Abstract
Naked mole rats are among the most hypoxia-tolerant mammals identified and live in chronic hypoxia throughout their lives. The physiological mechanisms underlying this tolerance, however, are poorly understood. Most vertebrates hyperventilate in acute hypoxia and exhibit an enhanced hyperventilation following acclimatization to chronic sustained hypoxia (CSH). Conversely, naked mole rats do not hyperventilate in acute hypoxia and their response to CSH has not been examined. In this study, we explored mechanisms of plasticity in the control of the hypoxic ventilatory response (HVR) and hypoxic metabolic response (HMR) of freely behaving naked mole rats following 8–10 days of chronic sustained normoxia (CSN) or CSH. Specifically, we investigated the role of the major inhibitory neurotransmitter γ-amino butyric acid (GABA) in mediating these responses. Our study yielded three important findings. First, naked mole rats did not exhibit ventilatory plasticity following CSH, which is unique among adult animals studied to date. Second, GABA receptor (GABAR) antagonism altered breathing patterns in CSN and CSH animals and modulated the acute HVR in CSN animals. Third, naked mole rats exhibited GABAR-dependent metabolic plasticity following long-term hypoxia, such that the basal metabolic rate was approximately 25% higher in normoxic CSH animals than CSN animals, and GABAR antagonists modulated this increase.
Keywords: hypoxic ventilatory response, hypoxic metabolic response, plethysmography, respirometry, ventilatory acclimatization to hypoxia
1. Introduction
Low O2 environments are common on the Earth and hypoxic stress has driven the evolution of novel adaptations in animals that inhabit such niches [1]. The key to tolerating hypoxia is to match O2 supply to O2 demand. To achieve this balance, the most hypoxia-tolerant animals, including some freshwater turtles and fish, reduce their metabolic rate sufficiently to rely entirely upon anaerobic energetic pathways while in hypoxia. This is typically accomplished by (i) entering into a torpor-like state, (i) drastically reducing body temperature (Tb) and most importantly, (iii) through novel strategies that mitigate accumulation of the acidic end products of anaerobic metabolism [2].
Perhaps a greater physiological challenge in hypoxia is to remain active, alert, and warm. Naked mole rats (Heterocephalus glaber) achieve this feat and are considered to be one of the most hypoxia-tolerant mammals. Subterranean naked mole rat burrows are poorly ventilated, which in combination with group respiration-mediated O2 depletion, creates a lifelong hypoxic environment [3]. Indeed, naked mole rats experience O2 levels within their burrow as low as 6.2% without detriment [3], and tolerate hypoxia of less than 3% O2 for several hours in the laboratory [4]. Owing to the constancy of their exposure to environmental hypoxia and also the relatively high ambient temperature in their burrows (approx. 28–30°C) [3], naked mole rats cannot easily use behavioural and thermoregulatory means to alter their metabolic rate and conserve energy. Indeed, we have recently reported that naked mole rats held in 7% O2 remain alert and warm [5]. However, despite sustained activity and Tb, naked mole rats also exhibit a robust hypoxic metabolic response (HMR), consisting of a 70% decrease in metabolic rate while breathing 7% O2. Concomitant to this HMR, naked mole rats also exhibit a 70% decrease in ventilation (in 7% O2) [5]. Such a hypoxia-mediated change in breathing is termed an acute hypoxic ventilatory response (acute HVR: i.e. a reflex change in ventilation that occurs when inspired O2 decreases). The acute HVR is ubiquitous among all adult vertebrates studied; however, in other species, ventilation increases relative to metabolism during hypoxia, either due to an increase in ventilation and/or a decrease in metabolic rate [6]. In other words, all other species examined to date exhibit hyperventilation in hypoxia, that is, an increase in the ratio of ventilation to metabolic rate. Therefore, among adult vertebrates, naked mole rats are unique among animals studied to date in that their HMR and HVR are equal and matched, and thus these animals successfully match O2 supply to O2 demand while staying active and warm in hypoxia without hyperventilating.
In other mammals, beyond the initial hyperventilatory response to acute hypoxic exposure, chronic sustained hypoxia (CSH) of days to weeks induces additional time-dependent increases in ventilation [6]. This secondary increase is termed ventilatory acclimatization to hypoxia (VAH) [6]. VAH is ubiquitous in all adult vertebrates studied to date and persists for days to weeks after the removal of the hypoxic stimulus, indicating the occurrence of plasticity within the ventilatory control circuits [7]. In adult mammals, the acute HVR and VAH are primarily mediated by the activation and subsequent remodelling of glutamatergic synapses, which upregulate the gain of excitatory signalling in ventilatory control circuits, resulting in increased breathing in hypoxia [8,9]. The occurrence of VAH has not been explored in naked mole rats but the ventilatory decrease observed during acute hypoxic exposure is partially mediated by an upregulation of inhibitory signalling, with early evidence implicating adenosine as a key regulator of this response [5]. Importantly, a role for γ-amino butyric acid (GABA), which is the primary inhibitory neurotransmitter in the adult mammalian central nervous system (CNS), and acts at 25–40% of the synapses within the CNS [10], has yet to be investigated. Relative to glutamate, GABA does not play a major role in the acute HVR or VAH in other small rodents [11,12], however, given the polarity between the HVR of naked mole rats and that of other small rodents (decreased versus increased breathing in acute hypoxia), we hypothesized that GABAergic signalling may be important in regulating the HVR or HMR of naked mole rats to both acute and prolonged hypoxia. Therefore, the aims of this study were to (i) evaluate the occurrence of plasticity in ventilatory and metabolic responses of naked mole rats after 8–10 days of chronic sustained normoxia (CSN: 21% O2) or CSH (8% O2) and (ii) evaluate a role for GABAergic neurotransmission in regulating the acute HVR, VAH and the HMR of naked mole rats.
2. Material and methodology
(a). Animals
Naked mole rats were bred in our facility and group-housed in interconnected multi-cage systems at 27°C and 21% O2 in 50% humidity under a 12 L : 12 D cycle. Animals were fed fresh tubers, vegetables, fruit and Pronutro cereal supplement ad libitum. Animals were not fasted prior to experimental trials.
(b). Experimental design
Thirty male and female adult naked mole rats weighing 35.1 ± 1.1 g were randomly divided into two experimental groups and acclimated to either (i) CSN (21% O2; n = 14) or (ii) CSH (8% O2; n = 16) for 8–10 days in their home cages. Following acclimatization, animals were individually placed, unrestrained, into an experimental chamber flushed with 21% O2 and held at approximately 27°C. Prior to experimentation, animals were allowed at least 30 min to acclimate, followed by 60 min of baseline recordings of ventilation (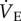 ) and metabolism in normoxia (21% O2). After baseline recordings, animals were quickly removed from the chamber and body temperature (Tb) was measured rectally using a thermocouple.
) and metabolism in normoxia (21% O2). After baseline recordings, animals were quickly removed from the chamber and body temperature (Tb) was measured rectally using a thermocouple.
CSN and CSH experimental groups were further divided into two treatment groups and received intraperitoneal 500 µl injections of either (i) saline (n = 7 and 11 for CSN and CSH, respectively) or (ii) a cocktail containing the GABAA receptor antagonist bicuculline (1 mg kg−1) and the GABAB receptor antagonist SCH50911 ((2S)-(+)-5,5-demethyl-2-morpholine acetic acid; 25 mg kg−1; n = 7 and 5 for CSN and CSH, respectively). Bicuculline and SCH50911 were purchased from Sigma-Aldrich (St Louis, MO, USA). The cocktail was first dissolved in dimethyl sulfoxide (DMSO) and then stock solutions were diluted into saline (final [DMSO] < 0.5%). The pharmacological doses of bicuculline and SCH50911 used in the present study are consistent with those used to block GABA receptors (GABARs) in similar studies conducted in small rodents (e.g. [12,13]). We observed no negative effects of drug administration: animals did not appear stressed and their behaviour was not altered. Immediately following injection, animals were returned to the recording chamber and ventilatory and metabolic measurements were collected in normoxia and during acute hypoxia (7% O2) under poikilocapnic conditions. Animals were held in each condition until metabolism and 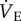 reached steady state (approx. 1 h). Tb was measured following injections and each exposure. The experimental trial took a total of approximately 4 h for each individual. The effects of bicuculline plus SCH50911 injection were determined by comparing data obtained from control animals (saline injection) relative to that collected from animals treated with the cocktail.
reached steady state (approx. 1 h). Tb was measured following injections and each exposure. The experimental trial took a total of approximately 4 h for each individual. The effects of bicuculline plus SCH50911 injection were determined by comparing data obtained from control animals (saline injection) relative to that collected from animals treated with the cocktail.
(c). Plethysmography and respirometry
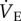 was measured using the barometric pressure method of plethysmography modified for continuous flow [14]. On the day of experimentation, individuals were placed into the experimental apparatus, which consisted of two rectangular 450 ml Plexiglas chambers, one being the animal chamber and the other the reference chamber. Calibrated rotameters were used to supply naked mole rats with an inflowing gas mixture set at a flow rate of 110 ml min−1. The total airflow ensured that neither O2, nor CO2 were altered by more than 1.5% by the animal's metabolism. Although 1.5% CO2 is high for most terrestrial mammals, this level of CO2 is actually reasonably low for naked mole rats. In their natural habitat, tunnel [CO2] is typically 2%, whereas in the nest chamber [CO2] is typically 5% [15]. Naked mole rats are adapted to this environment and do not respond physiologically or behaviourally to [CO2] of 1.5%. Fractional O2 and CO2 composition of inspired and expired gas were monitored using an O2 and CO2 analyser (Sable Systems, Las Vegas, NV, USA). The gas analyser was calibrated for O2 and CO2 before each trial with a premixed gas (21% O2; 1.55% CO2 balanced with N2). O2 consumption and CO2 production were calculated from the product of the constant airflow through the chamber and the difference between the outflow and inflow in the fractional concentrations of O2 and CO2 respectively. The air convection requirement (ACR) was calculated as the quotient of
was measured using the barometric pressure method of plethysmography modified for continuous flow [14]. On the day of experimentation, individuals were placed into the experimental apparatus, which consisted of two rectangular 450 ml Plexiglas chambers, one being the animal chamber and the other the reference chamber. Calibrated rotameters were used to supply naked mole rats with an inflowing gas mixture set at a flow rate of 110 ml min−1. The total airflow ensured that neither O2, nor CO2 were altered by more than 1.5% by the animal's metabolism. Although 1.5% CO2 is high for most terrestrial mammals, this level of CO2 is actually reasonably low for naked mole rats. In their natural habitat, tunnel [CO2] is typically 2%, whereas in the nest chamber [CO2] is typically 5% [15]. Naked mole rats are adapted to this environment and do not respond physiologically or behaviourally to [CO2] of 1.5%. Fractional O2 and CO2 composition of inspired and expired gas were monitored using an O2 and CO2 analyser (Sable Systems, Las Vegas, NV, USA). The gas analyser was calibrated for O2 and CO2 before each trial with a premixed gas (21% O2; 1.55% CO2 balanced with N2). O2 consumption and CO2 production were calculated from the product of the constant airflow through the chamber and the difference between the outflow and inflow in the fractional concentrations of O2 and CO2 respectively. The air convection requirement (ACR) was calculated as the quotient of 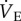 and O2 consumption. The % O2 extracted from each breath (EO2) was calculated as (
and O2 consumption. The % O2 extracted from each breath (EO2) was calculated as (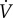 (FIo2-FEo2)/
(FIo2-FEo2)/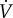 (FIo2)) × 100. Pressure fluctuations were compared to the pressure of the reference chamber and monitored with a differential pressure transducer (DP103-18, Validyne, Northridge, CA, USA) connected between the two chambers, amplified and electronically recorded to a computer. Respiratory frequency (fr) was calculated directly from the ventilation-induced pressure oscillations. Expiratory tidal volume (VT) was determined by integrating negative periods of ventilatory flow and then calculated using the equation of Drorbaugh & Fenn [16] and modified for flow-through plethysmography by Jacky [14,16]. To determine VT, the system was calibrated dynamically with the animal present in the chamber, as described previously [17]. Briefly, prior to each experiment known volumes of air (0.2, 0.5 and 1.0 ml) were injected into the chamber at a rate similar to the naked mole rats' inspiratory and expiratory times to produce pressure deflections at least 10 times as great as that of the animal's breathing. Resulting pressure deflections were linear across this range of calibration volumes.
(FIo2)) × 100. Pressure fluctuations were compared to the pressure of the reference chamber and monitored with a differential pressure transducer (DP103-18, Validyne, Northridge, CA, USA) connected between the two chambers, amplified and electronically recorded to a computer. Respiratory frequency (fr) was calculated directly from the ventilation-induced pressure oscillations. Expiratory tidal volume (VT) was determined by integrating negative periods of ventilatory flow and then calculated using the equation of Drorbaugh & Fenn [16] and modified for flow-through plethysmography by Jacky [14,16]. To determine VT, the system was calibrated dynamically with the animal present in the chamber, as described previously [17]. Briefly, prior to each experiment known volumes of air (0.2, 0.5 and 1.0 ml) were injected into the chamber at a rate similar to the naked mole rats' inspiratory and expiratory times to produce pressure deflections at least 10 times as great as that of the animal's breathing. Resulting pressure deflections were linear across this range of calibration volumes. 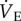 was measured with the system open and oscillations in recorded flow pressure were considered to be proportional to VT when corrected for calibration volume, chamber and body temperatures, and atmospheric and water vapour pressure at the altitude of our laboratory.
was measured with the system open and oscillations in recorded flow pressure were considered to be proportional to VT when corrected for calibration volume, chamber and body temperatures, and atmospheric and water vapour pressure at the altitude of our laboratory. 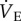 was calculated from the product of fr and VT.
was calculated from the product of fr and VT.
(d). Data collection and analysis
Ventilatory and metabolic variables were recorded and analysed on LabChart software (AD Instruments, Colorado Springs, CO, USA). Average values were calculated for each variable during the last 5 min of steady state of each experimental treatment. For 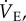 VT and fr, a series of at least 10 sets of 10 consecutive breaths were selected for analysis. Breaths were chosen from periods where the animal was awake but not active (i.e. not actively digging or exploring but also not asleep, as determined by visual examination).
VT and fr, a series of at least 10 sets of 10 consecutive breaths were selected for analysis. Breaths were chosen from periods where the animal was awake but not active (i.e. not actively digging or exploring but also not asleep, as determined by visual examination).
Statistical analysis was performed using commercial software (SPSS v. 15.0, SPSS Inc., Chicago, IL, USA). For all experiments, individual n values correspond to a single animal treated with either saline or bicuculline and SCH50911 dissolved in saline as described above, before and after exposure to 21% O2 and then acute hypoxia (7% O2). Values are presented as mean ± s.e.m. p < 0.05 was considered to achieve statistical significance. All data were normally distributed with equal variance (p > 0.05). Significance was evaluated using three-way ANOVA to test for significant interactions between the three independent variables: (i) the chronic inspired O2 level (i.e. 8 or 21% O2 for 8–10 days), (ii) the acute inspired O2 level (i.e. 7 versus 21%) and (iii) the treatment (i.e. saline versus cocktail injection). Bonferroni post hoc multiple comparisons tests were run on each of the dependent variables to compare the single point means of interest. The dependent variables analysed were: 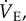 fr, Vt,
fr, Vt, 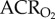 and
and 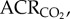 EO2, Tb and metabolic rate.
EO2, Tb and metabolic rate.
3. Results
(a). GABAR antagonism alters breathing patterns and reduces the acute HVR in CSN naked mole rats
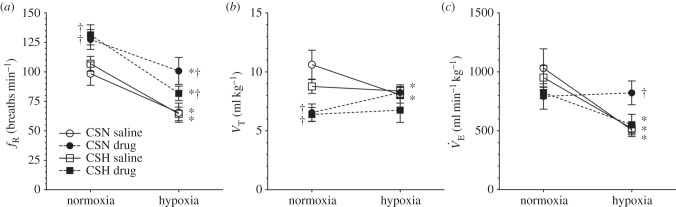
Ventilation and metabolism were measured in naked mole rats breathing normoxic gas mixtures before and after intraperitoneal injection of saline or a GABAR antagonist cocktail. Saline injections had no effect on any of the measured variables in normoxia relative to pre-injection measurements in either CSN or CSH experimental groups (p > 0.05; n = 7–11 each; data not shown). Thus, for the purposes of data presentation and statistical analysis we used post-injection normoxic measurements as the control in all datasets. Relative to when breathing normoxic gas mixtures, saline-treated CSN animals breathing 7% O2 exhibited a 51% reduction in 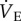 (from 1032 ± 163 to 507 ± 58 ml min−1 kg−1), which was due to significant decreases in both fr and VT (figure 1a–c, open circles). This change in
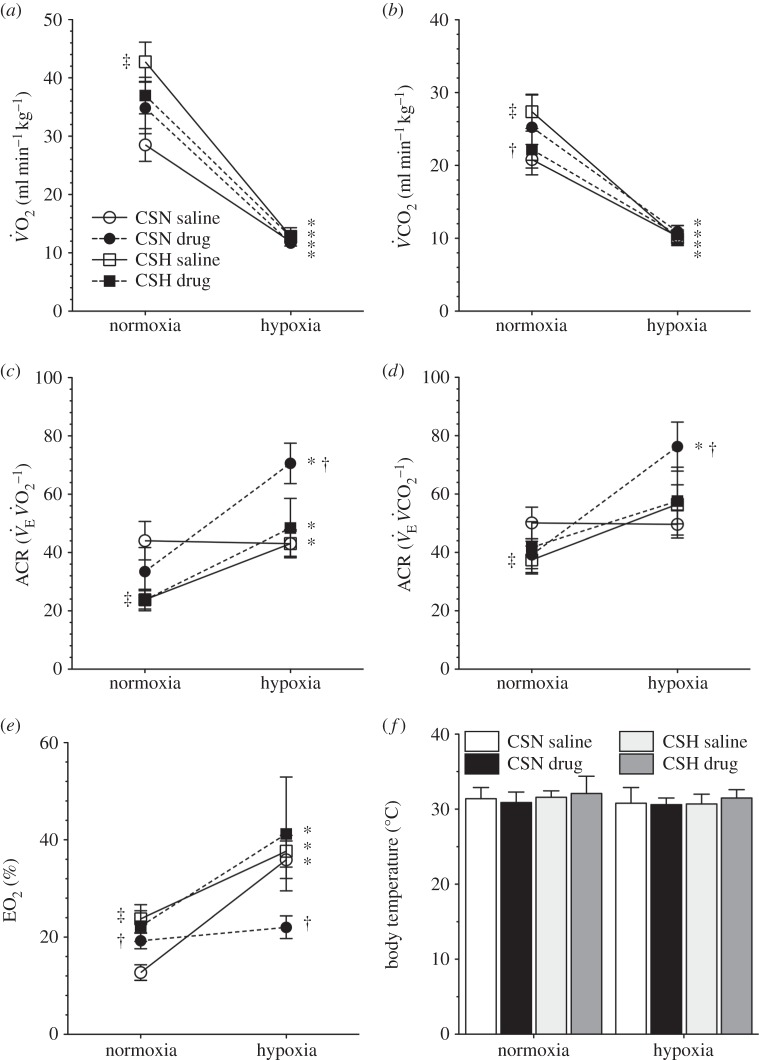
(from 1032 ± 163 to 507 ± 58 ml min−1 kg−1), which was due to significant decreases in both fr and VT (figure 1a–c, open circles). This change in 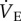 was matched by a 50–58% reduction in metabolic rate (from 28 ± 3 to 12 ± 1 ml O2 · min−1 kg−1, and from 21 ± 2 to 10 ± 1 ml CO2 · min−1 kg−1; figure 2a,b), and as a result of these equal magnitude changes, the
was matched by a 50–58% reduction in metabolic rate (from 28 ± 3 to 12 ± 1 ml O2 · min−1 kg−1, and from 21 ± 2 to 10 ± 1 ml CO2 · min−1 kg−1; figure 2a,b), and as a result of these equal magnitude changes, the 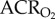 and
and 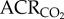 of CSN animals were not altered by acute hypoxic exposure (figure 2c,d). However, the O2 extraction efficiency of these animals increased nearly threefold in acute hypoxia (figure 2e). Tb was not different between any treatment groups or experimental protocols (figure 2f).
of CSN animals were not altered by acute hypoxic exposure (figure 2c,d). However, the O2 extraction efficiency of these animals increased nearly threefold in acute hypoxia (figure 2e). Tb was not different between any treatment groups or experimental protocols (figure 2f).
Figure 1.
Naked mole rats do not exhibit VAH to 8–10 days of CSH; GABA receptor antagonism alters breathing patterns and prevents the acute HVR in animals acclimated to chronic sustained normoxia (CSN). Effects of acute hypoxia (7% O2), saline (white circles: CSN; white squares: CSH), and a cocktail of the GABAA receptor antagonist bicuculline plus the GABAB receptor antagonist SCH50911 (black circles: CSN; black squares: CSH) on (a) breathing frequency (fR), (b) tidal volume (VT) and (c) total minute ventilation (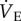 ) of naked mole rats. Data are mean ± s.e.m. from n = 7–11 naked mole rats per group. Asterisks indicate significant differences between normoxia and acute hypoxia values; daggers indicate significant differences between cocktail- and saline-treated animals (p < 0.05).
) of naked mole rats. Data are mean ± s.e.m. from n = 7–11 naked mole rats per group. Asterisks indicate significant differences between normoxia and acute hypoxia values; daggers indicate significant differences between cocktail- and saline-treated animals (p < 0.05).
Figure 2.
Naked mole rats exhibit metabolic plasticity following CSH. Effects of acute hypoxia (7% O2), saline (white circles: chronic sustained normoxia (CSN); white squares: CSH), and a cocktail of the GABAA receptor antagonist bicuculline plus the GABAB receptor antagonist SCH50911 (black circles: CSN; black squares: CSH) on (a) O2 consumption (V̇O2), (b) CO2 production (V̇CO2), (c,d) the air convection requirements of O2 (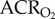 ) and CO2 (
) and CO2 (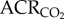 ), (e) O2 extraction efficiency (EO2) and (f) body temperature of naked mole rats following 8–10 days of constant exposure to 8% O2. Data are mean ± s.e.m. from n = 7–11 naked mole rats per group. Asterisks indicate significant differences between normoxia and acute hypoxia values; daggers indicate significant differences between cocktail- and saline-treated animals; double daggers indicate significant differences between CSN and CSH animals (p < 0.05).
), (e) O2 extraction efficiency (EO2) and (f) body temperature of naked mole rats following 8–10 days of constant exposure to 8% O2. Data are mean ± s.e.m. from n = 7–11 naked mole rats per group. Asterisks indicate significant differences between normoxia and acute hypoxia values; daggers indicate significant differences between cocktail- and saline-treated animals; double daggers indicate significant differences between CSN and CSH animals (p < 0.05).
Injection of the GABAR antagonist cocktail markedly changed breathing patterns in CSN animals breathing 21% O2; specifically, the cocktail increased fr by 27% and decreased Vt by 24% (figure 1a,b, black circles). These changes offset each other, and as a result, 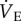 was not changed by GABAR antagonism (1032 ± 163 versus 792 ± 108 ml · min−1 kg−1 in saline- versus cocktail-treated CSN animals; figure 1c). GABAR antagonism had no effect on metabolic rate or the normoxic
was not changed by GABAR antagonism (1032 ± 163 versus 792 ± 108 ml · min−1 kg−1 in saline- versus cocktail-treated CSN animals; figure 1c). GABAR antagonism had no effect on metabolic rate or the normoxic 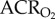 or
or 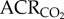 (figure 2a–d), but increased the O2 extraction efficiency of CSN animals breathing 21% O2 (figure 2e).
(figure 2a–d), but increased the O2 extraction efficiency of CSN animals breathing 21% O2 (figure 2e).
When cocktail-treated CSN animals were subject to acute hypoxia, fr decreased to a similar degree as in saline-treated CSN animals breathing acute hypoxia; however, Vt increased markedly (figure 1a,b). The magnitudes of these changes were similar but in the opposite direction, and as a result the net effect of breathing acute hypoxia on 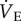 in cocktail-treated CSN animals was negligible and the acute HVR was inhibited (
in cocktail-treated CSN animals was negligible and the acute HVR was inhibited (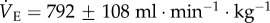 in normoxia versus 822 ± 102 ml min−1 kg−1 in hypoxia, figure 1c). Conversely, GABAR antagonism had no effect on the HMR: the change in metabolic rate was similar between cocktail- and saline-treated animals (figure 2a,b). As a result of GABAR antagonism inhibiting the HVR but not the HMR, the ACR of cocktail-treated CSN animals increased twofold in hypoxia and the increase in O2 extraction efficiency observed in saline-treated animals breathing acute hypoxia was prevented (figure 2c–e). These data suggest that GABAR antagonism inhibits non-ventilatory adaptations to hypoxia, decreasing the O2 extraction efficiency and forcing the animal to compensate by hyperventilating in acute hypoxia.
in normoxia versus 822 ± 102 ml min−1 kg−1 in hypoxia, figure 1c). Conversely, GABAR antagonism had no effect on the HMR: the change in metabolic rate was similar between cocktail- and saline-treated animals (figure 2a,b). As a result of GABAR antagonism inhibiting the HVR but not the HMR, the ACR of cocktail-treated CSN animals increased twofold in hypoxia and the increase in O2 extraction efficiency observed in saline-treated animals breathing acute hypoxia was prevented (figure 2c–e). These data suggest that GABAR antagonism inhibits non-ventilatory adaptations to hypoxia, decreasing the O2 extraction efficiency and forcing the animal to compensate by hyperventilating in acute hypoxia.
(b). Naked mole rats exhibit metabolic but not ventilatory plasticity following 8–10 days of CSH
To evaluate changes in the HVR and HMR, naked mole rats were group-housed in 21 or 8% O2 for 8–10 days. We observed no differences in fr, VT or 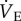 between CSN- and CSH-acclimated animals breathing either normoxic or hypoxic gases and the magnitude of the HVR was similar between experimental groups (figure 1a–c; white squares). These data suggest that, unlike in other vertebrates, synaptic strength in the circuits that mediate ventilatory responses to hypoxia is not modified in naked mole rats by exposure to CSH. Conversely, the normoxic metabolic rate of CSH animals was 25–33% higher than in CSN animals (43 ± 3 versus 28 ± 3 ml O2 · min−1 kg−1 and 27 ± 2 versus 21 ± 2 ml CO2 · min−1 kg−1; figure 2a,b) indicating that plasticity occurred in the control of metabolism following CSH. As a result of this difference, the ACR and O2 extraction efficiency of normoxic CSH animals were 20% lower and 50% higher, respectively, than in normoxic CSN animals (figure 2c–e). Conversely, these differences did not persist in animals breathing acute hypoxia and there were no differences between experimental groups with regard to metabolic rate, ACR or O2 extraction efficiency in hypoxia (figure 2c–e).
between CSN- and CSH-acclimated animals breathing either normoxic or hypoxic gases and the magnitude of the HVR was similar between experimental groups (figure 1a–c; white squares). These data suggest that, unlike in other vertebrates, synaptic strength in the circuits that mediate ventilatory responses to hypoxia is not modified in naked mole rats by exposure to CSH. Conversely, the normoxic metabolic rate of CSH animals was 25–33% higher than in CSN animals (43 ± 3 versus 28 ± 3 ml O2 · min−1 kg−1 and 27 ± 2 versus 21 ± 2 ml CO2 · min−1 kg−1; figure 2a,b) indicating that plasticity occurred in the control of metabolism following CSH. As a result of this difference, the ACR and O2 extraction efficiency of normoxic CSH animals were 20% lower and 50% higher, respectively, than in normoxic CSN animals (figure 2c–e). Conversely, these differences did not persist in animals breathing acute hypoxia and there were no differences between experimental groups with regard to metabolic rate, ACR or O2 extraction efficiency in hypoxia (figure 2c–e).
(c). GABAR antagonism alters breathing patterns but not the HVR or HMR following 8–10 days of CSH
In CSH-acclimatized animals, GABAR antagonism had similar effects on fr and Vt as in CSN animals. Specifically, breathing patterns were changed such that fr was increased and Vt decreased by the cocktail injection, resulting in no net change in 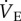 in normoxia (figure 1a–c; black squares). However, the magnitude of these changes was reduced relative to the CSN experimental group and the hypoxic increase in Vt observed in the CSN cocktail-injected animals was not observed in similarly treated CSH animals. As a result, the acute HVR was not sensitive to GABAR antagonism in CSH animals. Conversely, GABAR antagonism had a small but significant effect on the normoxic metabolic rate of CSH animals and reversed the effect of CSH acclimatization on normoxic metabolism (figure 2a,b). GABAR antagonism had no effect on the HMR in CSH animals when exposed to acute hypoxia (figure 2a,b), nor on the ACRs, or O2 extraction efficiency of these animals when breathing either 21 or 7% O2 (figure 2c–e).
in normoxia (figure 1a–c; black squares). However, the magnitude of these changes was reduced relative to the CSN experimental group and the hypoxic increase in Vt observed in the CSN cocktail-injected animals was not observed in similarly treated CSH animals. As a result, the acute HVR was not sensitive to GABAR antagonism in CSH animals. Conversely, GABAR antagonism had a small but significant effect on the normoxic metabolic rate of CSH animals and reversed the effect of CSH acclimatization on normoxic metabolism (figure 2a,b). GABAR antagonism had no effect on the HMR in CSH animals when exposed to acute hypoxia (figure 2a,b), nor on the ACRs, or O2 extraction efficiency of these animals when breathing either 21 or 7% O2 (figure 2c–e).
4. Discussion
Naked mole rats are among the most hypoxia-tolerant mammals identified. In this study, we explore mechanisms of plasticity in the control of the HVR and HMR of naked mole rats following 8–10 days of chronic normoxic or hypoxic exposure. We also investigate the role of the major inhibitory neurotransmitter GABA in mediating these responses. Our study yielded three important findings. First, naked mole rats do not exhibit ventilatory plasticity following long-term exposure to CSH, which is unique among adult vertebrates studied to date. Second, GABAR antagonism alters breathing patterns in CSN and CSH animals and prevents the acute HVR in CSN animals. Third, naked mole rats exhibit GABAR-dependent metabolic plasticity following long-term hypoxia, such that the basal metabolic rate is higher in normoxic CSH animals than CSN animals, and GABAR antagonists modulate this increase.
(a). Naked mole rats have a robust acute HVR and HMR
Consistent with our previous study in this species [5], we report that naked mole rats acclimated to normoxia exhibit a robust decrease in both 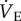 and metabolic rate when exposed to hypoxia for 1 h, which is a unique physiological response to hypoxia among adult mammals studied to date. The magnitudes of these hypoxia-mediated decreases are slightly less than those we reported in our previous study (50 versus 70% for
and metabolic rate when exposed to hypoxia for 1 h, which is a unique physiological response to hypoxia among adult mammals studied to date. The magnitudes of these hypoxia-mediated decreases are slightly less than those we reported in our previous study (50 versus 70% for 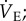 51 versus 70% for metabolism); however, in both studies, the magnitude of the HVR and HMR in response to acute hypoxia is equally matched. As a result, the ACR in acute hypoxia is unchanged and naked mole rats do not hyperventilate. Comparatively, hyperventilation in acute hypoxia, owing to increased ventilation and/or decreased metabolism, is observed in all other adult vertebrates studied [6,18], including other fossorial species, which are also typically tolerant to prolonged hypoxia [18–21]. Thus, a maintained ACR in acute hypoxia is a novel response that may represent a beneficial adaptation in naked mole rats. Instead, it is notable that the O2 extraction ratio of naked mole rats increases threefold in acute hypoxia. These data suggest that naked mole rats have developed non-ventilatory adaptations to hypoxia within the O2 transport cascade that ameliorate the need for hyperventilation in acute hypoxia. The mechanism underlying this increased efficiency is unclear but may involve hypoxia-mediated improvements to cardiac performance, blood-O2 carrying capacity, or O2 diffusivity between the lungs and blood or blood and tissues. Naked mole rats also exhibit an increase in their respiratory exchange ratio during acute hypoxia, consistent with a partial switch from primarily lipid-based metabolism towards a greater reliance on carbohydrate-based metabolism [22]. Carbohydrate metabolism requires less O2 in the breaking of chemical bonds to release energy and this may contribute to energy savings in hypoxia. Further research is required to test these hypotheses and determine the adaptive mechanisms that mitigate the need for hyperventilation during hypoxia in this species.
51 versus 70% for metabolism); however, in both studies, the magnitude of the HVR and HMR in response to acute hypoxia is equally matched. As a result, the ACR in acute hypoxia is unchanged and naked mole rats do not hyperventilate. Comparatively, hyperventilation in acute hypoxia, owing to increased ventilation and/or decreased metabolism, is observed in all other adult vertebrates studied [6,18], including other fossorial species, which are also typically tolerant to prolonged hypoxia [18–21]. Thus, a maintained ACR in acute hypoxia is a novel response that may represent a beneficial adaptation in naked mole rats. Instead, it is notable that the O2 extraction ratio of naked mole rats increases threefold in acute hypoxia. These data suggest that naked mole rats have developed non-ventilatory adaptations to hypoxia within the O2 transport cascade that ameliorate the need for hyperventilation in acute hypoxia. The mechanism underlying this increased efficiency is unclear but may involve hypoxia-mediated improvements to cardiac performance, blood-O2 carrying capacity, or O2 diffusivity between the lungs and blood or blood and tissues. Naked mole rats also exhibit an increase in their respiratory exchange ratio during acute hypoxia, consistent with a partial switch from primarily lipid-based metabolism towards a greater reliance on carbohydrate-based metabolism [22]. Carbohydrate metabolism requires less O2 in the breaking of chemical bonds to release energy and this may contribute to energy savings in hypoxia. Further research is required to test these hypotheses and determine the adaptive mechanisms that mitigate the need for hyperventilation during hypoxia in this species.
(b). Naked mole rats exhibit metabolic but not ventilatory plasticity following CSH
Beyond acute hypoxia, naked mole rats routinely experience periods of sustained hypoxia in their natural environment. Chronic hypoxia is an important driver of synaptic changes in the ventilatory control circuits that underlie plasticity of the HVR. VAH, which consists of a secondary increase in 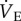 following CSH, has been reported in all species studied to date. VAH typically requires a few days to a week to fully manifest [6]. Plasticity changes related to VAH occur mainly at excitatory glutamatergic and dopaminergic synapses, both at the carotid body/carotid sinus nerve and within the CNS components of the ventilatory reflex, and build upon synaptic signalling mechanisms that underlie the acute HVR [8,9,23,24]. Given the otherwise ubiquitous occurrence of VAH in experimental models, it is a remarkable finding of our study that naked mole rats do not exhibit VAH or any apparent plasticity in their ventilatory responses following 8–10 days of CSH. Indeed, relative to CSN animals, no component of breathing was modified in the CSH experimental group when breathing either acute normoxia or hypoxia. It is possible that a longer hypoxic exposure to CSH or a deeper level of hypoxia might induce VAH in this species but this is unlikely given that one week of exposure to CSH is sufficient to induce VAH in all other species studied [6], and because 8% O2 is close to the deepest level of hypoxia naked mole rats experience in their natural environment [3].
following CSH, has been reported in all species studied to date. VAH typically requires a few days to a week to fully manifest [6]. Plasticity changes related to VAH occur mainly at excitatory glutamatergic and dopaminergic synapses, both at the carotid body/carotid sinus nerve and within the CNS components of the ventilatory reflex, and build upon synaptic signalling mechanisms that underlie the acute HVR [8,9,23,24]. Given the otherwise ubiquitous occurrence of VAH in experimental models, it is a remarkable finding of our study that naked mole rats do not exhibit VAH or any apparent plasticity in their ventilatory responses following 8–10 days of CSH. Indeed, relative to CSN animals, no component of breathing was modified in the CSH experimental group when breathing either acute normoxia or hypoxia. It is possible that a longer hypoxic exposure to CSH or a deeper level of hypoxia might induce VAH in this species but this is unlikely given that one week of exposure to CSH is sufficient to induce VAH in all other species studied [6], and because 8% O2 is close to the deepest level of hypoxia naked mole rats experience in their natural environment [3].
An important caveat of our study is that although naked mole rats live in a chronically hypoxic environment in nature, our experimental animals were bred in captivity and raised under normoxic conditions. It is well established that lifelong and multi-generational exposure to chronic hypoxia have different effects on the HVR than hypoxic exposures of days to weeks [6]. For example, when humans are exposed to prolonged chronic hypoxia for years or a lifetime, their acute HVR is blunted such that ventilatory sensitivity to changes in O2 availability are decreased relative to normal subjects who are acclimatized to hypoxia for a shorter period of time [25]. However, studies investigating the HVR of high altitude native species that have been raised at sea level, or of high altitude populations of humans that have lived at sea level for several years have consistently reported the occurrence of a robust VAH response to chronic hypoxic exposure [19,26]. Therefore, the impact on the occurrence of VAH of raising naked mole rats in a normoxic environment is difficult to predict. Further experiments are warranted to examine the acute HVR and VAH in naked mole rats that have been bred and raised in chronic hypoxia to determine if developing under hypoxic conditions induces a blunted acute HVR or reveals a VAH response to chronic hypoxia in this species.
Unlike the HVR, the HMR is typically shorter lived and not subject to plasticity following CSH. Indeed, any sustained change in metabolism after CSH is uncommon; in most other mammals, the initial HMR is reversed within hours to days of the onset of CSH [27]. Therefore, it is a surprising finding of our study that following acclimatization to CSH for 8–10 days the resting metabolism of naked mole rats in normoxia is elevated 25% relative to CSN animals. This is a fascinating result because it indicates that, beyond changes to breathing, additional systemic or cellular changes are occurring during CSH acclimatization within the systemic physiology of naked mole rats that enhances O2 extraction from the air and/or better facilitates its delivery to tissues. In support of this, the O2 extraction efficiency of normoxic CSH naked mole rats was threefold higher than in CSN animals. However, when CSH-acclimatized animals were exposed to acute hypoxia they exhibited metabolic rate depression to the same absolute level as CSN animals, indicating that metabolic plasticity occurs only when breathing normoxic gas mixtures following long-term acclimatization in hypoxia and does not impact the HMR. The mechanistic reason for this divergence is unclear but this adaptation may be related to the ecophysiology of this species. In naked mole rat burrows, hypoxia is a by-product of group respiration combined with poor gas diffusion through the soil. Therefore, the level of hypoxia is most severe in the nest chamber where the majority of the colony sleeps and huddles. Conversely, the extended tunnel networks of the burrow system are less populated and thus have higher O2 levels. Naked mole rats are more likely to do work while in the tunnels (e.g. digging, searching for sources of food and fresh air, etc.) and to rest in the nest chamber. Therefore, they likely experience more severe environmental hypoxia while at rest but relatively higher levels of O2 when they are working in the tunnels. In this context, our observation that naked mole rats have a greater metabolic scope in normoxia but not during acute hypoxia following CSH may represent a sensible adaptation.
An alternative possibility is that the elevated metabolism of naked mole rats following exposure to 8–10 days of CSH is due to the paying off of an O2 debt. O2 debt occurs as a result of the recruitment of anaerobic metabolic pathways during periods of deep hypoxia or intense exercise. This metabolic strategy typically leads to a build of acidic end products (e.g. lactate) in the muscle and other metabolically active tissues, which must be extruded from these tissues in the presence of O2 following reoxygenation. Because we assessed metabolism following the exposure of naked mole rats to CSH, we cannot conclude from our data that elevated metabolism in normoxia following acclimatization is not due to paying off of O2 debt; however, we consider this possibility unlikely due to the lifestyle of this species. As described above, naked mole rats live in a chronically hypoxic environment for their entire lifespan, with rare exposure to normoxic air. Therefore, it is unlikely that this species would have adapted to living and exercising in hypoxia by relying on anaerobic pathways that result in the accumulation of acidic end products because they would simply not be presented with an opportunity to pay off the resulting O2 debt. Furthermore, in pilot studies of animals exposed to acute hypoxia for several hours, we do not detect changes in tissue pH (ME Pamenter and WA Thompson 2015, unpublished data). Nonetheless, our metabolic data from CSH-acclimatized animals breathing normoxic gases should be interpreted with caution.
(c). GABAergic signalling contributes to breathing patternation and ventilatory and metabolic responses to hypoxia
GABA is the primary inhibitory neurotransmitter in the CNS of all adult vertebrates [10]. Given that increased breathing due to the acute HVR and VAH in other vertebrates is mediated by excitatory neurotransmitters [6,8], we hypothesized that decreased breathing in the hypoxic naked mole rat might be mediated by increased activity of this key inhibitory neurotransmitter. Our results support this hypothesis in CSN naked mole rats, in which the acute HVR is prevented by combined antagonism of GABAA and GABAB receptors. It is notable, however, that GABAR antagonism has no impact on the acute HVR following CSH, indicating that GABA no longer plays a signalling role in this response after acclimatization to CSH, and that other pathways become dominant. However, GABAR antagonism alters breathing patterns in both CSN and CSH animals, which is consistent with previous reports of GABARs modulating respiratory neuronal discharge in other species [28]. Interestingly, the pattern of this effect is different in naked mole rats than in other vertebrates. Specifically, GABAR antagonism increases fr and decreases VT in naked mole rats, but tends to decrease fr and increase VT in rats [12,29,30]. Nonetheless, the observed effects in rats are small and typically do not reach significance except in experimental preparations where the GABAergic system is potentiated, such as in obese Zucker rats [12]. Interestingly, the impact of GABAR antagonism on the breathing patterns of naked mole rats in acute hypoxia differs between CSN and CSH animals, such that following CSH, the hypoxia-mediated decrease in fr is greater than that observed in the CSN group, whereas Vt is unchanged by acute hypoxia in both groups. This change indirectly increases the relative contribution of breathing depth to total ventilation in acute hypoxia.
We have previously demonstrated that blockade of another major inhibitory neurotransmitter, adenosine, prevents the ventilatory decrease in acutely hypoxic naked mole rats [5]. Because blockade of either adenosinergic or GABAergic neurotransmission alone is sufficient to impair the acute HVR, it is likely that these two signalling molecules interact via the same cellular pathway in naked mole rats. This interaction is not surprising given that GABARs and adenosine receptors have been previously shown to interact in the control of breathing during development [31], presumably through adenosine-receptor mediated activation of GABAergic pathways since pre-treatment with GABAR antagonists prevents adenosine-mediated effects on ventilation in rats and piglets [32,33]. Taken together with our current results, marked similarities become apparent between respiratory controls in adult naked mole rats versus neonatal mammals. This is an important observation because the retention of neonatal synaptic characteristics into adulthood has been hypothesized as a key component of the remarkable hypoxia tolerance of naked mole rats [34]. Therefore, it is tempting to speculate that the same pathways as those that dominate breathing circuits in the neonatal CNS, namely adenosinergic and GABAergic, mediate the control of breathing in adult naked mole rats. In vitro electrophysiological experiments in naked mole rat carotid bodies and respiratory brainstem network preparations derived from CSN and CSH animals are required to examine the interaction of these and other neurotransmitters at the neuronal level in order to fully elucidate the underlying signalling mechanisms of these unique ventilatory responses to hypoxia, and to test the neotenic theory of hypoxia-tolerance in the control of ventilation in adult naked mole rats.
With regard to metabolism, GABAR antagonism in CSN animals results in a non-significant increase in normoxic metabolic rate, and, as a result of this trend, the normoxic O2 extraction efficiency of CSN naked mole rats is increased by GABAR antagonism. Conversely, because the cocktail prevents the acute HVR but has no effect on the HMR, GABAR antagonist-treated CSN animals hyperventilate in acute hypoxia, as evidenced by a doubling of the ACR in cocktail-treated CSN animals. However, the hypoxic increase in O2 extraction efficiency is prevented by GABAR antagonism, which would presumably impair hypoxia-tolerance in this species. The mechanism via which GABA impacts O2 extraction efficiency in CSN animals is unclear but may be due to changes in the sympathetic control of lung perfusion and ventilation/perfusion (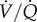 ) ratio, although a role for GABA in this
) ratio, although a role for GABA in this 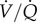 matching has not been explored in other species. Conversely, GABAR antagonism does not impact baseline O2 extraction efficiency in CSH animals, or the hypoxic increase in this parameter, which indicates that other mechanisms, probably outside of CNS control of ventilation or perfusion, mediate this change after acclimatization to CSH. This may be due to changes in blood-O2 binding affinity following CSH acclimatization and further experiments are warranted to investigate this possibility, as well as the effect of GABA and hypoxia on cardiac function and
matching has not been explored in other species. Conversely, GABAR antagonism does not impact baseline O2 extraction efficiency in CSH animals, or the hypoxic increase in this parameter, which indicates that other mechanisms, probably outside of CNS control of ventilation or perfusion, mediate this change after acclimatization to CSH. This may be due to changes in blood-O2 binding affinity following CSH acclimatization and further experiments are warranted to investigate this possibility, as well as the effect of GABA and hypoxia on cardiac function and 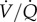 matching in naked mole rats.
matching in naked mole rats.
We also report that GABAR antagonism reverses the CSH-mediated increase in normoxic metabolic rate, but has no effect on the HMR during acute hypoxia. This is contrary to the typical activity of GABA in hypoxia-intolerant mammals, in which agonism of GABARs tends to decrease metabolic rate [35], and indicates that following CSH, GABA interferes with another inhibitory mechanism to increase resting metabolism in normoxia. In vitro experiments are required to elucidate the role of GABA in regulating metabolic plasticity following CSH.
(d). Conclusion
We report that naked mole rats, among the most hypoxia-tolerant mammals presently identified, exhibit metabolic but not ventilatory plasticity following 8–10 days of acclimatization to 8% O2. This is a unique response to chronic hypoxia among adult vertebrates studied to date. Furthermore, inhibition of GABAergic signalling alters breathing patterns in both CSN and CSH animals, prevents the acute HVR in CSN animals and modulates CSH-mediated metabolic plasticity. Our findings indicate that naked mole rats likely employ non-ventilatory adaptations during acute and chronic hypoxia that improve O2 extraction efficiency without the need for hyperventilation. These adaptations may allow naked mole rats to remain active and warm during hypoxic episodes of varying length without the benefit of increased O2 delivery via an energetically costly increase in their ACR. The specific synaptic mechanisms that regulate the acute HVR and metabolic plasticity following CSH remain unknown but likely involve a phenotype dominated by adenosinergic/GABAergic signalling such as is commonly found in neonatal mammals, indicating that naked mole rats may retain neonatal signalling characteristics that may contribute to their remarkable hypoxia tolerance.
Ethics
All protocols were performed with the approval of The University of British Columbia Animal Care Program (A13-0248) and in accordance with the relevant guidelines of the Canadian Council on Animal Care.
Data accessibility
Supporting data have been uploaded to Dryad (doi:10.5061/dryad.sq227) in keeping with the journal's policies.
Authors' contributions
M.E.P. conceived of and designed the study and wrote the manuscript. D.C., Y.A.D., A.S. and M.E.P. performed the respirometry experiments and analysed data. W.K.M. contributed to the experimental design and edited the manuscript. All authors gave final approval of the published version and agree to be accountable for all content therein.
Competing interests
We have no competing interests.
Funding
This work was supported by an NSERC Discovery grant to W.K.M. and a Parker B Francis PDF to M.E.P.
References
- 1.Dzal YA, Jenkin SE, Lague SL, Reichert MN, York JM, Pamenter ME. 2015. Oxygen in demand: how oxygen has shaped vertebrate physiology. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 186, 4–26. ( 10.1016/j.cbpa.2014.10.029) [DOI] [PubMed] [Google Scholar]
- 2.Bickler PE, Buck LT. 2007. Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annu. Rev. Physiol. 69, 145–170. ( 10.1146/annurev.physiol.69.031905.162529) [DOI] [PubMed] [Google Scholar]
- 3.Sherman PW, Jarvis JU, Alexander RD. 1991. The biology of the naked mole-rat. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Pamenter ME, Dzal YA, Milsom WK. 2014. Profound metabolic depression in the hypoxia-tolerant naked mole rat. FASEB J. 28, 879–872. [Google Scholar]
- 5.Pamenter ME, Dzal YA, Milsom WK. 2015. Adenosine receptors mediate the hypoxic ventilatory response but not the hypoxic metabolic response in the naked mole rat during acute hypoxia. Proc. R. Soc. B 282, 20141722 ( 10.1098/rspb.2014.1722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell FL, Milsom WK, Mitchell GS. 1998. Time domains of the hypoxic ventilatory response. Respir. Physiol. 112, 123–134. ( 10.1016/S0034-5687(98)00026-7) [DOI] [PubMed] [Google Scholar]
- 7.Teppema LJ, Dahan A. 2010. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol. Rev. 90, 675–754. ( 10.1152/physrev.00012.2009) [DOI] [PubMed] [Google Scholar]
- 8.Pamenter ME, Carr JA, Go A, Fu Z, Reid SG, Powell FL. 2014. Glutamate receptors in the nucleus tractus solitarius contribute to ventilatory acclimatization to hypoxia in rat. J. Physiol. 592, 1839–1856. ( 10.1113/jphysiol.2013.268706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pamenter ME, Nguyen J, Carr JA, Powell FL. 2014. The effect of combined glutamate receptor blockade in the NTS on the hypoxic ventilatory response in awake rats differs from the effect of individual glutamate receptor blockade. Physiol. Rep. 2, e12092 ( 10.14814/phy2.12092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong CG, Bottiglieri T, Snead OC III. 2003. GABA, gamma-hydroxybutyric acid, and neurological disease. Ann. Neurol. 54(Suppl 6), S3–S12. ( 10.1002/ana.10696) [DOI] [PubMed] [Google Scholar]
- 11.Chung S, Ivy GO, Reid SG. 2006. GABA-mediated neurotransmission in the nucleus of the solitary tract alters resting ventilation following exposure to chronic hypoxia in conscious rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1449–R1456. ( 10.1152/ajpregu.00645.2005) [DOI] [PubMed] [Google Scholar]
- 12.Yang AL, Lo MJ, Ting H, Chen JS, Huang CY, Lee SD. 2007. GABAA and GABAB receptors differentially modulate volume and frequency in ventilatory compensation in obese Zucker rats. J. Appl. Physiol. 102, 350–357. ( 10.1152/japplphysiol.01463.2005) [DOI] [PubMed] [Google Scholar]
- 13.Gannon RL, Millan MJ. 2011. Positive allosteric modulators at GABAB receptors exert intrinsic actions and enhance the influence of baclofen on light-induced phase shifts of hamster circadian activity rhythms. Pharmacol. Biochem. Behav. 99, 712–717. ( 10.1016/j.pbb.2011.06.029) [DOI] [PubMed] [Google Scholar]
- 14.Jacky JP. 1978. A plethysmograph for long-term measurements of ventilation in unrestrained animals. J. Appl. Physiol 45, 644–647. [DOI] [PubMed] [Google Scholar]
- 15.Bennett NC, Faulkes CG. 2000. African mole-rats: ecology and eusociality. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 16.Drorbaugh JE, Fenn WO. 1955. A barometric method for measuring ventilation in newborn infants. Pediatrics 16, 81–87. [PubMed] [Google Scholar]
- 17.Mcarthur MD, Milsom WK. 1991. Ventilation and respiratory sensitivity of euthermic Columbian and golden-mantled ground-squirrels (Spermophilus columbianus and Spermophilus lateralis) during the summer and winter. Physiol. Zool. 64, 921–939. ( 10.1086/physzool.64.4.30157949) [DOI] [Google Scholar]
- 18.Frappell P, Lanthier C, Baudinette RV, Mortola JP. 1992. Metabolism and ventilation in acute-hypoxia—a comparative-analysis in small mammalian-species. Am. J. Physiol. 262, R1040–R1046. [DOI] [PubMed] [Google Scholar]
- 19.Pichon A, Zhenzhong B, Favret F, Jin G, Shufeng H, Marchant D, Richalet JP, Ge RL. 2009. Long-term ventilatory adaptation and ventilatory response to hypoxia in plateau pika (Ochotona curzoniae): role of nNOS and dopamine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R978–R987. ( 10.1152/ajpregu.00108.2009) [DOI] [PubMed] [Google Scholar]
- 20.Arieli R, Ar A. 1979. Ventilation of a fossorial mammal (Spalax ehrenbergi) in hypoxic and hypercapnic conditions. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 47, 1011–1017. [DOI] [PubMed] [Google Scholar]
- 21.Tomasco IH, Del Rio R, Iturriaga R, Bozinovic F. 2010. Comparative respiratory strategies of subterranean and fossorial octodontid rodents to cope with hypoxic and hypercapnic atmospheres. J. Comp. Physiol. B 180, 877–884. ( 10.1007/s00360-010-0465-y) [DOI] [PubMed] [Google Scholar]
- 22.Frayn KN. 1983. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 55, 628–634. [DOI] [PubMed] [Google Scholar]
- 23.Pamenter ME, Go A, Fu Z, Powell FL. 2015. No evidence of a role for neuronal nitric oxide synthase in the nucleus tractus solitarius in ventilatory responses to acute or chronic hypoxia in awake rats. J. Appl. Physiol. 118, 750–759. ( 10.1152/japplphysiol.00333.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell FL, Huey KA, Dwinell MR. 2000. Central nervous system mechanisms of ventilatory acclimatization to hypoxia. Respir. Physiol. 121, 223–236. ( 10.1016/S0034-5687(00)00130-4) [DOI] [PubMed] [Google Scholar]
- 25.Beall CM, et al. 1997. Ventilation and hypoxic ventilatory response of Tibetan and Aymara high altitude natives. Am. J. Phys. Anthropol. 104, 427–447. ( 10.1002/(SICI)1096-8644(199712)104:4%3C427::AID-AJPA1%3E3.0.CO;2-P) [DOI] [PubMed] [Google Scholar]
- 26.Rivera-Ch M, Gamboa A, Leon-Velarde F, Palacios JA, O'Connor DF, Robbins PA. 2003. Selected contribution: high-altitude natives living at sea level acclimatize to high altitude like sea-level natives. J. Appl. Physiol. 94, 1263–1268; discussion 1253–1264 ( 10.1152/japplphysiol.00857.2002) [DOI] [PubMed] [Google Scholar]
- 27.Olson EB Jr, Dempsey JA. 1978. Rat as a model for humanlike ventilatory adaptation to chronic hypoxia. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 44, 763–769. [DOI] [PubMed] [Google Scholar]
- 28.Pierrefiche O, Foutz AS, Denavit-Saubie M. 1993. Effects of GABAB receptor agonists and antagonists on the bulbar respiratory network in cat. Brain Res. 605, 77–84. ( 10.1016/0006-8993(93)91358-Y) [DOI] [PubMed] [Google Scholar]
- 29.Reid SG, Powell FL. 2005. Effects of chronic hypoxia on MK-801-induced changes in the acute hypoxic ventilatory response. J. Appl. Physiol. 99, 2108–2114. ( 10.1152/japplphysiol.01205.2004) [DOI] [PubMed] [Google Scholar]
- 30.Tabata M, Kurosawa H, Kikuchi Y, Hida W, Ogawa H, Okabe S, Tun Y, Hattori T, Shirato K. 2001. Role of GABA within the nucleus tractus solitarii in the hypoxic ventilatory decline of awake rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1411–R1419. [DOI] [PubMed] [Google Scholar]
- 31.Picard N, Guenin S, Perrin Y, Hilaire G, Larnicol N. 2008. Prenatal diazepam exposure alters respiratory control system and GABAA and adenosine receptor gene expression in newborn rats. Pediatr. Res. 64, 44–49. ( 10.1203/PDR.0b013e31817445cf) [DOI] [PubMed] [Google Scholar]
- 32.Mayer CA, Haxhiu MA, Martin RJ, Wilson CG. 2006. Adenosine A2A receptors mediate GABAergic inhibition of respiration in immature rats. J. Appl. Physiol. 100, 91–97. ( 10.1152/japplphysiol.00459.2005) [DOI] [PubMed] [Google Scholar]
- 33.Wilson CG, Martin RJ, Jaber M, Abu-Shaweesh J, Jafri A, Haxhiu MA, Zaidi S. 2004. Adenosine A2A receptors interact with GABAergic pathways to modulate respiration in neonatal piglets. Respir. Physiol. Neurobiol. 141, 201–211. ( 10.1016/j.resp.2004.04.012) [DOI] [PubMed] [Google Scholar]
- 34.Penz OK, Fuzik J, Kurek AB, Romanov R, Larson J, Park TJ, Harkany T, Keimpema E. 2015. Protracted brain development in a rodent model of extreme longevity. Sci. Rep. 5, 11592 ( 10.1038/srep11592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor NC, Li A, Nattie EE. 2006. Ventilatory effects of muscimol microdialysis into the rostral medullary raphe region of conscious rats. Respir. Physiol. Neurobiol. 153, 203–216. ( 10.1016/j.resp.2005.11.005) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supporting data have been uploaded to Dryad (doi:10.5061/dryad.sq227) in keeping with the journal's policies.