Abstract
Plants display extensive intraspecific variation in secondary metabolites. However, the selective forces shaping this diversity remain often unknown, especially below ground. Using Taraxacum officinale and its major native insect root herbivore Melolontha melolontha, we tested whether below-ground herbivores drive intraspecific variation in root secondary metabolites. We found that high M. melolontha infestation levels over recent decades are associated with high concentrations of major root latex secondary metabolites across 21 central European T. officinale field populations. By cultivating offspring of these populations, we show that both heritable variation and phenotypic plasticity contribute to the observed differences. Furthermore, we demonstrate that the production of the sesquiterpene lactone taraxinic acid β-d-glucopyranosyl ester (TA-G) is costly in the absence, but beneficial in the presence of M. melolontha, resulting in divergent selection of TA-G. Our results highlight the role of soil-dwelling insects for the evolution of plant defences in nature.
Keywords: root herbivory, plant secondary metabolites, defence, selection, latex, fitness costs
1. Background
Heritable intraspecific variation is a common feature of many biological traits. Genetic variation results from heterogeneous selection pressures in relation to genetic architecture, population substructures and gene flow [1–4]. In plants, local differences in both abiotic and biotic factors may drive trait evolution [5]. Insect herbivores, the most abundant and diverse plant consumers, have long been suspected to play an important role in this context [6]. Recent studies demonstrate that herbivore abundance can covary with the expression of plant defence metabolites [7], that the exclusion of phytophagous insects can lead to a relaxation of defences within a few generations [8] and that defence genes are under differential selection across environments [9,10]. Together, these studies show that the temporal and spatial variation in above-ground herbivore communities can shape plant defensive chemistry.
In contrast with the ecological and evolutionary dynamics of above-ground plant–herbivore interactions, below-ground interactions have received little attention, despite the importance of roots for plant fitness and the high concentrations of secondary metabolites in below-ground organs [11–14]. The rhizosphere differs from the phyllosphere in both biotic and abiotic conditions [15], and the selective forces shaping variation in secondary metabolites may therefore differ between the two environments. The evolution of root secondary metabolites may for instance be driven by herbivores [11,12,16], pathogens [17] and symbionts [18], as well as nutrient availability [19], salt, drought and cold stress [20]. By comparing one mainland and two island populations, Watts et al. [21] showed that geographical isolation, including the escape from pocket gophers (Geomyidae), resulted in the evolutionary decline of root alkaloid concentrations of the host plant Eschscholzia californica (Papaveraceae). The specific potential of root herbivores to shape the abundance and geographical distribution of plant secondary metabolites, however, remains to be explored.
Among Europe's largest and most prevalent native root feeding insects are the larvae of the common cockchafer (Melolontha melolontha) (Coleoptera: Scarabaeidae). Owing to their pest status and conspicuous appearance in the adult stage, M. melolontha populations have been monitored closely by authorities, farmers and the public over recent decades [22]. Melolontha melolontha females lay their eggs close to their emergence site into meadows with high heat emission [23–25], resulting in stable geographical distribution patterns. Although the larvae are highly polyphagous, they preferentially oviposit close to and feed on the common dandelion (Taraxacum officinale agg., Flora Helvetica, 5th edn) during the final instar [26,27]. Taraxacum officinale is a perennial that relies heavily on its strong tap root for resprouting and flowering in spring. The plant is native to and widely distributed in Europe [28], and has recently gained a cosmopolitan distribution due to human dispersal. Taraxacum officinale is described as a species complex with sexual, outcrossing diploids in central and southern Europe and a multitude of apomictic, clonal triploids across the globe [29]. One of the factors that may explain the worldwide success of T. officinale is its capacity to produce latex in roots and other organs as a protection against herbivores. Latex is the cytoplasm of the specialized laticifer cells, and typically contains high concentrations of toxic and sometimes sticky secondary metabolites [30]. The latex of T. officinale is dominated by three secondary metabolite classes: phenolic inositol esters (PIEs), triterpene acetates (TritAcs) and the sesquiterpene lactone taraxinic acid β-d-glucopyranosyl ester (TA-G) [31]. In a recent study, we showed that TA-G protects plants against M. melolontha attack, opening up the possibility that this compound may be under natural selection by the root herbivore [32].
In this study, we investigated whether M. melolontha shapes variation in latex secondary metabolites in T. officinale. We profiled the latex chemistry of natural T. officinale populations growing under different M. melolontha herbivore pressure, and tested for inducibility, heritable variation and differential selection of the metabolites in the offspring of the field populations. Our results provide evidence that below-ground herbivores drive the ecology and evolution of chemical defences in plants.
2. Material and methods
(a). Identification and characterization of Taraxacum officinale field populations
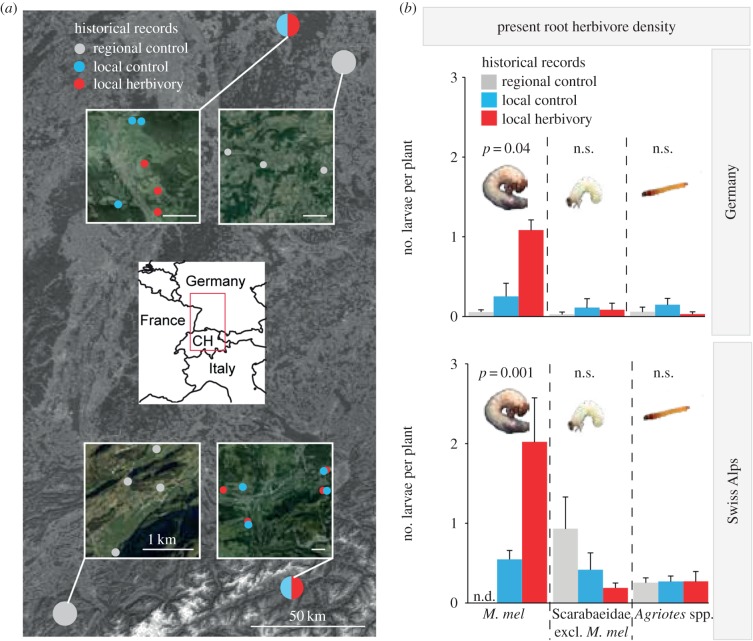
To investigate the influence of M. melolontha on the phenotypic variation of latex chemistry in natural T. officinale populations, we collected latex from a total of 252 T. officinale plants from 21 populations that evolved under different M. melolontha herbivory over the last 20 years. We identified two areas in which the presence of M. melolontha has been monitored by federal authorities: the Swiss Alps and Germany (figure 1a; electronic supplementary material, figure S2 and table S1). Within these two areas, we contacted local farmers who provided detailed information about the infestation histories of their fields over the last 20 years. Fields that repeatedly showed visible signs of M. melolontha feeding (reduction in plant growth and yield, brown patches, M. melolontha adult flights) were classified as historically M. melolontha infested (local herbivory). Fields at a distance of maximally 2 km that lacked visual signs of below-ground herbivory during the last 20 years were termed M. melolontha free (local control). As T. officinale seeds and pollen may disperse over 200–500 m [33,34], we identified additional T. officinale populations under low M. melolontha herbivory within the same geographical areas at a distance of 50–100 km to the local herbivory and local control populations (regional controls). Regional controls were identified based on data from Swiss and German authorities. The different populations were arranged in triplets so that each herbivore population had a matching local and regional control population in the same area with similar environmental conditions, including altitude, exposition, inclination, land use and climate. Detailed information about the information sources for the individual populations can be found in electronic supplementary material, table S1.
Figure 1.
Distribution map and present root herbivore abundance of different T. officinale populations across central Europe. (a) Map of identified natural T. officinale populations evolving under high (local herbivory) or low (‘local control’ and ‘regional control’) M. melolontha infestation levels over the last 20 years. Circles inside insets denote individual T. officinale populations. CH, Switzerland. (b) Mean number of root herbivore larvae per T. officinale plant found during a field survey in 2013. M. mel, M. melolontha. p-values of Kruskal–Wallis rank sum tests are shown. Error bars correspond to standard errors (+s.e.). n.s., not significant (p > 0.10).
The populations in Germany were shown earlier to consist mainly of triploids, while plants in the Swiss Alps are mostly diploid [29,35], allowing us to study the impact of M. melolontha on the evolution of secondary metabolites in both triploid apomictic and diploid obligate outcrossing plants. In both Germany and the Swiss Alps, we analysed three to four local herbivory, local control and regional control populations in spring 2013. For each population, we collected one fully ripened seed capsule per plant from 12 T. officinale individuals, with a minimal distance of 50 cm between plants. To characterize the present root herbivore community, we excavated a cube with 18 cm side length around each plant and recorded the identity and number of below-ground herbivores in each cube. Furthermore, we collected latex from the main root of each plant into Eppendorf tubes and glass vials, which were immediately frozen in dry ice. After transport to the laboratory, samples were stored at −80°C and extracted as described below.
(b). Secondary metabolite quantifications
The latex of T. officinale is dominated by three classes of secondary metabolites: TA-G, PIEs and TritAcs [31]. To quantify TA-G and PIEs, 1 ml methanol containing 10 µg ml−1 loganin and 100 µg ml−1 salicin as internal standards for TA-G and PIEs, respectively, was added to the Eppendorf tubes containing the sampled root latex. Tubes were vortexed for 5 min, centrifuged, and the supernatant was stored at −80°C until analysis. Methanol samples were measured on a high pressure liquid chromatograph (HPLC 1100 series equipment, Agilent Technologies), coupled to a photodiode array detector (G1315A DAD, Agilent Technologies). For quantification, peak areas were integrated at 245 nm for TA-G and at 275 nm for PIEs, and quantified using internal standards. For extraction of the TritAcs, 1 ml hexane containing 0.1 mg ml−1 cholesteryl acetate as internal standard was added to the glass vials. Vials were shaken for 5 min, centrifuged and the supernatant stored at −80°C until analysis. Hexane samples were analysed on an Agilent series 6890 gas chromatograph coupled to a flame ionization detector (GC-FID). Individual triterpene acetates were quantified based on the internal standard. Methodological details for the analytical procedures are described in Huber et al. [31].
(c). Analysis of field populations
Differences in the present root herbivore abundance between populations of different infestation histories were analysed with Kruskal–Wallis rank sum tests using the mean values of each population for each area separately. The effect of infestation history on the concentrations of TA-G, total PIEs and total TritAcs was analysed using mixed-effect models with maximum-likelihood estimations using the nlme package [36] for each area separately, with metabolite concentrations as response variable, infestation history as fixed effect and the population as a hierarchical random effect that includes region, village and plot number (electronic supplementary material, table S1). First, the hierarchical random effects were reduced stepwise by comparing two models with the original and simplified random effects using a likelihood ratio test. Second, two models—which after model simplification only contained the plot number as a random effect—with and without infestation history were compared with a likelihood ratio test for each area and metabolite separately. Summary statistics of the models are presented using the highly infested populations as the reference level. Results were visualized using ggplot2 [37] and gridExtra [38]. Maps were drawn with RgoogleMaps [39] and rworldmap [40]. All statistical analyses were performed in R v. 3.1.1 [41].
(d). Offspring growth and sampling
In order to investigate heritable variation, inducibility and fitness effects of latex secondary metabolites, as well as to verify the plant ploidy levels, we grew offspring of the plants from the field-sampled populations with and without M. melolontha larvae. To balance the number of populations between Germany and the Swiss Alps, the three populations with the most well-documented history of M. melolontha abundance for each infestation history were selected in both areas. Seeds were germinated in seedling trays in a growth chamber under standard conditions. After four weeks of growth in July 2013, the trays were placed outside for acclimatization (Jena, Germany: 50°54′34.8″ N; 11°34′00.1″ E). Each plant from the local herbivore populations was then potted together with either a plant from the matching regional control populations (regional pair) or a plant from the matching local control populations (local pair) inside a 2 l pot filled with soil (74% Klamann substrate TS1, 26% 0.7–1.2 mm sand, 1 g l−1 Osmocote Exact 3–4 M EG fertilizer), resulting in 144 combinations for each of the regional and local pairs. Two identical pots of each combination were set up; one of them was infested with one third instar M. melolontha larva seven weeks after germination, and the other was not manipulated (control). Melolontha melolontha larvae were collected from meadows in Spessart (Germany), and reared individually in 200 ml plastic beakers filled with potting soil and grated carrots in a phytotron in darkness operating under the following conditions: 12 L : 12 D cycle; temperature: day 13°C, night 11°C; humidity: 70%. Plants were watered whenever necessary. At the end of November 2013, plants were moved to a greenhouse (4–10°C) to overwinter. Plants were moved outside again in early March 2014, and seeds of mature capitula were collected every day and weighed. Plants were harvested at the end of May when seed production had ceased. Latex from the main roots was collected in Eppendorf tubes and glass vials for secondary metabolite analysis and immediately frozen in liquid nitrogen. Latex in Eppendorf tubes and glass vials was extracted, analysed and quantified as described above. The ploidy level of each accession was furthermore determined by flow cytometry as described by Bubner et al. [42].
(e). Offspring analysis
To investigate the inducibility of latex secondary metabolites, TA-G, total PIE and total TritAc concentrations were compared between control and M. melolontha-infested plants with Kruskal–Wallis rank sum tests. We analysed the inducibility of TA-G (TA-G concentration in herbivore-infested plants divided by TA-G concentration in control plants) according to the infestation history using Kruskal–Wallis rank sum tests for the local and regional pairs separately. To test for heritable variation in latex secondary metabolite concentrations, we analysed constitutive TA-G, total PIE and total TritAc concentrations according to infestation history for each area separately with Kruskal–Wallis rank sum tests for both the local and the regional pairs. The diploid individuals from Germany (5 of 108 plants) were excluded from the analysis to avoid confounding effects caused by their different ploidy levels. To measure selection on latex secondary metabolite concentrations in the presence and absence of M. melolontha herbivory, we calculated directional and disruptive/stabilizing selection gradients using regression coefficients for each metabolite class separately [43–45]. To obtain the regression coefficients, relative fitness was calculated by dividing total seed mass of each individual by the mean total seed mass of the population. Total seed mass is a good approximation for plant fitness, as it reflects total reproductive investment by integrating both seed number and seed weight [46]. The directional selection gradient was calculated by linear regression of the relative fitness to the concentration of latex secondary metabolites. The selective effect of M. melolontha on the latex secondary metabolite was determined by comparing the slope of the selection gradients in the presence and absence of this herbivore (significant interaction between treatment and secondary metabolites in a linear regression). The robustness of the selection gradients and of the net effect of M. melolontha on the selection was investigated by excluding the two indivduals with the highest TA-G concentration from the analysis. An estimate for stabilizing/disruptive selection gradient was obtained by the second-order coefficient of a quadratic regression of the relative fitness on the latex secondary metabolites [43–45]. The offspring from Germany in the local pair were excluded from the analysis to avoid non-independence of data points due to the replication of clonal individuals that were already present in the regional pair. Individuals that contained other non-identified sesquiterpene lactones or that did not produce seeds were excluded from the analysis. Broad sense heritability (H2), which we could estimate from different replicated asexual clones, was calculated using the replicated offspring from the German triploid populations (in the local and regional pairs). H2 of latex secondary metabolites was calculated as H2 = Vg/VT, where Vg is the total genetic variance and VT the total phenotypic variance (genetic and environmental variance) [47]. H2 was estimated for the control and herbivore-infested plants separately. Differences in ploidy levels between infestation histories were analysed with χ2-tests for each area separately. All statistical analyses were performed in R v. 3.1.1.
3. Results
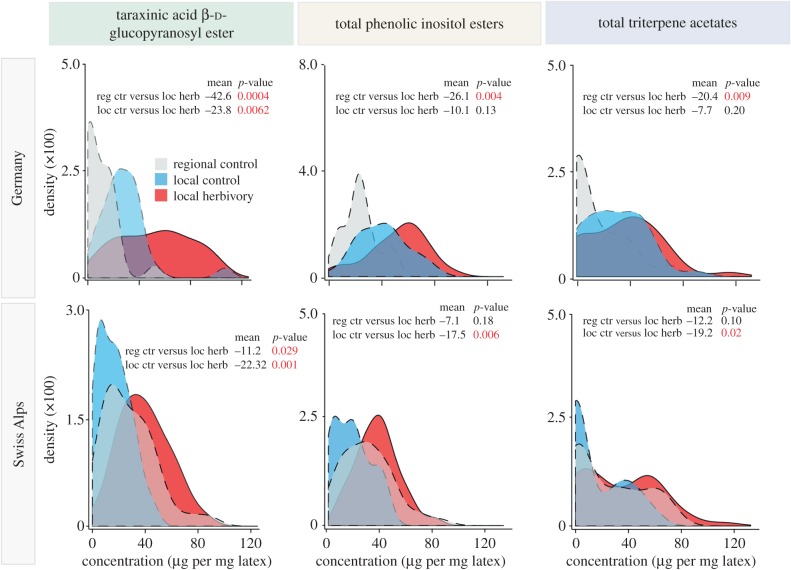
To explore the influence of M. melolontha abundance on the phenotypic variation in latex chemistry in nature, we surveyed the root herbivore community and latex secondary metabolites profiles in T. officinale populations that evolved under different M. melolontha pressure over the last 20 years in Germany and the Swiss Alps (figure 1a). As expected, M. melolontha was the major insect root herbivore in both number and size, and its present abundance matched the historical records (figure 1b; electronic supplementary material, table S2). The abundance of the other root feeding insects, including other Scarabaeidae and Agriotes spp. larvae, did not differ significantly between populations with different infestation histories and did not covary with M. melolontha abundance (figure 1b; electronic supplementary material, table S2). In both Germany and the Swiss Alps, all major latex secondary metabolite classes, TA-G, PIEs and TritAc, were positively associated with M. melolontha abundance (figure 2; electronic supplementary material, table S3). The concentration of TA-G was higher in the populations under strong M. melolontha pressure compared with both local and regional control populations with low M. melolontha abundance. Similarly, the total concentrations of PIEs and TritAcs were higher in the heavily infested populations than in either the regional or the local control populations, although the patterns were weaker than for TA-G. Taken together, these data indicate that M. melolontha shapes intraspecific phenotypic variation in the concentration of latex secondary metabolites in natural T. officinale populations.
Figure 2.
Latex secondary metabolite concentrations in T. officinale field populations. High latex secondary metabolite concentrations were associated with high M. melolontha abundance in both Germany and the Swiss Alps. Distributions show density estimates of latex secondary metabolite concentrations of T. officinale populations. p-values of linear mixed effect models are shown. reg ctr, regional control; loc ctr, local control; loc herb, local herbivory.
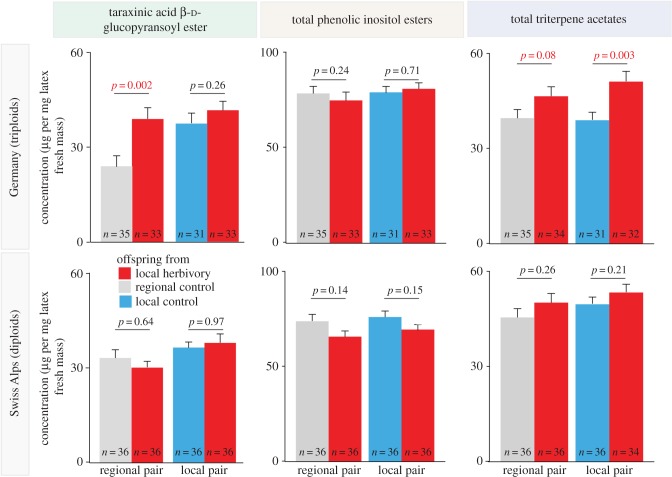
The observed differences in latex secondary metabolite concentrations in natural T. officinale populations may have resulted from different ploidy levels, phenotypic plasticity and/or heritable variation. To test these hypotheses, we analysed offspring from the field-sampled plants for ploidy, latex secondary metabolites and seed production in the presence and absence of M. melolontha larvae; 95% of the plants from the German populations were found to be triploid apomicts, while all plants from the Swiss Alps were diploid outcrossers. Plant ploidy levels did not differ between populations within Germany (herbivore : local control: 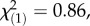 p = 0.35; local herbivore : regional control:
p = 0.35; local herbivore : regional control: 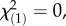 p = 1.0; χ2-tests) or the Swiss Alps (
p = 1.0; χ2-tests) or the Swiss Alps (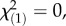 p = 1.0 for both local herbivore : local control and local herbivore : regional control, χ2-tests), showing that the herbivore-associated patterns were not confounded by differences in plant ploidy levels and that M. melolontha pressure had no influence on the abundance of diploids and triploids. Under controlled conditions, M. melolontha infestation increased TA-G concentration by 36% (
p = 1.0 for both local herbivore : local control and local herbivore : regional control, χ2-tests), showing that the herbivore-associated patterns were not confounded by differences in plant ploidy levels and that M. melolontha pressure had no influence on the abundance of diploids and triploids. Under controlled conditions, M. melolontha infestation increased TA-G concentration by 36% (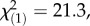 p < 4 × 10−6, Kruskal–Wallis rank sum test), while total PIE and total TritAc concentrations were reduced by 9–16% (figure 3; PIEs: 16%,
p < 4 × 10−6, Kruskal–Wallis rank sum test), while total PIE and total TritAc concentrations were reduced by 9–16% (figure 3; PIEs: 16%, 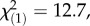 p = 0.0004, TritAcs: 9%,
p = 0.0004, TritAcs: 9%, 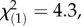 p = 0.04, Kruskal–Wallis rank sum tests). Inducibility of TA-G concentration did not differ between infestation histories (electronic supplementary material, figure S1). Inducibility of TA-G may therefore have contributed to the patterns observed in the field. Nevertheless, we also observed heritable variation of latex secondary metabolite concentrations among T. officinale populations with different infestation histories: in the triploid apomictic populations in Germany, constitutive TA-G concentrations were 60% higher in the populations with high M. melolontha abundance than the regional controls (figure 4;
p = 0.04, Kruskal–Wallis rank sum tests). Inducibility of TA-G concentration did not differ between infestation histories (electronic supplementary material, figure S1). Inducibility of TA-G may therefore have contributed to the patterns observed in the field. Nevertheless, we also observed heritable variation of latex secondary metabolite concentrations among T. officinale populations with different infestation histories: in the triploid apomictic populations in Germany, constitutive TA-G concentrations were 60% higher in the populations with high M. melolontha abundance than the regional controls (figure 4; 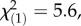 p = 0.02, Kruskal–Wallis rank sum test). No difference in constitutive TA-G concentrations was found between the populations with high M. melolontha abundance and the local control populations (figure 4;
p = 0.02, Kruskal–Wallis rank sum test). No difference in constitutive TA-G concentrations was found between the populations with high M. melolontha abundance and the local control populations (figure 4; 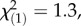 p = 0.26, Kruskal–Wallis rank sum test). Constitutive total TritAc concentrations were 20–25% higher in the heavily infested populations compared with both local and regional controls (local controls:
p = 0.26, Kruskal–Wallis rank sum test). Constitutive total TritAc concentrations were 20–25% higher in the heavily infested populations compared with both local and regional controls (local controls: 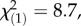 p = 0.003; regional controls:
p = 0.003; regional controls: 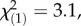 p = 0.08, Kruskal–Wallis rank sum tests). No difference in total PIE concentrations was observed between offspring populations (local controls:
p = 0.08, Kruskal–Wallis rank sum tests). No difference in total PIE concentrations was observed between offspring populations (local controls: 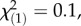 p = 0.71; regional controls:
p = 0.71; regional controls: 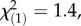 p = 0.24). In the sexual, obligate outcrossing populations from the Swiss Alps, the concentrations of the three latex metabolite classes did not differ between offspring populations (figure 4; TA-G: local controls:
p = 0.24). In the sexual, obligate outcrossing populations from the Swiss Alps, the concentrations of the three latex metabolite classes did not differ between offspring populations (figure 4; TA-G: local controls: 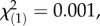 p = 0.97; regional controls:
p = 0.97; regional controls: 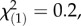 p = 0.64; PIEs: local controls:
p = 0.64; PIEs: local controls: 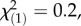 p = 0.15; regional controls:
p = 0.15; regional controls: 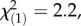 p = 0.14; TritAcs: local controls:
p = 0.14; TritAcs: local controls: 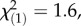 p = 0.21; regional controls:
p = 0.21; regional controls: 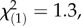 p = 0.26, Kruskal–Wallis rank sum tests).
p = 0.26, Kruskal–Wallis rank sum tests).
Figure 3.
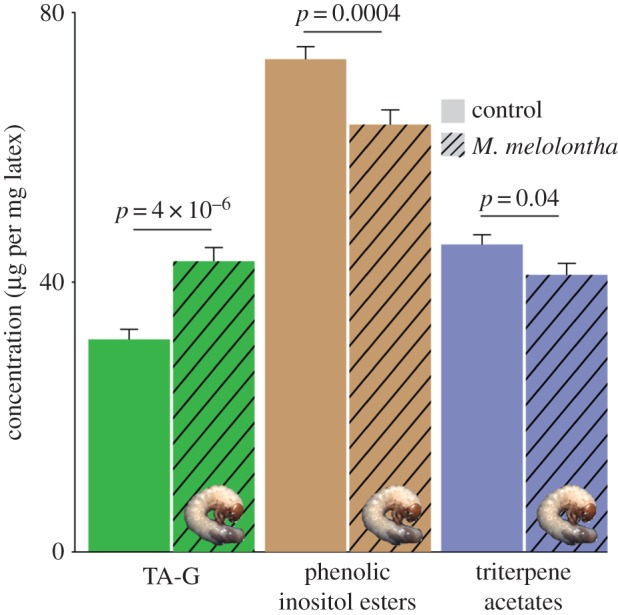
Induction of latex secondary metabolites upon M. melolontha infestation. Taraxinic acid β-d-glucopyranosyl ester (TA-G) concentration was induced, whereas total phenolic inositol ester and total triterpene acetate concentrations were reduced upon M. melolontha feeding in the offspring of the field populations. p-values from Kruskal–Wallis rank sum tests are shown for each metabolite class separately. Error bars correspond to standard errors (+s.e.). (Online version in colour.)
Figure 4.
Latex secondary metabolite concentrations in the offspring of the field populations. Heritable variation in the constitutive concentrations of taraxinic acid β-d-glucopyranosyl ester (TA-G) and total triterpene acetates were observed in the triploid, clonal offspring of the German populations but not in the diploid, sexual offspring of the populations of the Swiss Alps. Two offspring from either regional control and local herbivory (regional pair) or local control and local herbivory (local pair) were grown together in one pot. p-values from Kruskal–Wallis rank sum tests are shown. Sample size is shown inside each bar. Error bars correspond to standard errors (+s.e.).
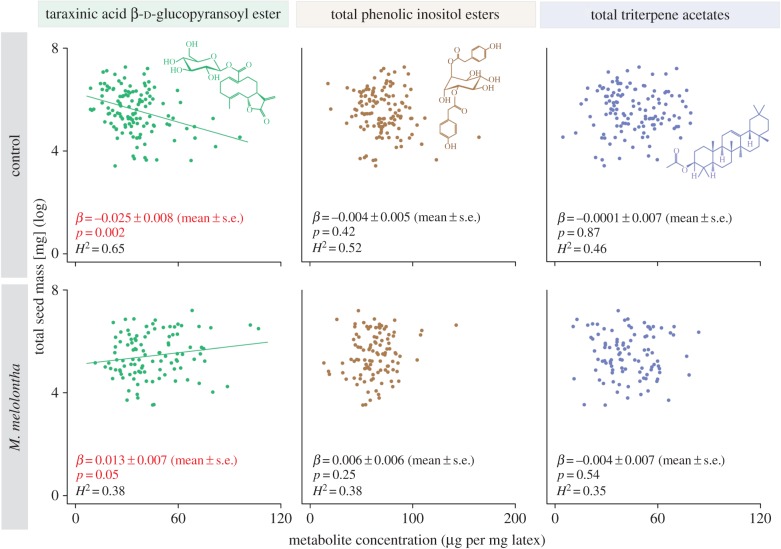
The fixation of phenotypic variation among populations requires divergent selection and heritability. We determined selection gradients by correlating secondary metabolite concentrations with total seed mass in the offspring from the field-sampled plants. For TA-G, the directional selection gradient (β) was positive in the presence (β = 0.013 ± 0.007 (mean ± s.e.), p = 0.05) of M. melolontha and negative in its absence (figure 5; β = −0.025 ± 0.008, p = 0.002), resulting in a strong effect of herbivory on the selection of TA-G (table 1). As the selection gradient in the presence of M. melolontha may be affected by two individuals with high TA-G concenration, we removed them from the analyses. Although the selection gradient of TA-G in the presence of M. melolontha was affected by the two individuals (β = −0.007 ± 0.006, p = 0.25), the net effect of herbivory on the selection of TA-G was robust to their removal (electronic supplementary material, table S4). For total PIEs and TritAcs, no significant directional selection gradients and no influence of herbivory were observed (table 1). For all major latex secondary metabolites, no signature of stabilizing or disruptive selection was found (electronic supplementary material, table S5). We further measured broad-sense heritability (H2) for all three metabolite classes. H2 ranged between 0.34 and 0.65 for the three metabolite classes in the absence and presence of M. melolontha, with highest heritability estimates found for TA-G (figure 5). Together, these experiments show that in T. officinale the concentrations of the three latex secondary metabolite classes are heritable, and that TA-G concentration but not total PIE or total TritAc concentrations are under M. melolontha-imposed divergent selection.
Figure 5.
Herbivory-dependent, divergent selection patterns of taraxinic acid β-d-glucopyranosyl ester (TA-G) concentrations. Correlations of TA-G, total phenolic inositol ester and total triterpene acetate concentrations with total seed mass (log-transformed) of different T. officinale genotypes in the absence (upper panel) and the presence (lower panel) of M. melolontha are shown. Broad-sense heritability (H2) and p-values of directional selection gradients (β) are shown. Representative chemical structures are depicted. s.e., standard error. (Online version in colour.)
Table 1.
Analysis of directional selection of M. melolontha on latex secondary metabolites. Relative fitness was analysed as a function of latex secondary metabolites in the absence and the presence of M. melolontha. Significant interaction terms indicate that M. melolontha exerts selection on the metabolite.
| estimate | s.e. | p-value | |
|---|---|---|---|
| herbivory | −1.1 | 0.72 | 0.14 |
| TA-G | −0.024 | 0.008 | 0.003** |
| total PIEs | 0.0046 | 0.006 | 0.44 |
| total TritAc | 9.2−5 | 0.006 | 0.99 |
| herbivory : TA-G | 0.034 | 0.01 | 0.001** |
| herbivory : total PIEs | −0.002 | 0.009 | 0.83 |
| herbivory : total TritAc | −0.004 | 0.01 | 0.69 |
4. Discussion
Plant roots display high concentrations and substantial intraspecific variation in secondary metabolites [12]. Despite the importance of roots for plant fitness, the function and evolution of these metabolites is often unknown [11]. We previously showed that the latex-derived sesquiterpene lactone TA-G is a major defensive compound in T. officinale roots that protects plants against M. melolontha attack [32]. Here, we demonstrate that M. melolontha can drive intraspecific variation of TA-G concentration in natural T. officinale populations. Three parallel lines of evidence support this hypothesis, as discussed below.
Taraxacum officinale populations that were growing under high M. melolontha pressure over the last 20 years had higher concentrations of the three major secondary metabolites in their root latex than plants growing under low M. melolontha herbivory. The pattern was most pronounced for the sesquiterpene lactone TA-G. As control and herbivore-infested populations of each area experienced similar abiotic and biotic conditions, and as no other root herbivore covaried with M. melolontha abundance, this screen suggests that M. melolontha can shape phenotypic variation of plant chemistry in nature. Such herbivore-associated patterns have often been predicted [48], but rarely been shown in nature either above [7,9] or below ground [21]. The positive association between TA-G concentration and M. melolontha abundance could be driven by two processes: first, TA-G may act as a defence and may be positively selected or induced by M. melolontha. Second, M. melolontha may preferentially occur on high TA-G containing plants. Previous experiments show that TA-G deters rather than attracts M. melolontha [32]. Furthermore, the data presented here provide evidence for both positive selection and induction of TA-G under M. melolontha attack. Therefore, the association between TA-G concentration and M. melolontha abundance in the field is most probably due to the defensive properties of TA-G.
We found that heritable variation contributed to the phenotypic differences of latex secondary metabolites in the field. TA-G and total TritAc levels were higher in the herbivore-infested populations compared with control populations in the offspring of the apomictic, triploid populations when grown in a common garden. Although confounding effects due to the geographical distance between the populations cannot fully be discounted, these data indicate that M. melolontha can affect heritable variation in TA-G concentration in the triploid populations. By contrast, offspring populations of the diploid populations did not differ in the concentration of any of the three metabolite classes. The lack of heritable variation between diploid populations may be due to outcrossing of the parental plants from heavily infested populations with plants growing under low M. melolontha pressure. Taraxacum officinale is visited by a broad range of pollinators [34]; thus, pollen transport over several kilometres is likely to occur [49]. By contrast, M. melolontha pressure can differ dramatically between sites that are only a few metres away from each other. Furthermore, recombination during meiosis in the diploid individuals may have led to the loss of allelic combinations that were responsible for the high TA-G concentrations in the mother plants. Our observation suggests that beneficial traits, such as protection against herbivores, can be selected faster in asexual than in sexual populations. This is in line with the general notion that differentiation between populations is established more rapidly in asexual than sexual populations [50], and highlights the importance of the plant reproductive strategy for herbivore-dependent natural selection.
The establishment of heritable variation between populations requires differential selection and heritability. Selection gradient analyses of the three metabolite classes in the presence and absence of M. melolontha showed negative selection of TA-G in the absence of M. melolontha and positive selection of TA-G in the presence of this herbivore, suggesting that M. melolontha attack leads to divergent selection of this sesquiterpene lactone. Negative correlations between the concentration of defensive metabolites and plant performance in the absence of herbivory are usually taken to indicate the potential fitness costs of compounds. However, the assumption that secondary metabolites are costly for plants is very controversial, with few examples of negative correlations between growth and defence [51,52] and many other examples with no apparent correlation [53,54]. Fitness costs can include both directs costs (i.e. costs of production of the metabolites) and indirect costs, such as decreases in mutualistic interactions [55]. Arbuscular mycorrhizal fungi for instance—which are often associated with T. officinale in the field [56]—may be hidden players in this context, as they affect plant fitness and resistance, and modulate primary and secondary metabolites [57–59]. Although mycorrhization was not measured in the present study, the common garden experiment is unlikely to be confounded by variation in mycorrhization, as the plants were grown simultaneously in the same soil and environment. Furthermore, TA-G accumulates specifically in the lacticifers [31], which are associated with the vascular tissues [60] and are very unlikely to come into direct contact with mycorrhizal fungi, which colonize the outer cell layers [61]. This spatial separation suggests that direct feedback effects between mycorrhizal colonization and TA-G abundance are unlikely. Although our experiments do not allow for a detailed assessment of the specific mechanisms behind the negative correlation of TA-G concentration and total seed mass in the absence of M. melolontha herbivory, they strongly suggest that TA-G production is costly for T. officinale growing under low root herbivore pressure.
The positive correlation between TA-G concentration and plant fitness indicates that the costs of TA-G are outweighed by its beneficial effect under high root herbivore pressure. Although this pattern was influenced by two individuals with high TA-G concentrations, the net effect of M. melolontha herbivory on the selection gradient was highly significant and robust to their removal. The beneficial effects of TA-G against root herbivores is further supported by our previous manipulative experiments demonstrating that TA-G deters M. melolontha feeding and thereby protects roots and improves plant performance [32]. Contrary to our previous observation that TA-G is not induced in different T. officinale genotypes after 11 days of M. melolontha herbivory in the greenhouse [32], we show here that TA-G is induced by 36% in plants that are attacked for 1 year under field conditions. Differences in infestation timing, growth conditions and plant genetic backgrounds may account for this result. The induction of TA-G upon prolonged M. melolontha infestation fits its role as a potentially costly defence, as inducibility is commonly regarded as a cost-saving strategy in the absence of herbivory [62]. Apart from divergent selection of TA-G in the presence and absence of M. melolontha, we also found high broad-sense heritability of TA-G concentration in the triploid plants. These data indicate that variation in M. melolontha abundance can lead to evolutionary changes of TA-G concentration over time.
Taken together, the association of TA-G concentration with M. melolontha abundance in the field, the heritable differences in TA-G concentration among triploids, the patterns of divergent selection of TA-G concentration in the absence and presence of M. melolontha, the relatively high heritability estimates of TA-G concentration and the defensive properties of TA-G provide evidence that M. melolontha shapes variation in T. officinale root latex chemistry in nature. As root herbivory can also affect plant growth and resistance indirectly via soil feedbacks [14], the evolution of plant defences in nature may be modulated by both direct and indirect effects of root herbivores on plant performance. Additional experiments involving reciprocal transplant experiments, population genetics data to track gene flow and the identification of potential TA-G-associated genes that are under selection may shed further light on the mechanistic underpinning of the observed patterns.
The tremendous diversity of plant secondary metabolites both within and between species has fascinated biologists for decades. Recent studies have shown that the heterogeneous distribution of herbivores can shape plant defensive chemistry above ground [7–9]. Moreover, it was found that heterogeneity in herbivore communities maintains intraspecific variation in defence genes [10]. By contrast, the potential of below-ground herbivores to shape chemical defences has remained elusive [21]. We show that variation in the abundance of a single root herbivore can contribute to the intraspecific variation in the concentration of a root secondary metabolite. We speculate here that secondary metabolites may be under strong selection by root herbivores for several reasons. First, many perennial plants depend on the roots as overwintering organs [63], which probably results in a strong selection for root defences. Second, herbivore avoidance strategies such as tissue shedding or nutrient reallocation may be deployed to a smaller extent in roots than in leaves due to the immediate requirement of roots for the survival of the plant (but see [64,65]). Third, roots are considered as an inferior food source compared with shoots, especially regarding nitrogen content [12]. Chemial defences that reduce the nutritional quality of the food source may therefore be especially effective in the roots. In general, the specific abiotic and biotic conditions that distinguish root herbivore interactions from leaf herbivore interactions [66] may result in distinct secondary metabolite selection patterns below ground. As the soil is rich in microbes [67], secondary metabolites that are active against both herbivores and pathogens may be under particularly strong positive selection, for instance. Integrating such additional factors will be important to further advance our understanding of natural variation in root secondary metabolite diversity and abundance. Taken together, the present study highlights the potential of root herbivores as evolutionary drivers of the chemical defences of their host plants. Thus, one of the central paradigms in plant–herbivore interactions can now be extended to below-ground environments.
Supplementary Material
Acknowledgements
We thank Lena Kurz, Christelle A. M. Robert and Huang Wei for their help during fieldwork. We are grateful to Helene Brändli and co-workers from Plantahof, Christian Schweizer from Agroscope Reckenholz, farmers in Switzerland and Germany, as well as Philipp Schlüter from the Institute for Systematic Botany at the University of Zurich for supporting the field survey. We are grateful to Shuqing Xu, Nadir Alvarez, Philipp Schlüter, Tobias Züst, Ted C. J. Turlings, Cris Kuhlemeier and Ian T. Baldwin for valuable discussions and suggestions on previous versions of the manuscript.
Data accessibility
The datasets supporting this article are available on Dryad: http://dx.doi.org/10.5061/dryad.7k1g1.
Authors' contributions
M.H. and M.E. conceived and designed the project and experiments. M.H., Z.B., J.F., T.B., Z.A. and M.E. performed experiments. M.H. analysed data. J.G. and M.E. provided reagents/materials and analysis tools. M.H. and M.E. wrote the manuscript. All authors contributed to previous versions of the manuscript.
Competing interests
The authors declare no competing interests.
Funding
The work of M.E. and Z.B. was supported by the Swiss National Science Foundation (grant no. 153517) and the European Commission (FP7-PEOPLE-2013-CIG-629134). The work of M.H., T.B., Z.A., J.G. and M.E. was supported by the Max Planck Society.
References
- 1.Rainey PB, Travisano M. 1998. Adaptive radiation in a heterogeneous environment. Nature 394, 69–72. ( 10.1038/27900) [DOI] [PubMed] [Google Scholar]
- 2.Lenormand T. 2002. Gene flow and the limits to natural selection. Trends Ecol. Evol. 17, 183–189. ( 10.1016/S0169-5347(02)02497-7) [DOI] [Google Scholar]
- 3.Newton AC, Allnutt TR, Gillies ACM, Lowe AJ, Ennos RA. 1999. Molecular phylogeography, intraspecific variation and the conservation of tree species. Trends Ecol. Evol. 14, 140–145. ( 10.1016/S0169-5347(98)01555-9) [DOI] [PubMed] [Google Scholar]
- 4.Sinervo B, Svensson E. 2002. Correlational selection and the evolution of genomic architecture. Heredity 89, 329–338. ( 10.1038/sj.hdy.6800148) [DOI] [PubMed] [Google Scholar]
- 5.Turner TL, Bourne EC, Von Wettberg EJ, Hu TT, Nuzhdin SV. 2010. Population resequencing reveals local adaptation of Arabidopsis lyrata to serpentine soils. Nat. Genet. 42, 260–263. ( 10.1038/ng.515) [DOI] [PubMed] [Google Scholar]
- 6.Mauricio R, Rausher MD. 1997. Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution 51, 1435–1444. ( 10.2307/2411196) [DOI] [PubMed] [Google Scholar]
- 7.Züst T, Heichinger C, Grossniklaus U, Harrington R, Kliebenstein DJ, Turnbull LA. 2012. Natural enemies drive geographic variation in plant defenses. Science 338, 116–119. ( 10.1126/science.1226397) [DOI] [PubMed] [Google Scholar]
- 8.Agrawal AA, Hastings AP, Johnson MTJ, Maron JL, Salminen JP. 2012. Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science 338, 113–116. ( 10.1126/science.1225977) [DOI] [PubMed] [Google Scholar]
- 9.Prasad KVSK, et al. 2012. A gain-of-function polymorphism controlling complex traits and fitness in nature. Science 337, 1081–1084. ( 10.1126/science.1221636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerwin R, et al. 2015. Natural genetic variation in Arabidopsis thaliana defense metabolism genes modulates field fitness. eLife 4 ( 10.7554/eLife.05604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmann S, Agrawal AA. 2008. In defense of roots: a research agenda for studying plant resistance to belowground herbivory. Plant Physiol. 146, 875–880. ( 10.1104/pp.107.112045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dam NM. 2009. Belowground herbivory and plant defenses. Annu. Rev. Ecol. Evol. Syst. 40, 373–391. ( 10.1146/annurev.ecolsys.110308.120314) [DOI] [Google Scholar]
- 13.Rasmann S, Erwin AC, Halitschke R, Agrawal AA. 2011. Direct and indirect root defences of milkweed (Asclepias syriaca): trophic cascades, trade-offs and novel methods for studying subterranean herbivory. J. Ecol. 99, 16–25. ( 10.1111/j.1365-2745.2010.01713.x) [DOI] [Google Scholar]
- 14.Sonnemann I, Hempel S, Beutel M, Hanauer N, Reidinger S, Wurst S. 2013. The root herbivore history of the soil affects the productivity of a grassland plant community and determines plant response to new root herbivore attack. PLoS ONE 8, e56524 ( 10.1371/journal.pone.0056524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown VK, Gange AC. 1990. Insect herbivory below ground. Adv. Ecol. Res. 20, 1–58. ( 10.1016/S0065-2504(08)60052-5) [DOI] [Google Scholar]
- 16.Erb M, Huber M, Robert CAM, Ferrieri AP, Machado RAR, Arce CCM. 2013. The role of plant primary and secondary metabolites in root-herbivore behaviour, nutrition and physiology. In Behaviour and physiology of root herbivores, Advances in Insect Physiology, vol. 45 (eds Johnson SN, Hiltpold I, Turlings TCJ), pp. 53–95. San Diego, CA: Elsevier Academic Press Inc. [Google Scholar]
- 17.Sarwar M, Kirkegaard JA, Wong PTW, Desmarchelier JM. 1998. Biofumigation potential of brassicas. III. In vitro toxicity of isothiocyanates to soil-borne fungal pathogens. Plant Soil. 201, 103–112. ( 10.1023/A:1004381129991) [DOI] [Google Scholar]
- 18.Mandal SM, Chakraborty D, Dey S. 2010. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 5, 359–368. ( 10.4161/psb.5.4.10871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon RA, Paiva NL. 1995. Stress-induced phenylpropanoid metabolism. Plant Cell. 7, 1085–1097. ( 10.1105/tpc.7.7.1085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramakrishna A, Ravishankar GA. 2011. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 6, 1720–1731. ( 10.4161/psb.6.11.17613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watts SM, Dodson CD, Reichman OJ. 2011. The roots of defense: plant resistance and tolerance to belowground herbivory. PLoS ONE 6, e0018463 ( 10.1371/journal.pone.0018463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller S. 2004. Engerlingsvorkommen und Bekämpfung in der Schweiz. Nachrbl. Deut. Pflanzensch. 56, 88–90. [Google Scholar]
- 23.Pener MP. 2013. Hormonal effects on flight and migration. In Endocrinology II, vol. 8 (ed Kerkut GA.), pp. 491–550. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 24.Blunck H. 1938. Über die Möglichkeit zur Bekämpfung der Maikäferengerlinge mittels landwirtschaftlicher Kulturmassnahmen. Z. Pflanzenkrank. Pflanzensch. 48, 253–272. [Google Scholar]
- 25.Hasler T. 1986. Abundanz- und Dispersionsdynamik von Melolontha melolontha (L.) in Intensivobstanlagen. PhD thesis, Eidgenössische Technische Hochschule Zürich, Zurich, Switzerland.
- 26.Hauss R, Schütte F. 1976. Experiments on polyphagous habits of white grubs Melolontha melolontha on plants of grassland. Anz. Schädlingsk. Pflanzensch. Umweltsch. 49, 129–132. ( 10.1007/BF01984983) [DOI] [Google Scholar]
- 27.Hauss R, Schütte F. 1978. Über die Eiablage des Maikäfers (Melolontha melolontha L.) in Abhängigkeit von den Wirtspflanzen des Engerlings. J. Appl. Entomol. 86, 167–174. [Google Scholar]
- 28.Scheenen TWJ, Vergeldt FJ, Heemskerk AM, Van As H. 2007. Intact plant magnetic resonance imaging to study dynamics in long-distance sap flow and flow-conducting surface area. Plant Physiol. 144, 1157–1165. ( 10.1104/pp.106.089250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verduijn MH, Van Dijk PJ, Van Damme JMM. 2004. Distribution, phenology and demography of sympatric sexual and asexual dandelions (Taraxacum officinale s.l.): geographic parthenogenesis on a small scale. Biol. J. Linn. Soc. 82, 205–218. ( 10.1111/j.1095-8312.2004.00325.x) [DOI] [Google Scholar]
- 30.Agrawal AA, Konno K. 2009. Latex: a model for understanding mechanisms, ecology, and evolution of plant defense against herbivory. Annu. Rev. Ecol. Evol. Syst. 40, 311–331. ( 10.1146/annurev.ecolsys.110308.120307) [DOI] [Google Scholar]
- 31.Huber M, et al. 2015. Identification, quantification, spatiotemporal distribution and genetic variation of major latex secondary metabolites in the common dandelion (Taraxacum officinale agg.). Phytochemistry 115, 89–98. ( 10.1016/j.phytochem.2015.01.003) [DOI] [PubMed] [Google Scholar]
- 32.Huber M, et al. 2016. A latex metabolite benefits plant fitness under root herbivore attack. PLoS Biol. 14, e1002332 ( 10.1371/journal.pbio.1002332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Hofsten CG. 1954. Studies on the genus Taraxacum Wigg with special reference to the group vulgaria DT in Scandinavia. Stockholm, Sweden: LTs Förlag.
- 34.Lázaro A, Totland O. 2010. Population dependence in the interactions with neighbors for pollination: a field experiment with Taraxacum officinale. Am. J. Bot. 97, 760–769. ( 10.3732/ajb.0900263) [DOI] [PubMed] [Google Scholar]
- 35.Menken SBJ, Smit E, DenNijs HCM. 1995. Genetical population structure in plants: gene flow between diploid sexual and triploid asexual dandelions (Taraxacum section Ruderalia). Evolution 49, 1108–1118. ( 10.2307/2410435) [DOI] [PubMed] [Google Scholar]
- 36.Pinheiro J, Bates D, DebRoy S, Sarkar S, orpteam Rc 2014. nlme: Linear and nonlinear mixed effects models. R package version 3.1–117 ed. See http://CRAN.R-project.org.
- 37.Wickham H. 2009. ggplot2: elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 38.Auguie B. 2012 gridExtra: Functions in grid graphics. R package version 0.9.1 ed. See http://CRAN.R-project.org/package=gridExtra . [Google Scholar]
- 39.Loecher M.2014. RgoogleMaps: overlays on Google map tiles in R. See http://cran.r-project.org/web/packages/RgoogleMaps/index.html .
- 40.South A. 2011. rworldmap: a new R package for mapping global data. R J. 3, 35–43. [Google Scholar]
- 41.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 42.Bubner B, Gase K, Berger B, Link D, Baldwin I. 2006. Occurrence of tetraploidy in Nicotiana attenuata plants after Agrobacterium-mediated transformation is genotype specific but independent of polysomaty of explant tissue. Plant Cell Rep. 25, 668–675. ( 10.1007/s00299-005-0111-4) [DOI] [PubMed] [Google Scholar]
- 43.Rausher MD. 1992. The measurement of selection on quantitative traits—biases due to environmental covariances between traits and fitness. Evolution 46, 616–626. ( 10.2307/2409632) [DOI] [PubMed] [Google Scholar]
- 44.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 45.Mitchell-Olds T, Shaw RG. 1987. Regression analysis of natural selection: statistical inference and biological interpretation. Evolution 41, 1149–1161. ( 10.2307/2409084) [DOI] [PubMed] [Google Scholar]
- 46.Venable DL. 1992. Size-number trade-offs and the variation of seed size with plant resource status. Am. Nat. 140, 287–304. ( 10.1086/285413) [DOI] [Google Scholar]
- 47.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer. [Google Scholar]
- 48.Bernhardsson C, Robinson KM, Abreu IN, Jansson S, Albrectsen BR, Ingvarsson PK. 2013. Geographic structure in metabolome and herbivore community co-occurs with genetic structure in plant defence genes. Ecol. Lett. 16, 791–798. ( 10.1111/ele.12114) [DOI] [PubMed] [Google Scholar]
- 49.Chifflet R, Klein EK, Lavigne C, Le Feon V, Ricroch AE, Lecomte J, Vaissière BE. 2011. Spatial scale of insect-mediated pollen dispersal in oilseed rape in an open agricultural landscape. J. Appl. Ecol. 48, 689–696. ( 10.1111/j.1365-2664.2010.01904.x) [DOI] [Google Scholar]
- 50.Heywood JS. 1991. Spatial analysis of genetic variation in plant populations. Annu. Rev. Ecol. Syst. 22, 335–355. ( 10.1146/annurev.es.22.110191.002003) [DOI] [Google Scholar]
- 51.Züst T, Joseph B, Shimizu KK, Kliebenstein DJ, Turnbull LA. 2011. Using knockout mutants to reveal the growth costs of defensive traits. Proc. R. Soc. B 278, 2598–2603. ( 10.1098/rspb.2010.2475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul-Victor C, Züst T, Rees M, Kliebenstein DJ, Turnbull LA. 2010. A new method for measuring relative growth rate can uncover the costs of defensive compounds in Arabidopsis thaliana. New Phytol. 187, 1102–1111. ( 10.1111/j.1469-8137.2010.03325.x) [DOI] [PubMed] [Google Scholar]
- 53.Koricheva J. 2002. Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83, 176–190. ( 10.1890/0012-9658(2002)083%5B;0176:MAOSOV%5D;2.0.CO;2) [DOI] [Google Scholar]
- 54.Almeida-Cortez JS, Shipley B, Arnason JT. 1999. Do plant species with high relative growth rates have poorer chemical defences? Funct. Ecol. 13, 819–827. ( 10.1046/j.1365-2435.1999.00383.x) [DOI] [Google Scholar]
- 55.Strauss SY, Rudgers JA, Lau JA, Irwin RE. 2002. Direct and ecological costs of resistance to herbivory. Trends Ecol. Evol. 17, 278–285. ( 10.1016/S0169-5347(02)02483-7) [DOI] [Google Scholar]
- 56.Becklin KM, Galen C. 2009. Intra- and interspecific variation in mycorrhizal associations across a heterogeneous habitat gradient in alpine plant communities. Arct. Antarc. Alp. Res. 41, 183–190. ( 10.1657/1938-4246-41.2.183) [DOI] [Google Scholar]
- 57.Gange AC, Brown VK, Sinclair GS. 1994. Reduction of black vine weevil larval growth by vesicular-arbuscular mycorrhizal infection. Entomol. Exp. Appl. 70, 115–119. ( 10.1111/j.1570-7458.1994.tb00739.x) [DOI] [Google Scholar]
- 58.Vannette RL, Rasmann S. 2012. Arbuscular mycorrhizal fungi mediate below-ground plant–herbivore interactions: a phylogenetic study. Funct. Ecol. 26, 1033–1042. ( 10.1111/j.1365-2435.2012.02046.x) [DOI] [Google Scholar]
- 59.Cameron DD, Neal AL, van Wees SCM, Ton J. 2013. Mycorrhiza-induced resistance: more than the sum of its parts? Trends Plant Sci. 18, 539–545. ( 10.1016/j.tplants.2013.06.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Razak A, Bahri SB. 2000. Development and distribution of laticifers in plants. Doctoral thesis, Durham University; See http:etheses.dur.ac.uk/4485. [Google Scholar]
- 61.Smith SE, Read D. 2008. Mycorrhizal symbiosis, 3rd edn London, UK: Academic Press. [Google Scholar]
- 62.Baldwin I, Hamilton W III. 2000. Jasmonate-induced responses of Nicotiana sylvestris results in fitness costs due to impaired competitive ability for nitrogen. J. Chem. Ecol. 26, 915–952. ( 10.1023/A:1005408208826) [DOI] [Google Scholar]
- 63.Crawley M. 2009. The structure of plant communities. In Plant ecology, vol. 2 (ed Crawley M.), pp. 475–531. New York, NY: Wiley. [Google Scholar]
- 64.Robert CAM, et al. 2014. Induced carbon reallocation and compensatory growth as root herbivore tolerance mechanisms. Plant Cell Environ. 37, 2613–2622. ( 10.1111/pce.12359) [DOI] [PubMed] [Google Scholar]
- 65.Robert CAM, Schirmer S, Barry J, Wade French B, Hibbard BE, Gershenzon J. 2015. Belowground herbivore tolerance involves delayed overcompensatory root regrowth in maize. Entomol. Exp. Appl. 157, 113–120. ( 10.1111/eea.12346) [DOI] [Google Scholar]
- 66.Johnson SN, Erb M, Hartley SE In press. Roots under attack: contrasting plant responses to below- and aboveground insect herbivory. New Phytol. ( 10.1111/nph.13807) [DOI] [PubMed] [Google Scholar]
- 67.Curtis TP, Sloan WT, Scannell JW. 2002. Estimating prokaryotic diversity and its limits. Proc. Natl Acad. Sci USA 99, 10 494–10 499. ( 10.1073/pnas.142680199) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available on Dryad: http://dx.doi.org/10.5061/dryad.7k1g1.