Abstract
Social insects use cuticular hydrocarbons (CHCs) to convey different social signals, including colony or nest identity. Despite extensive investigations, the exact source and identity of CHCs that act as nest-specific identification signals remain largely unknown. Perhaps this is because studies that identify CHC signals typically use organic solvents to extract a single sample from the entire animal, thereby analysing a cocktail of chemicals that may serve several signal functions. We took a novel approach by first identifying CHC profiles from different body parts of ants (Iridomyrmex purpureus), then used behavioural bioassays to reveal the location of specific social signals. The CHC profiles of both workers and alates varied between different body parts, and workers paid more attention to the antennae of non-nest-mate and the legs of nest-mate workers. Workers responded less aggressively to non-nest-mate workers if the CHCs on the antennae of their opponents were removed with a solvent. These data indicate that CHCs located on the antennae reveal nest-mate identity and, remarkably, that antennae both convey and receive social signals. Our approach and findings could be valuably applied to chemical signalling in other behavioural contexts, and provide insights that were otherwise obscured by including chemicals that either have no signal function or may be used in other contexts.
Keywords: nest-mate recognition, cuticular hydrocarbons, antennae
1. Introduction
The vast majority of signals in social insect colonies are mediated by chemical compounds [1], among which cuticular hydrocarbons (CHCs) play the central role in various signalling contexts. The glandular source and chemical identity of signals associated with alarm pheromones [2,3], sex pheromones [4–6] and trail pheromones [7] are well known. By contrast, the source and identity of CHCs associated with nest-mate recognition signals are poorly understood, despite their crucial significance in maintaining the social integrity of the colony [8–10]. While the variation in nest-mate recognition signals has been investigated extensively [11–13], the identity and function of the specific chemical compounds remain elusive, perhaps because the source of these signals is rarely considered. Typically, chemical analyses of CHC signals in social and other insects involve using organic solvents to extract a single sample from the entire animal [10,14,15]. A disadvantage of this approach is that it results in an unnecessarily complex pool of chemicals from all body parts, comprising a cocktail of signals that are likely to have diverse functions in different contexts.
By contrast, the variety and roles of the exocrine glands across the ant body have long been described [16], including Dufour's gland, the pygidial gland, the postpharyngeal gland (PPG) and various subepithelial glands. The PPG is thought to be the primary source of CHCs that subsequently disperse across the body [17]. The subepithelial glands, which are distributed across different body parts of ants, were suspected to produce hydrocarbons, but their role in hydrocarbon signalling was not confirmed [18]. The distribution and diversity of these exocrine glands suggest the possibility of differentiated hydrocarbon profiles on the cuticle of the ant. To the best of our knowledge, this possibility has not been examined comprehensively.
Here, we examine the location-specific diversity of the CHC profile across the body of Australian meat ants Iridomyrmex purpureus, and the roles of antennae as a source and in the detection of chemical social signals, using chemical analyses and behavioural experiments.
2. Material and methods
Workers of I. purpureus were collected from near the entrances of 20 mature colonies (electronic supplementary material S1). We compared the variation in the CHC profile of different body parts with the profile of the whole body, by pooling dissected antennae, heads, legs and abdomens from each of 20 workers from six colonies. The CHCs on the dissected body parts and the whole bodies were extracted with hexane, and analysed with GC-FID and GC-MS (electronic supplementary material S1). We also compared between-colony differences in the CHC profile for each body part by taking individual samples of the antennae, legs and abdomens from 15 workers collected from each of five colonies. The CHC profiles were analysed using a procedure described earlier (electronic supplementary material S1). CHC profiles on antennae, heads, wings, legs and abdomens of alates (males and females) of I. purpureus were analysed using the protocols described above (electronic supplementary material S3).
We examined whether workers direct their antennation behaviour towards different body parts of other workers by conducting two-on-two aggression assays in containers lined with fluon. Workers from 12 colonies, assigned into six colony pairs, were tested against nest-mates and non-nest-mates. The first 3 min of the behaviour of the marked ants were recorded, allowing us to calculate the number of antennations of the focal individuals towards different body parts of their opponents (electronic supplementary material S1).
We examined the role of antennae as a source of chemical social signals in nest-mate recognition behaviour by conducting two-on-two aggression assays (as above) in two experiments. First, we placed two marked, focal ants from one colony of each colony pair (n = 6 pairs of colonies) in the assay container with two other, unmarked ants. The unmarked ants were nest-mates with intact antennae, non-nest-mates with intact antennae, and non-nest-mates or nest-mates whose antennae had been amputated at the base. The second experiment followed the same protocol as above, except that in the antennal manipulation treatments, the antennae of the workers were dipped in either hexane (to remove surface chemicals) or in pure water (as controls) (electronic supplementary material S1). The behaviour of the marked individuals was recorded and analysed with the observer blind to the origin of the opposing ants (electronic supplementary material S1).
3. Results
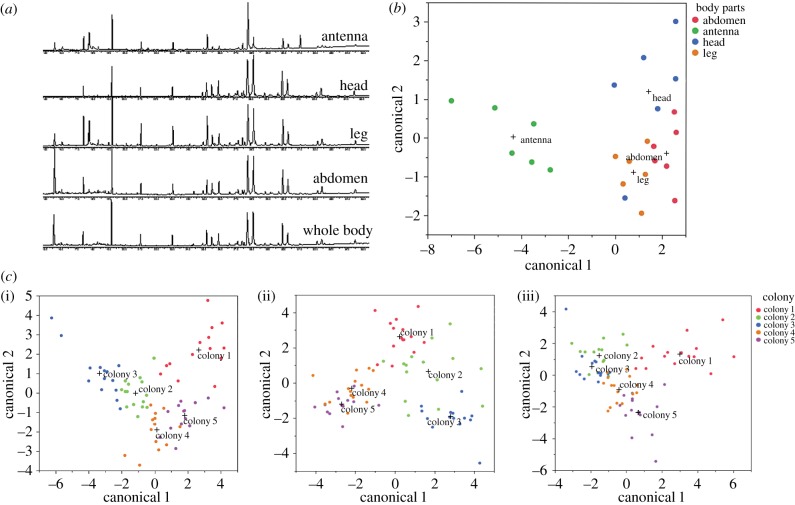
There is significant variation in the CHC profiles between the antennae, heads, legs and abdomen of I. purpureus (figure 1a). Discriminant analysis indicated that the chemical profile of antennae strongly separates from that of the other body parts (function 1 explains 86.7% of the variation, canonical correlation = 0.94, Wilks's λ = 0.043, χ2 = 6.35, d.f. = 15, p < 0.001; function 2 explains 7.6% of the variation, canonical correlation = 0.64, Wilks's λ = 0.383, χ2 = 2.61, d.f. = 8, p = 0.02; figure 1b). The model correctly classifies 87.5% of the body parts, with the antennae profile clearly distinguishable from that of the other body parts, supporting the use of our isolated body part approach. In the pooled body part samples, this variation is due to differences in relative quantities, rather than the types of components (figure 1a; electronic supplementary material, table S2.1). Unsurprisingly, a whole-body extract yielded the greatest variety of CHCs, and the antennae yielded the least (figure 1a; electronic supplementary material, table S2.2; the diagnostic power of each component on whole body, antenna, leg and abdomen samples is provided in the electronic supplementary material, table S2.2).
Figure 1.
Discriminant analysis using chemical signals. (a) Chromatogram (GC-FID) of the CHCs from different ant body parts and from the entire ant, all from the same colony. (b) Discriminant analysis plot of pooled body parts extractions (20 individuals from each of five colonies). Signals on each body part are clustered, and antennal signals are well separated from the rest of the body parts. This model correctly classifies 87.5% body parts, despite the colony difference (cross indicates group centroid). (c) Discriminant analysis plots of (i) antennae (correctly classifies 90.0% individuals), (ii) legs (correctly classifies 93.1% individuals) and (iii) abdomens (correctly classifies 88.89% individuals). Each point represents the body part profile from a single ant from five different colonies distinguished by colours. (For statistics see the electronic supplementary material. Online version in colour.)
Our discriminant analysis showed that it is possible to distinguish between colonies with the chemical profile on the antennae, legs and abdomens (figure 1c) from individual ants (antennae: 90.0% correct classification; legs: 93.1% correct classification; abdomens: 88.9% correct classification; electronic supplementary material S2). It is likely that not all of the body parts are involved in nest-mate recognition signalling. Conventionally, the whole-body wash of ants is used to separate colonies [11–13,19], but clearly, studying the whole-animal CHC components cannot discern the sources of individual body part variation.
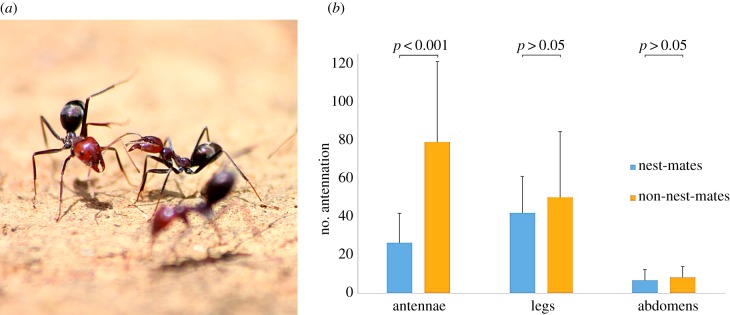
When workers encounter other workers, they brush their antennae over the body of the other individual [20]. When workers encounter non-nest-mates, they sometimes engage in a ritualized display behaviour [20] (figure 2a). The frequency with which I. purpureus workers antennate different body parts reflected the between-body variation in chemical profiles. Over 90% of the antennation was directed to the antennae or legs. Workers antennate the antennae (but not the legs or abdomens) of non-nest-mates more frequently than those of nest-mates (F2,862 = 269.5, p < 0.001; figure 2b). This suggests that although colonies may be distinguished statistically by extractions from various body parts, the antennae provide the important colony identity cues.
Figure 2.
Worker antennation interest in different body parts of nest-mates and non-nest-mates. (a) Display behaviour of I. purpureus. (b) The variation in antennation frequency was explained by a significant colony identity × body part interaction (error bars indicate s.e.m.), with differences between nest-mates and non-nest-mates when the behaviour is directed to the antennae only (F2,862 = 269.5, p < 0.001). (Online version in colour.)
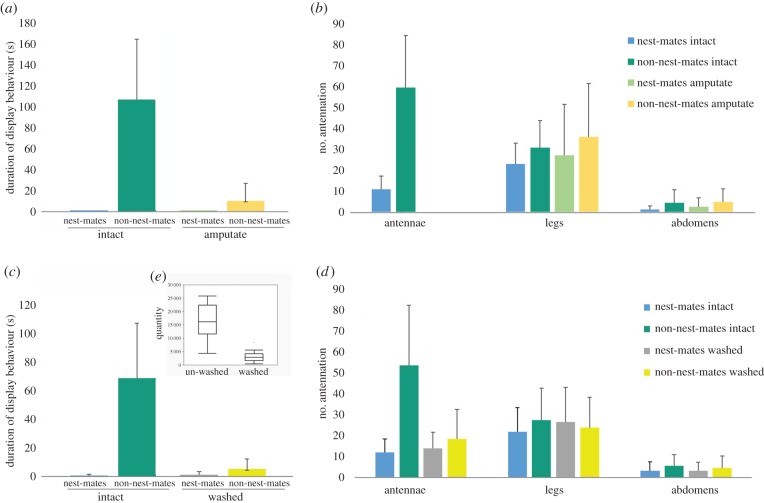
The antennae ablation experiments confirmed that the CHCs used in colony identity are sourced on the antennae. Workers of I. purpureus failed to respond aggressively to non-nest-mates whose antennae had been amputated (figure 3a; F2,94 = 23.51, p < 0.001) or washed with hexane (figure 3c; F2,94 = 21.70, p < 0.001), and instead their reaction was similar to that shown towards unmanipulated nest-mates (figure 3b,e). Chemical analysis confirmed that the quantity of CHC on the antennae was significantly reduced after washing with the solvent (figure 3d).
Figure 3.
Workers fail to distinguish non-nest-mates after manipulation of their opponents' antennae. (a,b) The duration of display behaviour and number of antennations, when intact ants encounter nest-mates and non-nest-mates with antennae amputated. The duration of display behaviour and antennation frequency were compared by ANOVA (significant differences, *p < 0.001; error bars indicate s.e.m.). (c,d) Duration of display behaviour and antennation frequency, when intact ants encounter nest-mates and non-nest-mates with antennal signals washed (the duration of display behaviour, and antennation frequency were compared by ANOVA (significant differences, *p < 0.001; error bars indicate s.e.m.). (e) Box plot of quantity of total CHCs on intact and washed antennae (line, median; box, 25th and 75th percentiles; whiskers, data range). (Online version in colour.)
4. Discussion
We discovered significant location-specific diversity of CHC profile of both worker and reproductive castes of ants, and that the antennae of workers can act as both a receiver and source of social chemical signals. These results considerably narrow the search to identify social identity signals in ants and, given that I. purpureus is unremarkable in the context of nest-mate recognition, probably also other clades of social insects. Our approach of identifying where the ‘receiver’ directs most interest in detecting cuticular signals, and then isolating the identity of the CHCs, could be applied to chemical signalling in other social contexts, including task cues [21]. More broadly, it may also reveal patterns that were otherwise obscured by including chemicals that either have no signal function or may be used in other contexts. Indeed, location-specific chemical signals also seem likely in solitary insects, where hydrocarbon-based contact or short-range pheromones may provide multiple signals, including for mate choice [22,23], species recognition [24] and other behavioural contexts [25–27].
Although it is possible to distinguish between colonies based on CHCs from the antennae, legs and abdomens, our behaviour assays clearly show that nest-mate recognition in I. purpureus is achieved through differences in the CHCs found only on the antennae. The compounds on the antennae are not inherently better sources of information, but are just used differently. Indeed, compounds located on the antennae were also found on other body parts (electronic supplementary material, table S2.1) and the signalling functions of these chemicals have not yet been identified. The presence of the same compounds on different body parts raises the intriguing possibility that these compounds may provide different signals when detected on different body parts—an efficient means of differentiating cuticle-derived chemical signals, given a finite number of metabolic products [28].
The typical method of identifying CHC signals usually involves extracting the hydrocarbons of the entire individual insect. Our study clearly shows that the diversity of CHC profiles on different body parts of a single ant exceeds that of ants from different colonies, indicating a more complicated CHC signalling than previously recognized [13]. We suspect that location-specific CHC signals will be a common feature of social insects: we found similar differences in male and female alates of I. purpureus (electronic supplementary material S3, figure S3.1), where the profiles of different body parts can be clearly separated, regardless of the sex of the individual. Interestingly, earlier reports of CHC profiles on the body parts of two formicine ants [29,30] are also consistent with this view.
Conventional models of nest-mate recognition signals [31] suggest that colonies acquire a distinctive gestalt colony odour, as hydrocarbons are distributed across the body parts of individuals [17,32] and among individuals within the colony [33,34] by trophallaxis, self-grooming, allo-grooming and physical contact. These models predict a largely invariant chemical profile between the body parts of individuals and among individuals within the colony. Our data suggest a more precise mechanism of colony identity signals that are sourced on the antennae. Other social signals may be similarly derived from various exocrine glands found on other body parts [35,36]. It also raises the fascinating possibility that antennation behaviour, especially in species like I. purpureus that are characterized by colony-wide displays [37], allows a worker to simultaneously obtain and convey information from her opponents.
Over 125 years ago, Auguste Forel demonstrated that antennae detect social signals by experimentally removing the antennae of workers of four species of ants, and then placing the ants together in a container. He found the ants huddled ‘piously together, one on top of another, despite the diversity of species’ [38, p. 88]. Since then, it has become widely understood that the antennae comprise the key sensory organ specialized to receive chemical signals in ants [39] and other insects [40]. Our study makes the novel discovery that antennae can act as both a source and sensor of chemical social signals.
Supplementary Material
Acknowledgements
We thank Doug Hilton, Dany Zemeitat, Jocelyn Miller and Lina Shan for their helpful comments on the manuscript, and Penny Orbell for her help with the field experiments.
Data accessibility
The datasets supporting this article have been made available at Dryad.
Authors' contributions
Q.W. designed the study, behavioural experiments, chemical analyses and manuscript preparation; J.Q.D.G. assisted with chemical analyses, experimental design and manuscript preparation; I.E.W. assisted with manuscript preparation; M.A.E. designed the study, data analysis and manuscript preparation.
Competing interests
The authors declare no conflict of interest.
Funding
The research was supported by the Australian Research Council (DP120100162 to M.A.E., LP110100138 to I.E.W.).
References
- 1.Hölldobler B, Wilson EO. 2009. The superorganism: the beauty, elegance, and strangeness of insect societies. New York, NY: WW Norton & Company. [Google Scholar]
- 2.Alonso L, Vander Meer R. 2002. Queen primer pheromone affects conspecific fire ant (Solenopsis invicta) aggression. Behav. Ecol. Sociobiol. 51, 122–130. ( 10.1007/s002650100417) [DOI] [Google Scholar]
- 3.Billen J, Ito F, Maile R, Morgan ED. 1998. The mandibular gland, probably the source of the alarm substance in Leptanilla sp. (Hymenoptera, Formicidae). Naturwissenschaften 85, 596–597. ( 10.1007/s001140050557) [DOI] [Google Scholar]
- 4.Greenberg L, Aliabadi A, McElfresh JS, Topoff H, Millar JG. 2004. Sex pheromone of queens of the slave-making ant, Polyergus breviceps. J. Chem. Ecol. 30, 1297–1303. ( 10.1023/b:joec.0000030300.11787.01) [DOI] [PubMed] [Google Scholar]
- 5.Dietemann V, Peeters C, Liebig J, Thivet V, Holldobler B. 2003. Cuticular hydrocarbons mediate discrimination of reproductives and nonreproductives in the ant Myrmecia gulosa. Proc. Natl Acad. Sci. USA 100, 10 341–10 346. ( 10.1073/pnas.1834281100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Oystaeyen A, et al. 2014. Conserved class of queen pheromones stops social insect workers from reproducing. Science 343, 287–290. ( 10.1126/science.1244899) [DOI] [PubMed] [Google Scholar]
- 7.Mitra A. 2013. Function of the Dufour's gland in solitary and social Hymenoptera. J. Hymenopt. Res. 35, 33–58. ( 10.3897/jhr.35.4783) [DOI] [Google Scholar]
- 8.Symonds MR, Elgar MA. 2008. The evolution of pheromone diversity. Trends Ecol. Evol. 23, 220–228. ( 10.1016/j.tree.2007.11.009) [DOI] [PubMed] [Google Scholar]
- 9.van Zweden JS, d'Ettorre P. 2010. Nestmate recognition in social insects and the role of hydrocarbons. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds GJ Blomquist, A-G Bagnères), pp. 222–243. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 10.Martin SJ, Vitikainen E, Helantera H, Drijfhout FP. 2008. Chemical basis of nest-mate discrimination in the ant Formica exsecta. Proc. R. Soc. B 275, 1271–1278. ( 10.1098/rspb.2007.1708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newey P. 2011. Not one odour but two: a new model for nestmate recognition. J. Theor. Biol. 270, 7–12. ( 10.1016/j.jtbi.2010.10.029) [DOI] [PubMed] [Google Scholar]
- 12.Sturgis SJ, Gordon DM. 2012. Nestmate recognition in ants (Hymenoptera: Formicidae): a review. Myrmecol. News. 16, 101–110. [Google Scholar]
- 13.van Wilgenburg E, Ryan D, Morrison P, Marriott PJ, Elgar MA. 2006. Nest- and colony-mate recognition in polydomous colonies of meat ants (Iridomyrmex purpureus). Naturwissenschaften 93, 309–314. ( 10.1007/s00114-006-0109-y) [DOI] [PubMed] [Google Scholar]
- 14.Sturgis SJ, Gordon DM. 2013. Aggression is task dependent in the red harvester ant (Pogonomyrmex barbatus). Behav. Ecol. 24, 532–539. ( 10.1093/beheco/ars194) [DOI] [Google Scholar]
- 15.Ichinose K, Cerdá X, Jean-Philippe C, Lenoir A. 2005. Detecting nestmate recognition patterns in the fission-performing ant Aphaenogaster senilis: a comparison of different indices. J. Insect Behav. 18, 633–650. ( 10.1007/s10905-005-7016-5) [DOI] [Google Scholar]
- 16.Bagneres A-G, Blomquist GJ. 2010. Site of synthesis, mechanism of transport and selective deposition of hydrocarbons. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds GJ Blomquist, A-G Bagnères), pp. 75–99. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 17.Lucas C, Pho DB, Fresneau D, Jallon JM. 2004. Hydrocarbon circulation and colonial signature in Pachycondyla villosa. J. Insect Physiol. 50, 595–607. ( 10.1016/j.jinsphys.2004.04.006) [DOI] [PubMed] [Google Scholar]
- 18.Gobin B, Ito F, Billen J. 2003. The subepithelial gland in ants: a novel exocrine gland closely associated with the cuticle surface. Acta Zool. 84, 285–291. ( 10.1046/j.1463-6395.2003.00149.x) [DOI] [Google Scholar]
- 19.Esponda F, Gordon DM. 2015. Distributed nestmate recognition in ants. Proc. R. Soc. B 282, 20142838 ( 10.1098/rspb.2014.2838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Wilgenburg E, van Lieshout E, Elgar MA. 2005. Conflict resolution strategies in meat ants (Iridomyrmex purpureus): ritualised displays versus lethal fighting. Behaviour 142, 701–716. ( 10.1163/1568539054729150) [DOI] [Google Scholar]
- 21.Martin SJ, Drijfhout FP. 2009. Nestmate and task cues are influenced and encoded differently within ant cuticular hydrocarbon profiles. J. Chem. Ecol. 35, 368–374. ( 10.1007/s10886-009-9612-x) [DOI] [PubMed] [Google Scholar]
- 22.Higgie M, Chenoweth S, Blows MW. 2000. Natural selection and the reinforcement of mate recognition. Science 290, 519–521. ( 10.1126/science.290.5491.519) [DOI] [PubMed] [Google Scholar]
- 23.Panhuis TM, Butlin R, Zuk M, Tregenza T. 2001. Sexual selection and speciation. Trends Ecol. Evol. 16, 364–371. ( 10.1016/s0169-5347(01)02160-7) [DOI] [PubMed] [Google Scholar]
- 24.Blows MW, Allan RA. 1998. Levels of mate recognition within and between two Drosophila species and their hybrids. Am. Nat. 152, 826–837. ( 10.1086/286211) [DOI] [PubMed] [Google Scholar]
- 25.McGaha TW, et al. 2015. Identification of communal oviposition pheromones from the Black Fly Simulium vittatum. PLoS ONE 10, 15 ( 10.1371/journal.pone.0118904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everaerts C, Farine JP, Cobb M, Ferveur JF. 2010. Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PLoS ONE 5, e9607 ( 10.1371/journal.pone.0009607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tillman JA, Seybold SJ, Jurenka RA, Blomquist GJ. 1999. Insect pheromones: an overview of biosynthesis and endocrine regulation. Insect Biochem. Mol. Biol. 29, 481–514. ( 10.1016/s0965-1748(99)00016-8) [DOI] [PubMed] [Google Scholar]
- 28.Oi CA, Van Oystaeyen A, Caliari Oliveira R, Millar JG, Verstrepen KJ, van Zweden JS, Wenseleers T. 2015. Dual effect of wasp queen pheromone in regulating insect sociality. Curr. Biol. 25, 1638–1640. ( 10.1016/j.cub.2015.04.040) [DOI] [PubMed] [Google Scholar]
- 29.Lenoir A, Depickere S, Devers S, Christides JP, Detrain C. 2009. Hydrocarbons in the ant Lasius niger: from the cuticle to the nest and home range marking. J. Chem. Ecol. 35, 913–921. ( 10.1007/s10886-009-9669-6) [DOI] [PubMed] [Google Scholar]
- 30.Bonavita-Cougourdan A, Clement JL, Lange C. 1993. Functional subcaste discrimination (foragers and brood-tenders) in the ant Camponotus vagus Scop—polymorphism of cuticular hydrocarbon patterns. J. Chem. Ecol. 19, 1461–1477. ( 10.1007/bf00984890) [DOI] [PubMed] [Google Scholar]
- 31.Lenoir A, Hefetz A, Simon T, Soroker V. 2001. Comparative dynamics of gestalt odour formation in two ant species Camponotus fellah and Aphaenogaster senilis (Hymenoptera: Formicidae). Physiol. Entomol. 26, 275–283. ( 10.1046/j.0307-6962.2001.00244.x) [DOI] [Google Scholar]
- 32.Soroker V, Hefetz A. 2000. Hydrocarbon site of synthesis and circulation in the desert ant Cataglyphis niger. J. Insect Physiol. 46, 1097–1102. ( 10.1016/s0022-1910(99)00219-x) [DOI] [PubMed] [Google Scholar]
- 33.Dahbi A, Hefetz A, Cerda X, Lenoir A. 1999. Trophallaxis mediates uniformity of colony odor in Cataglyphis iberica ants (Hymenoptera, Formicidae). J. Insect Behav. 12, 559–567. ( 10.1023/A:1020975009450) [DOI] [Google Scholar]
- 34.Lenoir A, D'Ettorre P, Errard C, Hefetz A. 2001. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46, 573–599. ( 10.1146/annurev.ento.46.1.573) [DOI] [PubMed] [Google Scholar]
- 35.Marques-Silva S, Matiello-Guss CP, Delabie JH, Mariano CS, Zanuncio JC, Serrao JE. 2006. Sensilla and secretory glands in the antennae of a primitive ant: Dinoponera lucida (Formicidae: Ponerinae). Microsc. Res. Techniq. 69, 885–890. ( 10.1002/jemt.20356) [DOI] [PubMed] [Google Scholar]
- 36.Billen J. 2009. Occurrence and structural organization of the exocrine glands in the legs of ants. Arth. Struct. Dev. 38, 2–15. ( 10.1016/j.asd.2008.08.002) [DOI] [PubMed] [Google Scholar]
- 37.Thomas ML, Parry LJ, Allan RA, Elgar MA. 1999. Geographic affinity, cuticular hydrocarbons and colony recognition in the Australian meat ant Iridomyrmex purpureus. Naturwissenschaften 86, 87–92. ( 10.1007/s001140050578) [DOI] [Google Scholar]
- 38.Forel A. 1928. The social world of the ants compared with that of man. New York, NY: A & C Boni. [Google Scholar]
- 39.Ozaki M, Wada-Katsumata A, Fujikawa K, Iwasaki M, Yokohari F, Satoji Y, Nisimura T, Yamaoka R. 2005. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 309, 311–314. ( 10.1126/science.1105244). [DOI] [PubMed] [Google Scholar]
- 40.Chapman RF. 1998. The insects: structure and function. Cambridge, UK: Cambridge University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been made available at Dryad.