Botulinum toxin is a mainstay therapy for dystonia. Formulations available are three types of botulinumtoxinA and one type of botulinumtoxinB.1 Antibodies can develop against the toxin, leading to treatment failure. IncobotulinumtoxinA (Xeomin; Merz Pharmaceuticals GmbH, Frankfurt, Germany) is differentiated from other types of botulinumtoxinA preparations by being free from complexing proteins, speculated to make the product less antigenic.2
Methods
We report on a patient with musician's cramp with good therapeutic response to incobotulinumtoxinA after there was a loss of clinical benefit in the patient and a negative frontalis test with onabotulinumtoxinA (BOTOX; Allergan, Inc., Irvine, CA) and rimabotulinumtoxinB (Myobloc/Neurobloc; Solstice Neurosciences, San Francisco, CA). Though there were different injectors, the supervising attendings were consistent and electromyography (EMG) and ultrasound (US) were utilized.
Results
A 65‐year‐old man had musician's cramp since age 30, with left‐hand fourth finger metacarpophalangeal joint flexion and interphalangeal joint extension on pressing violin strings (see Video). His treatment course is described in Table 1. He received onabotulinumtoxinA, mostly into the second and third lumbricals. He initially reported fair benefit (20%–50%), using a self‐reported visual analog scale ranging from 0% (no improvement) to 100% (normal use). Some variability between treatment cycles was noted in magnitude and duration of responses, including postinjection weakness. The injection interval was determined by the patient's symptoms, as well as need for high‐level performance. Between the sixth to mid‐seventh years of treatment, the benefit reached 50% to 60%. He then skipped injections for 6 months because he was doing well. After resuming at the previously effective dose of onabotulinumtoxinA, there was 0% benefit and no weakness, despite injection at a higher dose. Frontalis testing with a single 15‐unit dose injection showed resistance to onabotulinumtoxinA. He was switched to rimabotulinumtoxinB from the eighth to the mid‐ninth year of treatment, with 10% to 35% benefit. Late in the ninth year of treatment, he reported 0% benefit and no weakness with doses up to 1,500 units. Frontalis testing with a 500‐unit dose injection of rimabotulinumtoxinB showed resistance. Frontalis testing with a 15‐unit dose injection of incobotulinumtoxinA showed a positive response (Fig. 1). He was switched to incobotulinumtoxinA and has had 50% to 60% benefit in four cycles over 1.5 years and has been able to continue playing as a professional violinist.
Table 1.
Treatment course with botulinum toxin over 10 yr
| Date of Treatment | Toxin Type | Dose (Units) | % Weakness | % Benefit |
|---|---|---|---|---|
| August 2001 | Ona | 45 | 0 | 50 |
| December 2001 | 50 | 50 | 20 | |
| February 2002 | 65 | 30 | 20 | |
| June 2002 | 50 | 20 | 50 | |
| August 2002 | 45 | 20 | 50 | |
| December 2002 | 35 | 25 | 50 | |
| July 2003 | 40 | |||
| April 2004 | 30 | 60 | 20 | |
| December 2004 | 20 | 50 | 40 | |
| January 2006 | 15 | 10 | 10 | |
| July 2006 | 20 | 40 | 60 | |
| August 2007 | 15 | 35 | 55 | |
| December 2007 | 17.5 | 20 | 50 | |
| June 2008 | 22.5 | 0 | 0 | |
| December 2008 | 30 | 0 | 0 | |
| 15 unitsFrontalis test | Negative | |||
| January 2009 | Rima | 900 | 10 | 20 |
| March 2009 | 1000 | 70 | 25 | |
| September 2009 | 1000 | 25 | 25 | |
| December 2009 | 600 | 15 | 10 | |
| March 2010 | 700 | 10 | 10 | |
| September 2010 | 800 | 10 | 35 | |
| November 2010 | 1000 | 0 | 0 | |
| January 2011 |
500 unitsFrontalis test 1500 |
Negative | ||
| February 2011 | Inco |
15 units Frontalis test 17 |
Positive | |
| May 2011 | 20 | 25 | 55 | |
| December 2011 | 20 | 50 | 60 | |
| July 2012 | 17.5 | 10 | 60 | |
| January 2013 | 16 | 25 | 50 |
Figure 1.
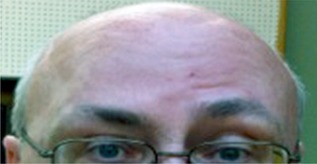
Positive frontalis test. Asymmetric brow raising resulting from weakness of the right frontalis muscle after injection with incobotulinumtoxinA.
Discussion
Musician's cramp is a task‐specific dystonia, with patients typically unable to continue careers as professional musicians.3 Botulinum toxin injection is safe and effective in the long‐term treatment of patients with focal hand dystonia.2 Response to subsequent botulinum toxin injections is reliably predicted by the frontalis test,4 which guided the decision to switch botulinum toxin formulations twice in this case. The frontalis test is a sensitive biological test for immunoresistance, correlating well with the presence of neutralizing antibodies detected by the in vivo mouse protection bioassay and western blotting assay.4
IncobotulinumtoxinA has not been associated with development of neutralizing antibodies, possibly because of the absence of complexing proteins.5, 6 A patient with poststroke spasticity responded well to incobotulinumtoxinA, after being a secondary nonresponder to onabotulinumtoxinA, as evidenced by the extensor digitorum brevis test.7 Neither this patient nor our patient had laboratory testing for antibodies; but, in both cases, resistance was demonstrated by frontalis testing, which is more clinically valuable. Whereas the development of antibodies may be associated with the complexing proteins, the neutralizing antibodies detected by lab assays are reported to be against the toxin serotype (A or B), rather than the complexing proteins. In such a case, however, it would seem unlikely that incobotulinumtoxinA would restore response.
The long interval between the last onabotulinumtoxinA and the initiation of incobotulinumtoxinA might be relevant. We acknowledge the potential for a decline in resistance with time and/or the risk of redevelopment of immunoresistance, and that it is not known whether the patient would have responded again to onabotulinumtoxinA or rimabotulinumtoxinB. When there is immunoresistance to onabotulinumtoxinA, after a long interval, the person may respond again for at least one cycle, but then often quickly redevelops immunoresistance.8 Antibody level may drop and then return. However, if incobotulinumtoxinA is less antigenic, in this situation, the antibodies might not return. Our patient demonstrated steady and continued response to incobotulinumtoxinA for more than 1.5 years.
We no longer test for antibodies in our patients showing signs of nonresponse and go directly to frontalis testing. This is because we are interested in clinical responsivity, which the frontalis test directly measures. Patients can become nonresponders even without demonstration of antibodies.
Factors other than neutralizing antibodies can explain treatment failure, such as errors related to toxin preparation, storage or reconstitution, muscle selection, inadequate dosing per injection site, or changes in disease presentation or expectations,7 and injection skill. In this case, however, these factors have been stable and maximal doses have been tried, with US and EMG guidance for adequate muscle localization.
It could be that our patient simply had a fluctuating response, which is not uncommon in musician's dystonia,3 but such patients are usually unable to continue performing. This is important to consider, especially given that the patient's incobotulinumtoxinA dose is significantly less than commonly used equivalent dosing with his previous onabotulinumtoxinA. Recent meta‐analyses show no difference in potencies between onabotulinumtoxinA and incoboutlinumtoxinA.9 More studies are necessary to determine clinically relevant differences in biological activity and potency of the different toxin types.10 EMG showing lack of denervation to confirm the case as a true resistance would have been useful.
In conclusion, this case report suggests that switching to other types of botulinum toxin should be studied further in larger studies given that such a strategy might be considered as a viable treatment option.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
V.F.M.L.R.: 1A, 1B, 1C, 2B, 2C, 3A, 3B
B.I.K: 1A, 1B, 1C, 2B, 2C, 3B
C.L: 1A, 1B, 1C, 2B, 2C, 3B
K.A.: 1A, 1B, 1C, 2B, 2C, 3B
M.H.: 1A, 1B, 1C, 2B, 2C, 3B
Disclosures
Funding Sources and Conflicts of Interest: This work was supported by a training grant from the Dystonia Medical Research Foundation. The authors report no conflicts of interest.
Financial Disclosures for previous 12 months: Dr. Ramos is a federal government employee, working for the National Institutes of Health (NIH). This work was undertaken as part of her official duty. Her research at the NIH is supported by the NIH Intramural Program. Dr. Karp is a federal government employee, working for the NIH. This work was undertaken as part of her official duty. Her research at the NIH is supported by the NIH Intramural Program. Dr. Karp is an investigator on a study that receives research support from Allergan through a Clinical Trials Agreements (CTA) with the NIH. Dr. Lungu is a federal government employee, working for the NIH. This work was undertaken as part of his official duty. Dr. Lungu's research at the NIH is supported by the NIH Intramural Program. Dr. Alter is an employee of Mount Washington Pediatric Hospital and a federal government contractor, working for the NIH. This work was undertaken as part of her official duty at the NIH. She has received consulting honoraria from Allergan and speaking honoraria from Ipsen. She has received royalties from Demos Medical Publishing. Dr. Hallett is a federal government employee, working for the NIH. This work was undertaken as part of his official duty. Dr. Hallett's research at the NIH is largely supported by the NIH Intramural Program. Dr. Hallett serves as Chair of the Medical Advisory Board for, and receives honoraria and funding for travel from, the Neurotoxin Institute. He may accrue revenue on U.S. Patent #6,780,413 B2 (issued: 24 August 2004): Immunotoxin (MAB‐Ricin) for the treatment of focal movement disorders, and US Patent #7,407,478 (issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H‐coil); in relation to the latter, he has received license fee payments from the NIH (from Brainsway) for licensing of this patent. He is on the editorial board of 22 journals and received royalties from publishing from Cambridge University Press, Oxford University Press, John Wiley & Sons, Wolters Kluwer, and Elsevier. He has received honoraria for lecturing from Columbia. Supplemental research funds came from the Kinetics Foundation, for studies of instrumental methods to monitor Parkinson's disease, BCN Peptides, S.A., for treatment studies of blepharospasm, and Medtronics, Inc., for studies of DBS, through Clinical Trials Agreements (CTA) with the NIH.
Supporting information
A video accompanying this article is available in the supporting information here.
Video. Musician's dystonia in the left hand, with fourth finger metacarpophalangeal joint flexion and third interphalangeal joint extension on pressing violin strings.
Acknowledgments
The authors thank Elaine Considine, RN, for her help with coordinating the elements of the manuscript. The authors acknowledge that this manuscript was prepared as part of their official duties as employees of the Department of Health and Human Services (DHHS). The work presented here does not officially express the opinion of the DHHS.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Abrams SB, Hallett M. Clinical utility of different botulinum neurotoxin preparations. Toxicon 2013;67:81–86. [DOI] [PubMed] [Google Scholar]
- 2. Frevert J, Dressler D. Complexing proteins in botulinum toxin type A drugs: a help or a hindrance? Biologics 2010;4:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schuele S, Lederman RJ. Long‐term outcome of focal dystonia in string instrumentalists. Mov Disord 2004;19:43–48. [DOI] [PubMed] [Google Scholar]
- 4. Lungu C, Karp BI, Alter K, Zolbrod R, Hallett M. Long‐term follow‐up of botulinum toxin therapy for focal hand dystonia: outcome at 10 years or more. Mov Disord 2011;26:750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanna PA, Jankovic J. Mouse bioassay versus Western blot assay for botulinum toxin antibodies: correlation with clinical response. Neurology 1998;50:1624–1629. [DOI] [PubMed] [Google Scholar]
- 6. Kanovsky P, Slawek J, Denes Z, et al. Efficacy and safety of treatment with incobotulinum toxin A (botulinum neurotoxin type A free from complexing proteins; NT 201) in post‐stroke upper limb spasticity. J Rehabil Med 2011;43:486–492. [DOI] [PubMed] [Google Scholar]
- 7. Santamato A, Ranieri M, Panza F, et al. Effectiveness of switching therapy from complexing protein‐containing botulinum toxin type A to a formulation with low immunogenicity in spasticity after stroke: a case report. J Rehabil Med 2012;44:795–797. [DOI] [PubMed] [Google Scholar]
- 8. Sankhla C, Jankovic J, Duane D. Variability of the immunologic and clinical response in dystonic patients immunoresistant to botulinum toxin injections. Mov Disord 1998;13:150–154. [DOI] [PubMed] [Google Scholar]
- 9. Jandhyala R. Relative potency of incobotulinumtoxinA vs onabotulinumtoxinA a meta‐analysis of key evidence. J Drugs Dermatol 2012;11:731–736. [PubMed] [Google Scholar]
- 10. Bonaparte JP, Ellis D, Quinn JG, Ansari MT, Rabski J, Kilty SJ. A comparative assessment of three formulations of botulinum toxin A for facial rhytides: a systematic review and meta‐analyses. Syst Rev 2013;2:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video accompanying this article is available in the supporting information here.
Video. Musician's dystonia in the left hand, with fourth finger metacarpophalangeal joint flexion and third interphalangeal joint extension on pressing violin strings.


