Abstract
Primary cavity excavators, such as woodpeckers, are ecosystem engineers in many systems. Associations between cavity excavators and fungi have long been hypothesized to facilitate cavity excavation, but these relationships have not been experimentally verified. Fungi may help excavators by softening wood, while excavators may facilitate fungal dispersal. Here we demonstrate that excavators facilitate fungal dispersal and thus we report the first experimental evidence of a symbiosis between fungi and a cavity excavator, the red-cockaded woodpecker (RCW, Picoides borealis). Swab samples of birds showed that RCWs carry fungal communities similar to those found in their completed excavations. A 26-month field experiment using human-made aseptically drilled excavations in live trees, half of which were inaccessible to RCWs, demonstrated that RCWs directly alter fungal colonization and community composition. Experimental excavations that were accessible to RCWs contained fungal communities similar to natural RCW excavations, whereas inaccessible experimental excavations contained significantly different fungal communities. Our work demonstrates a complex symbiosis between cavity excavators and communities of fungi, with implications for forest ecology, wildlife management, and conservation.
Keywords: cavity excavator, cavity nester, fungal communities, multipartite symbiosis, Picoides borealis, wood decay fungi
1. Background
Symbioses are important components of biological systems, directly or indirectly affecting many levels of ecological organization, from individual organisms to entire ecosystems. While the traditional view of symbiosis was of pair-wise species interactions, the emerging perspective is that symbioses often involve diverse suites of interacting organisms, with this diversity being integral to the outcome of symbiotic interactions. Symbioses are defined as ‘the living together of unlike organisms’ [1], that is, a close association, and can operate along a continuum that ranges from parasitism to mutualism [2,3]. Some symbioses maintain entire ecosystems, such as the multipartite symbiotic interaction between cnidarians, zooxanthellae algae, bacteria, and various other organisms that enable the existence of coral reefs [4,5]. Other symbioses function at the level of individual organisms and range from the well known, such as the human microbiome, which is necessary for proper digestion, immunity, and development [6,7], to the more obscure, such as the interactions between individual crayfish and communities of ectosymbiotic worms [8]. Understanding complex symbiotic interactions is fundamental to understanding biological processes at many scales, from individuals to entire communities of symbionts, and a community perspective is necessary to characterize these multipartite symbiotic associations. In this study, we examine one such multipartite association between woodpeckers and diverse fungal communities.
Many woodpeckers are primary cavity excavators and ecosystem engineers who construct nest and roost cavities that are used by many species of secondary cavity users including non-excavating, cavity-nesting birds, termed secondary cavity nesters, as well as a variety of mammals, herpetofauna, and invertebrates. In forest systems where naturally formed, or non-excavated, cavities are rare, cavity excavators are keystone species [9,10]. Some excavating species depend on decayed wood in living or dead trees, while others require relatively healthy, living trees. Although traditionally woodpeckers are viewed as the providers of cavities, this service may depend on associations with fungi, especially in cases where excavators favour relatively healthy wood. Thus, wood decay fungi are also ecosystem engineers [11], because they not only facilitate the formation of non-excavated cavities, but also soften wood to create potential excavation sites [12–18], the availability of which may limit cavity excavation. Cavity excavators in turn may facilitate fungal dispersal and colonization by carrying a combination of fungal spores and hyphal fragments from existing cavities to future excavation sites [17–19]. Thus, the ecosystem function served by excavators may rely upon mutualistic associations with fungi.
Although frequently hypothesized to occur, direct interactions between cavity excavators and fungi have yet to be experimentally demonstrated. Birds have long been thought to be capable of fungal spore dispersal [19–23], and cavity excavators have been speculated to be capable of dispersing wood decay fungi. Two recent studies provide evidence that both black woodpeckers (Dryocopus martius) and red-cockaded woodpeckers (RCWs, Picoides borealis), two species separated by geography and phylogeny, may facilitate the progression of decay in their excavations [16,17].
RCWs are federally endangered in the USA and are cooperatively breeding birds that live in family groups. They are primary excavators in the longleaf pine ecosystem of southeastern North America, to which they are endemic [24,25]. Within each RCW family group, every bird requires an RCW-excavated roost cavity in the heartwood of a living pine tree. In addition to the roost cavities, each family group also maintains a number of incomplete excavations in various stages of construction, termed cavity starts. All excavations belonging to one RCW family group constitute a cavity tree cluster. RCWs are the only birds that exclusively excavate through the sapwood and into the heartwood of living pine trees and the time to complete the excavation process can range from less than one year to decades [26]. Thus, excavation constitutes a major investment of time and energy. However, once a cavity is complete, the woodpeckers can use it for many years, during which time the birds maintain active resin wells in the wood surrounding the excavation. This prolonged excavation period and the prolonged period of use of completed excavations contrasts with the behaviour of many woodpecker species that excavate cavities in dead trees in a matter of weeks, and use the cavity for a single year [13]. Additionally, once an RCW cavity is no longer in use by RCWs, a diverse community of secondary cavity users may then use it [9]. Thus, understanding the unusual excavation behaviour of RCWs is critical to management efforts for these endangered birds [27] and for the communities their cavities support.
In order to disperse into the wood of a healthy living tree, fungi need a point of access. RCW cavity starts are wounds in the trunks of trees and thus are possible infection courts for fungi. Because RCWs intermittently visit their cavity starts throughout the excavation process, the birds may carry fungi from their roost cavities into their cavity starts during the excavation process. If RCWs inoculate their excavations with wood decay fungi, they could gain decreased excavation time and increased access to a limited resource—trees available for relatively rapid excavation; the fungi would gain an additional method of dispersal beyond sporulation. RCWs are thought to seek out trees infected with a particular fungus, Porodaedalea pini sensu lato, for excavation [12,13,18], though there is little evidence to support this beyond the presence of the fungus in many RCW excavations [17]. We found in an earlier study that in addition to P. pini s.l., there are at least 28 other taxa of wood decay fungi associated with RCW excavations [17]. Our previous work demonstrated that trees containing RCW excavations are inhabited by distinct fungal communities, suggesting that the birds either: (i) select trees already colonized by these fungal communities for excavation (the tree selection hypothesis), (ii) facilitate establishment of these fungi through their excavation behaviour, either directly by carrying fungi on their bodies, or indirectly by changing the microhabitat within the tree during excavation (the bird facilitation hypothesis), or (iii) use a combination of tree selection and bird facilitation (the combined hypothesis).
In this study, we tested the bird facilitation hypothesis by first conducting a field survey to determine whether RCWs carry fungi found in their excavations by externally swabbing the birds and comparing fungal communities on their bodies to those in their complete and incomplete excavations. Second, we conducted a field experiment to test whether RCWs facilitate fungal infection in longleaf pine trees during cavity excavation by aseptically drilling cavity starts into non-excavated trees, restricting RCW access to half of these trees, and comparing the change in fungal communities over time in excavations accessible and inaccessible to RCWs.
2. Material and methods
(a). Field site
The study was conducted on Marine Corps Base Camp Lejeune (MCBCL), in Onslow County on the central coast of North Carolina, USA (34°38′7.25″ N, 77°18′9.44″ W). MCBCL is composed of 105 km2 of water and 445 km2 of land, including roughly 227 km2 of pine habitat considered suitable for RCWs [28]. The RCW population on MCBCL has been intensively monitored since 1986 and has grown from a low of 26 groups in 1991 to 105 in 2015. As part of this on-going study, complete RCW cavities and RCW cavity starts are documented as they are located on the landscape and monitored thereafter.
(b). Red-cockaded woodpecker swabbing
During 2009, we opportunistically swabbed the beaks, wings, and feet of adult RCWs captured as part of population monitoring, using sterile cotton swabs. Capture was accomplished by flushing birds from their roost cavities into a net on the end of an extendable pole. Care was taken not to touch the areas of the bird that were being swabbed; if there was any question of contamination, swabs were discarded. Prior to removing the bird from the net, the individual handling the swabs cleaned their hands with sanitizer, and only the cardboard end of the swab was handled. Once the RCW was in hand, the targeted sample area was gently wiped with the cotton end of a swab, and after swabbing, the swab was placed into a sterile 15 ml tube, with the cotton tip in the bottom of the tube and the cardboard end oriented towards the cap. We used this procedure to swab the beak, left wing, and left foot of each bird. After swabbing and additional processing related to population monitoring, the bird was released. We also included three field control swabs, which were handled in exactly the same way, in the same setting, but in the absence of a bird. All swabs were kept on wet ice, and then transferred into a −20° C freezer before processing.
(c). Red-cockaded woodpecker exclusion experiment
In September 2009, we selected 15 active RCW clusters on MCBCL containing at least four non-excavated longleaf pine trees with a heartwood diameter large enough to house an RCW cavity. All active complete (n = 36) and incomplete (n = 42) RCW excavations within these 15 clusters were sampled following the protocols in Jusino et al. [29]; additional details on these trees may be found in Jusino et al. [17]. From the non-excavated trees within each of the 15 clusters, four with attributes similar to cavity trees were selected. In September and October 2009, artificial 5.1 cm diameter cavity starts [30] were aseptically drilled through the sapwood and into the heartwood in each of these 60 previously non-excavated trees at average cavity height (5–6 m). After drilling, all starts were aseptically sampled by collecting wood scrapings in two locations from within the start with a sharpened sample collection device [29]. Additionally, each tree was aseptically cored using a 4.35 mm diameter, 3-threaded increment borer approximately 20 cm above the start following the protocols described by Jusino et al. [29]. The heartwood of these cores was kept, and the sapwood portion was sterilely re-inserted into the core site to prevent the artificial introduction of pathogenic organisms. Initially, galvanized steel screens with 0.64 × 0.64 cm openings were placed over all of the drilled starts in order to prevent birds and other animals from entering the start and being injured or killed by leaking resin. Once the resin stopped flowing copiously (July 2010), two of the four starts in each cluster were unscreened, allowing birds access. Twenty-six months post-drilling, in December 2011, all drilled starts were re-sampled and cored at cavity height.
(d). DNA extraction, PCR amplification, cloning, and sequencing
DNA was extracted from each drilled start sample following the protocol described by Brazee & Lindner [31] with the modifications described by Jusino et al. [29]. To extract DNA from the swabs, the cotton tips were removed and placed into a sterile 2 ml tube containing 300 µl of filtered cell lysis solution (CLS; [32]). These tubes were sealed and placed in a −80°C freezer to await further processing. Frozen samples were then placed in a 65°C water bath for 2 h, after which each swab was removed from its tube and placed in a sterile 0.5 ml tube with the cap and bottom tip removed. The 0.5 ml tubes containing the swabs were then placed inside sterile 2 ml tubes, thus creating a small ‘funnel’ that could be used to suspend swab tips in the 2 ml collection tubes. The assembly was centrifuged for 15 s intervals at 3 000 relative centrifugal force (rcf) until at least 290 µl of CLS was recovered. DNA was extracted from 100 µl of the recovered CLS following the protocol described by Brazee & Lindner [31] with the modifications described by Jusino et al. [29].
After DNA extraction, all samples were processed and subjected to PCR amplification, cloning, and sequencing of fungal DNA. We performed PCR on the internal transcribed spacer (ITS) region with the Basidiomycota-specific primer pair ITS1F [33] and ITS4b-21 [29] following the protocol described by Jusino et al. [29]. All samples with PCR products that were visible on an ethidium bromide stained gel were then cloned and sequenced following the protocol described by Lindner & Banik [32]. DNA sequences were edited using Sequencher 4.9 and identifications were obtained via Genbank NCBI BLAST (National Center for Biotechnology Information; Basic Local Alignment Search Tool), using a 97% identity match cut-off for species level identification. In addition to traditional negative controls and field swab controls, we included laboratory controls that underwent each step of the molecular process (i.e. our laboratory extraction controls underwent each downstream step).
(e). Analysis of red-cockaded woodpecker swabs
We visualized Basidiomycota communities found on RCWs, in their excavations and in non-excavated trees in ordination space using non-parametric multidimensional scaling (NMDS) performed with the metaMDS function in the Vegan package of R [34], with the modified Raup–Crick dissimilarity metric [35], calculated by the raupcrick function in Vegan. We then statistically compared Basidiomycota community composition on RCW swabs, in complete and incomplete RCW excavations, and in non-excavated trees using non-parametric permutational multivariate ANOVA (PERMANOVA) tests [36] with the adonis function in the Vegan package of R [34], using the modified Raup–Crick distance metric. We tested for differences in multivariate dispersion among fungal communities using a multivariate homogeneity of group dispersion test [37], the betadisper function in the Vegan package of R [34].
(f). Analysis of red-cockaded woodpecker exclusion experiment
The samples taken from each tree were pooled for analysis. We examined the change in Basidiomycota prevalence from pre-drilling to 26 months after drilling (the end of the experiment) using a χ2-test. We compared the Basidiomycota species richness from pre-drilling of artificial cavity starts to the end of the experiment using species accumulation curves based on rarefaction equations [38] generated by the R package, Species [39]. We compared Basidiomycota prevalence in the RCW accessible and RCW inaccessible experimental drilled starts (at the end of our experiment) using a χ2-analysis, and we compared Basidiomycota species richness across these two treatments using the Species package in R.
We then compared Basidiomycota communities in RCW accessible and RCW inaccessible drilled starts at the end of the experiment using PERMANOVA and betadisper tests, with the modified Raup–Crick distance metric. We also visualized the Basidiomycota communities found in complete RCW cavities, RCW cavity starts, the 60 (non-excavated) experimental trees at the start of the experiment, and the RCW accessible and inaccessible drilled starts at the end of the experiment, using the NMDS ordination procedure described for RCW swabs. We also performed PERMANOVA and betadisper to test for differences in the fungal community composition among these groups.
3. Results
(a). Red-cockaded woodpecker swabs
We swabbed 11 birds and found that RCWs carry a variety of Basidiomycota fungi associated with their excavations. Basidiomycota fungi were detected on all 11 birds, but on none of our negative control swabs (field or laboratory). There was no difference in the fungal communities found on different areas of the birds (PERMANOVA, p = 0.75), so all areas per swabbed bird were pooled for all other analyses. We identified 33 Basidiomycota taxa on RCWs. Consistent with the bird facilitation hypothesis, 27% (9/33) of the fungi we found on RCWs were also found in RCW excavations and only 12% (4/33) were found in trees without excavations (electronic supplementary material, S1). Of the four fungal taxa found on RCWs and in trees without excavations, only one (Irpex lacteus) was not also found in RCW excavations. In total, 61% (20/33) of the fungi we detected on RCWs were putative wood decay fungi, of which 40% (8/20) were also found in RCW excavations. We detected P. pini s.l., the fungus previously implicated as important to RCWs, on one bird out of the 11 we swabbed. We detected other putative wood decay fungi on all 11 birds. The communities of Basidiomycota fungi found on RCWs are similar to those found in complete RCW cavities, and have some overlap with those found in RCW cavity starts, but are compositionally different from those found in non-excavated trees (PERMANOVA r2 = 0.20, pseudo-F = 6.40, p < 0.0001; figure 1), and there were significant differences in multivariate dispersion among groups (betadisper F = 4.68, p = 0.006; figure 1).
Figure 1.
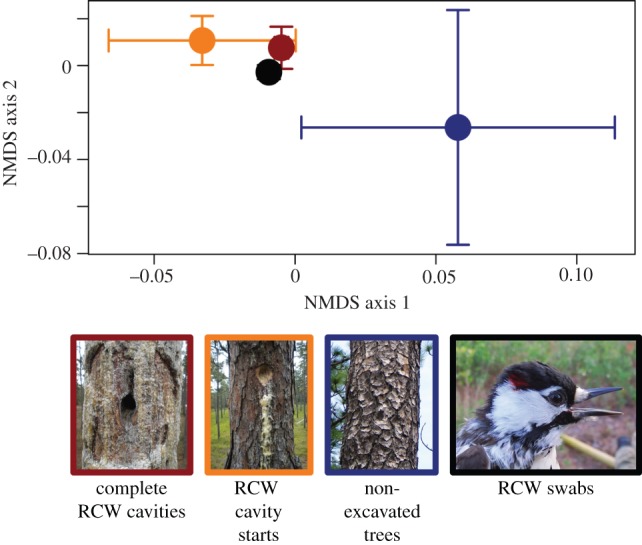
NMDS ordinations of the communities of Basidiomycota fungi found on RCWs and in RCW cavities, RCW cavity starts, and non-excavated trees. The dots in the centre represent the means of the points on the two NMDS axes, and the bars represent 1 s.e. from the mean. NMDS stress = 0.0069, two dimensions.
(b). Red-cockaded woodpecker exclusion experiment
Prior to the creation of the experimental drilled starts, 27% of the non-excavated trees used in the experiment were already infected with Basidiomycota fungi. From these baseline samples, we identified 17 taxa of Basidiomycota, of which 14 were putative wood decay taxa [17]. After 26 months, 75% of the drilled cavity starts tested positive for Basidiomycota, and the diversity increased to 46 taxa, 30 of which were putative wood decay fungi (electronic supplementary material, S1). Seventeen putative wood decay Basidiomycota were detected in RCW accessible drilled starts and 16 in the inaccessible drilled starts, only three of which were found in both treatment groups. Prevalence and species richness of Basidiomycota in drilled starts increased significantly from the beginning to the end of the experiment (Basidiomycota prevalence x2 = 26.16, p < 0.0001, species richness p < 0.0001, Shannon index p = 0.038), but treatment (i.e. RCW access) did not affect fungal richness or prevalence (Basidiomycota prevalence x2 = 0.09, p = 0.765, species richness p = 0.584, Shannon index p = 0.265).
While Basidiomycota fungal species richness and prevalence rates were similar between the treatment groups, fungal community composition differed (PERMANOVA r2 = 0.10, pseudo-F = 4.56, p = 0.002), with no significant effect on multivariate dispersion (betadisper F = 3.80, p = 0.062). Furthermore, we found significant differences in fungal community structure between the five types of trees sampled (PERMANOVA r2 = 0.18, pseudo-F = 5.97, p < 0.0001), with some effects on community dispersion (betadisper F = 2.95, p = 0.03). These differences in community composition are consistent with the bird facilitation hypothesis. The Basidiomycota communities found in the accessible drilled starts at the end of our experiment were compositionally more similar to those found in completed RCW-made cavities than those found in RCW-made starts, non-excavated trees at the beginning of the experiment, and inaccessible drilled starts at the end of our experiment (figure 2). Furthermore, the Basidiomycota communities detected in the accessible drilled starts were more similar to those found in RCW starts than the communities detected in inaccessible drilled starts, and the fungal communities present in any type of excavation were compositionally different from those found in non-excavated trees. Finally, there was no evidence of RCW activity on any of the drilled starts at the beginning of the experiment but by the end of the experiment there was evidence of RCW activity in the excavation (work to the entrance tunnel and/or deeper excavation of the start and in one case a fully complete cavity) or in the surrounding wood (resin wells) of 26 of the 30 (86.7%) accessible starts, and evidence of RCW activity (resin wells) in the wood surrounding 10 of the 30 (33.3%) inaccessible starts.
Figure 2.
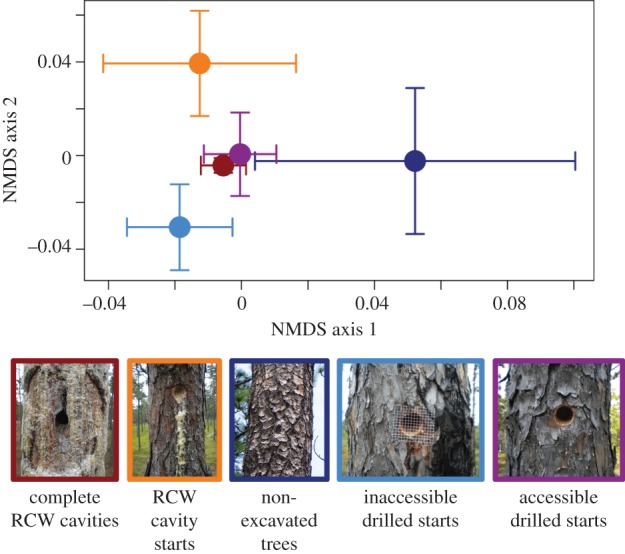
NMDS ordinations of communities of Basidiomycota fungi found in completed RCW cavities, RCW cavity starts, non-excavated trees, drilled starts accessible to RCWs, and drilled starts inaccessible to RCWs. The dots in the centre represent the means of the points on the two NMDS axes, and the bars represent 1 s.e. from the mean. NMDS stress = 0.0062, two dimensions.
4. Discussion
This study sought to test the ‘bird facilitation hypothesis', which states that RCWs facilitate the establishment of fungi in their excavations through their excavation behaviour. Our results provide strong support for this hypothesis. Consistent with the bird facilitation hypothesis, we have demonstrated that RCWs carry DNA from a diversity of fungi, including many responsible for wood decay, and that many of these fungi are found in RCW excavations. This result demonstrates that RCWs can act as agents of fungal dispersal. Also consistent with the bird facilitation hypothesis, our experiment demonstrated that access by RCWs influences the fungal community composition in excavations in living pine trees. The results from our RCW exclusion experiment demonstrated that excavations accessible to RCWs are colonized by different fungal communities than excavations that are inaccessible to RCWs, and that fungal communities in both accessible and inaccessible excavations are more prevalent, more diverse, and different in composition compared to those in non-excavated trees. Thus, RCWs appear to influence fungal colonization and fungal community composition, including wood decay fungi, in living trees in two ways: (i) indirectly, by making a wound in a tree and (ii) directly, by facilitating the establishment of particular fungi associated with their completed cavities.
Although we cannot completely rule out the possibility that vertebrate vectors other than RCWs facilitated development of the fungal communities observed in accessible starts, evidence suggests that RCWs played a dominant or exclusive role. Whereas we found evidence that RCWs were visiting and working on almost all (26/30) of the accessible starts, and showed interest in some (10/30) of the inaccessible starts, we did not detect evidence of any other vertebrate entering the accessible starts.
The fungal communities found on RCWs were compositionally similar to those found in their complete cavities. Interestingly, the fungal community composition of RCW cavity starts was compositionally different from the fungal communities detected on RCWs and in their completed cavities. This result suggests that most of the fungi recovered from the swabbed birds were acquired from RCW roost cavities. The RCWs that were sampled were swabbed immediately after departing their roost cavities, and thus had recent exposure to completed cavities but not necessarily to cavity starts. All of the swabbed birds were captured during the autumn months, but the peak of the RCW excavation season is the summer months (June–August; JRW 2015, personal observation).
Historically, RCWs were thought to have an association with one fungus, P. pini s.l., but RCW excavations have recently been shown to house a diversity of fungi [17,29], suggesting that these birds have not one, but many fungal associates. The most prevalent Basidiomycota taxa detected in RCW cavities and starts, Exobasidiomycetes sp. 2 and P. pini s.l., were also detected on the birds. We detected P. pini s.l. on only one of the 11 birds we swabbed. It is possible that P. pini s.l. was not in the cavities these particular birds were using, or that not all birds carry this species at all times, or that this species of fungus does not consistently rely on RCWs for transmission. In addition to P. pini s.l., we detected eight other wood decay fungi on the birds that also occurred in their excavations, as well as 12 other wood decay fungi not detected in their excavations. We also detected many other fungi on RCWs from the phylum Basidiomycota whose ecological functions are largely unknown. In addition to being exposed to fungi within their excavations, these birds come into contact with many trees on a daily basis while foraging, and likely encounter spores in flight and on the invertebrates they consume.
During our RCW exclusion experiment, the fungal communities in accessible starts developed to resemble fungal communities in RCW cavities more so than fungal communities in inaccessible starts. The inaccessible starts developed communities dominated by Acaromyces ingoldii, a fungus present, but much less prevalent, in RCW cavities, RCW starts, and our accessible experimental starts. The Basidiomycota communities in the accessible drilled starts more closely resemble those in completed RCW cavities than those in RCW-excavated cavity starts, a result that may be related to excavation depth. All of our drilled cavity starts were ‘advanced starts' that penetrated through the sapwood and into the heartwood of the trees, leaving only the cavity chamber unexcavated. Many of the RCW-excavated starts sampled were much less advanced, consisting of only a cavity entrance tunnel that had not yet reached the heartwood. The process of tunnelling through the sapwood is a time-limiting step in RCW cavity excavation [26] and thus some of the fungi detected in recently initiated RCW starts may represent early successional species and/or species adapted to living in sapwood rather than heartwood. The Basidiomycota communities in RCW starts to become more compositionally similar to those in RCW cavities as they become more advanced over time [17]. By tunnelling through the sapwood and into the heartwood of the experimental starts, we appear to have ‘artificially advanced’ fungal community succession by allowing fungi to quickly access the heartwood. However, RCW access was necessary for succession to advance to the state characteristic of RCW cavities, strongly supporting the bird facilitation hypothesis. Together, the results of our RCW swabbing survey and RCW exclusion experiment suggest that RCWs cause predictable changes in the fungal community composition of the trees they excavate. These results provide evidence for a symbiotic association between an ecosystem engineer and entire communities of fungi.
While we have shown that RCWs influence fungal community colonization and composition in their excavations, we do not yet fully understand the implications of this association for the birds. Further research could reveal this association to be a mutualism if the fungal communities found in RCW accessible starts decrease excavation time for RCWs. This could be investigated by tracking changes in wood hardness over time, perhaps following methods similar to those used by Lorenz et al. [40]. This could prove challenging, however, due to the length of the excavation process and the fact that living rather than dead trees are involved. Further, differences may be subtle, as the fungal communities found in inaccessible as well as accessible starts could be responsible for softening wood. It may even be that wood hardness does not reflect the complexity of the relationship between the fungi and the birds. Perhaps the fungal communities associated with the woodpeckers produce a more favourable rate of decay than the fungal communities found in inaccessible starts, which perhaps produce decay too quickly or aggressively. Additional complexities are possible, for example, the birds may carry fungi into their starts (and the accessible starts) that are antagonistic to some of the fungi found in inaccessible starts. Further detailed experimental and observational work is needed to fully understand the extent and nature of the interactions among RCWs and fungal communities.
Although our results provide strong evidence for the bird facilitation hypothesis, some elements of tree selection could be operating in this system as well. Indeed, the high prevalence of P. pini s.l. in RCW excavated starts lends support to the combined bird facilitation/tree selection hypothesis [17]. Tree selection is the historical and generally accepted hypothesis for woodpecker excavation, and could certainly be the standard for some primary cavity excavators, but the tree selection hypothesis and the bird facilitation hypothesis are not mutually exclusive. Martin et al. [41] showed that excavators in interior British Columbia preferentially select quaking aspen (Populus tremuloides) for excavation, though it only represents 15% of the available trees. Zahner et al. [16] demonstrated that black woodpeckers preferentially excavate cavity starts in trees with evidence of decay. Indeed, these findings support the tree selection hypothesis, but further research on the fungal communities in these systems is needed to determine whether bird facilitation also applies. Bednarz et al. [42] tested tree selection by inoculating living trees with known decay fungi and monitoring excavation activity for nine years. They found no support for the preferential selection of nest excavation sites in inoculated living trees in their system, though they did show some support for the selection of inoculated living trees for general use by picids. We recommend a test similar to that used by Bednarz et al. [42] to test the tree selection hypothesis in RCWs.
Lorenz et al. [40] recently demonstrated that wood hardness in snags was an important predictor of nest site selection for six picid species in Washington, USA, and suggested that tree selection explained excavation patterns in this system, specifically the selection of trees with soft interiors. It may be that tree selection plays a larger role in the relatively rapid excavation of cavities in dead trees than in the lengthy excavation process in living pines in our system. However, Lorenz et al. [40] did not identify the fungi responsible for decay in their system or investigate the possible role of cavity starts as potential infection courts for decay fungi. Thus, bird facilitation could also play a role in such systems.
5. Conclusion
Through a test of the bird facilitation hypothesis, we have provided the first experimental evidence that a primary cavity excavator facilitates fungal colonization of living trees. The possible relationships between cavity excavators and the fungi that aid excavation have interested researchers for decades, and many researchers have suggested that woodpeckers select trees infected by certain fungi for excavation [12–16,41,43]. We have demonstrated that the relationships between woodpeckers and fungi may be far more complex than previously imagined, and stretch beyond habitat selection to symbiotic and potentially mutualistic relationships between woodpeckers and multiple species of fungi. Our system is unique in that RCWs excavate solely through the sapwood and into the heartwood of living pine trees, but the symbiosis observed in this system may not be a singular, isolated example. Even excavators that complete cavities very quickly could introduce fungi into trees. Furthermore, the process of bird facilitation of fungal establishment may not be mutually exclusive from tree selection by the birds; that both occur could be the norm. Because so many organisms depend on the cavities created by excavators, multipartite symbioses between excavators and fungi may be critically important to native biodiversity in forested systems worldwide, and therefore further knowledge of these interactions may be vital to understanding the habitat requirements of all the organisms that rely on the tree cavities produced by these ecosystem engineers.
Supplementary Material
Acknowledgements
We sincerely thank James Skelton for helpful comments, and assistance with statistical analyses, Dana M. Hawley, Robert H. Jones, and David G. Schmale III for thoughtful guidance throughout this project, and two anonymous reviewers whose comments greatly improved this manuscript.
Ethics
RCW work was authorized by United States Fish and Wildlife Service (USFWS) permit TE070846-2 and Institutional Animal Care and Use Committee (IACUC) permit 10-138-BIOL.
Data accessibility
Data are presented in the supplementary materials and/or will be made available upon request from the corresponding author. GenBank accession numbers KM103937–KM104154.
Authors' contributions
M.A.J., D.L.L., M.T.B., K.R.R., and J.R.W. designed research; M.A.J. and K.R.R. performed research; M.A.J. analysed data; M.A.J. and J.R.W. wrote the paper.
Competing interests
The authors declare no competing interests.
Funding
Funding for fieldwork was provided by the US Department of Defense, Marine Corps Base Camp Lejeune. Funding for the analysis of samples was provided by the Harold H. Bailey fund at Virginia Tech and the US Forest Service, Northern Research Station. Further funding for this work was provided by the American Ornithologists' Union, the Mycological Society of America, the Society for Integrative and Comparative Biology and the Virginia Tech Graduate Research and Development Program. Further support for M.A.J. was provided by the US Department of Defense, Strategic Environmental Research and Development Program (RC-1471 and RC-1413, Defense Coastal/Estuarine Research Program).
References
- 1.De Bary A. 1879. Die erscheinung der symbiose. Strasburg, Germany: Verlag von Karl J. Trübner. [Google Scholar]
- 2.Ewald PW. 1987. Transmission modes and evolution of the parasitism-mutualism continuum. Ann. NY Acad. Sci. 503, 295–306. ( 10.1111/j.1749-6632.1987.tb40616.x) [DOI] [PubMed] [Google Scholar]
- 3.Bronstein JL. 1994. Conditional outcomes in mutualistic interactions. Trends Ecol. Evol. 9, 214–217. ( 10.1016/0169-5347(94)90246-1) [DOI] [PubMed] [Google Scholar]
- 4.Rohwer F, Seguritan V, Azam F, Knowlton N. 2002. Diversity and distribution of coral-associated bacteria. Mar. Ecol. Progress Ser. 243, 1–10. ( 10.3354/meps243001) [DOI] [Google Scholar]
- 5.Goodrich-Blair H, Hussa E. 2013. It takes a village: ecological and fitness impacts of multipartite mutualism. Annu. Rev. Microbiol. 67, 161–178. ( 10.1146/annurev-micro-092412-155723) [DOI] [PubMed] [Google Scholar]
- 6.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. 2005. Host-bacterial mutualism in the human intestine. Science 307, 1915–1920. ( 10.1126/science.1104816) [DOI] [PubMed] [Google Scholar]
- 7.Grice EA, et al. 2009. Topographical and temporal diversity of the human skin microbiome. Science 324, 1190–1192. ( 10.1126/science.1171700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skelton J, Creed RP, Brown BL. 2014. Ontogenetic shift in host tolerance controls initiation of a cleaning symbiosis. Oikos 123, 677–686. ( 10.1111/j.1600-0706.2013.00963.x) [DOI] [Google Scholar]
- 9.Blanc LA, Walters JR. 2008. Cavity-nest webs in a longleaf pine ecosystem. The Condor 110, 80–92. ( 10.1525/cond.2008.110.1.80) [DOI] [Google Scholar]
- 10.Martin K, Eadie JM. 1999. Nest webs: a community-wide approach to the management and conservation of cavity-nesting forest birds. For. Ecol. Manag. 115, 243–257. ( 10.1016/S0378-1127(98)00403-4) [DOI] [Google Scholar]
- 11.Jones CG, Lawton JH, Shachak M. 1996. Organisms as ecosystem engineers. Oikos 69, 373–386. ( 10.2307/3545850) [DOI] [Google Scholar]
- 12.Conner RN, Orson K, Adkisson CS. 1976. Woodpecker dependence on trees infected by fungal heart rots. Wilson Bull. 88, 575–581. [Google Scholar]
- 13.Jackson JA, Jackson BJS. 2004. Ecological relationships between fungi and woodpecker cavity sites. The Condor 106, 37–49. ( 10.1650/7483) [DOI] [Google Scholar]
- 14.Witt C. 2010. Characteristics of aspen infected with heartrot: implications for cavity-nesting birds. For. Ecol. Manag. 260, 1010–1016. ( 10.1016/j.foreco.2010.06.024) [DOI] [Google Scholar]
- 15.Cockle KL, Martin K, Robledo G. 2012. Linking fungi, trees, and hole-using birds in a Neotropical tree-cavity network: Pathways of cavity production and implications for conservation. For. Ecol. Manag. 264, 210–219. ( 10.1016/j.foreco.2011.10.015) [DOI] [Google Scholar]
- 16.Zahner V, Sikora L, Pasinelli G. 2012. Heart rot as a key factor for cavity tree selection in the black woodpecker. For. Ecol. Manag. 271, 98–103. ( 10.1016/j.foreco.2012.01.041) [DOI] [Google Scholar]
- 17.Jusino MA, Lindner DL, Banik MT, Walters JR. 2015. Heart rot hotel: fungal communities in red-cockaded woodpecker excavations. Fungal Ecol. 14, 33–43. ( 10.1016/j.funeco.2014.11.002) [DOI] [Google Scholar]
- 18.Jackson JA. 1977. Red-cockaded woodpeckers and pine red heart disease. The Auk 94, 160–163. [Google Scholar]
- 19.Farris KL, Huss MJ, Zack S. 2004. The role of foraging woodpeckers in the decomposition of ponderosa pine snags. The Condor 106, 50–59. ( 10.1650/7484) [DOI] [Google Scholar]
- 20.Heald FD, Studhalter RA. 1913. Preliminary note on birds as carriers of the chestnut blight fungus. Science 38, 278–280. ( 10.2307/1639930) [DOI] [PubMed] [Google Scholar]
- 21.Warner GM, French D. 1970. Dissemination of fungi by migratory birds: survival and recovery of fungi from birds. Can. J. Bot. 48, 907–910. ( 10.1139/b70-127) [DOI] [Google Scholar]
- 22.Francesca N, Carvalho C, Almeida PM, Sannino C, Settanni L, Sampaio JP, Moschetti G. 2013. Wickerhamomyces sylviae f.a., sp. nov., an ascomycetous yeast species isolated from migratory birds. Int. J. Syst. Evol. Microbiol. 63, 4824–4830. ( 10.1099/ijs.0.056382-0) [DOI] [PubMed] [Google Scholar]
- 23.Alfonzo A, Francesca N, Sannino C, Settanni L, Moschetti G. 2013. Filamentous fungi transported by birds during migration across the Mediterranean sea. Curr. Microbiol. 66, 236–242. ( 10.1007/s00284-012-0262-9) [DOI] [PubMed] [Google Scholar]
- 24.Walters JR, Doerr PD, Carter JH. 1988. The cooperative breeding system of the red-cockaded woodpecker. Ethology 78, 275–305. ( 10.1111/j.1439-0310.1988.tb00239.x) [DOI] [Google Scholar]
- 25.Ligon JD. 1970. Behavior and breeding biology of the red-cockaded woodpecker. The Auk 87, 255–278. ( 10.2307/4083919) [DOI] [Google Scholar]
- 26.Harding S, Walters J. 2004. Dynamics of cavity excavation by red-cockaded woodpeckers. In Red-cockaded woodpecker: road to recovery (eds Costa R, Daniels S), pp. 412–422. Blaine, Washington: Hancock House. [Google Scholar]
- 27.Walters JR. 1991. Application of ecological principles to the management of endangered species: the case of the red-cockaded woodpecker. Annu. Rev. Ecol. Syst. 22, 505–523. ( 10.1146/annurev.es.22.110191.002445) [DOI] [Google Scholar]
- 28.MCBCL. 2006. Marine Corps Base, Camp Lejeune Integrated Natural Resources Management Plan (2007–2011). Environmental Management Division, Marine Corps Base Camp Lejeune. See http://www.lejeune.marines.mil/OfficesStaff/EnvironmentalMgmt/INRMP.aspx.
- 29.Jusino MA, Lindner DL, Cianchetti JC, Grisé AT, Brazee NJ, Walters JR. 2014. A minimally invasive method for sampling nest and roost cavities for fungi: a novel approach to identify the fungi associated with cavity-nesting birds. Acta Ornithol. 49, 233–242. ( 10.3161/173484714X687127) [DOI] [Google Scholar]
- 30.Copeyon CK. 1990. A technique for constructing cavities for the red-cockaded woodpecker. Wildlife Soc. Bull. 18, 303–311. ( 10.2307/3782218) [DOI] [Google Scholar]
- 31.Brazee NJ, Lindner DL. 2013. Unravelling the Phellinus pini s.l. complex in North America: a multilocus phylogeny and differentiation analysis of Porodaedalea. For. Pathol. 43, 132–143. ( 10.1111/efp.12008) [DOI] [Google Scholar]
- 32.Lindner DL, Banik MT. 2009. Effects of cloning and root-tip size on observations of fungal ITS sequences from Picea glauca roots. Mycologia 101, 157–165. ( 10.3852/08-034) [DOI] [PubMed] [Google Scholar]
- 33.Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol. Ecol. 2, 113–118. ( 10.1111/j.1365-294X.1993.tb00005.x) [DOI] [PubMed] [Google Scholar]
- 34.Oksanen J, et al. 2015. vegan: Community Ecology Package. R package version 2.3-0. http://CRAN.R-project.org/package=vegan.
- 35.Chase JM, Kraft NJ, Smith KG, Vellend M, Inouye BD. 2011. Using null models to disentangle variation in community dissimilarity from variation in α-diversity. Ecosphere 2, 1–11. ( 10.1890/ES10-00117.1) [DOI] [Google Scholar]
- 36.Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austr. Ecol. 26, 32–46. [Google Scholar]
- 37.Anderson MJ. 2006. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62, 245–253. ( 10.1111/j.1541-0420.2005.00440.x) [DOI] [PubMed] [Google Scholar]
- 38.Hurlbert SH. 1971. The nonconcept of species diversity: a critique and alternative parameters. Ecology 52, 577–586. ( 10.2307/1934145) [DOI] [PubMed] [Google Scholar]
- 39.Czederplitz DLL. 2001. Forest management and the diversity of wood-inhabiting polyporoid and corticioid fungi. Madison, WI: University of Wisconsin. [Google Scholar]
- 40.Lorenz TJ, Vierling KT, Johnson TR, Fischer PC. 2015. The role of wood hardness in limiting nest site selection in avian cavity excavators. Ecol. Appl. 25, 1016–1033. ( 10.1890/14-1042.1) [DOI] [PubMed] [Google Scholar]
- 41.Martin K, Aitken KE, Wiebe KL. 2004. Nest sites and nest webs for cavity-nesting communities in interior British Columbia, Canada: nest characteristics and niche partitioning. The Condor 106, 5–19. ( 10.1650/7482) [DOI] [Google Scholar]
- 42.Bednarz JC, Huss MJ, Benson TJ, Varland DE. 2013. The efficacy of fungal inoculation of live trees to create wood decay and wildlife-use trees in managed forests of western Washington, USA. For. Ecol. Manag. 307, 186–195. ( 10.1016/j.foreco.2013.06.041) [DOI] [Google Scholar]
- 43.Blanc LA, Martin K. 2012. Identifying suitable woodpecker nest trees using decay selection profiles in trembling aspen (Populus tremuloides). For. Ecol. Manag. 286, 192–202. ( 10.1016/j.foreco.2012.08.021) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are presented in the supplementary materials and/or will be made available upon request from the corresponding author. GenBank accession numbers KM103937–KM104154.


