity in wild populations [54,55]. Significant deviations from Hardy–Weinberg equilibrium for these nine microsatellite loci was also calculated [53] (see the electronic supplementary material, table S1, for observed and expected heterozygosities).
(c). Non-competitive fertilization trials and embryo survival
We conducted 59 in vitro non-competitive fertilization trials using the milt of each male combined with the unfertilized ova from each female that he was paired with in the competitive fertilization trials (in which 14 males were paired with only one female, five males with two different females, nine males with three females, and two males with four females). These non-competitive fertilization trials were not replicated but were handled as for the competitive trials, and at the same time. We confirmed that all males were reproductively fertile because they had fertilized some ova in at least one trial. Thus, any differences in male fertilization success in the competitive in vitro fertilization trials could not be attributed to male infertility. On average, 115 (108, 122; 95% CL) eggs were examined to determine the proportion of surviving embryos in each of these 59 batches of eggs, and an average of 57% (49, 64; 95% CL) of eggs in each batch were successfully fertilized. Dead eggs were counted and removed twice weekly. We used this measure of embryo survival as a direct measure of early life reproductive success, as this has previously been used in other salmonid studies [49,56].
(d). Measuring sperm quality traits
We measured sperm swimming speed for each male at 10 s post-activation using a CEROS sperm tracker (v. 12, Hamilton-Thorne Research, Beverly, MA, USA) with the sperm in OF (50% in 2010, 100% in 2011) from those females used in that male's competitive fertilization trials, employing methods previously described [7,27,30]. In brief, < 1 µl of milt was pipetted onto a 20 µl Leja slide (Leja Products B.V., Nieuw-Vennep, The Netherlands), on a temperature-controlled stage cooler (TS-4 Thermal Microscope Stage, Physitemp, USA) set to 12.5°C to match the natural spawning water temperature.
We used average path velocity (VAP, µm s−1)—which estimates the average velocity of a sperm cell for 0.5 s over a smoothed cell path—as our measure of sperm swimming speed [7,27,29,30]. VAP was measured twice for each milt sample in each activation medium, and we used the mean in further statistical analyses. Thus, for the milt sample from each male in each of the 90 replicated competitive fertilization trials, we measured the VAP in river water and in either 50% (in 2010) or 100% OF (in 2011) from the female in that trial. VAP was calculated from an average of 94 (86, 102; 95% CL) sperm tracks per milt sample (n = 360 VAP estimates, comprising four samples—activated in OF—from the milt of each male in each of the replicated 90 competitive fertilization trials). Within-male repeatability (ICC = intraclass correlation coefficient) of VAP was high, with up to four measures of VAP per male (n = 28 experimental males) from their milt activated in (i) 50% OF (ICC = 0.8, p ≤ 0.0001) or (ii) 100% OF (ICC = 0.7, p < 0.0001). VAP was positively correlated with other sperm velocity parameters (see the electronic supplementary material).
(e). Statistical analyses
All statistical analyses were performed using R v. 3.1.2 [57]; descriptive statistics are presented as mean (95% confidence limits). See the electronic supplementary material, statistical appendix, for details with regard to the choice of methods and comprehensive results of all analyses, including tests of statistical assumptions and alternative methods of analysis.
We used an information-theoretic approach to model evaluation [58], wherein we consider all models with Akaike Information Criterion (AICc) values within 2.0 of the best-fitting model to be statistically equivalent, given the data. We report all sets of top models, as well as the methods used for evaluating the significance of fixed effects in the electronic supplementary material.
3. Results
(a). Genetic analyses
Excluding one locus (Ots-104) in the 2010 spawning season, we did not detect significant deviations from Hardy–Weinberg equilibrium for the nine microsatellite loci used in this study (see the electronic supplementary material, table S1). Individual male MLH was similar for both spawning seasons (2010; MLH = 0.79 [0.70, 0.88] n = 13 males, 2011: MLH = 0.82 [0.76, 0.87] n = 13 males; F1,26 = 0.31, p = 0.59). Mean pairwise genetic relatedness (rMF, using the TrioML estimator) of male–female dyads was also similar for both spawning seasons (2010: rMF = 0.029 (0.01, 0.05) n = 35 unique female–male dyads (15 males and 4 females), 2011: rMF = 0.047 (0.03, 0.06) n = 55 unique female–male dyads (13 males and 6 females); F1,88 = 1.7, p = 0.18).
(b). Sperm competition trials
In the 90 replicated competitive fertilization trials, the male that obtained the majority of fertilizations (the ‘winner’) realized, on average, 75.7% (72.4, 78.9; 95% CL) (range 50–100%) of the fertilizations. This fertilization advantage (additional number of offspring sired) of the winner in these trials was significantly predicted by the difference in the sperm swimming speeds (VAP) between the two competing males (table 1 and figure 1), controlling for the non-significant effects of both OF concentration and the difference in MLH between the males. One of the top models in this analysis also included the difference in male–female relatedness as a predictor (electronic supplementary material, table S2), suggesting that this variable might be worth investigating further in a study with a larger sample size. Thus, in competitive fertilization trials, the fertilization advantage for the male that fertilized the majority of eggs increased linearly with the difference in the competing males' sperm swimming speeds in both 50% and 100% OF concentrations (figure 1). Only about 50% of the eggs were fertilized in each competitive fertilization trial, as we used a much lower than natural sperm : egg ratio (100 ova per 108 spermatozoa) in each trial to ensure that fertilizations were not swamped by the sperm of one male.
Table 1.
Best-fitting generalized linear mixed effect model (GLMM) to predict the fertilization advantage by the winner (number of eggs fertilized in excess of those fertilized by the loser) in each of 90 competitive fertilization trials from predictor variables calculated as the differences (Δ) between the two males with respect to (i) individual male multilocus heterozygosity (MLH) and (ii) their sperm velocities (VAP in µm s–1) measured in that female's OF. This model also controls for OF concentration used in the trial as this was different in the 2 years of the study, as well as for the random effects of male and female identities. P-values were calculated using the Kenward–Rogers method; VAP and MLH were standardized (std) in this analysis. See the electronic supplementary material for (i) details of top models (AICc < 2) in this set (electronic supplementary material, table S2) and (ii) a separate best-fitting model for each ovarian fluid (OF) concentration (see the electronic supplementary material, table S3).
| parameters (fixed effects only) | estimate (95% CL) p |
|---|---|
| intercept | 29.2 (22.94, 35.39) |
| std Δ MLH | 6.4 (–0.38, 13.10) 0.11 |
| std ΔVAP in OF (µm s−1) | 11.2 (4.79, 17.52) 0.003 |
| OF concentration (50 or 100%) | –7.5 (–15.80, 0.81) 0.12 |
Figure 1.
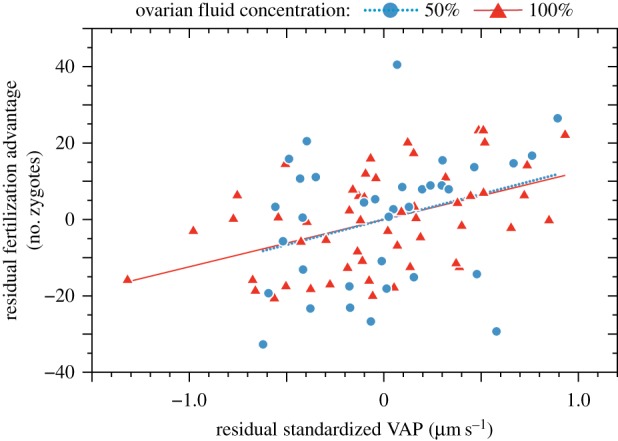
Partial regression plot to illustrate the association between sperm competition success (fertilization advantage of male that obtained the majority of fertilizations) and the difference in the mean sperm swimming speed (VAP in µm s−1) of the two competing males, as measured in 50% and 100% ovarian fluid (OF) at 10 s after sperm activation (analysed separately, electronic supplementary material, table S3). Each data point is the pooled data from a replicated two-male sperm competition trial (n = 35 and 55 for 50% and 100% OF concentrations, respectively). Both linear models shown here control for the difference in male MLH, but not for the random effects of male and female identities (see the electronic supplementary material, appendix). (Online version in colour.)
This fertilization advantage for the winning male was not related to the difference in the competitors' sperm swimming speeds measured in fresh water (std beta = –0.50 (–7.49, 6.50), F1.0,72.1 = 0.02, p = 0.90), controlling for the effects of OF concentration and MLH as well as the random effects of male and female identities. VAP was not included in any of the top models in this set (see the electronic supplementary material, table S4).
(c). Embryo survival
In the non-competitive fertilization trials, where there was no rival male sperm, the proportion of fertilized eggs that developed successfully (i.e. were still viable at 28 d) was significantly related both to that male's sperm swimming speed in the female's OF (figure 2a) and his MLH (figure 2b and table 2), controlling for the significant effect of year in which the male and female were sampled (see also the electronic supplementary material, table S5). Although different OF concentrations were used each year, it seems reasonable to attribute the differences between years to differences in parental and offspring quality, rather than to the effects of OF concentration. In these trials, embryo survival was not significantly related to sperm swimming (VAP) measured in fresh water (GLMM: std beta = 0.65 [–0.48,1.78] p = 0.26), while controlling for fixed effects of year and both male and female MLH, and the random effects of male and female identity (see the electronic supplementary material, table S4).
Figure 2.
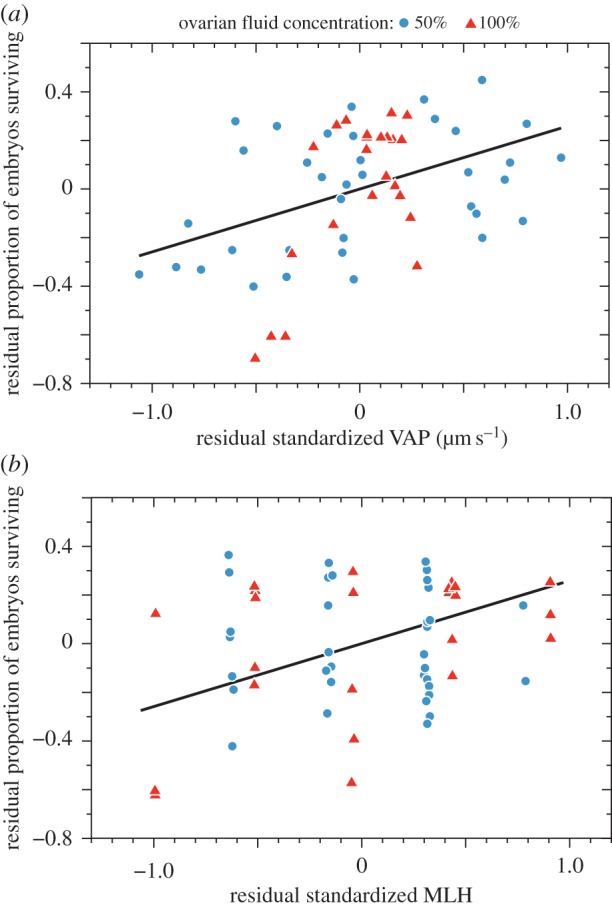
Partial regression plots to illustrate the relationship between embryo survival (the proportion of eggs that survived to 28 days after fertilization) in the non-competitive fertilization trials and that male's (a) sperm swimming speed (VAP) in the female's ovarian fluid (OF) and (b) individual male multilocus heterozygosity (MLH). Each data point is from a single non-competitive fertilization trial, with the same number of spermatozoa used from each male in each trial. These plots are based on a linear model, using the proportion of embryos that survived as the response while controlling for OF concentration, and either (a) VAP or (b) MLH but not for the random effects of male and female identities (see the electronic supplementary material, appendix). (Online version in colour.)
Table 2.
Best-fitting GLMM to predict embryo survival (number of eggs survived versus died) in the non-competitive fertilization trials (n = 59) from (i) the male's mean sperm velocity (VAP in µm s–1) measured in the female's OF, (ii) individual male multilocus heterozygosity (MLH) estimated from nine polymorphic microsatellite loci, and (iii) his genetic relatedness (TrioML) to the female (all standardized (std) in this analysis). There was only one top model (AICc < 2) in this set and it did not include TrioML. We used fish from two different spawning seasons so the model controls for potential differences between years in embryo survival, as well as the random effects of male and female identities. See the electronic supplementary material, table S4, for an Markov chain Monte Carlo Generalized Linear Mixed Models (MCMC)-GLMM with this same model structure, showing the same pattern of results.
| parameters (fixed effects only) | estimate (95% CL) p |
|---|---|
| intercept | –0.52 (–1.31, 0.27) 0.20 |
| std MLH | 0.89 (0.10, 1.67) 0.03 |
| std VAP in OF (µm s−1) | 1.59 (0.66, 2.52) 0.0008 |
| OF concentration (50 or 100%) | 2.50 (1.91, 3.81) 0.0002 |
4. Discussion
(a). Evidence for cryptic female choice
In this study, we found that when the sperm of two male chinook salmon compete to fertilize a clutch of eggs, one of the males was, on average, 50% more successful, even when there were (experimentally) the same number of sperm in each male's ‘ejaculate’. Siring success was substantially biased towards the male whose sperm swam faster in the presence of that female's OF, and was significantly related to the difference between the males' sperm swimming speeds in both 50% and 100% OFs (figure 1 and table 1; electronic supplementary material, table S3). Despite the difference in spawning seasons with respect to fishes and OF concentrations, the relationships between the winning male's fertilization advantage and the relative (standardized) difference between his and his rival's sperm swimming speeds was remarkably similar (figure 1; see the electronic supplementary material, table S3a). However, the effect of actual VAP (i.e. not standardized) was almost three times as high in 50% OF (slope = 0.42) as in 100% OF solution (slope = 0.16), though the difference in estimates between the two OF concentrations was not significant (see the electronic supplementary material, table S3b). A stronger effect of OF on VAP might be expected in the more dilute (50%) solution due to the high viscosity of pure OF [29], but differences between years might also have been important in this study. Because the fertilization advantage of the winner was not related to the difference between the males' sperm swimming speeds in fresh water, we conclude that male fertilization success was largely the result of interactions between his sperm and the female's OF, and not solely due to the sperm's traits.
We have previously shown that, in chinook salmon, OF surrounding unfertilized ova has a positive effect on sperm swimming speed [30], that the effect of a given female's OF on sperm behaviour varies from male to male [27], and that the difference in the sperm swimming speed of competing males predicts differential male fertilization success [7]. This study confirms that the effect of OF on sperm velocity is likely to be a mechanism of CFC. These findings contribute to a growing body of work demonstrating that female-derived fluids surrounding the unfertilized ova can mediate CFC prior to gamete contact in a range of external and internal fertilizers (e.g. in guppies [20], frogs [59], and salmon [18]).
Interestingly, males with faster sperm swimming speed did not ‘win’ (i.e. have higher fertilization success) in all of the competitive fertilization trials, nor did they fertilize all of the female's ova in most trials. Overall, the male with the faster swimming sperm ‘won’ 61% of trials, whereas the male with the slower swimming sperm won 27%, and in 12% of trials males with different sperm swimming speeds fertilized the same proportion of eggs. We did not measure other factors that may have resulted in non-random mating, including: (i) male–female compatibility at the major histocompatibility (MHC) complex, which may confer higher pathogen resistance again egg disease [60–62] and (ii) between-male variation in the quality of seminal fluid, which is known to contain components that influence embryo development and viability [63].
The mechanistic basis for the variation in sperm velocity that we [27] and others [31,34,35] have observed—between spermatozoa from different males activated in the same female's OF—remains unknown. While OF had a strong effect on fertilization outcomes in our competitive fertilization experiments, our results indicate that sperm velocity in OF was not mediated by the pairwise genetic relatedness between males and females. These results contrast with a study in lake trout, Salvelinus namaycush, wherein a 20% OF solution mediated sperm velocity according to the pairwise genetic relatedness between males and females [33].
Recently, we have identified 174 proteins in the OF of female chinook, with variation among females in both the number and concentrations of proteins [64]. These proteins may diffuse from the unfertilized ovum's outer layer to interact with proteins present on the plasma membrane of the spermatozoa, resulting in differential modification of the ion channels responsible for flagellar beating and sperm velocity. Small peptides that diffuse from the egg have been isolated in a wide variety of echinoderm species (e.g. sea urchins and starfish) and have been shown to alter sperm behaviour [65,66]. Recent research in mammals has also identified several chemokines present in human follicular endometrial fluid that interact with a receptor common to several chemoattractant peptides present in mouse and human sperm—peptides that modify sperm velocity and chemotaxis [67].
(b). Is cryptic female choice adaptive?
The survival of embryos fertilized by each male—in separate, non-competitive fertilization trials—was also significantly related to the male's sperm swimming speed in that female's OF (figure 2a and table 2), as well as to his MLH (figure 2b and table 2). Thus, whatever the influence of OF on CFC that was mediated by its effect on sperm behaviour, the result is that this CFC was adaptive as it enhanced female reproductive success, as measured by embryo survival. This is the first evidence that we know of showing that CFC results in fitness benefits to the female, but more work will be needed to elucidate the genetic and biochemical mechanisms underlying that choice, and the reasons for the resulting increase in embryo survival.
Our measure of female (and male) reproductive success—embryo survival at day 28 (350 ATUs)—is obviously a crude surrogate for fitness, in that it measures only survival over a tiny, albeit extremely important, fraction of the offspring's life. It is unknown how that measure of success might influence true fitness and even whether survival in the benign incubator environment tells us anything about embryo survival in the rough and tumble of the natural spawning environment. In one study, embryo survival to the eyed stage (about 280 ATUs or about 22 days for the conditions in our incubators) in a natural stream averaged 42.6% [68], suggesting that this is normally a period when the mortality rate is high.
The relationship between a male's MLH and the survival of the embryo that he has sired (figure 2b) suggests that females benefit from mating with ‘high-quality’, genetically diverse males, and supports theoretical predictions that females secure genetic benefits for their offspring by choosing sperm of males with particular genotypes [36–38]. Offspring survival may be enhanced when females produce offspring with high genetic heterozygosity, resulting in either a reduction in the likelihood of the expression of recessive deleterious alleles, or an increase in the number of potentially useful gene products. For example, offspring diversity at the MHC locus should allow an individual to respond to a greater range of pathogens [60,69–71]. Evidence is accruing that interactions between the maternal and paternal genomes is a strong fitness predictor across a range of species including birds, mice, and fish [40,69,72,73].
We are cautious about interpreting the correlation between embryo survival and the sire's MLH. We used nine, presumably neutral, microsatellite loci to estimate each male's MLH as a proxy for genome-wide heterozygosity [41,74] but MLH may not reflect heterozygosity at other loci and by extension genomic heterozygosity [75]. Further investigation is required into the pattern of increased embryo survival, and other fitness-related traits in the offspring such as growth rate, survival, and subsequent reproductive success [76].
Other researchers have interpreted a correlation between offspring survival and the sperm traits possessed by the winning male during sperm competition as evidence in favour of the ‘good sperm’ hypothesis (GSH) [77]. The GSH predicts that males with competitively superior sperm will produce offspring with better viability. While that is certainly what we found, the competitive superiority of male chinook sperm was mediated by the female's OF, a process that we interpret as CFC—those males did not have competitively superior sperm when activated either in fresh water or in the OF of some other females. This extension to the GSH has been acknowledged by others [78] but has not previously been documented, to the best of our knowledge. Given the increasing evidence for CFC in both internal and external fertilizers [14,18–20,79], the effects of that choice on sperm behaviour and morphology deserves further modelling and empirical study.
While the occurrence and mechanisms of CFC may be easiest to study in external fertilizers, we expect that in most organisms there will be the potential for male gametes to encounter female-specific biochemical environments whether those be OFs, mucous on vaginal and uterine walls, or, in the case of plants, stigmatic surfaces and stylar tissues [80]. We suggest that this is a fruitful avenue for further research, both to explore the biochemical basis for the CFC revealed by the correlation with male MLH that we observed, and to look at more comprehensive indices of offspring fitness to determine what benefits accrue to females making choices at the gamete level.
Supplementary Material
Acknowledgements
We are grateful to the hatchery staff at Salmon Smolt, NZ, in particular Ben Divett, Karl French, Errol White, Tom Gough, and Luke Price for providing facilities, fish husbandry, gamete handling assistance, and advice. We also thank Janine Wing, Tanya Blakely, Katherine McBride, Sara Ferreira for assistance in the field; Shinichi Nakagawa for statistical advice; Amy Osborne, Tammy Steeves, and Helen Taylor for assistance with genetic analyses; Jim Briskie for access to computer-assisted sperm analysis software.
Ethics
All animals were collected and maintained according to the standards of the Animal Ethics Committee for the University of Otago, New Zealand (permit no. AEC/13/10).
Data accessibility
The datasets analysed in this study, and the details of statistical analyses, are available on Dryad (http://dx.doi.org/10.5061/rspb.2016.0001).
Authors' contributions
P.R., R.M., and N.G. conceived and designed the study; P.R. conducted all of the fertilization experiments; P.R. and R.M. analysed the data and wrote the manuscript; all authors edited and approved the final manuscript.
Competing interests
The authors have no competing interests.
Funding
Study funded by a Royal Society of New Zealand Marsden Grants to N.G. and R.M. (grant no. UOO913) and P.R. (grant no. UOO1209), grants to N.G. from the University of Otago, and grants to R.M. from Queen's University (Research Chair) and the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Eberhard WG. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Birkhead TR, Pizzari T. 2002. Postcopulatory sexual selection. Nat. Rev. Genet. 3, 262–273. ( 10.1038/nrg774) [DOI] [PubMed] [Google Scholar]
- 3.Simmons LW. 2001. Sperm competition and its evolutionary consequences in the insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Parker GA. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567. ( 10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 5.Gage MJG, Macfarlane CP, Yeates S, Ward RG, Searle JB, Parker GA. 2004. Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 14, 44–47. ( 10.1016/j.cub.2003.12.028) [DOI] [PubMed] [Google Scholar]
- 6.Gasparini C, Simmons LW, Beveridge M, Evans JP. 2010. Sperm swimming velocity predicts competitive fertilization success in the green swordtail Xiphophorus helleri. PLoS ONE 5, e12146 ( 10.1371/journal.pone.0012146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans JP, Rosengrave P, Gasparini C, Gemmell NJ. 2013. Delineating the roles of males and females in sperm competition. Proc. R. Soc. B 280, 20132047 ( 10.1098/rspb.2013.2047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snook RR. 2005. Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 20, 46–53. ( 10.1016/j.tree.2004.10.011) [DOI] [PubMed] [Google Scholar]
- 9.Pizzari T, Parker GA. 2009. Sperm competition and sperm phenotype. In Sperm biology: an evolutionary perspective (eds Birkhead TR, Hoslen DJ, Pitnick SS), pp. 207–245. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Simmons LW, Fitzpatrick JL. 2012. Sperm wars and the evolution of male fertility. Reproduction 144, 519–534. ( 10.1530/REP-12-0285) [DOI] [PubMed] [Google Scholar]
- 11.Slatyer RA, Mautz BS, Backwell PR, Jennions MD. 2012. Estimating genetic benefits of polyandry from experimental studies: a meta-analysis. Biol. Rev. 87, 1–33. ( 10.1111/j.1469-185X.2011.00182.x) [DOI] [PubMed] [Google Scholar]
- 12.Oliver M, Evans JP. 2014. Chemically moderated gamete preferences predict offspring fitness in a broadcast spawning invertebrate. Proc. R. Soc. B 281, 20140148 ( 10.1098/rspb.2014.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitcher T, Neff B. 2007. Genetic quality and offspring performance in Chinook salmon: implications for supportive breeding. Conserv. Genet. 8, 607–616. ( 10.1007/s10592-006-9204-z) [DOI] [Google Scholar]
- 14.Løvlie H, Gillingham MA, Worley K, Pizzari T, Richardson DS. 2013. Cryptic female choice favours sperm from major histocompatibility complex-dissimilar males. Proc. R. Soc. B 280, 20131296 ( 10.1098/rspb.2013.1296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neff BD, Garner SR, Heath DD. 2008. The MHC and non-random mating in a captive population of Chinook salmon. Heredity 101, 175–185. ( 10.1038/hdy.2008.43) [DOI] [PubMed] [Google Scholar]
- 16.Garner SR, Bortoluzzi RN, Heath DD, Neff BD. 2010. Sexual conflict inhibits female mate choice for major histocompatibility complex dissimilarity in Chinook salmon. Proc. R. Soc. B 277, 885–894. ( 10.1098/rspb.2009.1639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landry C, Garant D, Duchesne P, Bernatchez L. 2001. Good genes as heterozygosity: the major histocompatibility complex and mate choice in Atlantic salmon (Salmo salar). Proc. R. Soc. Lond. B 268, 1279–1285. ( 10.1098/rspb.2001.1659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeates SE, Diamond SE, Einum S, Emerson BC, Holt WV, Gage MJG. 2013. Cryptic choice of conspecific sperm controlled by the impact of ovarian fluid on sperm swimming behaviour. Evolution 67, 3523–3536. ( 10.1111/evo.12208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward PI. 2000. Cryptic female choice in the yellow dung fly Scathophaga stercoraria (L.). Evolution 54, 1680–1686. ( 10.1111/j.0014-3820.2000.tb00712.x) [DOI] [PubMed] [Google Scholar]
- 20.Gasparini C, Pilastro A. 2011. Cryptic female preference for genetically unrelated males is mediated by ovarian fluid in the guppy. Proc. R. Soc. B 278, 2495–2501. ( 10.1098/rspb.2010.2369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birkhead T. 1998. Cryptic female choice: criteria for establishing female sperm choice. Evolution 52, 1212–1218. ( 10.2307/2411251) [DOI] [PubMed] [Google Scholar]
- 22.Pitnick S, Brown WD. 2000. Criteria for demonstrating female sperm choice. Evolution 54, 1052–1056. ( 10.1111/j.0014-3820.2000.tb00107.x) [DOI] [PubMed] [Google Scholar]
- 23.Bishop JDD, Jones CS, Noble LR. 1996. Female control of paternity in the internally fertilizing compound Ascidian Diplosoma listerianum. II. Investigation of male mating success using RAPD markers. Proc. R. Soc. Lond. B 263, 401–407. ( 10.1098/rspb.1996.0061) [DOI] [Google Scholar]
- 24.Vacquier VD, Swanson WJ, Hellberg ME. 1995. What have we learned about sea urchin sperm bindin? Develop. Growth Differ 37, 1–10. ( 10.1046/j.1440-169X.1995.00001.x) [DOI] [PubMed] [Google Scholar]
- 25.Palumbi SR. 1999. All males are not created equal: fertility differences depend on gamete recognition polymorphisms in sea urchins. Proc. Natl Acad. Sci. USA 96, 12 632–12 637. ( 10.1073/pnas.96.22.12632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palumbi SR. 2008. Speciation and the evolution of gamete recognition genes: pattern and process. Heredity 102, 66–76. ( 10.1038/hdy.2008.104) [DOI] [PubMed] [Google Scholar]
- 27.Rosengrave P, Gemmell NJ, Metcalf V, McBride K, Montgomerie R. 2008. A mechanism for cryptic female choice in chinook salmon. Behav. Ecol. 19, 1179–1185. ( 10.1093/beheco/arn089) [DOI] [Google Scholar]
- 28.Berejikian B, Tezak E, LaRae A. 2000. Female mate choice and spawning behaviour of chinook salmon under experimental conditions. J. Fish Biol. 57, 647–661. ( 10.1111/j.1095-8649.2000.tb00266.x) [DOI] [Google Scholar]
- 29.Rosengrave P, Taylor H, Montgomerie R, Metcalf V, McBride K, Gemmell NJ. 2009. Chemical composition of seminal and ovarian fluids of chinook salmon (Oncorhynchus tshawytscha) and their effects on sperm motility traits. Comp. Biochem. Physiol. A 152, 123–129. ( 10.1016/j.cbpa.2008.09.009) [DOI] [PubMed] [Google Scholar]
- 30.Rosengrave P, Montgomerie R, Metcalf VJ, McBride K, Gemmell NJ. 2009. Sperm traits in Chinook salmon depend upon activation medium: implications for studies of sperm competition in fishes. Can. J. Zool. 87, 920–927. ( 10.1139/z09-081) [DOI] [Google Scholar]
- 31.Turner E, Montgomerie R. 2002. Ovarian fluid enhances sperm movement in Arctic charr. J. Fish Biol. 60, 1570–1579. ( 10.1111/j.1095-8649.2002.tb02449.x) [DOI] [Google Scholar]
- 32.Wojtczak M, Dietrich GJ, Słowińska M, Dobosz S, Kuźmiński H, Ciereszko A. 2007. Ovarian fluid pH enhances motility parameters of rainbow trout (Oncorhynchus mykiss) spermatozoa. Aquaculture 270, 259–264. ( 10.1016/j.aquaculture.2007.03.010) [DOI] [Google Scholar]
- 33.Butts IAE, Johnson K, Wilson CC, Pitcher TE. 2012. Ovarian fluid enhances sperm velocity based on relatedness in lake trout, Salvelinus namaycush. Theriogenology 78, 2105–2109. ( 10.1016/j.theriogenology.2012.06.031) [DOI] [PubMed] [Google Scholar]
- 34.Dietrich GJ, Wojtczak M, Słowińska M, Dobosz S, Kuźmiński H, Ciereszko A. 2008. Effects of ovarian fluid on motility characteristics of rainbow trout (Oncorhynchus mykiss Walbaum) spermatozoa. J. Appl. Ichthyol. 24, 503–507. ( 10.1111/j.1439-0426.2006.01130.x) [DOI] [Google Scholar]
- 35.Urbach D, Folstad I, Rudolfsen G. 2005. Effects of ovarian fluid on sperm velocity in Arctic charr (Salvelinus alpinus). Behav. Ecol. Sociobiol. 57, 438–444. ( 10.1007/s00265-004-0876-4) [DOI] [Google Scholar]
- 36.Tregenza T, Wedell N. 2000. Genetic compatibility, mate choice and patterns of parentage: invited review. Mol. Ecol. 9, 1013–1027. ( 10.1046/j.1365-294x.2000.00964.x) [DOI] [PubMed] [Google Scholar]
- 37.Neff BD, Pitcher TE. 2005. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 14, 19–38. ( 10.1111/j.1365-294X.2004.02395.x) [DOI] [PubMed] [Google Scholar]
- 38.Zeh JA, Zeh DW. 1997. The evolution of polyandry II: post-copulatory defences against genetic incompatibility. Proc. R. Soc. Lond. B 264, 69–75. ( 10.1098/rspb.1997.0010) [DOI] [Google Scholar]
- 39.Firman RC, Simmons LW. 2015. Gametic interactions promote inbreeding avoidance in house mice. Ecol. Lett. 18, 937–943. ( 10.1111/ele.12471) [DOI] [PubMed] [Google Scholar]
- 40.Ryder TB, Tori WP, Blake JG, Loiselle BA, Parker PG. 2010. Mate choice for genetic quality: a test of the heterozygosity and compatibility hypotheses in a lek-breeding bird. Behav. Ecol. 21, 203–210. ( 10.1093/beheco/arp176) [DOI] [Google Scholar]
- 41.Forstmeier W, Schielzeth H, Mueller JC, Ellegren H, Kempenaers B. 2012. Heterozygosity–fitness correlations in zebra finches: microsatellite markers can be better than their reputation. Mol. Ecol. 21, 3237–3249. ( 10.1111/j.1365-294X.2012.05593.x) [DOI] [PubMed] [Google Scholar]
- 42.Unwin M, Quinn T, Kinnison M, Boustead N. 2000. Divergence in juvenile growth and life history in two recently colonized and partially isolated chinook salmon populations. J. Fish Biol. 57, 943–960. ( 10.1111/j.1095-8649.2000.tb02203.x) [DOI] [Google Scholar]
- 43.Heath DD, Fox CW, Heath JW. 1999. Maternal effects on offspring size: early development of chinook salmon. Evolution 53, 1605–1611. ( 10.2307/2640906) [DOI] [PubMed] [Google Scholar]
- 44.Bobe J, Labbé C. 2010. Egg and sperm quality in fish. Gen. Comp. Endocrinol. 165, 535–548. ( 10.1016/j.ygcen.2009.02.011) [DOI] [PubMed] [Google Scholar]
- 45.Walsh PS, Metzger DA, Higuchi R. 1991. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10, 506–513. [PubMed] [Google Scholar]
- 46.Condrey MJ, Bentzen P. 1998. Characterization of coastal cutthroat trout (Oncorhynchus clarki clarki) microsatellites and their conservation in other salmonids. Mol. Ecol. 7, 787–789. [PubMed] [Google Scholar]
- 47.Beacham TD, Jonsen KL, Supernault J, Wetklo M, Deng L, Varnavskaya N. 2006. Pacific Rim population structure of Chinook salmon as determined from microsatellite analysis. Trans. Am. Fish. Soc. 135, 1604–1621. ( 10.1577/T06-071.1) [DOI] [Google Scholar]
- 48.Banks MA, Blouin MS, Baldwin BA, Rashbrook VK, Fitzgerald HA. 1999. Isolation and inheritance of novel microsatellites in Chinook salmon (Oncorhynchus tschawytscha). J. Hered. 90, 281–282. ( 10.1093/jhered/90.2.281) [DOI] [Google Scholar]
- 49.Heath DD, Bryden CA, Shrimpton JM, Iwama GK, Kelly J. 2002. Relationships between heterozygosity, allelic distance (d2), and reproductive traits in chinook salmon. Can. J. Fish. Aquat. Sci. 59, 77–84. ( 10.1139/f01-192) [DOI] [Google Scholar]
- 50.Kalinowski ST, Taper ML, Marshall TC. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106. ( 10.1111/j.1365-294X.2007.03089.x) [DOI] [PubMed] [Google Scholar]
- 51.Wang J. 2011. COANCESTRY: a program for simulating, estimating and analysing relatedness and inbreeding coefficients. Mol. Ecol. Resour. 11, 141–145. ( 10.1111/j.1755-0998.2010.02885.x) [DOI] [PubMed] [Google Scholar]
- 52.Taylor HR. 2015. The use and abuse of genetic marker-based estimates of relatedness and inbreeding. Ecol. Evol. 5, 3140–3150. ( 10.1002/ece3.1541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller M, Peakall R, Smouse P. 2012. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 28, 2537–2539. ( 10.1093/bioinformatics/bts460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slate J, David P, Dodds K, Veenvliet B, Glass B, Broad T, McEwan J. 2004. Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: theoretical expectations and empirical data. Heredity 93, 255–265. ( 10.1038/sj.hdy.6800485) [DOI] [PubMed] [Google Scholar]
- 55.Kirk H, Freeland JR. 2011. Applications and implications of neutral versus non-neutral markers in molecular ecology. Int. J. Mol. Sci. 12, 3966–3988. ( 10.3390/ijms12063966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Houde AL, Wilson CC, Neff BD. 2013. Genetic architecture of survival and fitness-related traits in two populations of Atlantic salmon. Heredity 111, 513–519. ( 10.1038/hdy.2013.74) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.R Core Team. 2015. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/) [Google Scholar]
- 58.Burnham KP, Anderson DR, Huyvaert KP. 2011. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65, 23–35. ( 10.1007/s00265-010-1029-6) [DOI] [Google Scholar]
- 59.Simmons LW, Roberts JD, Dziminski MA. 2009. Egg jelly influences sperm motility in the externally fertilizing frog, Crinia georgiana. J. Evol. Biol. 22, 225–229. ( 10.1111/j.1420-9101.2008.01628.x) [DOI] [PubMed] [Google Scholar]
- 60.Consuegra S, de Leaniz CG. 2008. MHC-mediated mate choice increases parasite resistance in salmon. Proc. R. Soc. B 275, 1397–1403. ( 10.1098/rspb.2008.0066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evans ML, Dionne M, Miller KM, Bernatchez L. 2012. Mate choice for major histocompatibility complex genetic divergence as a bet-hedging strategy in the Atlantic salmon (Salmo salar). Proc. R. Soc. B 279, 379–386. ( 10.1098/rspb.2011.0909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pitcher TE, Neff BD. 2006. MHC class IIB alleles contribute to both additive and nonadditive genetic effects on survival in Chinook salmon. Mol. Ecol. 15, 2357–2365. ( 10.1111/j.1365-294X.2006.02942.x) [DOI] [PubMed] [Google Scholar]
- 63.Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. 2014. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc. Natl Acad. Sci. USA 111, 2200–2205. ( 10.1073/pnas.1305609111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson SL, Villarroel M, Rosengrave P, Carne A, Kleffmann T, Lokman PM, Gemmell NJ. 2014. Proteomic analysis of chinook salmon (Oncorhynchus tshawytscha) ovarian fluid. PLoS ONE 9, e104155 ( 10.1371/journal.pone.0104155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki N. 1995. Structure, function and biosynthesis of sperm-activating peptides and fucose sulfate glycoconjugate in the extracellular coat of sea urchin eggs. Zool. Sci. 12, 13–27. ( 10.2108/zsj.12.13) [DOI] [PubMed] [Google Scholar]
- 66.Cook SP, Brokaw CJ, Muller CH, Babcock DF. 1994. Sperm chemotaxis: egg peptides control cytosolic calcium to regulate flagellar responses. Dev. Biol. 165, 10–19. ( 10.1006/dbio.1994.1229) [DOI] [PubMed] [Google Scholar]
- 67.Caballero-campo P, Buffone MG, Benencia F, Conejo-garcía JR, Rinaudo PF, Gerton GL. 2014. A role for the chemokine receptor CCR6 in mammalian sperm motility and chemotaxis. J. Cell. Physiol. 229, 68–78. ( 10.1002/jcp.24418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Legard CD. 2013. Embryonic survival of brown trout (Salmo trutta L.) in the Salmon River. New York, NY: State University of New York Col. of Environmental Science & Forestry. [Google Scholar]
- 69.Penn DJ, Damjanovich K, Potts WK. 2002. MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc. Natl Acad. Sci. USA 99, 11 260–11 264. ( 10.1073/pnas.162006499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dionne M, Miller KM, Dodson JJ, Bernatchez L. 2009. MHC standing genetic variation and pathogen resistance in wild Atlantic salmon. Phil. Trans. R. Soc. B 364, 1555–1565. ( 10.1098/rstb.2009.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacob A, Evanno G, Von Siebenthal BA, Grossen C, Wedekind C. 2010. Effects of different mating scenarios on embryo viability in brown trout. Mol. Ecol. 19, 5296–5307. ( 10.1111/j.1365-294X.2010.04884.x) [DOI] [PubMed] [Google Scholar]
- 72.Reusch TB, HaÈberli MA, Aeschlimann PB, Milinski M. 2001. Female sticklebacks count alleles in a strategy of sexual selection explaining MHC polymorphism. Nature 414, 300–302. ( 10.1038/35104547) [DOI] [PubMed] [Google Scholar]
- 73.Yeates SE, Einum S, Fleming IA, Mens H-J, Stet RJM, Hindar K, Holt WV, Van Look KJW, Gage MJG. 2009. Atlantic salmon eggs favour sperm in competition that have similar major histocompatibility alleles. Proc. R. Soc. B 276, 559–566. ( 10.1098/rspb.2008.1257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller JM, Malenfant RM, David P, Davis CS, Poissant J, Hogg JT, Festa-Bianchet M, Coltman DW. 2014. Estimating genome-wide heterozygosity: effects of demographic history and marker type. Heredity 112, 240–247. ( 10.1038/hdy.2013.99) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chapman J, Nakagawa S, Coltman D, Slate J, Sheldon B. 2009. A quantitative review of heterozygosity–fitness correlations in animal populations. Mol. Ecol. 18, 2746–2765. ( 10.1111/j.1365-294X.2009.04247.x) [DOI] [PubMed] [Google Scholar]
- 76.Borrell Y, Carleos C, Sánchez J, Vázquez E, Gallego V, Asturiano J, Blanco G. 2011. Heterozygosity–fitness correlations in the gilthead sea bream Sparus aurata using microsatellite loci from unknown and gene-rich genomic locations. J. Fish Biol. 79, 1111–1129. ( 10.1111/j.1095-8649.2011.03099.x) [DOI] [PubMed] [Google Scholar]
- 77.Yasui Y. 1997. A ‘good-sperm’ model can explain the evolution of costly multiple mating by females. Am. Nat. 149, 573–584. ( 10.1086/286006) [DOI] [Google Scholar]
- 78.McNamara KB, van Lieshout E, Simmons LW. 2014. A test of the sexy-sperm and good-sperm hypotheses for the evolution of polyandry. Behav. Ecol. 25, 989–995. ( 10.1093/beheco/aru067) [DOI] [Google Scholar]
- 79.Peretti AV, Aisenberg A. 2015. Cryptic female choice in arthropods. Berlin, Germany: Springer. [Google Scholar]
- 80.Willson MF, Burley N. 1983. Mate choice in plants: tactics, mechanisms, and consequences. Princeton, NJ: Princeton University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed in this study, and the details of statistical analyses, are available on Dryad (http://dx.doi.org/10.5061/rspb.2016.0001).


