Abstract
Purpose
Although vaginal stenosis (VS) is a recognized toxicity in women who receive pelvic radiation therapy (RT), the relationship between RT dose and the volume and extent of toxicity has not been analyzed. We modeled this relationship to identify predictors of VS.
Methods and Materials
We evaluated 54 women, aged 29 to 78 years, who underwent pelvic RT for rectal or anal cancer during 2008 to 2011 and were enrolled in a prospective study evaluating vaginal dilator use. Maximum dilator size was measured before RT (baseline) and 1 month and 12 months after RT. Dilator use was initiated at 1 month. The difference (D) in dilator size before and after RT was recorded. Those with D ≤−1 were classified as having VS (n = 35); those with D ≥0 were classified as having no VS (n = 19 at 1 month). Dose-volume parameters were extracted, and the generalized equivalent uniform dose (gEUD) was used to build a predictive model.
Results
The mean vaginal doses were 50.0 Gy and 36.8 Gy for anal and rectal cancer patients, respectively. One month after RT, a gEUD model using a wide range of a values suggests that sparing of vaginal volume to a low dose may be important. When gEUD (a = −1) was <35 Gy and the mean vaginal dose was <43 Gy, severe VS was reduced (P = .02). A 1-year analysis suggests increasingly negative D values with increasing mean dose. However, patients with compliance <40% were more likely to have toxicity.
Conclusions
Vaginal stenosis is influenced by multiple RT dose-volume characteristics. Mean dose and gEUD constraints together may reduce the risk of severe VS.
Introduction
Vaginal stenosis (VS) can be a long-term complication among women undergoing pelvic radiation therapy (RT). The physical changes have been well described, including adhesions of vaginal walls, narrowing and shortening of the vaginal barrel, and loss of elasticity. A concomitant decrease in vaginal mucosal secretions leads to dyspareunia (1) and a significant effect on quality of life (2).
Current literature regarding RT-induced toxicity to the vagina is primarily from the gynecologic cancer population (3–5). However, there are fewer reports of this late effect in women treated for lower gastrointestinal cancers. In the United States, there will be an estimated 7210 new cases of anal cancer (6) and 40,000 new cases of rectal cancer in 2014 (7), with women accounting for 64% of anal cancers and 42% of rectal cancers (8). The standard management of these tumors includes pelvic RT with concurrent chemotherapy in most patients. The aim of this study was to investigate dosimetric predictors of the development and severity of VS in patients with lower gastrointestinal cancers undergoing pelvic RT who participated in a prospective study evaluating the incidence of VS.
Methods and Materials
Patient characteristics
Fifty-four women with histologically confirmed rectal or anal cancer who underwent pelvic RT at our institution between February 2008 and June 2011 were enrolled in an Institutional Review Board–approved prospective study investigating compliance with vaginal dilator use and risk factors for development of VS. Exclusion criteria included prior RT to the pelvis and use of hormonal therapy. Patients were enrolled before treatment, at which time the maximum size of a dilator that could be easily inserted into the vagina (pre-RT dilator size) was recorded in a baseline survey before the initiation of RT. One month (approximately 5 weeks) after the completion of RT, the same survey was given at the patients’ first follow-up clinic visit. The post-RT dilator size was determined at this timepoint as this was when women were instructed to regularly start using the vaginal dilator 3 times per week, and compliance with dilator use would potentially confound further assessments of the degree of VS. Surveys were again administered 6 and 12 months after RT over the phone or in the clinic. Diary recordings of dilator use were performed at home and collected at the end of each month. Compliance was calculated by dividing the total number of times a patient used the dilator out of the 156 possible times during the 52 weeks on study (3 times/week × 52 weeks = 156). There were 5 vaginal dilator sizes, with dimensions as follows: 7 cm (length) × 1.5 cm (diameter) for size 0, 9 cm × 2 cm for size 1, 11 cm × 2.5 cm for size 2, 14 cm × 3 cm for size 3, and 16 cm × 3.5 cm for size 4 (9).
Patient characteristics are described in Table 1. The pre-RT dilator sizes were as follows: size 1 (n = 4), size 2 (n = 9), size 3 (n = 20), and size 4 (n = 21). Almost all patients (94%) received concurrent chemotherapy with concurrent 5-fluorouracil and mitomycin C (anal cancer patients) or 5-fluorouracil (rectal or anal adenocarcinoma patients). One patient received cisplatin and etoposide for small cell carcinoma of the rectum. One patient received concurrent FOLFOX (5-FU, oxaliplatin, and leucovorin). The patient with leiomyosarcoma did not receive concurrent chemotherapy. All 27 rectal cancer patients received chemoradiation preoperatively, and 5 received induction FOLFOX before chemoradiation.
Table 1.
Patient characteristics one month after radiation therapy (n = 54)
| Characteristic | All patients | No stenosis (D ≥0) | Stenosis (D <0) | P value |
|---|---|---|---|---|
| Median age (y) | 55 (range, 29–78) | 60 | 55 | .5025 |
| Menopausal status | .2470 | |||
| Premenopausal | 13 (24%) | 4 (21%) | 9 (26%) | |
| Peri- or postmenopausal | 41 (76%) | 15 (79%) | 26 (74%) | |
| Primary site of origin | .0017 | |||
| Anal cancer | 27 (50%) | 4 (21%) | 23 (66%) | |
| Adenocarcinoma | 2 (4%) | |||
| Squamous cell carcinoma | 25 (46%) | |||
| Rectal cancer | 27 (50%) | 15 (79%) | 12 (34%) | |
| Adenocarcinoma | 25 (46%) | |||
| Leiomyosarcoma | 1 (2%) | |||
| Small cell carcinoma | 1 (2%) | |||
| Baseline sexual activity | .2058 | |||
| No | 17 (31%) | 5 (26%) | 12 (34%) | |
| Yes | 37 (69%) | 14 (74%) | 23 (66%) | |
| Mean dose to vagina (Gy) | 43.4 | 38.4 | 45.9 | .0035 |
Abbreviation: D = postradiation dilator size at 1 month – preradiation dilator size.
Radiation therapy planning
All patients underwent computed tomography (CT) simulation with intravenous contrast medium in the prone position with marker placed at the anal verge. Target volumes and normal tissues were contoured on each axial slice (2.5- to 3-mm thickness) of the treatment planning CT based on the RT Oncology Group Anorectal Contouring Atlas (10). All anal cancer patients received intensity modulated RT (IMRT), and rectal cancer patients received IMRT or 3-dimensional conformal RT. Full details are in the Supplementary text (available online at www.redjournal.org).
Efforts were made to keep the dose going to 85% of the volume of the vagina to <45 Gy, although this was not a strict planning goal. Vaginal contours were reviewed and edited by 1 author (C.S.) with the use of radiology atlases and supervision of an experienced radiation oncologist (K.G.) for consistency because of the lack of a consensus definition in the literature. Specifically, the soft tissue extending from the vaginal meatus to the inferior aspect of the uterus as visible on the CT simulation scan was contoured. Vaginal doses for each patient were recalculated using the original treatment plan, and dose-volume parameters were extracted for modeling using our in-house CERR (Computational Environment for Radiotherapy Research) (11) and DREES (Dose Response Explorer System) (12) software.
Analysis of outcome
The difference between the 2 vaginal dilator sizes, D, was defined as D = (post-RT dilator size) − (pre-RT dilator size). A larger negative value for D would correspond to increasing severity of VS. Patients were split into 2 groups: the toxicity group (D ≤−1) and the no toxicity group (D ≥0). Univariate and multivariable analyses were performed to test the association of various dosimetric and clinical prognostic factors (including age, menopausal status, primary site, and selected dosimetric parameters) to the likelihood of VS (D ≤−1). Outcomes analyzed on a continuous scale (ie, D) were analyzed using linear regression, and the dichotomous outcomes of presence or absence of stenosis were analyzed using logistic regression. Outcomes were evaluated 1 month and 1 year after RT. The statistical software package MATLAB version 7.11 (MathWorks, Natick, MA) was used.
Generalized equivalent uniform dose model
The generalized equivalent uniform dose (gEUD) model provides a single metric for reporting nonuniform dose distributions, thus reducing the dose-volume histogram into a single index, taking into account dose heterogeneities. It is defined as “the uniform dose that, if delivered over the same number of fractions as the nonuniform dose distribution of interest, yields the same radiobiological effect” (13). The gEUD is defined as
| (1) |
where N is the number of voxels, vi is the fractional volume, Di is the dose at that volume, and a is an adjusting parameter that describes the dose-volume effect (14). For negative and positive a values, cold and hot spots, respectively, result in large effects on gEUD. The gEUD value with a = 1 is equal to the mean dose. When a becomes a large negative value, gEUD approaches the minimum dose. Conversely, when a becomes a large positive value, gEUD approaches the maximum dose. To assess predictive power, we used the Spearman rank correlation (Rs) coefficient and the area under the receiver operating characteristic curve (AUC).
Results
The distribution of pre-RT (baseline) dilator size was as follows: size 1 (n = 4), size 2 (n = 9), size 3 (n = 20), and size 4 (n = 21). The mean vaginal dose was 50.0 Gy for anal cancer patients and 36.8 Gy for rectal cancer patients (Fig. 1) (Supplementary text, available online at www.redjournal.org). Table 2 details the dose-volume parameters for the vagina. The mean vaginal dilator size at baseline was 3.1 (standard error [SE] = 0.13). It was 2.2 (SE = 0.12) at 1 month, 2.7 (SE = 0.14) at 3 months, and 3.2 (SE = 0.14) at 12 months after RT.
Fig. 1.
Representative radiation plans for (A) a rectal cancer patient treated with 3-dimensional conformal radiation therapy and (B) an anal cancer patient treated with IMRT.
Table 2.
Descriptive statistics of dose-volume parameters
| Vagina dose-volume parameter |
Anal cancer, n = 27 |
Rectal cancer, n = 27 |
P value |
|---|---|---|---|
| Volume (cm3) | 40.3 (16.9) | 38.5 (11.9) | .6529 |
| Mean dose (Gy) | 50.0 (3.3) | 36.8 (10.0) | <.0001 |
| Minimum dose (Gy) | 30.4 (13.3) | 8.7 (11.0) | <.0001 |
| Maximum dose (Gy) | 55.8 (3.5) | 51.3 (2.4) | <.0001 |
| V5 Gy (%) | 100 (0) | 86.4 (17.7) | .0002 |
| V10 Gy (%) | 100 (0) | 82.0 (19.7) | <.0001 |
| V20 Gy (%) | 98.2 (3.9) | 77.9 (21.1) | <.0001 |
| V30 Gy (%) | 96.5 (5.9) | 73.1 (22.3) | <.0001 |
| V40 Gy (%) | 93.1 (8.9) | 66.2 (22.9) | <.0001 |
| V50 Gy (%) | 58.0 (29.7) | 20.3 (22.2) | <.0001 |
Abbreviation: Vx = percentage volume receiving ≥x Gy.
Values in parenthesis indicate standard deviation. A 2-sample independent t test was used for calculating P values.
Analysis 1 month after RT
One month after RT, there were 35 patients in the stenosis group (D ≤−1) and 19 patients in the no stenosis group (D ≥0). Clinical variables were not significant for predicting VS (Table 1). There was a significant difference (P = .003) between patients with anal and rectal cancers in terms of the severity of VS.
Figure 2 demonstrates D (as a continuous variable) as a function of the mean vaginal dose. As mean dose increases, D becomes increasingly negative, corresponding to a larger decrease in dilator size from before RT to after RT. The original dilator difference against mean dose data was used to calculate a line of best fit, resulting in the slope of −0.0449 (Fig. 2A). Note that the fitted line (R2 = 0.89) is well fitted to the binned values (mean dilator difference for samples falling into every 10-Gy interval, ranging from 20 to 60 Gy) and R2 = 0.16 for the original dilator difference.
Fig. 2.
Vaginal dilator size differences (D) plotted against mean vaginal dose 1 month after RT (A) and 12 months after RT (B). Red circles indicate the mean dilator difference (± standard deviation) for samples falling into 10-Gy intervals. Blue line indicates the fitted line to original dilator difference against mean dose data. The regression correlation is weak but statistically significant (P = .003, R2 = 0.16 at 1 month and P = .048, R2 = 0.1 at 12 months). A color version of this figure is available at www.redjournal.org.
In univariate analysis using individual dosimetric variables, the gEUD variables had higher Rs and AUC than other dosimetric variables, including mean dose, maximum dose, Vx (percentage volume receiving at least x Gy), and Dx (minimum dose to the x% highest dose volume): the best dosimetric variables were mean dose (Rs = 0.40, P = .0026, AUC = 0.76, odds ratio [OR] = 1.08), V20 (Rs = 0.39, P = .0036, AUC = 0.73, OR = 1.04) and D55 (Rs = 0.38, P = .0044, AUC = 0.73, OR = 1.08), whereas the highest gEUD was obtained when a = −1 (Rs = 0.42, P = .0014, AUC = 0.76, OR = 1.04). Figure 3 shows the results of univariate analysis for gEUD variables with different a values ranging from −20 to 30. Multivariate analysis revealed that the best-performing logistic regression model includedMOC5 (mean dose to the coldest 5% of the vagina) and tumor site, resulting in Rs = 0.45 (P = .0006), AUC = 0.77, and OR = 1.03 (MOC5) and 0.25 (tumor site) where tumor sites were coded as 0 and 1 for anal cancer and rectal cancer, respectively. This is similar to the performance of our gEUD model, suggesting that the gEUD model could be the best model in this analysis.
Fig. 3.
Univariate Spearman correlation analysis with different a values in the generalized equivalent uniform dose (gEUD) equation. In this model, geometric mean dose was used for gEUD with a = 0.
In gEUD, hot and cold spot doses are emphasized with large positive and large negative a values, respectively (14). In further analysis, the patients were split into 3 groups: severe stenosis (D <−1), mild stenosis (D = −1), and no stenosis (D ≥0). When gEUD (a = −1) was <35 Gy and the mean vaginal dose was <43 Gy, severe VS was significantly reduced (P = .02 using χ2 test), as demonstrated in Figure 4.
Fig. 4.
Distribution of mean dose against generalized equivalent uniform dose (gEUD) (a = −1) by dilator difference, D. The patients were split into 3 groups—severe stenosis (D <−1), mild stenosis (D = −1), and no stenosis (D ≥0)—where D = (postradiation dilator size at 1 month) – (preradiation dilator size). The thick solid lines are at gEUD = 35 Gy and mean dose = 43 Gy.
Analysis 1 year after RT
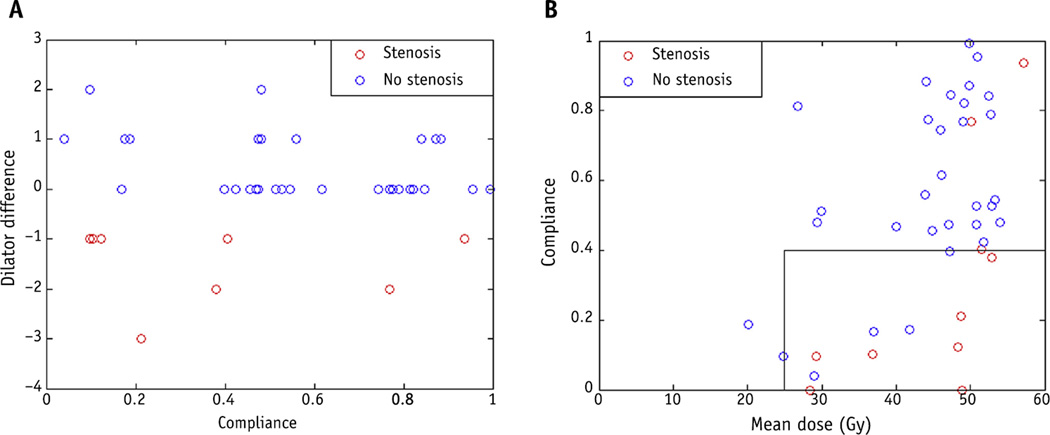
At 1 year, 10 patients had D ≤−1 (stenosis) and 31 patients had D ≥0 (no stenosis), and no data were available for 13 patients. Attrition in study participation over the 1-year period was most commonly due to personal reasons and other treatment-related toxicities. Univariate analysis and multivariate analysis did not demonstrate any association of the presence of stenosis (D ≤−1) with clinical or dosimetric variables other than stage (data not shown). Figure 2B demonstrates D (as a continuous variable) as a function of mean dose to the vagina 12 months after RT: as mean dose increases, the dilator difference also becomes increasing negative (P = .048). Univariate analysis using gEUD values did not show statistically significant association with the presence of or absence of stenosis (D ≤−1) using logistic regression. The impact of dilator use was also evaluated. Figure 5A demonstrates the effect of compliance on D, with a lower likelihood of VS as compliance increases. The 2 patients with toxicity who had compliance >60% received mean vaginal doses >50 Gy. Figure 5B demonstrates the effect of mean dose and compliance on toxicity. Patients with compliance <40% were more likely to have toxicity. In those receiving a mean dose >40 Gy, compliance with dilator use should be >40% to have the greatest likelihood of avoiding VS.
Fig. 5.
(A) Dilator difference versus compliance with dilator use 12 months after radiation therapy. (B) Vaginal stenosis as a function of compliance and mean vaginal dose 12 months after radiation therapy.
Discussion
Several dosimetric and clinical studies have documented the prevalence of VS and its impact on women with gynecologic cancer (4, 5, 15). However, women undergoing pelvic RT for nongynecologic cancers have historically not been counseled about the risk of vaginal toxicity, and there has been less concern about limiting vaginal RT doses. Nonetheless, these women still experience an impact on quality of life from VS. By using prospectively collected vaginal dilator size data before and after pelvic RT among women with rectal and anal cancers, we have quantified the extent of stenosis and suggest potential dosimetric guidelines to reduce its severity.
The concept of the gEUD was favored to present our results because it may reflect the dose tolerance for a tissue more accurately than a dose-volume histogram for a given structure, especially when data are limited regarding toxicity over dose-volume—based constraints. The results of the gEUD model clearly implicate sparing of some substantial volume of the vagina to a low dose as an important risk factor. However, Figure 4 also indicates a role for the mean dose as well.
Using more conventional dose-volume metrics, our results (Fig. 2) suggest that patients began experiencing VS at a mean dose of roughly 25 Gy. In particular, our analysis with 3 groups, severe stenosis (D <−1), mild stenosis (D = −1), and no stenosis (D ≥0), found that a combination of mean dose and gEUD (a = −1) could be used as a potential guideline to reduce the risk of VS. As seen in Figure 4, when gEUD (a = −1) is less than 35 Gy, and the mean vaginal dose is less than 43 Gy, there is a significantly reduced risk of severe VS (P = .02, χ2 test). We therefore suggest the use of mean dose as a potential treatment planning consideration until more comprehensive datasets can be analyzed. The suggested threshold is not discontinuous, and reducing either the gEUD or mean dose values below this threshold (or both) is likely to further reduce the risk and extent of VS. We anticipate that analysis of our plans by dose to a specific region of the vagina (such as the proximal or distal aspects of the vagina) would demonstrate similar results, inasmuch as the dose to the vagina (including the proximal vagina) is higher in general for patients with anal cancer.
There are no defined dosimetric guidelines or published prospective evaluation of VS in women who receive pelvic RT. Studies in the gynecologic cancer population do note worsening toxicity with the use of higher brachytherapy doses per fraction (16). However, Bahng et al (15) did not note a difference in toxicity based on dose, but only a minority of patients received a fractionation other than 7 Gy × 3 in their retrospective analysis.
The patients in our study were enrolled in a prospective trial examining use of vaginal dilators, allowing us to obtain baseline quantitative measures of vaginal “size” and reducing subjectivity. Although this study depends partially on patient self-report, its prospective design minimizes the likely underreporting that occurs because of the sensitive nature of the subject, which may be reflected in a retrospective chart review.
Although our results indicate that toxicity may be mitigated over time with the use of vaginal dilators, our analysis 1 month after RT also emphasizes the early development of stenosis. Even shortly after RT, women experienced a change in their vaginal anatomy. Although this may be partially confounded by persistent acute mucositis from the pelvic RT, the development of anatomic changes in the vaginal wall at an early timepoint is supported by other studies in the gynecologic patient population, indicating that vaginal shortening was noted even during the course of RT (17).
Conversely, our data may underestimate the severity and presence of stenosis, given the relatively good compliance with vaginal dilator use in our patients. It is likely that compliance would be lower in the general population, although patients with acute treatment-related toxicity may have decreased compliance 1 month after RT. Likewise, there may be limited generalizability of our results to other populations, including those who are non–English-speaking, who are of other cultural or ethnic heritage, or who have varying personal preferences regarding vaginal dilator use. Earlier studies reported that vaginal symptoms can occur up to 1 year after RT (4), and our 1-month data suggest that nearly two thirds of women had early toxicity. Certainly, the data obtained here should be correlated with patient-reported outcomes; there is an ongoing study at our institution studying vaginal dilator use. Although there is likely some error in self-measurement and reporting, intrapersonal discrepancy in dilator size measurement should be minimized.
Investigations of other clinical factors associated with stenosis in the gynecologic cancer population have found conflicting data on correlation with the stage or primary site of disease (4, 5), and Bahng et al (15) reported an association of shorter vaginal length and vaginal dilator use with decreased risk of VS. It is difficult to separate the direct effect of chemoradiation on the vagina from the secondary effects from ovarian dysfunction. Flay and Matthews (18) noted in a small series of patients that stenosis was more likely with the use of multiple treatment modalities.
Unlike previous studies demonstrating the significance of age on the rates of VS (4, 15), age was not predictive in our data. Similarly, menopausal status was not significant in our data, suggesting that the effects of ovarian dysfunction may be outweighed by radiation to the vagina. However, the majority of patients in this study were postmenopausal, and the overall number of patients in this study was limited.
In conclusion, in this study we designed a model to predict early VS in anal and rectal cancer patients treated with pelvic RT. The difference between dilator sizes before and after RT was used as an indicator of VS severity. Modeling suggests that consideration of a gEUD with an a parameter that weights lower doses (a = −1) and the mean dose could reduce VS. Maintaining gEUD [a = −1] <35 Gy may effectively spare a significant fraction of the vagina. The early prediction of VS based on this model could assist physicians to provide patients at high risk with a customized treatment. Our results suggest that higher rates of compliance with vaginal dilators are needed in the setting of higher mean doses to the vagina. Further testing with other patient cohorts and clinically significant (or patient-reported) outcomes would increase confidence. Any efforts to limit vaginal dose must be balanced against the necessary coverage of target structures to preserve optimal oncologic outcomes.
Supplementary Material
Summary.
Vaginal stenosis is a known complication of pelvic radiation therapy. Data on dose-volume relationship and extent of toxicity are lacking. Using prospectively collected vaginal dilator size information before and after radiation therapy, we quantified the extent of stenosis and studied its association with clinical and dosimetric variables. The results demonstrate the importance of minimizing the low-dose regions and mean dose to the vagina.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Grigsby PW, Russell A, Bruner D, et al. Late injury of cancer therapy on the female reproductive tract. Int J Radiat Oncol Biol Phys. 1995;31:1281–1299. doi: 10.1016/0360-3016(94)00426-L. [DOI] [PubMed] [Google Scholar]
- 2.Mirabeau-Beale KL, Viswanathan AN. Quality of life (QOL) in women treated for gynecologic malignancies with RT: A literature review of patient-reported outcomes. Gynecol Oncol. 2014;134:403–409. doi: 10.1016/j.ygyno.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Bergmark K, Avall-Lundqvist E, Dickman PW, et al. Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med. 1999;340:1383–1389. doi: 10.1056/NEJM199905063401802. [DOI] [PubMed] [Google Scholar]
- 4.Brand AH, Bull CA, Cakir B. Vaginal stenosis in patients treated with radiotherapy for carcinoma of the cervix. Int J Gynecol Cancer. 2006;16:288–293. doi: 10.1111/j.1525-1438.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 5.Bruner DW, Lanciano R, Keegan M, et al. Vaginal stenosis and sexual function following intracavitary radiation for the treatment of cervical and endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1993;27:825–830. doi: 10.1016/0360-3016(93)90455-5. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N, Krapcho M, Garshell J, et al., editors. SEER Cancer Statistics Review, 1975–2011, 2014. [Accessed June 10, 2015]; based on November 2013 SEER data submission, posted to the SEER web site. Available at: http://seer.cancer.gov/csr/1975_2011/.
- 7.American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 8.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed June 9, 2015]; http://www.owenmumford.com/en/range/21/amielle-comfort.html. [Google Scholar]
- 10.Myerson R, Naqa I, Abrams R, et al. Elective Clinical Target Volumes in Anorectal Cancer: An RTOG Consensus Panel Contouring Atlas. [Accessed June 10, 2015]; Available at: http://www.rtog.org/CoreLab/ContouringAtlases/Anorectal.aspx. [Google Scholar]
- 11.Deasy JO, Blanco AI, Clark VH. CERR: A computational environment for radiotherapy research. Med Phys. 2003;30:979–985. doi: 10.1118/1.1568978. [DOI] [PubMed] [Google Scholar]
- 12.El Naqa I, Suneja G, Lindsay PE, et al. Dose response explorer: An integrated open-source tool for exploring and modelling radiotherapy dose-volume outcome relationships. Phys Med Biol. 2006;51:5719–5735. doi: 10.1088/0031-9155/51/22/001. [DOI] [PubMed] [Google Scholar]
- 13.Niemierko A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med Phys. 1997;24:103–110. doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- 14.Park CS, Kim Y, Lee N, et al. Method to account for dose fractionation in analysis of IMRT plans: Modified equivalent uniform dose. Int J Radiat Oncol Biol Phys. 2005;62:925–932. doi: 10.1016/j.ijrobp.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 15.Bahng AY, Dagan A, Bruner DW, et al. Determination of prognostic factors for vaginal mucosal toxicity associated with intravaginal high-dose rate brachytherapy in patients with endometrial cancer. Int J Radiat Oncol Biol Phys. 2012;82:667–673. doi: 10.1016/j.ijrobp.2010.10.071. [DOI] [PubMed] [Google Scholar]
- 16.Sorbe B, Straumits A, Karlsson L. Intravaginal high-dose-rate brachytherapy for stage I endometrial cancer: A randomized study of two dose-per-fraction levels. Int J Radiat Oncol Biol Phys. 2005;62:1385–1389. doi: 10.1016/j.ijrobp.2004.12.079. [DOI] [PubMed] [Google Scholar]
- 17.Katz A, Njuguna E, Rakowsky E, et al. Early development of vaginal shortening during RT for endometrial or cervical cancer. Int J Gynecol Cancer. 2001;11:234–235. doi: 10.1046/j.1525-1438.2001.01019.x. [DOI] [PubMed] [Google Scholar]
- 18.Flay LD, Matthews JH. The effects of radiotherapy and surgery on the sexual function of women treated for cervical cancer. Int J Radiat Oncol Biol Phys. 1995;31:399–404. doi: 10.1016/0360-3016(94)E0139-B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.