Abstract
Reticulitermes, Heterotermes and Coptotermes form a small termite clade with partly overlapping distributions. Although native species occur across all continents, the factors influencing their distribution are poorly known. Here, we reconstructed the historical biogeography of these termites using mitochondrial genomes of species collected on six continents. Our analyses showed that Reticulitermes split from Heterotermes + Coptotermes at 59.5 Ma (49.9–69.5 Ma 95% CI), yet the oldest split within Reticulitermes (Eurasia and North America) is 16.1 Ma (13.4–19.5 Ma) and the oldest split within Heterotermes + Coptotermes is 36.0 Ma (33.9–40.5 Ma). We detected 14 disjunctions between biogeographical realms, all of which occurred within the last 34 Ma, not only after the break-up of Pangaea, but also with the continents in similar to current positions. Land dispersal over land bridges explained four disjunctions, oceanic dispersal by wood rafting explained eight disjunctions, and human introduction was the source of two recent disjunctions. These wood-eating termites, therefore, appear to have acquired their modern worldwide distribution through multiple dispersal processes, with oceanic dispersal and human introduction favoured by the ecological traits of nesting in wood and producing replacement reproductives.
Keywords: Isoptera, long distance dispersal, molecular clock, Rhinotermitidae
1. Introduction
Termites are a small insect clade comprising about 3000 described species [1], yet they are important beyond their modest diversity, due to their ability to digest lignocellulose, the most abundant biomolecule on the Earth [2,3]. Consequently, they are hugely abundant and important ecologically, due to their essential role in decomposition and nutrient recycling [4–6]. Termites are also economically important, as they are significant pests of trees in agriculture, timber in forestry and human construction [7–9].
Pest species are concentrated in a few clades of termites. There are 97 species that are considered to be major pests of trees in agriculture, forestry and/or human-made wooden structures [1]. Of these major pests, 39 species are found in the clade comprising Reticulitermes, Heterotermes and Coptotermes [1,10]. Although native species in these three genera are found around the world, about half of the 28 species of invasive termites belong to these genera, compounding their international economic significance [11,12].
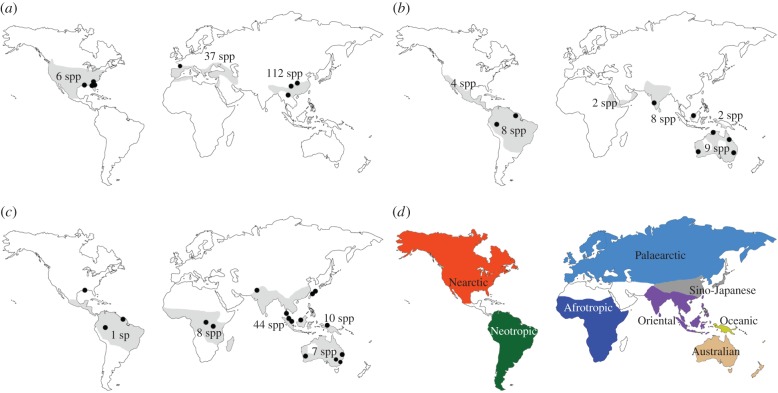
Despite their enormous economic importance, the factors influencing the distribution of Reticulitermes, Heterotermes and Coptotermes species around the world have not been investigated in detail. Reticulitermes has a temperate, holarctic distribution, although it is absent from the drier areas of north Asia east of the Caspian Sea [13,14], with the highest diversity found in China and then around the Mediterranean (although many species are likely synonyms [1,15]). Heterotermes and Coptotermes are pantropical, although Heterotermes is absent from Africa except a small area around Ethiopia (figure 1) [13,14]. The diversity of Heterotermes is about the same in the Australian, Oriental and Neotropical regions, and that of Coptotermes is highest in Oriental Asia then Papua (although many species are likely synonyms [1,18]). The oldest known fossils are not found in the same locations as the highest diversity. Those of Reticulitermes are from Europe (Baltic amber), dated from the Late Eocene, approximately 34 Ma [13], those of Heterotermes from Mexico and those of Coptotermes from the Dominican Republic (Dominican amber), dated from the Late Oligocene to Early Miocene, 14–26 Ma [13,19].
Figure 1.
Distribution map of (a) Reticulitermes, (b) Heterotermes and (c) Coptotermes. Light grey areas are the native range of each genus, black circles represent the sampling locations of the specimens used in this study, and species numbers are given as per Krishna et al. [1] and Scheffrahn et al. [16]. As C. formosanus was sampled from introduced populations, a few black circles occur outside the native range of Coptotermes. (d) Map of the main zoogeographic realms considered in this study [17]. White areas are regions from which no samples were analysed in this study.
There are three mechanisms that may explain these distributions and lack of correlation with fossil ages: vicariance and long distance dispersal either by oceanic rafting or by human transport. Vicariance would split groups, due to splitting of landmasses or the raising of mountains. Long distance dispersal could occur over land and sea by natural means in the past, or by human introduction recently. Land dispersal may be through natural flight of winged alates; this would require considerable time, as alates typically fly less than 1 km [20,21], and generation times may be years [22]. Oceanic rafting occurs when floating wood carries termite occupants across otherwise non-traversable space [23]. The floating wood can become a propagule when it contains whole colonies, or even groups of foragers, so long as they can produce replacement reproductives from wingless individuals [24]. Human transport mimics these conditions, just more quickly, and is responsible for the spread of invasive species [11,12].
It is possible to use the timing of splits between species to identify the most likely mechanism behind distribution patterns: vicariance is old and concurs with past geophysical events, human transport young and ocean rafting sometime between. Vicariance may explain some aspects of relatively separate distributions among the genera: molecular-clock analyses estimated that Reticulitermes + Heterotermes + Coptotermes split from Termitidae about 67 Ma, that Reticulitermes split up from Coptotermes + Heterotermes about 50–56 Ma, and that the most recent common ancestor of Coptotermes and Heterotermes dated back to 27–29 Ma [10,25]. Note that Reticulitermes occupies a relatively separate distribution from Heterotermes + Coptotermes (figure 1d), which may be explained by its adaptation to a temperate climate.
The dispersal mechanisms that shaped the distribution within genera are unclear, with the best information for Reticulitermes. Intriguingly, although Reticulitermes is an old lineage, modern species of Reticulitermes shared a common ancestor relatively recently, about 18 Ma [10,15,25]. The modern distribution of Reticulitermes can be explained by dispersal using land bridges, possibly over the Pacific via Beringia, as is the case for other insects [26–29]. Alternatively, they may have dispersed between the two biogeographic realms through rafting. By contrast, the pantropical distribution of Heterotermes and Coptotermes must be mostly the result of ocean dispersal by wood rafting or human transportation, as there has been just one land bridge (between Africa and Asia) since they evolved. Unfortunately, data found in existing studies are insufficient to make conclusions, due to low sampling of species and geographical regions, or use of relatively small DNA regions, thus preventing any conclusions to be drawn [30–35].
Here, we aimed to identify the mechanisms explaining the worldwide distribution of Reticulitermes, Heterotermes and Coptotermes. We did so by determining the full mitochondrial genome sequences of 31 Coptotermes, 14 Heterotermes and 13 Reticulitermes samples, collected along their entire distribution range (figure 1). Termite mitochondrial genomes are about 16 kb, of which 15 kb is phylogenetically informative, encoding 13 protein-coding genes, two ribosomal RNA genes and 22 transfer RNA genes [10,36–38]. Mitochondrial genomes are now becoming a common marker for phylogenetic studies, thanks to the progress of next generation sequencing and their resulting phylogenies have successfully resolved the relationships of several insect groups [38]. With this information, we built a phylogeny based on the mitochondrial genome data and calculated dates for evolutionary splits based on fossils of Coptotermes, Heterotermes and Reticulitermes. We used this phylogeny to resolve the relationships between species of the group and to investigate the origin and timing of the disjunctions in their distribution across biogeographic realms. We then discuss the relative role of vicariance, oceanic rafting and human transport in shaping the modern global distribution pattern of subterranean termites.
2. Material and methods
(a). Mitochondrial genome sequencing
We collected 44 samples of termites of the genera Coptotermes, Heterotermes and Reticulitermes around the world, and used 14 samples from these genera sequenced in previous studies [10,36,37,39–41], for a total of 37 species (electronic supplementary material, table S1). We stored all samples in RNA-later® at −80°C until DNA extraction. We extracted whole genomic DNA using the phenol–chloroform extraction procedure, from about five individual specimens per sample, after removing the digestive tract. We amplified the complete mitochondrial genomes with TaKaRa LA Taq in two long PCR reactions using primers that we specifically designed for termites (electronic supplementary material, table S2). We determined the concentration of both long PCR fragments using Qubit v. 3.0 fluorometer, then mixed them in equimolar concentration and then multiplexed and paired-end sequenced them with Illumina HiSeq2000.
We assembled separately 88 bp paired-end reads using the CLC suite of programs, as described in Bourguignon et al. [10]. In all cases of polymorphic bases, we selected the base with the highest representation. We omitted control regions of the mitochondrial genomes from the final matrix, as they present repetitive patterns that are generally poorly assembled with short reads, and thus provide no useful information. We annotated the 22 tRNAs, the 13 protein-coding genes and the two ribosomal RNAs by eye, aided by previously published sequences that we aligned on each mitochondrial genome using the Muscle algorithm [42] implemented in MEGA v. 5.2.1 [43].
(b). Alignment
We carried out alignments for the 45 mitochondrial genomes sequenced in this study with those from an additional 66 termite species, whose mitochondrial genome sequences have been deposited in GenBank [10,36,37,39–41]. Among these species, there were nine Reticulitermes, four Coptotermes and one Heterotermes species (see electronic supplementary material, table S1). Additionally, we included the sequences of five other polyneopteran insect outgroups whose mitochondrial genomes have been deposited in GenBank: two cockroaches, Periplaneta fuliginosa and Cryptocercus relictus; a mantis, Tamolanica tamolana; a phasmid, Megacrania alpheus; and a locust Locusta migratoria. We pruned all tips but those of the 13 Reticulitermes, 31 Coptotermes and 14 Heterotermes after phylogenetic analyses for depicting tree topologies. We aligned each gene individually using the Muscle algorithm [42] implemented in MEGA 5.2 [43]. We aligned protein-coding genes as codons, and tRNA and ribosomal RNA genes as DNA, then we concatenated the resulting alignments with SequenceMatrix [44].
(c). Phylogenetic analyses and molecular dating
We determined the partitioning scheme with PartitionFinder [45]. We used a GTR model with gamma-distributed rate variation across sites for all partitions. We found between-species heterogeneity in base composition for the third codon position of Coptotermes, Heterotermes and Reticulitermes (χ2 = 181.18; d.f. = 54; p < 10−6). To determine the effect of third codon position on the tree topology and timing, phylogenetic analyses were carried out twice independently, once with the third codon position included in the analysis (hereafter PF3+) and once without the third codon position (PF3−). PF3+ comprised 21 partitions and PF3− comprised 13 partitions (electronic supplementary material, table S3).
We analysed the concatenated DNA sequence alignment with a relaxed molecular-clock model using the Bayesian phylogenetic software BEAST v. 1.8.0 [46] (see electronic supplementary material, datasets S1 and S2). Rate variation was modelled among branches using uncorrelated lognormal relaxed clocks [46,47], with a single model for all genes, that allows for a different relative rate for each partition. We used a Yule speciation process for the tree prior [48] and estimated posterior distributions of parameters, including the tree, using MCMC sampling. We performed two replicate MCMC runs, with the tree and parameter values sampled every 10 000 steps over a total of 100 million generations. We obtained a maximum clade credibility tree using Tree Annotator within the BEAST software package with a burn-in of the first 10 millions generations as determined with Tracer v. 1.5 [49]. We checked acceptable sample sizes and convergence to the stationary distribution using Tracer v. 1.5.
We calibrated the molecular clock using 13 minimum age constraints (electronic supplementary material, table S4). Minimum age constrains were determined based on the fossil record, and we systematically selected the youngest possible age for each fossil as mentioned in the Paleobiology Database (PaleoBioDB, www.paleobiodb.org). We implemented fossil calibrations as exponential priors on node times (electronic supplementary material, table S4). Some of these dates differ from previous calibrations we used [10,25], which were based on dates reported in Emerson [13]. This is because the ages of certain periods, such as the Oligocene, have been revised over time.
We used the RAxML v. 7.7.1 (black-box webserver; http://embnet.vital-it.ch/raxml-bb/) [50] in order to test the effect of phylogenetic method on tree topology. We carried out two analyses, one on PF3+ and one of PF3−, and we used a Gamma model of rate heterogeneity. Bootstrap supports were computed using 100 replications.
We used mitochondrial genomes and phylogenetic analyses to explain the biogeographic patterns of Reticulitermes, Heterotermes and Coptotermes. Although mitochondrial genomes are large compared with regular PCR-amplified markers, all their genes are linked and transmitted as a single package maternally. Mitogenome phylogenies can potentially be discordant to species phylogenies, in the case of hybridization-introgression or lineage sorting. However, because alleles coalesce with time, discordances generally occur only between closely related species and deep nodes are generally adequately inferred [51]. The rare cases of discordance in deep nodes are associated with ancient rapid radiations, which are characterized by very short branches with low posterior probabilities, and require enormous datasets to be resolved [52]. We can, therefore, reasonably conclude that nodes recovered with 100% Bayesian posterior probabilities and 100% bootstrap in all our analyses adequately reflect the relationships among taxa.
(d). Biogeographic analyses
We reconstructed the evolution of termite geographical ranges using a Bayesian binary model implemented in the RASP v. 2.1 software [53,54]. We used two models of state frequencies, fixed (JC) and estimated (F81); and two models of among-site rate variation, equal and gamma (+G), with the default chain parameters (namely 50 000 cycles, 10 chains, with a sampling every 100 generations and a temperature of 0.1) for the Bayesian analysis. Root distribution was set to null and the maximum number of areas for each node was set to 2. Sample locations were used to give each tip one biogeographic area. We distinguished eight biogeographic realms as determined in Holt et al. [17]: Australian, Afrotropical, Oriental, Nearctic, Neotropical, Oceanian (New Guinea), Palaearctic and Sino-Japanese.
3. Results
(a). Tree topology
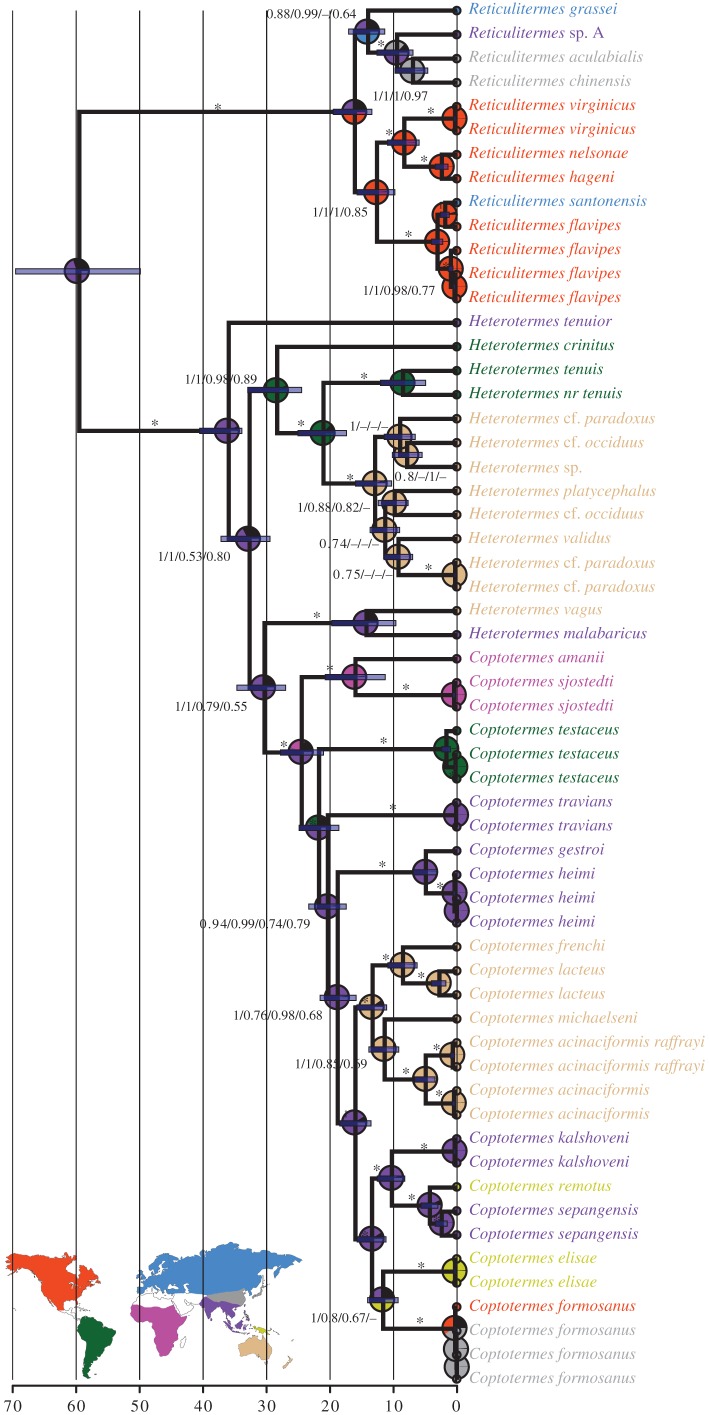
Our Bayesian analyses supported the monophyly of Reticulitermes and Coptotermes, but Heterotermes was paraphyletic with respect to Coptotermes (figure 2). The tree topology was unaffected by exclusion of the third codon position, except for the branching patterns of Australian species of Heterotermes. Likelihood analyses yielded the same topology as the Bayesian analyses with three exceptions: (i) for the PF3+ partition, Reticulitermes formed a polytomy divided into three groups: R. grassei, R. sp. A + R. aculabialis + R. chinensis, and the Nearctic Reticulitermes; (ii) C. elisae was the sister group of C. formosanus + C. kalshoveni + C. remotus + C. sepengensis in PF3−. (iii) The branching patterns of Australian species of Heterotermes were variable among analyses.
Figure 2.
Phylogenetic chronogram of termites based on the full mitochondrial genome, with third codon position included (PF3+), reconstructed using BEAST. Scale bar estimates age in millions of years. Node bars represent 95% CIs. Branch labels are support for the four analyses in the following order: Bayesian posterior probabilities of the Bayesian trees with PF3+ and PF3− partition schemes, bootstrap supports of the maximum-likelihood trees with PF3+ and PF3− partition schemes; dashes (–) indicate that the node is absent for a given analysis; asterisks (*) indicate 100% support for all four analyses. Node pie charts are the inferred ancestral distribution reconstructed using a Bayesian binary model. The map shows the zoogeographic realms considered in the analyses [17].
(b). Molecular dating
The two chronograms recovered very similar divergence date estimates, with a maximum difference of 1.9 Ma between analyses, therefore, we display the results of PF3+ only (figure 2). With PF3+, the divergence between Reticulitermes and Heterotermes + Coptotermes was estimated at 59.5 Ma (49.9–69.5 Ma 95% CI) and the most recent common ancestor of Reticulitermes at 16.1 Ma (13.4–19.5 Ma 95% CI). The most recent common ancestor of Heterotermes + Coptotermes was dated at 36.0 Ma (33.9–40.5 Ma 95% CI) and the most recent common ancestor of Coptotermes at 24.5 Ma (21.0–27.8 Ma 95% CI; figure 2).
(c). Biogeographic reconstruction
We reconstructed the ancestral range distribution on all trees independently in order to assess the effect of uncertainties in phylogenetic reconstruction. The Bayesian tree computed with the PF3+ partition scheme was illustrative of the variation in ancestral state reconstruction (figure 2). The Bayesian binary models of state frequencies and site variation only marginally differ, so we present the results of the analysis with fixed (JC) state frequencies and equal among-site rate variation only. Overall, our analyses retrieved 14 disjunctions between biogeographic areas (as determined by Holt et al. [17]).
We inferred four disjunctions for the genus Reticulitermes, including one of human origin. Reticulitermes was characterized by an early split between Nearctic and Old World species estimated at 12.6–16.1 Ma (9.8–19.5 Ma 95% CI). Old World Reticulitermes species were monophyletic but the origin of the distribution patterns between Palaearctic, Sino-Japanese and Oriental species was unresolved. The Palaearctic (France) R. santonensis, synonymized with R. flavipes [55], was nested within the Nearctic clade, supporting its recent human introduction.
We inferred three dispersal events for the genus Heterotermes. The most recent common ancestor of modern Heterotermes species was inferred to have existed in the Oriental realm, with an origin around 36.0 Ma (33.9–40.5 Ma 95% CI). Heterotermes appears to have dispersed from the Oriental realm twice, once to the Neotropical realm around 28.3–32.7 Ma (24.5–37.2 Ma 95% CI) and once to the Australian realm around 14.3 Ma (19.7 Ma 95% upper bound). There was a second dispersal to Australia from the Neotropics around 12.9–21.0 Ma (10.3–25.1 Ma 95% CI), based on the nested position of the larger Australian Heterotermes clade (figure 2).
We inferred seven dispersal events for the genus Coptotermes, but these events may be construed in two different scenarios. In the first scenario, the ancestral Coptotermes was from the Oriental realm, with an origin around 24.5 Ma (21.0–27.8 Ma 95% CI). Coptotermes dispersed five times from there, to the Afrotropics around 16.0–24.5 Ma (11.3–27.8 Ma 95% CI), to the Neotropics (ambiguous support) around 1.7–21.7 Ma (1.1–27.8 Ma 95% CI), to Australia around 13.3–16.0 Ma (11.1–18.5 Ma 95% CI), and twice independently to New Guinea, once less than 4.3 Ma (5.7 Ma 95% upper bound) and once less than 11.6 Ma (14.1 Ma 95% upper bound). In the second scenario, the ancestral Coptotermes evolved in the Afrotropics, and then dispersed to the Neotropics then the Oriental realm independently, and from the Oriental realm to Australia and New Guinea independently (figure 2). The ancestor of the major invasive pest C. formosanus was found to have either a New Guinean or Oriental origin, although the modern C. formosanus is known to be native to the Sino-Japanese realm, and was introduced to the Nearctic realm from there [56].
4. Discussion
In this study, we confirmed the relationship pattern and dating of the three genera as found in earlier molecular studies [10,15,25,37]. We found that the split between Reticulitermes and Heterotermes + Coptotermes occurred around 59 Ma (49.9–69.5 Ma 95% CI), the most recent common ancestor of the clade Heterotermes + Coptotermes arose 36 Ma (33.9–40.5 Ma 95% CI), and Coptotermes split from sister Heterotermes species around 24.5 Ma (21.0–27.8 Ma 95% CI). Therefore, the entire clade Reticulitermes + Heterotermes + Coptotermes originated after the break-up of Pangaea, and after the partial break-up of Gondwana. Consequently, the current native distribution of modern taxa cannot be explained by the past distribution of their ancestors spread across the earlier super continent. All three genera must have dispersed over oceans, either by land bridges or by rafting in wood.
Our results cannot identify the centre of origin of Reticulitermes, but we found a split between Old World and New World species dating to around 16.1 Ma (13.4–19.5 Ma 95% CI). The low nodal support for the early branching of Reticulitermes, combined with low sampling of species from the Palaearctic, Sino-Japanese and Oriental realms, prevent us from drawing a definitive scenario of the historical biogeography of the genus. However, one scenario consistent with our results is that the native distribution is relictual from a past Holarctic distribution, followed by a gradual retraction as the Earth cooled down since the Middle Miocene, 14 Ma [57]. The Palaearctic, Sino-Japanese and most of the Oriental realms are part of a single continental mass on one tectonic plate. Reticulitermes is distributed in two distinct areas on this continent: an area roughly including South Europe and Western Asia (Palaearctic); and an area including the Sino-Japanese realm and the Northernmost Oriental realm (figure 1a). The most recent common ancestor of European and Sino-Japanese species of Reticulitermes was dated to the Middle Miocene, 14.0 Ma (11.4–17.1 Ma 95% CI), concurring with previous estimations [15], when temperate forests stretched from Europe to Far East Asia [58,59], therefore, we consider it likely that Reticulitermes declined in central Asia as the climate became cooler and dryer [60].
The other major disjunction in the distribution of Reticulitermes is between the Old World and the New World species, whose most recent common ancestor was dated to the Early Miocene, 16.1 Ma (13.4–19.5 Ma 95% CI), coinciding with the end of a global climatic warming 25–15 Ma [57]. Dedeine et al. [15] also dated the origin of extant Reticulitermes species during the Early Miocene, 18.4 Ma. The Bering Land Bridge was covered by coniferous forests similar to the present taiga [29], through which cold-adapted insects, such as bumblebees [26], may have dispersed between the Nearctic and Palaearctic realms during that period of time. In this scenario, the modern distribution of Reticulitermes was shaped by vicariance from a Holarctic distribution which gradually retracted southward as forests were replaced by grassland during the second half of the Miocene [29]. However, modern species of Reticulitermes are not adapted to taiga, suggesting that Reticulitermes dispersed between the Nearctic and the Palaearctic realms by rafting across ocean, or using the land bridge across Iceland that possibly existed during the Miocene [61]. Our ancestral range reconstruction concurred with previous studies that showed that the French R. santonensis is a synonym of the American R. flavipes and was introduced into France by humans [55,62].
The most recent common ancestor of Heterotermes may have appeared in the Orient around 36.0 Ma (33.9–40.6 Ma 95% CI) (in the Eocene), after the initial collision of the Indian and Eurasian plates [63]. The oldest fossil species of Heterotermes, H. eocenicus, lived in Europe around 34 Ma, suggesting that Heterotermes was more widely distributed in the past and went extinct in Europe, and possibly in other nowadays cooler realms. The spread from the Orient to Africa may have been over land, however, the spread to the Neotropics and Australia must have been over oceans. Sampling of additional species of Heterotermes from India, Ethiopia and from the Neotropics could improve the reconstruction of the genus' ancestral range.
The direction of more recent dispersal events received stronger support, and was consistently recovered in all our analyses. Our ancestral state reconstruction showed that Heterotermes colonized Australia at least once from the Neotropical realm 12.9–21.0 Ma (10.3–25.1 Ma 95% CI), and possibly from the Oriental realm less than 14.3 Ma (19.7 Ma 95% CI upper bound). Southeast Asia and Australia have never been connected by land bridges, although chains of volcanic island arcs may have existed, over which termites might have island hopped [64]. Additionally, the collision of the Australian and Asian plates closed deep water passages between the Pacific and Indian oceans about 20 Ma, causing major changes in oceanic currents [65], and possibly favouring the colonization of Australia by Neotropical Heterotermes in the same time period.
The most recent common ancestor of modern Coptotermes species may have evolved in either Africa or the Orient around 24.5 Ma (21.0–27.8 Ma 95% CI) (in the Oligocene), and the movement between Africa and Asia may have been over the ocean before the land bridge connecting Africa and Eurasia formed, around 18 Ma, or over land afterwards [66]. However, the dispersal events to the Neotropics, Australia and New Guinea were all over water, considering that these regions were isolated at the time dispersals took place.
For modern Coptotermes, our analyses inferred an Oriental origin, with subsequent dispersal to the Afrotropics, Neotropics, Australia and New Guinea. However, support for this scenario was not strong, and we cannot exclude an African origin. Additional sampling within the sister groups of Coptotermes may help, as it did for Asian and Australian Coptotermes alone (cf. [25] and [33]). However, additional sampling within basal Coptotermes species is unlikely to provide resolution for either the node including all Coptotermes or the node including all non-African Coptotermes, because there are few additional extant species of Coptotermes in Africa and none in South America.
Coptotermes species diversity may have changed markedly in Africa and South America over time. Three Coptotermes species were described from Dominican amber [19], but only C. testaceus occurs today in the Neotropics [16]. Although the fossil record from the Afrotropics is non-existent for Coptotermes, only three described modern species have solid evidence for validity [18], two of which were included in this study. Coptotermes, therefore, appears to have experienced relatively high extinction rates in the Neotropics, and possibly in the Afrotropics, that blurred the signal and left too few modern species to resolve the early dispersal events of the genus based on phylogenetic methods.
The direction of more recent dispersal events received stronger support and was consistently recovered in all our analyses. We found that Coptotermes colonized Australia once from the Oriental realm 13.3–16.0 Ma (11.1–18.5 Ma 95% CI), which matched the estimate of Lee et al. [25].
We found that the New Guinean species of Coptotermes considered in this study have the most recent origin, also from the Orient, concurring with Bourguignon & Roisin [67], who proposed that the termite fauna from New Guinea originated from the Orient. This may be due to the reappearance of New Guinea and the creation of the high mountains from the collision of the Australian plate with the Asian plate in the Miocene [65]. Finally, our results also placed C. formosanus as the sister group of the New Guinean C. elisae, both genera being nested within Oriental Coptotermes. C. elisae shares many morphological similarities with the Oriental C. curvignathus (T Bourguignon 2013, personal observation) supporting the notion that C. elisae originated from the Oriental realm. C. formosanus is currently believed to be native to southern China [56] and our results show it is descended from an Oriental ancestor.
For all three genera, the dispersal over oceans was probably by rafting, i.e. termites living in floating dead wood. Lower termites, including those in the Rhinotermitidae, often nest in dead wood [25,68,69]. Such wood may be washed into rivers and thence into oceans. These wood rafts can become functional propagules with a complete colony (that is, one containing the reproductive king and queen), or without, as secondary reproductives can easily be generated in these genera [70]. The relative amount of over ocean dispersal may be related to the types of food targeted by the different genera. Reticulitermes and Heterotermes target relatively small pieces of dead, fallen wood, whereas Coptotermes targets entire trees, often nesting inside the tree trunk [25,68,69]. One tree floating on sea would probably contain enough food for a termite colony to survive for many months, allowing longer distance colonization events. Such rafting is usually used to explain the origin of the biota of oceanic islands [71] and was inferred many times from dated phylogenies [72–74].
The process of crossing an ocean in a wooden raft is analogous to human introduction. Termites introduced by humans are all wood-eating and wood-nesting species. Some of the worst invaders are species of Reticulitermes, Heterotermes and Coptotermes. Humans simply assist dispersal over oceans and make it more efficient [11,12]. Interestingly, various Coptotermes species have invaded more locations than either Reticulitermes or Heterotermes, mimicking the pattern from natural oceanic crossings.
Our sampling covered most of the realms in which each genus occurs, and is therefore likely to have identified most disjunctions arising without the influence of humans. However, additional sampling will possibly reveal further examples. Multispecies sampling within a biogeographical realm can reveal multiple colonization events, such as in the case of Heterotermes, which appears to have colonized Australia twice. More dispersal events, therefore, cannot be excluded, especially in the genera Heterotermes and Coptotermes in view of their high dispersal ability. In addition, more future dispersal events assisted by humans are likely.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Brian Forschler, Naeem Iqbal and Tamara Hartke for providing specimens, Barbora Křižková, Maria Lee An and Martin Jendryka for laboratory assistance, Jan Křeček for help with species identification and Simon Ho for assistance with analyses.
Data accessibility
Mitochondrial genome sequences have been deposited in GenBank (see electronic supplementary material, table S1 for accession numbers).
Authors' contributions
T.B. and T.A.E. conceived the study, T.B. designed the study, T.B., J.S., D.S.D., Y.R. and T.A.E. collected the material, and T.B. and N.L. analysed the data. All authors contributed significantly to the text of the manuscript and approved the final version.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the LHK fund of the National University of Singapore; by the Singapore–MIT Alliance for Research and Technology; the Alliance National University of Singapore, Université Sorbonne Paris Cité, the Internal Grant Agency of Faculty of Forestry and Wood Sciences, CULS (IGA B03/15) and the Czech Science Foundation (project no. 15–07015Y). T.B. was supported by the University of Sydney through a postdoctoral fellowship.
References
- 1.Krishna K, Grimaldi DA, Engel MS. 2013. Treatise on the Isoptera of the world, vol. 1. Bull. Am. Mus. Nat. Hist. 377, 1–196. ( 10.1206/377.1) [DOI] [Google Scholar]
- 2.Béguin P, Aubert JP. 1994. The biological degradation of cellulose. FEMS Microbiol. Rev. 13, 25–58. ( 10.1111/j.1574-6976.1994.tb00033.x) [DOI] [PubMed] [Google Scholar]
- 3.Dixon RK, Solomon AM, Brown S, Houghton RAM, Trexier C, Wisniewski J. 1994. Carbon pools and flux of global forest ecosystems. Science 263, 185–190. ( 10.1126/science.263.5144.185) [DOI] [PubMed] [Google Scholar]
- 4.Bignell DE. 2006. Termites as soil engineers and soil processors. In Intestinal microorganisms of soil invertebrates (eds König H, Varma A), pp. 183–220. Berlin, Germany: Springer. [Google Scholar]
- 5.Evans TA, Dawes TZ, Ward PR, Lo N. 2011. Ants and termites increase crop yield in a dry climate. Nat. Commun. 2, 1257 ( 10.1038/ncomms1257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jouquet P, Traoré S, Choosai C, Hartmann C, Bignell DE. 2011. Influence of termites on ecosystem functioning. Ecosystem services provided by termites. Eur. J. Soil Biol. 47, 215–222. ( 10.1016/j.ejsobi.2011.05.005) [DOI] [Google Scholar]
- 7.Hickin NE. 1971. Termites, a world problem. London, UK: The Rentokil Library, Hutchinson. [Google Scholar]
- 8.Su NY, Scheffrahn RH. 2000. Termites as pests of buildings. In Termites: evolution, sociality, symbioses, ecology (eds Abe T, Bignell DE, Higashi M), pp. 437–453. Dordrecht, The Netherlands: Kluwer. [Google Scholar]
- 9.Rouland-Lefèvre C. 2011. Termites as pests of agriculture. In Biology of termites: a modern synthesis (eds Bignell DE, Roisin Y, Lo N), pp. 499–517. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 10.Bourguignon T, et al. 2015. The evolutionary history of termites as inferred from 66 mitochondrial genomes. Mol. Biol. Evol. 32, 406–421. ( 10.1093/molbev/msu308) [DOI] [PubMed] [Google Scholar]
- 11.Evans TA. 2011. Invasive termites. In Biology of termites: a modern synthesis (eds Bignell DE, Roisin Y, Lo N), pp. 519–562. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 12.Evans TA, Forschler BT, Grace JK. 2013. Biology of invasive termites: a worldwide review. Annu. Rev. Entomol. 58, 455–474. ( 10.1146/annurev-ento-120811-153554) [DOI] [PubMed] [Google Scholar]
- 13.Emerson AE. 1971. Tertiary fossil species of the Rhinotermitidae (Isoptera), phylogeny of genera, and reciprocal phylogeny of associated Flagellata (Protozoa) and the Staphylinidae (Coleoptera). Bull. Am. Mus. Nat. Hist. 146, 243–304. [Google Scholar]
- 14.Eggleton P. 2000. Global patterns of termite diversity. In Termites: evolution, sociality, symbioses, ecology (eds Abe T, Bignell DE, Higashi M), pp. 25–51. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 15.Dedeine F, Dupont S, Guyot S, Matsuura K, Wang C, Habibpour B, Bagnères AG, Mantovani B, Luchetti A. 2016. Historical biogeography of Reticulitermes termites (Isoptera: Rhinotermitidae) inferred from analyses of mitochondrial and nuclear loci. Mol. Phylogenet. Evol. 94, 778–790. ( 10.1016/j.ympev.2015.10.020) [DOI] [PubMed] [Google Scholar]
- 16.Scheffrahn RH, Carrijo TF, Křeček J, Su NY, Szalanski AL, Austin JW, Chase JA, Mangold JR. 2015. A single endemic and three exotic species of the termite genus Coptotermes (Isoptera, Rhinotermitidae) in the New World. Arthropod. Syst. Phylogenet. 73, 333–348. [Google Scholar]
- 17.Holt BG, et al. 2013. An update of Wallace's zoogeographic regions of the world. Science 339, 74–79. ( 10.1126/science.1228282) [DOI] [PubMed] [Google Scholar]
- 18.Chouvenc T, et al. 2016. Revisiting Coptotermes (Isoptera: Rhinotermitidae): a global taxonomic roadmap for species validity and distribution of an economically important subterranean termite genus. Syst. Entomol. 41, 299–306. ( 10.1111/syen.12157) [DOI] [Google Scholar]
- 19.Krishna K, Grimaldi D. 2009. Diverse Rhinotermitidae and Termitidae (Isoptera) in Dominican Amber. Am. Mus. Novit. 3640, 1–48. ( 10.1206/633.1) [DOI] [Google Scholar]
- 20.Messenger MT, Mullins AJ. 2005. New flight distance recorded for Coptotermes formosanus (Isoptera: Rhinotermitidae). Fla Entomol. 88, 99–100. ( 10.1653/0015-4040(2005)088%5B0099:NFDRFC%5D2.0.CO;2) [DOI] [Google Scholar]
- 21.Hu J, Zhong JH, Guo MF. 2007. Alate dispersal distances of the black-winged subterranean termite Odontotermes formosanus (Isoptera: Termitidae) in southern China. Sociobiology 50, 1–8. [Google Scholar]
- 22.Keller L. 1998. Queen lifespan and colony characteristics in ants and termites. Insect. Soc. 45, 235–246. ( 10.1007/s000400050084) [DOI] [Google Scholar]
- 23.Thiel M, Haye PA. 2006. The ecology of rafting in the marine environment. III. Biogeographical and evolutionary consequences. Oceanogr. Mar. Biol. An Annu. Rev. 43, 323–429. ( 10.1201/9781420006391.ch7) [DOI] [Google Scholar]
- 24.Bourguignon T, Chisholm RA, Evans TA. 2016. The termite worker phenotype evolved as dispersal strategy for fertile wingless individuals before eusociality. Am. Nat. 187, 372–387. ( 10.1086/684838) [DOI] [PubMed] [Google Scholar]
- 25.Lee TRC, Cameron SL, Evans TA, Ho SYW, Lo N. 2015. The origins and radiation of Australian Coptotermes termites: from rainforest to desert dwellers. Mol. Phylogenet. Evol. 82, 234–244. ( 10.1016/j.ympev.2014.09.026) [DOI] [PubMed] [Google Scholar]
- 26.Hines HM. 2008. Historical biogeography, divergence times, and diversification patterns of bumble bees (Hymenoptera: Apidae: Bombus). Syst. Biol. 57, 58–75. ( 10.1080/10635150801898912) [DOI] [PubMed] [Google Scholar]
- 27.Vila R, et al. 2011. Phylogeny and palaeoecology of Polyommatus blue butterflies show Beringia was a climate-regulated gateway to the New World. Proc. R. Soc. B 278, 2737–2744. ( 10.1098/rspb.2010.2213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meseguer AS, Coeur d'acier A, Genson G, Jousselin E. 2015. Unravelling the historical biogeography and diversification dynamics of a highly diverse conifer-feeding aphid genus. J. Biogeogr. 88, 1482–1492. ( 10.1111/jbi.12531) [DOI] [Google Scholar]
- 29.Sanmartín I. 2001. Patterns of animal dispersal, vicariance and diversification in the Holarctic. Biol. J. Linn. Soc. 73, 345–390. ( 10.1111/j.1095-8312.2001.tb01368.x) [DOI] [Google Scholar]
- 30.Austin JW, Szalanski AL, Uva P, Bagnères AG, Kence A. 2002. A comparative genetic analysis of the subterranean termite genus Reticulitermes (Isoptera: Rhinotermitidae). Ann. Entomol. Soc. Am. 95, 753–760. ( 10.1603/0013-8746(2002)095%5B0753:ACGAOT%5D2.0.CO;2) [DOI] [Google Scholar]
- 31.Austin JW, Szalanski AL, Cabrera BJ. 2004. Phylogenetic analysis of the subterranean termite family Rhinotermitidae (Isoptera) by using the mitochondrial cytochrome oxidase II gene. Ann. Entomol. Soc. Am. 97, 548–555. ( 10.1603/0013-8746(2004)097%5B0548:PAOTST%5D2.0.CO;2) [DOI] [Google Scholar]
- 32.Luchetti A, Trenta M, Mantovani B, Marini M. 2004. Taxonomy and phylogeny of north mediterranean Reticulitermes termites (Isoptera, Rhinotermitidae): a new insight. Insect. Soc. 51, 117–122. ( 10.1007/s00040-003-0715-z) [DOI] [Google Scholar]
- 33.Lo N, Eldridge RH, Lenz M. 2006. Phylogeny of Australian Coptotermes (Isoptera: Rhinotermitidae) species inferred from mitochondrial COII sequences. Bull. Entomol. Res. 96, 433–437. [PubMed] [Google Scholar]
- 34.Yeap BK, Othman AS, Lee CY. 2009. Molecular systematics of Coptotermes (Isoptera: Rhinotermitidae) from East Asia and Australia. Ann. Entomol. Soc. Am. 102, 1077–1090. ( 10.1603/008.102.0616) [DOI] [Google Scholar]
- 35.Velonà A, Ghesini S, Luchetti A, Marini M, Mantovani B. 2010. Starting from Crete, a phylogenetic re-analysis of the genus Reticulitermes in the Mediterranean area. Mol. Phylogenet. Evol. 56, 1051–1058. ( 10.1016/j.ympev.2010.04.037) [DOI] [PubMed] [Google Scholar]
- 36.Cameron SL, Whiting MF. 2007. Mitochondrial genomic comparisons of the subterranean termites from the genus Reticulitermes (Insecta: Isoptera: Rhinotermitidae). Genome 202, 188–202. [DOI] [PubMed] [Google Scholar]
- 37.Cameron SL, Lo N, Bourguignon T, Svenson GJ, Evans TA. 2012. A mitochondrial genome phylogeny of termites (Blattodea: Termitoidae): robust support for interfamilial relationships and molecular synapomorphies define major clades. Mol. Phylogenet. Evol. 65, 163–173. ( 10.1016/j.ympev.2012.05.034) [DOI] [PubMed] [Google Scholar]
- 38.Cameron SL. 2014. Insect mitochondrial genomics: implications for evolution and phylogeny. Annu. Rev. Entomol. 59, 95–117. ( 10.1146/annurev-ento-011613-162007) [DOI] [PubMed] [Google Scholar]
- 39.Tokuda G, Isagawa H, Sugio K. 2011. The complete mitogenome of the Formosan termite, Coptotermes formosanus Shiraki. Insect. Soc. 59, 17–24. ( 10.1007/s00040-011-0182-x) [DOI] [Google Scholar]
- 40.Chen Q, Wang K, Tan Y, Xing L. 2016. The complete mitochondrial genome of the subterranean termite, Reticulitermes chinensis Snyder (Isoptera: Rhinotermitidae). Mitochondrial DNA 27, 1428–1429. ( 10.3109/19401736.2014.953077) [DOI] [PubMed] [Google Scholar]
- 41.Kai W, Xiao-Hui G, Chun-Hua D, Lian-Xi X, Jiang-Li T, Xiao-Hong S. In press Complete mitochondrial genome of a parthenogenetic subterranean termite, Reticulitermes aculabialis Tsai et Hwang (Isoptera: Rhinotermitidae). Mitochondrial DNA. [DOI] [PubMed] [Google Scholar]
- 42.Edgar RC. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 5, 113 ( 10.1186/1471-2105-5-113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. ( 10.1093/molbev/msr121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaidya G, Lohman DJ, Meier R. 2011. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27, 171–180. ( 10.1111/j.1096-0031.2010.00329.x) [DOI] [PubMed] [Google Scholar]
- 45.Lanfear R, Calcott B, Ho SYW, Guindon S. 2012. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 29, 1695–1701. ( 10.1093/molbev/mss020) [DOI] [PubMed] [Google Scholar]
- 46.Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 ( 10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biol. 4, e88 ( 10.1371/journal.pbio.0040088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gernhard T. 2008. The conditioned reconstructed process. J. Theor. Biol. 253, 769–778. ( 10.1016/j.jtbi.2008.04.005) [DOI] [PubMed] [Google Scholar]
- 49.Rambaut A, Drummond AJ. 2007. Tracer. http://tree.bio.ed.ac.uk/software/tracer/.
- 50.Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML Web servers. Syst. Biol. 57, 758–771. ( 10.1080/10635150802429642) [DOI] [PubMed] [Google Scholar]
- 51.Degnan JH, Rosenberg NA. 2009. Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends Ecol. Evol. 24, 332–340. ( 10.1016/j.tree.2009.01.009) [DOI] [PubMed] [Google Scholar]
- 52.Whitfield JB, Lockhart PJ. 2007. Deciphering ancient rapid radiations. Trends Ecol. Evol. 22, 258–265. ( 10.1016/j.tree.2007.01.012) [DOI] [PubMed] [Google Scholar]
- 53.Yu Y, Harris AJ, Blair C, He X. 2015. RASP (Reconstruct Ancestral State in Phylogenies): a tool for historical biogeography. Mol. Phylogenet. Evol. 87, 46–49. ( 10.1016/j.ympev.2015.03.008) [DOI] [PubMed] [Google Scholar]
- 54.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. ( 10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- 55.Austin J, Szalanski AL, Scheffrahn RH, Messenger MT, Dronnet P, Bagnères AG. 2005. Genetic evidence for the synonymy of two Reticulitermes species: Reticulitermes flavipes and Reticulitermes santonensis. Ann. Entomol. Soc. Am. 98, 395–401. ( 10.1603/0013-8746(2005)098%5B0395:GEFTSO%5D2.0.CO;2) [DOI] [Google Scholar]
- 56.Husseneder C, Simms DM, Delatte JR, Wang C, Grace JK, Vargo EL. 2011. Genetic diversity and colony breeding structure in native and introduced ranges of the Formosan subterranean termite, Coptotermes formosanus. Biol. Invasions 14, 419–437. ( 10.1007/s10530-011-0087-7) [DOI] [Google Scholar]
- 57.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–694. ( 10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]
- 58.Zhilin SG. 2001. Structure of the Turgayan flora in the Oligocene and Miocene and its palaeoclimatic features. Acta Palaeobot. 41, 141–146. [Google Scholar]
- 59.Tang ZH, Ding ZL. 2013. A palynological insight into the Miocene aridification in the Eurasian interior. Palaeoworld 22, 77–85. ( 10.1016/j.palwor.2013.05.001) [DOI] [Google Scholar]
- 60.Miao Y, Herrmann M, Wu F, Yan X, Yang S. 2012. What controlled Mid–Late Miocene long-term aridification in Central Asia?—global cooling or Tibetan Plateau uplift: a review. Earth Sci. Rev. 112, 155–172. ( 10.1016/j.earscirev.2012.02.003) [DOI] [Google Scholar]
- 61.Denk T, Grímsson F, Zetter R. 2010. Episodic migration of oaks to Iceland: evidence for a North Atlantic ‘land bridge’ in the latest Miocene. Am. J. Bot. 97, 276–287. ( 10.3732/ajb.0900195) [DOI] [PubMed] [Google Scholar]
- 62.Perdereau E, Bagnères AG, Bankhead-Dronnet S, Dupont S, Zimmermann M, Vargo EL, Dedeine F. 2013. Global genetic analysis reveals the putative native source of the invasive termite, Reticulitermes flavipes, in France. Mol. Ecol. 22, 1105–1119. ( 10.1111/mec.12140) [DOI] [PubMed] [Google Scholar]
- 63.van Hinsbergen DJJ, Lippert PC, Dupont-Nivet G, McQuarrie N, Doubrovine PV, Spakman W, Torsvik TH. 2012. Greater India Basin hypothesis and a two-stage Cenozoic collision between India and Asia. Proc. Natl Acad. Sci. USA 109, 7659–7664. ( 10.1073/pnas.1117262109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moss SJ, Wilson MEJ. 1998. Biogeographic implications of the Tertiary palaeogeographic evolution of Sulawesi and Borneo. In Biogeography and geological evolution of SE Asia (eds Hall R, Holloway JD), pp. 133–163. Leiden, The Netherlands: Backhuys Publishers. [Google Scholar]
- 65.Hall R. 1998. The plate tectonics of Cenozoic SE Asia and the distribution of land and sea. In Biogeography and geological evolution of SE Asia (eds Hall R, Holloway JD), pp. 99–131. Leiden, The Netherlands: Backhuys Publishers. [Google Scholar]
- 66.Rögl F. 1999. Mediterranean and Paratethys. Facts and hypotheses of an Oligocene to Miocene paleogeography (short review). Geol. Carpathica 50, 339–349. [Google Scholar]
- 67.Bourguignon T, Roisin Y. 2011. Revision of the termite family Rhinotermitidae (Isoptera) in New Guinea. ZooKeys 148, 55–103. ( 10.3897/zookeys.148.1826) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noirot C. 1970. The nests of termites. In Biology of termites, vol. II (eds Krishna K, Weesner FM), pp. 311–350. New York, NY: Academic Press. [Google Scholar]
- 69.Noirot C, Darlington JPEC. 2000. Termite nests: architecture, regulation and defence. In Termites: evolution, sociality, symbioses, ecology (eds Abe T, Bignell DE, Higashi M), pp. 121–139. Dordrecht, The Netherlands: Kluwer Academic Publishing. [Google Scholar]
- 70.Myles TG. 1999. Review of secondary reproduction in termites (Insecta: Isoptera) with comments on its role in termite ecology and social evolution. Sociobiology 33, 1–88. [Google Scholar]
- 71.de Queiroz A. 2005. The resurrection of oceanic dispersal in historical biogeography. Trends Ecol. Evol. 20, 68–73. ( 10.1016/j.tree.2004.11.006) [DOI] [PubMed] [Google Scholar]
- 72.Ward PS, Brady SG, Fisher BL, Schultz TR. 2015. The evolution of myrmicine ants: phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Syst. Entomol. 40, 61–81. ( 10.1111/syen.12090) [DOI] [Google Scholar]
- 73.Friedman M, Keck BP, Dornburg A, Eytan RI, Martin CH, Hulsey CD, Wainwright PC, Near TJ. 2013. Molecular and fossil evidence place the origin of cichlid fishes long after Gondwanan rifting. Proc. R. Soc. B 280, 20131733 ( 10.1098/rspb.2013.1733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antoine PO, et al. 2012. Middle Eocene rodents from Peruvian Amazonia reveal the pattern and timing of caviomorph origins and biogeography. Proc. R. Soc. B 279, 1319–1326. ( 10.1098/rspb.2011.1732) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Mitochondrial genome sequences have been deposited in GenBank (see electronic supplementary material, table S1 for accession numbers).