Abstract
BACKGROUND
Recent studies suggest that the decision to undergo breast reconstruction and contralateral prophylactic mastectomy (CPM) are closely related. Here we describe the relationship between method of reconstruction and decision to undergo CPM. We also evaluate recent trends in CPM use in the context of literature questioning its oncologic benefit.
STUDY DESIGN
Female patients with unilateral breast cancer were identified and data extracted from the Surveillance, Epidemiology, and End Results (SEER) database from 2000 through 2010. Logistic regression analyses were performed to study the relationship between having CPM and key demographic, oncologic and reconstructive factors among women with unilateral breast cancer.
RESULTS
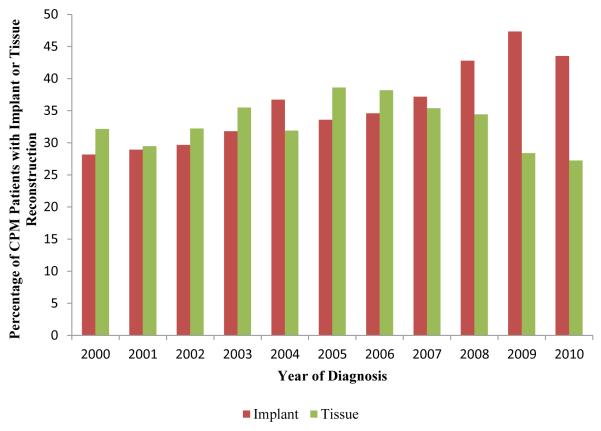
A total of 157,042 patients with unilateral breast cancer were included. CPM rate increased from 7.7% to 28.3% during the study period, and the proportion of reconstructed patients who underwent CPM increased from 19% to 46%. Reconstruction was associated with higher odds of CPM (odds ratio (OR) 2.79, 95% CI 2.70-2.88, p<0.0001) after controlling for oncologic and demographic factors. Among women who had reconstruction, implant-based reconstruction was associated with significantly higher odds of CPM than autologous tissue reconstruction (OR 1.38, p<0.0001). Over the study period Implant reconstruction rates increased from 28.2% to 43.5% while autologous reconstruction rates decreased from 32.2% to 27.3% in CPM patients.
CONCLUSIONS
The frequency of CPM continues to increase in spite of literature questioning its oncologic benefit. Our study confirms that reconstruction and the decision to undergo CPM are closely related, with implant reconstruction dominating in patients who undergo CPM. Given the relationship between reconstruction and the choice for CPM, plastic surgeons should play an active role in educating patients to avoid decisions made based on inaccurate information.
Keywords: Breast Reconstruction, Contralateral Prophylactic Mastectomy, Unilateral Mastectomy, National Trends, Surveillance, Epidemiology, End Results
INTRODUCTION
Contralateral prophylactic mastectomy (CPM) is performed with the intention of reducing the risk of contralateral breast cancer in select patients with unilateral breast cancer. Patients at increased risk for contralateral breast cancer including those with a genetic predisposition to developing breast cancer, those with a strong family history of breast cancer, and patients with previous chest wall irradiation may be offered CPM1-3. The survival benefit of CPM however remains a subject of debate with some studies suggesting no survival advantage4-6. A subject of further debate is the utility of CPM in patients without a known increased risk for contralateral disease.
In spite of concerns about the absence of a survival benefit and the morbidity associated with such a major surgical procedure, CPM rates have risen at an alarming rate over the past decade. King et al reported close to a 300% increase in CPM rates from 1997 to 2005 at Memorial-Sloan Kettering1. Their institution specific data suggests that young patient age, family history of breast cancer, Caucasian ethnicity, and immediate breast reconstruction are all predictors of CPM use1. Using the Surveillance, Epidemiology, and End Results (SEER) database, Tuttle et al showed a nearly 200% increase in CPM use for ductal carcinoma in situ (DCIS) between 1998 and 2005.7,8 Again, patients of younger age had higher odds of undergoing CPM. Although these and other studies have shown increases in CPM during the early part of the last decade, it is unclear whether concerns over the procedures’ questionable survival benefit have affected the trends of its use in recent years. Also requiring further attention are reconstructive factors which may contribute to the decision to undergo CPM. The availability of reconstruction was cited as a factor in favor of deciding to undergo CPM in a recent survey of 200 patients9. A separate retrospective study also showed that women who undergo CPM are more likely to pursue breast reconstruction10. The role of varying reconstructive strategies on decisions made for CPM is yet to be evaluated.
This study aims to assess recent trends in CPM use, given the growing body of literature suggesting that this procedure may be overused. In addition, we explore the relationship between CPM and breast reconstruction to help inform efforts geared at better counseling patients with early stage unilateral breast cancer.
METHODS
Data
Population-level de-identified data were extracted from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) cancer database (November 2012 submission) for the years 2000 through 2010. The SEER database collects patient-level data for all index malignant tumors in 18 cancer registries across the United States and captures 28% of the nation’s population11. This database is regarded as nationally representative and contains detailed demographic, socioeconomic, oncologic, and therapeutic information. To ensure data accuracy, chart abstracters undergo extensive training. Malignant tumors are encoded by use of the ninth revision of the International Classification of Diseases for Oncology. In addition to demographic and oncologic data, the SEER database has recently included specific data on multiple breast reconstruction techniques. Data on reconstruction in this database is limited to procedures performed within 4 months of mastectomy. Thus, data on delayed reconstructions performed greater than 4 months after mastectomy was not available.
Patient Inclusion/Exclusion
Female patients aged 18 years to 80 years from 2000 to 2010 with American Joint Committee on Cancer (AJCC) stage I to III breast cancer were eligible for selection. Patients with a diagnosis of unilateral, ductal and/or lobular carcinoma (histology codes: 8500, 8501, 8503, 8504, 8520, 8524), who had undergone either unilateral mastectomy alone (surgery codes 40,41,43-46, 50,51,53-56) or unilateral mastectomy with CPM (surgery codes 42,47-49,50,52,57-59,63,75) were included. Only patients with new primary breast cancers were included. Patients with stage IV disease, recurrent, and/or bilateral breast cancer were excluded.
Statistical Analysis
Categorical demographic characteristics accounted for in our analysis included age (18-35, 36-55, 56-70, and 71-80 years old), race (White, Black, Native American, Asian American, Other, Unknown), and year of diagnosis (2000-2010). Median county household income (in thousands) and percentage of women in the county with less than 12 years of education were treated as continuous demographic variables. Categorical oncologic variables included tumor stage (I, II, III), tumor size (< 1 cm, 1-1.9 cm, 2-4.9 cm, 5+ cm, unknown), tumor grade (I, II, III, IV), node status (positive, negative, unknown), ER status (positive, negative, unknown/borderline), PR status (positive, negative, unknown/borderline), radiation (yes, no, unknown), and whether this was a first primary (yes or no). Significant associations with CPM and reconstruction status were determined using Pearson’s chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables. Logistic regression analyses were performed to evaluate the odds of CPM among patients who had mastectomy with or without reconstruction, and among patients who had mastectomy with reconstruction only. All statistical analyses were performed with SAS version 9.3 (SAS Institute Inc). Tests were deemed statistically significant at level 0.05.
RESULTS
Population Characteristics
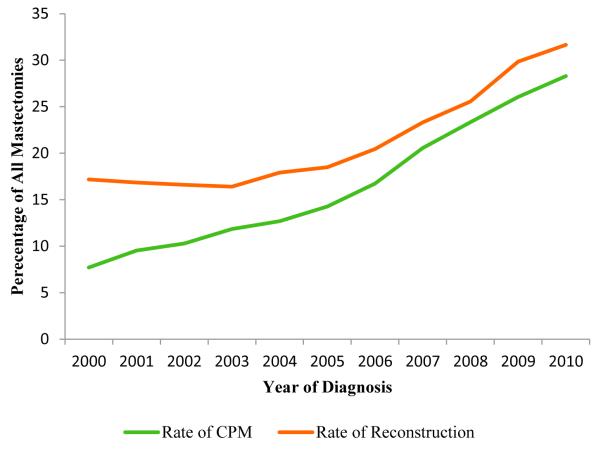
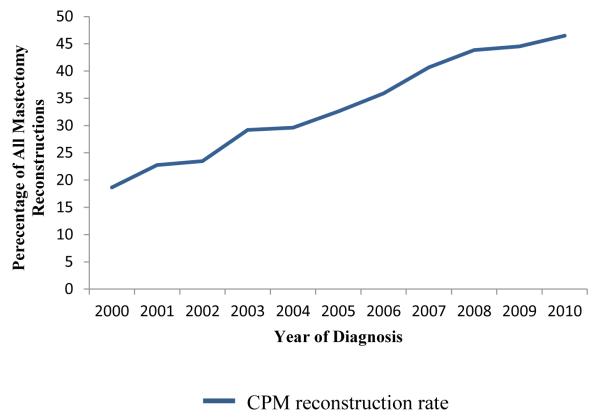
There were a total of 157,042 patients included for analysis. The demographic breakdown and tumor characteristics are outlined in Table 1. CPM was performed in 26,418 patients (16.8%) and unilateral mastectomies were performed in 130,624 patients (83.2%). The CPM rate increased throughout the period from 7.7% in 2000 to 28.3% in 2010 (Fig 1). The rate of reconstruction in all unilateral breast cancer patients also increased from 17.2% to 31.7% during the same timeframe (Fig 1). The percentage of reconstructed patients who had undergone CPM increased from 18.7% to 46.5% (Fig 2). Overall, 16.6% of non-CPM mastectomy patients underwent reconstruction while 46.1% of CPM patients underwent reconstruction (p<0.0001) (Table 2). Among CPM patients, implant reconstruction rates increased from 28.2% to 43.5% while autologous reconstruction rates decreased from 32.2% to 27.3% (figure 3). There was a statistically significant difference in the proportion of patients who had unilateral mastectomy with or without reconstruction and CPM with or without reconstruction based on all key demographic and oncologic features (Table 3).
Table 1.
Patient Demographics and Tumor Characteristics.
| Characteristic | N | % of total |
|---|---|---|
| Age, years | ||
| 18-35 | 5895 | 3.8 |
| 36-55 | 68,017 | 43.3 |
| 56-70 | 56,443 | 35.9 |
| 71-80 | 26,687 | 17.0 |
| Race | ||
| White | 127,131 | 81.0 |
| Black | 15,657 | 10.0 |
| Native American | 845 | 0.5 |
| Asian American | 12,764 | 8.1 |
| Other | 226 | 0.1 |
| Unknown | 419 | 0.3 |
| Year of Diagnosis | ||
| 2000 | 13,296 | 8.5 |
| 2001 | 14,423 | 9.2 |
| 2002 | 14,090 | 9.0 |
| 2003 | 13,536 | 8.6 |
| 2004 | 13,653 | 8.7 |
| 2005 | 13,186 | 8.4 |
| 2006 | 13,655 | 8.7 |
| 2007 | 14,684 | 9.4 |
| 2008 | 15,357 | 9.8 |
| 2009 | 15,761 | 10.0 |
| 2010 | 15,401 | 9.8 |
| Stage | ||
| I | 54,808 | 34.9 |
| II | 65,853 | 41.9 |
| III | 36,381 | 23.2 |
| Tumor Size | ||
| <1 | 23,955 | 15.3 |
| 1-1.9 | 44,914 | 28.6 |
| 2-4.9 | 23,622 | 15.0 |
| 5+ | 62,393 | 39.7 |
| Unknown | 2,158 | 1.4 |
| Grade | ||
| I | 22,566 | 14.4 |
| II | 62,628 | 39.9 |
| III | 60,452 | 38.5 |
| IV | 2,355 | 1.5 |
| Unknown | 9,041 | 5.8 |
| Node Status | ||
| Negative | 82,321 | 52.4 |
| Positive | 74,632 | 47.5 |
| Unknown | 89 | 0.1 |
| ER Status | ||
| Negative | 33,748 | 21.5 |
| Positive | 110,021 | 70.1 |
| Borderline/Unknown | 13,273 | 8.4 |
| PR Status | ||
| Negative | 49,715 | 31.7 |
| Positive | 91,505 | 58.3 |
| Borderline/Unknown | 15,822 | 10.1 |
| Radiation | ||
| No | 115,150 | 73.3 |
| Yes | 37,038 | 23.6 |
| Unknown | 4,854 | 3.1 |
| First Primary | ||
| No | 14,487 | 9.2 |
| Yes | 142,555 | 90.8 |
| Surgery | ||
| Unilateral, no reconstruction | 108,916 | 69.4 |
| Unilateral, reconstruction | 21,708 | 13.8 |
| Unilateral CPM, no reconstruction | 14,243 | 9.1 |
| Unilateral CPM, reconstruction | 12,175 | 7.8 |
| 2007-2011 Summary County Variables | Median | |
| Median Household Income (in thousands) | 56.55 | |
| % of women with <12 years education | 40.54 |
Figure 1.
Increasing trends in contralateral prophylactic mastectomy and immediate breast reconstruction use from 2000-2010.
Figure 2.
An increasing percentage of reconstructed patients underwent CPM.
Table 2.
Breast Reconstruction and CPM Rates
| Reconstruction |
|||
|---|---|---|---|
| No | Yes | p-value |
|
| CPM | n (%) | n (%) | |
| No | 108,916 (83.4) | 21,708 (16.6) | <0.0001 |
| Yes | 14,243 (53.9) | 12,175 (46.1) | |
Figure 3.
Trend of increasing implant reconstruction and decreasing autologous tissue reconstruction in CPM patients.
Table 3.
Characteristics of patients undergoing unilateral mastectomy or CPM with and without reconstruction.
| Characteristic | Unilateral, No Reconstruction |
Unilateral, Reconstruction |
CPM, No Reconstruction |
CPM, Reconstruction |
|||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | % | N | % | N | % | N | % | p | |
| Age, years | |||||||||
| 18-35 | 2,826 | 2.6 | 1,105 | 5.0 | 863 | 6.1 | 1,101 | 9.0 | <0.001 |
| 36-55 | 39,252 | 36.0 | 13,184 | 60.7 | 7,291 | 51.2 | 8,290 | 68.1 | |
| 56-70 | 42,453 | 39.0 | 6,518 | 30.0 | 4,860 | 34.1 | 2,612 | 21.5 | |
| 71-80 | 24,385 | 22.4 | 901 | 4.2 | 1,229 | 8.6 | 172 | 1.4 | |
| Race | |||||||||
| White | 84,452 | 78.5 | 18,217 | 83.9 | 12,540 | 88.0 | 10,922 | 89.7 | <0.001 |
| Black | 12,090 | 11.1 | 2,017 | 9.3 | 852 | 6.0 | 698 | 5.7 | |
| Native American | 644 | 0.6 | 71 | 0.3 | 96 | 0.7 | 34 | 0.3 | |
| Asian American | 10,292 | 9.5 | 1,315 | 6.1 | 693 | 4.9 | 464 | 3.8 | |
| Other | 150 | 0.1 | 35 | 0.2 | 22 | 0.2 | 19 | 0.2 | |
| Unknown | 288 | 0.3 | 53 | 0.2 | 40 | 0.3 | 38 | 0.3 | |
| Year of Diagnosis | |||||||||
| 2000 | 10,414 | 9.6 | 1,858 | 8.6 | 598 | 4.2 | 426 | 3.5 | <0.001 |
| 2001 | 11,171 | 10.3 | 1,877 | 8.7 | 822 | 5.8 | 553 | 4.5 | |
| 2002 | 10,850 | 10.0 | 1,790 | 8.3 | 901 | 6.3 | 549 | 4.5 | |
| 2003 | 10,362 | 9.5 | 1,572 | 7.2 | 954 | 6.7 | 648 | 5.3 | |
| 2004 | 10,199 | 9.4 | 1,721 | 7.9 | 1,009 | 7.1 | 724 | 6.0 | |
| 2005 | 9,569 | 8.9 | 1,645 | 7.6 | 1,087 | 7.6 | 795 | 6.5 | |
| 2006 | 9,580 | 8.8 | 1,790 | 8.3 | 1,282 | 9.0 | 1,003 | 8.2 | |
| 2007 | 9,635 | 8.9 | 2,030 | 9.4 | 1,626 | 11.4 | 1,393 | 11.4 | |
| 2008 | 9,569 | 8.8 | 2,205 | 10.2 | 1,861 | 13.1 | 1,722 | 14.1 | |
| 2009 | 9,043 | 8.3 | 2,611 | 12.0 | 2,012 | 14.1 | 2,095 | 17.2 | |
| 2010 | 8,434 | 7.7 | 2,609 | 12.0 | 2,091 | 14.7 | 2,267 | 18.6 | |
| Stage | |||||||||
| I | 35,385 | 32.5 | 8,926 | 41.1 | 5,120 | 35.9 | 5,377 | 44.2 | <0.001 |
| II | 45,864 | 42.1 | 9,119 | 42.0 | 5,825 | 40.9 | 5,045 | 41.4 | |
| III | 27,667 | 25.4 | 3,663 | 16.9 | 3,298 | 23.2 | 1,753 | 14.4 | |
| Tumor Size | |||||||||
| <1 | 14,430 | 13.3 | 4,662 | 21.5 | 2,218 | 15.6 | 2,645 | 21.7 | <0.001 |
| 1-1.9 | 30,015 | 27.6 | 6,609 | 30.4 | 4,273 | 30.0 | 4,017 | 33.0 | |
| 2-4.9 | 18,738 | 17.2 | 2,814 | 13.0 | 1,303 | 9.2 | 767 | 6.3 | |
| 5+ | 44,039 | 40.4 | 7,477 | 34.4 | 6,192 | 43.5 | 4,685 | 38.5 | |
| Unknown | 1,694 | 1.6 | 146 | 0.7 | 257 | 1.8 | 61 | 0.5 | |
| Grade | |||||||||
| I | 15,065 | 13.8 | 3,351 | 15.4 | 2,152 | 15.1 | 1,998 | 16.4 | <0.001 |
| II | 42,929 | 39.4 | 9,077 | 41.8 | 5,666 | 39.8 | 4,956 | 40.7 | |
| III | 42,914 | 39.4 | 7,778 | 35.8 | 5,376 | 37.7 | 4,384 | 36.0 | |
| IV | 1,736 | 1.6 | 289 | 1.3 | 189 | 1.3 | 141 | 1.2 | |
| Unknown | 6,272 | 5.8 | 1,213 | 5.6 | 860 | 6.0 | 696 | 5.7 | |
| Node Status | |||||||||
| Negative | 55,145 | 50.6 | 12,376 | 57.0 | 7,516 | 52.8 | 7,284 | 59.8 | <0.001 |
| Positive | 53,691 | 49.3 | 9,327 | 43.0 | 6,724 | 47.2 | 4,890 | 40.2 | |
| Unknown | 80 | 0.1 | 5 | <0.1 | 3 | <.1 | 1 | <0.1 | |
| ER Status | |||||||||
| Negative | 24,006 | 22.0 | 4,086 | 18.8 | 3,150 | 22.1 | 2,506 | 20.6 | <0.001 |
| Positive | 74,650 | 68.5 | 16,120 | 74.3 | 10,205 | 71.7 | 9,046 | 74.3 | |
| Borderline/Unknown | 10,260 | 9.4 | 1,502 | 6.9 | 888 | 6.2 | 623 | 5.1 | |
| PR Status | |||||||||
| Negative | 35,407 | 32.5 | 6,202 | 28.6 | 4,515 | 31.7 | 3,591 | 29.5 | <0.001 |
| Positive | 61,203 | 56.2 | 13,786 | 63.5 | 8,636 | 60.6 | 7,880 | 64.7 | |
| Borderline/Unknown | 12,306 | 11.3 | 1,720 | 7.9 | 1,092 | 7.7 | 704 | 5.8 | |
| Radiation | |||||||||
| No | 78,662 | 72.2 | 16,735 | 77.1 | 10,191 | 71.6 | 9,562 | 78.5 | <0.001 |
| Yes | 26,689 | 24.5 | 4,341 | 20.0 | 3,694 | 25.9 | 2,314 | 19.0 | |
| Unknown | 3,565 | 3.3 | 632 | 2.9 | 358 | 2.5 | 299 | 2.5 | |
| First Primary | |||||||||
| No | 10,669 | 9.8 | 1,652 | 7.6 | 1,232 | 8.6 | 934 | 7.7 | <0.001 |
| Yes | 98,247 | 90.2 | 20,056 | 92.4 | 13,011 | 91.4 | 11,241 | 92.3 | |
|
2007-2011
Summary County Variables |
median | median | median | median | p | ||||
| Median Household Income (in thousands) |
56.27 | 61.82 | 57.58 | 61.11 | <0.001 | ||||
| % of women with <12 years education |
42.56 | 37.38 | 39.60 | 36.88 | <0.001 | ||||
Association of CPM with oncologic and demographic factors in all women with unilateral breast cancer
Younger age, white race, reconstruction, later year of diagnosis, lower stage, smaller tumor size, node negative status, first primary cancer, higher median county income and increased county percentage of women with greater than 12 years of education all independently increased the odds of CPM (Table 4). Significantly higher odds of CPM were found in younger patients (O.R. 7.25, 95% C.I. 6.68-7.88 in patients 18-35). White race was also associated with higher odds of CPM when compared with Black race (O.R. 2.17, 95% C.I. 2.04-2.30). Patients who were reconstructed had significantly higher odds of CPM compared with those that were not reconstructed (O.R. 2.79, 95% C.I. 2.70-2.88). There was no significant difference in odds of CPM based on lymph node status or radiation use.
Table 4.
Results from multiple logistic regression model for odds of CPM among all patients (n=157,042)
| Factor | OR | 95% CI | p |
|---|---|---|---|
| Age, years | |||
| 18-35 | 7.25 | 6.68-7.88 | <0.0001 |
| 36-55 | 4.05 | 3.81-4.30 | |
| 56-70 | 2.28 | 2.14-2.42 | |
| 71-80 | 1.00 | Referent | |
| Race | |||
| White | 2.17 | 2.04-2.30 | <0.0001 |
| Black | 1.00 | Referent | |
| Native American | 1.78 | 1.41-2.24 | |
| Asian American | 0.89 | 0.82-0.97 | |
| Other or Unknown | 1.70 | 1.37-2.12 | |
| Year of Diagnosis | 1.16 | 1.15-1.16 | <0.0001 |
| Reconstruction | |||
| No | 1.00 | Referent | <0.0001 |
| Yes | 2.79 | 2.70-2.88 | |
| Stage | |||
| I | 1.17 | 1.08-1.27 | <0.0001 |
| II | 1.11 | 1.06-1.16 | |
| III | 1.00 | Referent | |
| Tumor Size | |||
| <1 | 1.10 | 1.04-1.16 | <0.0001 |
| 1-1.9 | 1.15 | 1.10-1.20 | |
| 2-4.9 | 0.97 | 0.92-1.03 | |
| 5+ | 1.00 | Referent | |
| Unknown | 1.31 | 1.15-1.49 | |
| Tumor Grade | |||
| I | 1.06 | 0.92-1.19 | <0.0001 |
| II | 1.00 | 0.89-1.14 | |
| III | 0.97 | 0.85-1.10 | |
| IV | 1.00 | Referent | |
| Unknown | 1.26 | 1.10-1.45 | |
| Node Status | |||
| Negative | 1.00 | Referent | 0.004 |
| Positive | 0.93 | 0.89-0.98 | |
| Unknown | 0.43 | 0.15-1.19 | |
| ER Status | |||
| Negative | 1.00 | Referent | 0.08 |
| Positive | 0.95 | 0.90-0.99 | |
| Borderline/Unknown | 0.97 | 0.85-1.11 | |
| PR Status | |||
| Negative | 1.00 | Referent | 0.15 |
| Positive | 1.03 | 0.98-1.07 | |
| Borderline/Unknown | 0.93 | 0.83-1.05 | |
| Radiation | |||
| No | 1.00 | Referent | 0.002 |
| Yes | 1.01 | 0.97-1.05 | |
| Unknown | 0.85 | 0.78-0.93 | |
| First Primary | |||
| No | 0.95 | 0.90-1.00 | 0.034 |
| Yes | 1.00 | Referent | |
| Median County Household Income (in thousands) |
1.01 | 1.01-1.01 | <0.0001 |
| Average County % of women with <12 years education |
0.99 | 0.99-1.00 | 0.0001 |
| Registry | - | 17 df | <0.0001 |
Association of CPM with oncologic and demographic factors in women with unilateral breast cancer who were reconstructed
Among patients who had reconstruction, younger age, White race, and later year of diagnosis continued to be independently associated with significantly higher odds of CPM (Table 5). In addition, patients who had implant-based reconstruction had significantly higher odds of undergoing CPM when compared with patients undergoing autologous tissue reconstruction (O.R. 1.38, 95% C.I. 1.31-1.47).
Table 5.
Results from multiple logistic regression model for odds of CPM among reconstructed patients (n=33,883)
| Factor | OR | 95% CI | p |
|---|---|---|---|
| Age, years | |||
| 18-35 | 6.57 | 5.43-7.94 | <0.0001 |
| 36-55 | 3.79 | 3.19-4.49 | |
| 56-70 | 2.13 | 1.79-2.53 | |
| 71-80 | 1.00 | Referent | |
| Race | |||
| White | 1.98 | 1.80-2.19 | <0.0001 |
| Black | 1.00 | Referent | |
| Native American | 1.50 | 0.96-2.35 | |
| Asian American | 0.95 | 0.82-1.10 | |
| Other or Unknown | 1.85 | 1.29-2.64 | |
| Year of Diagnosis | 1.14 | 1.13-1.15 | <0.0001 |
| Stage | |||
| I | 1.19 | 1.04-1.36 | 0.028 |
| II | 1.11 | 1.02-1.20 | |
| III | 1.00 | Referent | |
| Tumor Size | |||
| <1 | 0.94 | 0.86-1.03 | <0.0001 |
| 1-1.9 | 1.06 | 0.98-1.14 | |
| 2-4.9 | 0.84 | 0.76-0.93 | |
| 5+ | 1.00 | Referent | |
| Unknown | 1.00 | 0.73-1.37 | |
| Tumor Grade | |||
| I | 1.01 | 0.81-1.26 | 0.001 |
| II | 0.96 | 0.78-1.19 | |
| III | 0.97 | 0.78-1.20 | |
| IV | 1.00 | Referent | |
| Unknown | 1.21 | 0.96-1.53 | |
| Node | |||
| Negative | 1.00 | Referent | 0.63 |
| Positive | 0.97 | 0.90-1.05 | |
| Unknown | 0.54 | 0.06-4.77 | |
| ER Status | |||
| Negative | 1.00 | Referent | 0.004 |
| Positive | 0.88 | 0.81-0.95 | |
| Borderline/Unknown | 1.03 | 0.81-1.30 | |
| PR Status | |||
| Negative | 1.00 | Referent | 0.57 |
| Positive | 1.00 | 0.93-1.08 | |
| Borderline/Unknown | 0.89 | 0.72-1.11 | |
| Radiation | |||
| No | 1.00 | Referent | 0.14 |
| Yes | 0.98 | 0.91-1.05 | |
| Unknown | 0.86 | 0.74-1.00 | |
| First Primary | |||
| No | 0.90 | 0.82-0.98 | 0.018 |
| Yes | 1.00 | Referent | |
| Median County Household Income (in 1,000s) |
1.01 | 1.01-1.02 | <0.0001 |
| Average County % of women with <12 years education |
1.01 | 1.00-1.01 | 0.004 |
| Registry | - | 17 df | <0.0001 |
| Type of Reconstruction | |||
| Tissue | 1.00 | Referent | <0.0001 |
| Implant | 1.38 | 1.31-1.47 | |
| Combined | 1.10 | 1.01-1.19 | |
| Not otherwise specified | 1.39 | 1.30-1.49 |
DISCUSSION
In this study, we use the updated SEER database to report recent trends in CPM use, and to identify key demographic and oncologic factors associated with higher odds of CPM. Also explored are breast reconstruction trends in this patient population. Previous studies have performed similar analyses on CPM trends using the SEER database though with data that is currently almost a decade old7,8. A majority of the studies on this subject have not explored the role reconstruction plays alongside observed CPM trends. To our knowledge, no studies have specifically delved into differences in odds of CPM based on reconstructive method. Understanding the relationship between CPM and reconstruction method should provide additional insight into the role played by breast reconstruction. This information can then be used to help guide discussions with patients, on expected outcomes of unilateral and bilateral breast reconstruction, while addressing potential misconceptions related to reconstruction.
Throughout our analyses, four main factors were associated with significantly higher odds of CPM – younger age, White race, year of diagnosis, and reconstruction. Although lower tumor stage was associated with higher odds of CPM in the whole group and the subset of reconstructed patients, the contribution of this factor was less marked. Other oncologic factors such as lymph node status and radiation delivery did not strongly associate with CPM.
The lifetime risk of contralateral breast cancer in breast cancer patients is approximately 3-4%12. Morrow et al have suggested that the survival benefit of CPM is minimal, likely due to the small risk of contralateral breast cancer4. Furthermore, a study by Tuttle et al showed that breast cancer patients have a tendency to overestimate their risk of contralateral breast cancer. Using a survey approach in 74 patients, they found that patients estimate a mean 10-year risk of contralateral breast cancer of over 30%13. Similarly, Rosenberg et al have shown that non-carriers of a breast cancer gene mutation estimated the 5-year risk of contralateral breast cancer at over 15%14. This misperception of risk may contribute to patients’ choices for CPM14. Interestingly however, Tuttle et al did not find a significant difference in the perceived risk of contralateral breast cancer reported by patients who had CPM and those that did not. In our study we cannot account for patients’ beliefs about risk of contralateral breast cancer. However we did find that primarily demographic factors, and not oncologic factors, are associated with higher odds of CPM. Of note, our finding that higher tumor stage does not associate with significantly higher odds of CPM, suggests that perceptions of contralateral breast cancer risk may not be related to tumor severity or other oncologic factors. Fear of disease recurrence in the absence of clinical indications has been shown to be a strong motivator for decisions to undergo CPM15-17.
Younger patient age may be associated with a higher perceived risk for contralateral breast cancer, although a difference between perceived risks based on patient age has not been confirmed in the literature to our knowledge. In the survey administered by Tuttle et al13, patients who underwent CPM were younger than those that did not, although this only approached statistical significance (p = 0.09). They found no significant difference in age when stratifying patients based on low or high perceived risk. Our analysis also shows that recent year of diagnosis is associated with higher odds of CPM, similar to trends shown by others7,8. In spite of evidence suggesting overuse of CPM, low contralateral cancer risk, and minimal survival benefit in early stage unilateral breast cancer patients, its use has continued to increase as recently as 20104,12. As even more recent data becomes available, it will be important to understand how findings from published literature have affected patterns of CPM use.
The role of reconstruction in the decision to undergo CPM is receiving increasing attention in the literature. A study of patient and surgeon characteristics associated with CPM showed that patients with female surgeons had three times the odds of receiving CPM as patients with male surgeons in a single healthcare system2. Alderman et al found that female surgeons are also more likely to refer their patients to plastic surgeons to discuss reconstruction18. Therefore, it is possible that decisions to undergo CPM and reconstruction are related, especially in patients with female surgeons. In a survey of over 200 CPM patients, Soran et al showed that in addition to concerns for development of contralateral breast cancer, the availability of reconstruction was cited as a reason for undergoing CPM9. The possible link between decisions to undergo CPM and decisions for breast reconstruction are alluded to in a retrospective study of 446 mastectomy patients including 174 CPM patients performed by Pinell-White et al, which showed that women who underwent CPM were more likely to pursue breast reconstruction10.
Our study uses a large cohort of patients from the SEER database to confirm a strong relationship between CPM use and breast reconstruction. We found that the increased odds of CPM among reconstructed patients approaches 2.8, and only young patient age has a stronger association with odds of CPM. These findings are confirmed by a recent study by Ashfaq et al who found similarly elevated odds of CPM in reconstructed patients (O.R. 3.6)19. The reason for the difference in odds of CPM described by their study and ours is likely due to a difference in the years included, as ours includes a longer and more recent time span. Adding to the findings provided by Ashfaq et al, we perform a separate analysis of odds of CPM among reconstructed patients, to identify whether a specific reconstruction method is associated with higher odds of CPM. In our analysis, we found that odds of CPM were significantly different based on method of reconstruction, suggesting a complex relationship between CPM and reconstruction. Patients with implant reconstructions had a higher odds of CPM compared to patient who underwent autologous reconstruction. The trend towards increasing use of implant reconstructions in bilateral mastectomy patients, not limited to CPM, was highlighted by Albornoz et al using the National Inpatient Sample20. It is possible that patients who desire implant-based reconstruction rather than autologous reconstruction choose to undergo CPM based on misconceptions about the ability to obtain symmetry after unilateral implant-based breast reconstruction. It is also possible that patients who desire CPM may not be candidates for bilateral reconstruction with autologous tissue. Achieving symmetry with a unilateral implant based reconstruction is possible but can be challenging, especially in cases with a significantly ptotic contralateral breast. Maneuvers used to improve on symmetry include contralateral mastopexy, reduction or an augmentation with a smaller implant than is used for the reconstruction. The recent introduction of form stable anatomic gel implants to the United States market may help further improve on our ability to achieve symmetry with implants in unilateral mastectomies.
The association between CPM and breast reconstruction does not indicate whether the decision to undergo CPM is affected by a primary desire to undergo reconstruction, or whether the decision to undergo reconstruction is affected by a primary desire to undergo CPM. In our experience, decision-making related to the surgical management of breast cancer may involve several patient visits with the surgical oncologist and plastic surgeon; patients must choose among a variety of options including breast conservation therapy, unilateral mastectomy without reconstruction, unilateral mastectomy with reconstruction, mastectomy with CPM, or mastectomy with CPM and reconstruction. It is likely that the availability of reconstruction to restore the final chest wall appearance, in addition to perceived risk of contralateral breast cancer, has provided an incentive for patients to undergo bilateral mastectomy. This would be consistent with the nearly parallel, upward trends in CPM and reconstruction rates observed (figure 1). By demonstrating that the method of reconstruction is associated with odds of CPM, we are able to uniquely show that not only the decision to undergo reconstruction, but also the decision of which reconstructive method to undergo deserves attention when counseling patients. This suggests that plastic surgeons may have a palpable role in guiding patients during the decision-making process, both with respect to CPM and reconstruction.
This study has a number of limitations. With use of the SEER database, granular information which provides a rationale for treatment strategies or choices stemming from issues such as patient education about their disease, access to a multidisciplinary cancer team, challenges associated with establishing an initial diagnosis, and family history are unknown. Also not possible to account for with a large database are patient factors, such as level of anxiety, which play an important role in the decisions patients make for cancer therapy. Of note, breast reconstruction in this database is limited to immediate and early delayed reconstruction; as such all delayed reconstruction performed later than 4 months after mastectomy are not accounted for and reconstruction rates are likely underestimated as a result. The strength of this study however, stems from the large sample size and diverse population which supports the generalizability of our findings. In our analysis, we were unable to adjust for factors which may confer a higher risk of contralateral breast cancer, such as BRCA status or family history.
CPM rates are on the rise in spite of concerns about its oncologic benefits. Alongside the upward trend of CPM rates is an increase in the utilization of breast reconstruction among CPM patients. The importance of understanding the relationship between CPM and reconstruction cannot be overstated. CPM alone poses a risk for significant complications21, as does bilateral breast reconstruction following bilateral mastectomy22. Additionally, there is a significant economic burden associated with CPM and bilateral reconstruction, as opposed to a unilateral mastectomy with reconstruction. As we strive to provide our patients with the optimal oncologic care, we must also be mindful of the risks of aggressive surgery while taking into account patient’s desires. With the potential influence of reconstruction on choices made for CPM, plastic surgeons should play an active role, alongside other members of the oncologic team, in helping educate patients about reconstruction to ensure that decisions are made based on accurate information.
Acknowledgments
Support for this study was provided in part by grants from the Plastic Surgery Foundation (to A.O.M) and by a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120) (to K.C.C.).
Footnotes
To be presented at Plastic Surgery The Meeting, October 10 through 14, 2014, in Chicago, Illinois
Disclosure Statement: None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript
REFERENCES
- 1.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29:2158–64. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- 2.Arrington AK, Jarosek SL, Virnig BA, et al. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol. 2009;16:2697–2704. doi: 10.1245/s10434-009-0641-z. [DOI] [PubMed] [Google Scholar]
- 3.Jones NB, Wilson J, Kotur L, et al. Contralateral prophylactic mastectomy for unilateral breast cancer: An increasing trend at a single institution. Ann Surg Oncol. 2009;16:2691–2696. doi: 10.1245/s10434-009-0547-9. [DOI] [PubMed] [Google Scholar]
- 4.Morrow M. Prophylactic mastectomy of the contralateral breast. The Breast. 2011;S3:S108–10. doi: 10.1016/S0960-9776(11)70306-X. [DOI] [PubMed] [Google Scholar]
- 5.Rosen PP, Groshen S, Kinne DW, Norton L. Factors influencing prognosis in node negative breast carcinoma: analysis of 767 T1N0M0/T2N0M0 patients with long term follow-up. J Clin Oncol. 1993;11:2090–100. doi: 10.1200/JCO.1993.11.11.2090. [DOI] [PubMed] [Google Scholar]
- 6.Fayanju OM, Stoll CR, Fowler S, Colditz GA, Margenthaler JA. Contralateral prophylactic mastectomy after unilateral breast cancer: A systematic review and meta-analysis. Ann Surg. 2014 Jun 19; doi: 10.1097/SLA.0000000000000769. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27:1362–7. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 8.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25:5203–9. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 9.Soran A, Kamali Polat A, Johnson R, McGuire KP. Increasing trend of contralateral prophylactic mastectomy: what are the factors behind this phenomenon? Surgeon. 2014 doi: 10.1016/j.surge.2014.02.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Pinell-White XA, Kolegraff K, Carlson GW. Predictors of contralateral prophylactic mastectomy and the impact on breast reconstruction. Ann Plast Surg. 2014 doi: 10.1097/SAP.0000000000000099. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology, and End Results Program. National Cancer Institute; [Accessed 3 May, 2014]. Overview of the SEER Program. http://seer.cancer.gov/about/overview.html. [Google Scholar]
- 12.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003;56:1038–45. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 13.Abbott A, Rueth N, Pappas-Varco S, et al. Perceptions of contralateral breast cancer: an overestimation of risk. Ann Surg Oncol. 2011;18:3129–36. doi: 10.1245/s10434-011-1914-x. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SM, Tracy MS, Meyer ME, et al. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Ann Intern Med. 2013;159:373–81. doi: 10.7326/0003-4819-159-6-201309170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi M, Hunt KK, Arun BK, et al. Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer Prev Res (Phila) 2010;3:1026–1034. doi: 10.1158/1940-6207.CAPR-09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brewster AM, Parker PA. Current knowledge on contralateral prophylactic mastectomy among women with sporadic breast cancer. Oncologist. 2011;16:935–941. doi: 10.1634/theoncologist.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley ST, Jagsi R, Morrow M, et al. Social and clinical determinants of contralateral prophylactic mastectomy. JAMA Surg. 2014 May 21; doi: 10.1001/jamasurg.2013.5689. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alderman AK, Hawley ST, Waljee J, Morrow M, Katz SJ. Correlates of referral practices of general surgeons to plastic surgeons for mastectomy reconstruction. Cancer. 2007;109:1715–20. doi: 10.1002/cncr.22598. [DOI] [PubMed] [Google Scholar]
- 19.Ashfaq A, McGhan LJ, Pockaj BA, et al. Impact of Breast Reconstruction on the Decision to Undergo Contralateral Prophylactic Mastectomy. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-3712-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 21.Miller ME, Czechura T, Martz B, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Ann Surg Oncol. 2013;20:4113–4120. doi: 10.1245/s10434-013-3108-1. [DOI] [PubMed] [Google Scholar]
- 22.Osman F, Saleh F, Jackson TD, Corrigan MA, Cil T. Increased postoperative complications in bilateral mastectomy patients compared to unilateral mastectomy: an analysis of the NSQIP database. Ann Surg Oncol. 2013;20:3212–3217. doi: 10.1245/s10434-013-3116-1. [DOI] [PubMed] [Google Scholar]