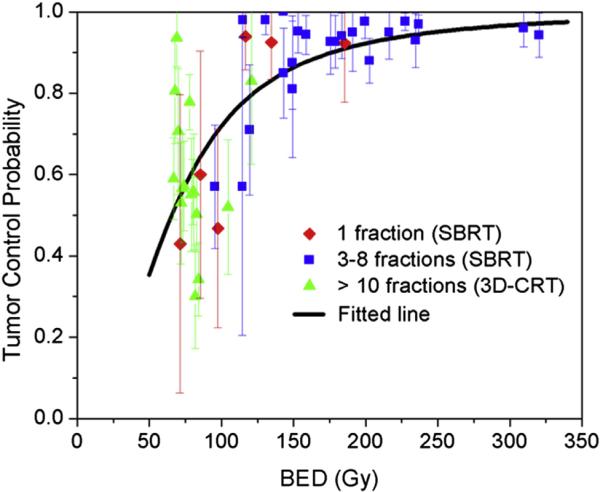
To the Editor: With the increasing use of radiosurgery and stereotactic body radiation therapy (SBRT) in radiation oncology, there has been a growing need to understand the radiobiology contributing to the remarkably high tumor control rates seen with the large fraction sizes used. We therefore read with great interest the recent editorial by Brown et al regarding whether “New Biology” was needed to understand SBRT dose response in lung cancer (1), and their more recent paper revisiting the analysis with the same conclusions (2). The authors presented a fitted tumor control probability (TCP) curve based on a wide range of local control rates from published series, including conventional fractionation (3-dimensional conformal radiation therapy [3DCRT]; >10 fractions), hypofractionation (SBRT; 3-8 fractions), and single-dose radiation (SBRT; 1 fraction), according to their linear quadratic (LQ)-based biologically effective doses (BEDs) (1, 3). The stated conclusion was that “there is no indication from these data that SBRT and 3DCRT produce different TCP probabilities when adjusted for BED” and “it follows there is no need to invoke a new biology” (1). We disagree, however, that the data presented can support this conclusion.
For their analysis, the authors coalesced the included series into average data points, but ignored the actual statistical spread of the data (based on sample size). Figure 1 shows the data presented by Brown et al, but with the addition of 95% confidence interval bars associated with each published data point. Using a simple χ2 test, we can ask if the spread of the original reports is consistent with the hypothesis that all the data are drawn from a single LQ model in BED, assumed to be the best fit model for all of the data together. If not, there is no evidence that all of the data are consistently drawn from the same distribution. With this type of analysis, the null hypothesis is that the distribution of studies follows a fitted curve, and the hypothesis is typically rejected when its probability falls below .05. In fact, using each report as its own bin in a χ2 analysis yields a χ2 value of 66.9 with 44 degrees-of-freedom and an associated probability of observing variations at least this large, if the data really were drawn from the best fit LQ curve, of only 1.4% (P=.014). For this reason, we reject the curve fit as a good representation for all of the data, and therefore disagree with the authors’ claim that “there is no indication from these data that SBRT and 3DCRT produce different TCP probabilities when adjusted for [LQ derived] BED.”
Fig. 1.
Tumor control probability (TCP) versus biologically effective dose (BED) using the linear quadratic (LQ) model. Data are from Mehta et al (3). The fitted line was obtained using a logistic regression model. The vertical lines indicate the 95% confidence intervals for each cohort. Three-dimensional conformal radiation therapy (3D-CRT; >10 fractions), stereotactic body radiation therapy (SBRT; 3-8 fractions), and SBRT (1 fraction) are shown in green, blue and red, respectively.
We followed up this initial statistical analysis with a review of the original dataset. The details are given in the Supplemental Materials, including a discussion of the sources of heterogeneity of the dataset. Even when the data are filtered to improve homogeneity, according to our own judgments, we reach a similar conclusion: The data across fractionation regimens are not consistent with the hypothesis that they are drawn from the same BED-based function.
To further clarify SBRT tumor response, higher quality data, that is, data gathered in a more consistent and comprehensive fashion, will need to be collected and analyzed.
Supplementary Material
Contributor Information
Shyam S. Rao, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, New York, New York.
Jung Hun Oh, Department of Medical Physics, Memorial Sloan-Kettering Cancer Center, New York, New York.
Andrew Jackson, Department of Medical Physics, Memorial Sloan-Kettering Cancer Center, New York, New York.
Joseph O. Deasy, Department of Medical Physics, Memorial Sloan-Kettering Cancer Center, New York, New York.
References
- 1.Brown JM, Brenner DJ, Carlson DJ. Dose escalation, not “new biology,” can account for the efficacy of stereotactic body radiation therapy with non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85:1159–1160. doi: 10.1016/j.ijrobp.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88:254–262. doi: 10.1016/j.ijrobp.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta N, King CR, Agazaryan N, et al. Stereotactic body radiation therapy and 3-dimensional conformal radiotherapy for stage I non-small cell lung cancer: A pooled analysis of biological equivalent dose and local control. Pract Radiat Oncol. 2012;2:288–295. doi: 10.1016/j.prro.2011.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.