Summary
Background
Safe and effective treatments are urgently needed for patients with relapsed/refractory acute myeloid leukaemia (AML). We investigated the efficacy and safety of vosaroxin, a first-in-class anticancer quinolone derivative, plus cytarabine in patients with relapsed/refractory AML.
Methods
VALOR was a phase 3, double-blind, placebo-controlled trial conducted at 101 international sites. Patients were randomised 1:1 to vosaroxin (90 mg/m2 IV days 1,4) plus cytarabine (1 g/m2 IV days 1–5) (vos/cyt) or placebo plus cytarabine (pla/cyt) using a permuted block procedure stratified by disease status, age, and geographic location. All participants were blind to treatment assignment. Primary endpoints were overall survival (OS) and 30- and 60-day mortality. Efficacy analyses were by intention-to-treat; safety analyses included all treated patients. This study is registered at clinicaltrials.gov (NCT01191801).
Findings
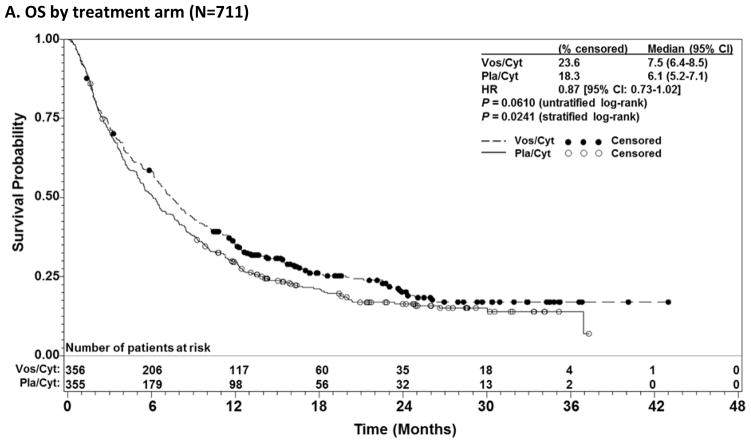
Between December 2010 and September 2013, 711 patients were randomised to vos/cyt (n=356) or pla/cyt (n=355). Median OS was 7·5 months with vos/cyt and 6·1 months with pla/cyt (hazard ratio 0·87; unstratified log-rank p=0·061; stratified p=0·0241) and was supported by a sensitivity analysis censoring for subsequent transplant (6·7 and 5·3 months; p=0·0243). Complete remission (CR) rate was higher with vos/cyt vs pla/cyt (30·1% vs 16·3%, p<0·0001). Early mortality rates were equivalent (vos/cyt vs pla/cyt: 30-day, 7·9% vs 6·6%; 60-day, 19·7% vs 19·4%). Treatment-related deaths occurred at any time in 18 patients (5·1%) with vos/cyt and 8 (2·3%) with pla/cyt. Grade ≥3 adverse events more frequent with vos/cyt included febrile neutropenia (167/355 [47%] vs 117/350 [33%]), stomatitis (54 [15%] vs 10 [3%]), hypokalaemia (52 [15%] vs 21 [6%]), sepsis (42 [12%] vs 18 [5%]), and pneumonia (39 [11%] vs 26 [7%]).
Interpretation
Addition of vosaroxin to cytarabine prolonged survival in patients with relapsed/refractory AML, increasing CR rates with equivalent early mortality. These results support vos/cyt as an option for salvage therapy in AML patients.
Funding
Sunesis Pharmaceuticals
Introduction
The prognosis of patients with relapsed or refractory acute myeloid leukaemia (AML) is poor; median survival is less than 1 year.1,2 High-dose cytarabine monotherapy or cytarabine-based combination regimens are often used as salvage therapy with limited efficacy; in a recent randomised trial, salvage therapy with the investigator’s choice of one of seven commonly used regimens (including high-dose cytarabine) produced complete remission (CR) in 12% of patients and a median survival of 3.3 months, with no significant difference between regimens.3 Toxicity is also a concern, particularly in patients over 60 years of age, who constitute the majority of the AML population.1,4–6
Vosaroxin is an anticancer quinolone derivative that intercalates DNA and inhibits topoisomerase II activity.7 In contrast to traditional topoisomerase inhibitors, vosaroxin is minimally metabolised due to its stable core quinolone structure and is not associated with significant formation of free radicals, reactive oxygen species, or toxic metabolites.7–9 Vosaroxin is not a substrate of P-glycoprotein and can induce apoptosis independent of p53.7,10 Vosaroxin and cytarabine exhibit non-overlapping primary mechanisms of action and demonstrate synergistic antiproliferative activity in preclinical assays.10,11 In a phase 2 study, vosaroxin plus cytarabine demonstrated clinical activity and good tolerability in patients with relapsed/refractory AML, producing a 25% CR rate with low (2·5%) 30-day all-cause mortality.12
These results supported the initiation of a phase 3 trial. The cytarabine dose and schedule selected was based on several factors, including the phase 2 study results, regimens used in recent clinical registration studies,4 results from ex vivo studies,13 and published clinical studies14–17 suggesting that intermediate-dose cytarabine may provide similar clinical benefit with less toxicity than high-dose cytarabine. The phase 3 VALOR (“Vosaroxin and Ara-C combination evaLuating Overall survival in Relapsed/refractory AML”) study was designed to assess whether the addition of vosaroxin to cytarabine confers a survival benefit in patients with relapsed/refractory AML, compared with cytarabine alone.
Methods
Study Design
VALOR was a phase 3, randomised, double-blind, placebo-controlled trial conducted at 101 sites in North America, Europe, South Korea, and Australia/New Zealand. The study protocol was approved by an ethics review board at each participating institution. The study was conducted in accordance with the Declaration of Helsinki.
Patients
Written informed consent was provided by all patients. Eligible patients were ≥18 years of age with a diagnosis of AML, as defined by World Health Organization criteria,18 and were in first relapse or had refractory disease. Patients with acute promyelocytic leukaemia were excluded. Relapse was defined as reemergence of ≥5% leukaemia blasts in bone marrow or ≥1% blasts in peripheral blood 90 days to 24 months after first CR or CR without complete platelet recovery (CRp). Refractory AML was defined as persistent disease ≥28 days after initiation of induction therapy or relapse <90 days after first CR or CRp. All patients must have received prior induction therapy with an anthracycline (or anthracenedione) plus cytarabine; no more than two prior induction cycles were allowed. Additional inclusion criteria included Eastern Cooperative Oncology Group performance status of 0–2 and adequate liver (total bilirubin ≤1·5 × the upper limit of normal [ULN], aspartate aminotransferase ≤2·5 × ULN, alanine aminotransferase ≤2·5 × ULN), renal (serum creatinine ≤2·0 mg/dL) and cardiac (left ventricular ejection fraction ≥40%) function. Patients were excluded if they had received cytarabine-containing treatment at a total dose ≥5 g/m2 within 90 days of randomisation. Other AML therapies were not permitted while the patient was receiving study medication. Hydroxyurea was permitted up to 24 hours prior to randomisation. (See Supplementary Appendix for complete exclusion criteria.)
Randomisation and masking
Patients were assigned to a treatment group using a permuted block randomisation procedure. Randomisation was stratified by disease status (refractory, first relapse at ≥90 days and <12 months, or first relapse at ≥12 months and ≤24 months), age (<60 years or ≥60 years), and geographic location (US sites or non-US sites). The study sponsor and all participants were blinded to treatment group assignment; only the data and safety monitoring board (DSMB) and independent statistics provider (ISP) had access to unblinded data in a controlled and limited manner during the study. Treatment assignments remained blinded until follow-up was completed for the final analysis.
Procedures
Patients were randomised 1:1 to receive vosaroxin (90 mg/m2 in cycle 1 and 70 mg/m2 in subsequent cycles by short [within 10 minutes] intravenous [IV] infusion on days 1 and 4) or placebo in combination with cytarabine (1 g/m2, 2-h infusion on days 1–5) (Supplementary Figure S1). A second induction cycle could be initiated between 14 days and 8 weeks after initiation of cycle 1, at the discretion of the investigator, in patients with residual leukemia upon bone marrow assessment during initial induction, provided all drug-related toxicity had resolved to grade ≤1. Up to two additional cycles could be administered as consolidation therapy in patients who achieved CR or CRp within 12 weeks after therapy initiation, at the discretion of the investigator. Growth factor support and/or transfusions were permitted according to institutional guidelines.
Results of cytogenetic and molecular marker assessments were collected if available at screening, but were not required. Complete blood counts were obtained at least weekly to monitor haematologic recovery. Bone marrow biopsy or aspirate was obtained at screening, approximately day 15 during induction, and at haematologic recovery (to absolute neutrophil count >1000 cells/μL) or by day 57 of induction cycles for response assessment. Safety, including documentation of adverse events (AEs), was assessed at each visit; severity was graded per National Cancer Institute Common Terminology Criteria for Adverse Events, version 4·0.
Outcomes
Primary endpoints were overall survival (OS) and all-cause mortality at 30 and 60 days. The secondary endpoint was CR rate; tertiary endpoints included combined CR rate (CR/CRp/CR with incomplete recovery of platelets or neutrophils [CRi]), overall remission rate (ORR; CR/CRp/CRi plus partial response); event-free survival (EFS), leukaemia-free survival (LFS), rate of post-treatment allogeneic stem cell transplant (alloSCT), and safety/tolerability. Responses were categorised based on revised International Working Group response criteria19 and were reviewed by a blinded, independent review panel.
Statistical analysis
This was an event-driven study incorporating an innovative adaptive trial design that permitted a prespecified, one-time increase in OS events, to ensure adequate statistical power to detect a clinically meaningful survival benefit over a range of potential outcomes. Initial target accrual was 375 OS events in 450 patients, providing 90% power (at two-sided level α=0·05) to detect an improvement in median survival from 5 months in the placebo/cytarabine group to 7 months in the vosaroxin/cytarabine group (hazard ratio [HR], 0·71). A formal interim survival analysis was performed by the ISP after 173 events (approximately 50% of required events) according to predefined statistical procedures stipulated and evaluated by the DSMB. Based on this interim analysis, the DSMB could recommend: 1) study termination based on efficacy or futility; 2) continuation as planned; or 3) a 50% increase in planned OS event accrual (to 562 events), with a corresponding increase in sample size (to 675 patients). After reviewing the interim results, the DSMB recommended option 3.
Primary efficacy analyses were conducted after 562 deaths using an unstratified log-rank test. A stratified log-rank test using factors at randomisation was pre-specified as supportive of efficacy. Because the adaptive trial design resulted in a data-dependent increase in the total number of events, a weighted log-rank statistic was employed for both unstratified and stratified tests to control type I error. The independent statistic at stage 1 (prior to interim analysis) was combined with its independent increment at stage 2 (post-interim analysis) using prespecified weights of √(187/375) (i.e., equal to the square root of the planned proportion of events expected if there were no adaptation in design).20,21 The use of the weighted statistic ensured that the type 1 error α=0·05 would be preserved notwithstanding the adaptive increase in total events. Additional log-rank tests were performed within each stratum defined at randomisation. Kaplan-Meier methods and Cox proportional hazard modeling were used to analyse the time-to-event endpoints, both overall and within subsets of patients defined by randomisation strata. All p values were two-sided.
OS was defined as the time between randomisation and death; surviving patients were censored from the OS analysis at the analysis cutoff date or the last date known to be alive, whichever occurred first. In the primary OS analysis, patients were not censored for administration of subsequent nonprotocol AML therapy, including transplantation. A preplanned sensitivity analysis was performed for OS in which patients were censored at the start of a transplant conditioning regimen; patients with subsequent non-transplant AML therapy were not censored. EFS was defined as the time from randomisation to treatment failure, relapse, or death due to any cause. LFS was defined as the time from CR to relapse or death due to any cause.
Response rates and all-cause mortality rates were calculated for each treatment group and corresponding 95% CIs were calculated using the Clopper-Pearson method. Rates were compared between treatment groups using a chi-square test and by Cochran-Mantel-Haenszel tests stratified by factors used at randomisation, and corresponding 95% CIs were calculated using the normal approximation to the binomial distribution.
The intent-to-treat population consisted of all randomised patients, and was used for the analysis of efficacy endpoints. The safety population comprised all patients who received any amount of study drug.
Role of the funding source
This study was sponsored by Sunesis Pharmaceuticals. Sunesis employees were involved in the trial design, were responsible for all data collection, management, and analysis, and were involved in the writing of the report. The corresponding author had full access to the data and had final responsibility for the decision to submit for publication.
Results
Between December 16, 2010 and September 25, 2013, 711 patients were enrolled and randomised to receive vosaroxin plus cytarabine (vos/cyt; n=356) or placebo plus cytarabine (pla/cyt; n=355) (Supplementary Figure S2). Baseline characteristics were well balanced between treatment arms (Table 1).
Table 1.
Baseline characteristics of patients treated with vosaroxin plus cytarabine or placebo plus cytarabine (N=711)
| Vosaroxin + cytarabine (n=356) | Placebo + cytarabine (n=355) | |
|---|---|---|
|
| ||
| Sex, n (%) | ||
| Male | 202 (56·7) | 192 (54·1) |
| Female | 154 (43·3) | 163 (45·9) |
| Age, yr | ||
| Mean | 61·0 | 60·2 |
| Median (range) | 64 (20–80) | 63 (18–82) |
| Age subgroup*, n (%) | ||
| <60 | 130 (36·5) | 130 (36·6) |
| ≥60 | 226 (63·5) | 225 (63·4) |
| Disease status*, n (%) | ||
| Early relapse† | 127 (35·7) | 129 (36·3) |
| Late relapse‡ | 77 (21·6) | 77 (21·7) |
| Refractory | 152 (42·7) | 149 (42·0) |
| Region*, n (%) | ||
| US | 161 (45·2) | 159 (44·8) |
| Outside US | 195 (54·8) | 196 (55·2) |
| Race, n (%)§ | ||
| White | 253 (71·1) | 242 (68·2) |
| Black | 21 (5·9) | 11 (3·1) |
| Asian | 20 (5·6) | 18 (5·1) |
| Other | 4 (1·1) | 7 (2·0) |
| Multiple | 0 | 3 (0·8) |
| Type of AML, n (%)¶ | ||
| AML not otherwise specified | 188 (52·8) | 180 (50·7) |
| AML with myelodysplasia-related changes | 103 (28·9) | 93 (26·2) |
| AML with recurrent genetic abnormalities | 54 (15·2) | 71 (20·0) |
| Myeloid sarcoma | 2 (0·6) | 1 (0·3) |
| Therapy-related myeloid neoplasm | 9 (2·5) | 10 (2·8) |
| ECOG PS, n (%)# | ||
| 0 | 156 (44·1) | 143 (40·5) |
| 1 | 158 (44·6) | 162 (45·9) |
| 2 | 40 (11·3) | 48 (13·6) |
| Cytogenetic risk, n (%)^ | ||
| Favorable | 7 (2·9) | 9 (3·8) |
| Intermediate | 175 (72·9) | 155 (64·9) |
| Unfavorable | 58 (24·2) | 75 (31·4) |
| Number of prior induction cycles, n (%) | ||
| 1 | 274 (77·0) | 259 (73·0) |
| 2 | 82 (23·0) | 95 (26·8) |
| >2 | 0 | 1 (0·3) |
Pre-planned randomisation strata.
First complete remission duration of 90 days to 12 months.
First complete remission duration of 12 months to 24 months.
Race not reported in 132 patients
Per World Health Organization 2008 criteria.18
ECOG PS missing in 4 patients.
Per National Comprehensive Cancer Network Treatment Guidelines, AML, 2014; cytogenetic risk not available in 232 patients.
AML=acute myeloid leukaemia; ECOG PS=Eastern Cooperative Oncology Group performance status.
Nearly all patients (99%) completed a first induction cycle; 148 (21%) completed a second induction cycle (70 [20%] in the vos/cyt arm and 78 [22%] in the pla/cyt arm). Twenty-seven percent of patients in the vos/cyt arm and 14% in the pla/cyt arm completed at least one consolidation cycle; 13% and 6%, respectively, received two consolidation cycles. Discontinuation prior to maximum allowed treatment was primarily attributed to treatment failure (n=434; 50% vos/cyt; 73% pla/cyt); patient/physician decision (n=76; 13% vos/cyt; 8% pla/cyt); death (n=42; 9% vos/cyt; 3% pla/cyt); or AEs (n=17; 3% vos/cyt; 2% pla/cyt).
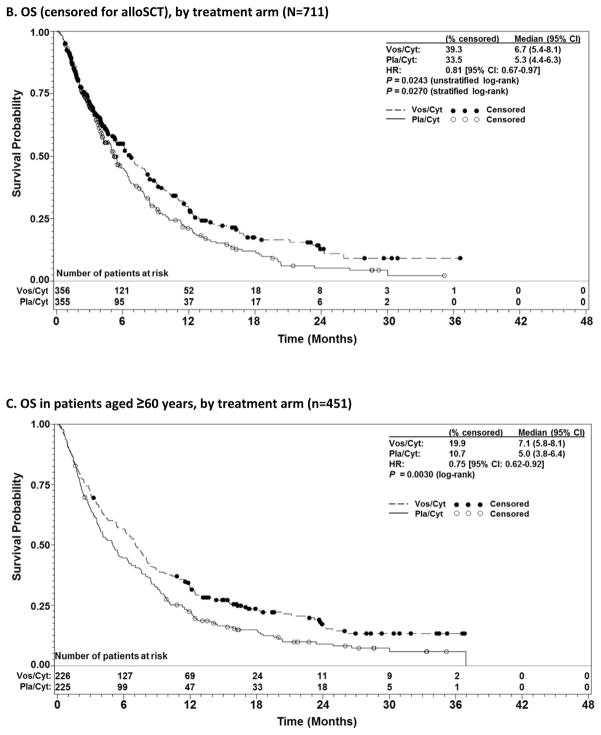
Median OS was 7·5 months (95% CI: 6·4–8·5) for vos/cyt vs 6·1 months (95% CI: 5·2–7·1) for pla/cyt (HR 0·87 [95% CI: 0·73–1·02]; unstratified log-rank p=0·0610) (Figure 1A). Notably, OS was significantly prolonged in a predefined secondary analysis that stratified by factors used in randomisation (stratified log-rank p=0·0241). In a predefined analysis censoring for subsequent alloSCT, median OS was improved with vos/cyt (6·7 vs 5·3 months; HR 0·81 [95% CI: 0·67–0·97]; p=0·0243; stratified p=0·0270) (Figure 1B). Overall, 210 patients (29·5%) underwent alloSCT following the study. Transplant rates were comparable between treatment arms (30·1% vos/cyt; 29·0% pla/cyt), but markedly higher in younger patients (119/260 [45·8%] <60 years; 91/451 [20·2%] ≥60 years). Median time to transplantation was similar between arms (3.3 months [95% CI 3.0, 4.0] with vos/cyt and 3.1 months [95% CI 2.8, 3.5] with pla/cyt). Any subsequent non-protocol AML therapy (including transplant) was administered in 68.6% of patients (80.0% of patients <60 years and 62.1% of patients ≥60 years; Supplementary Table S1).
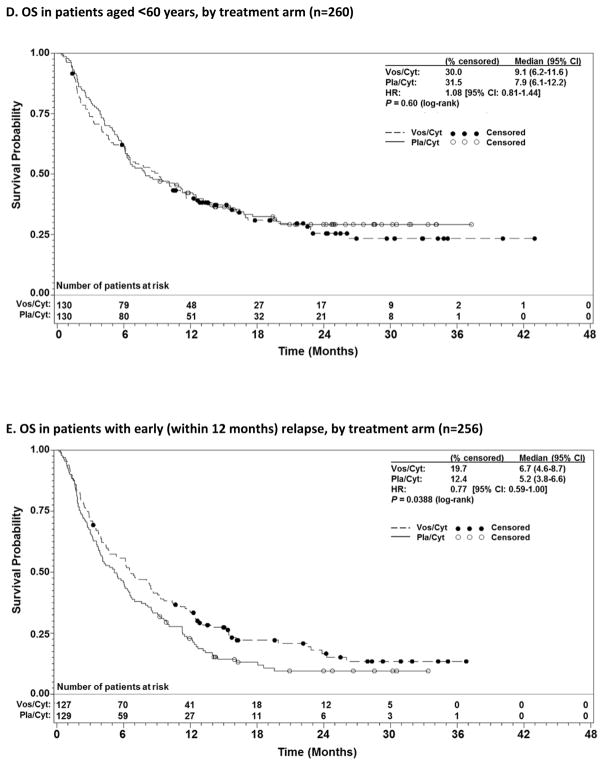
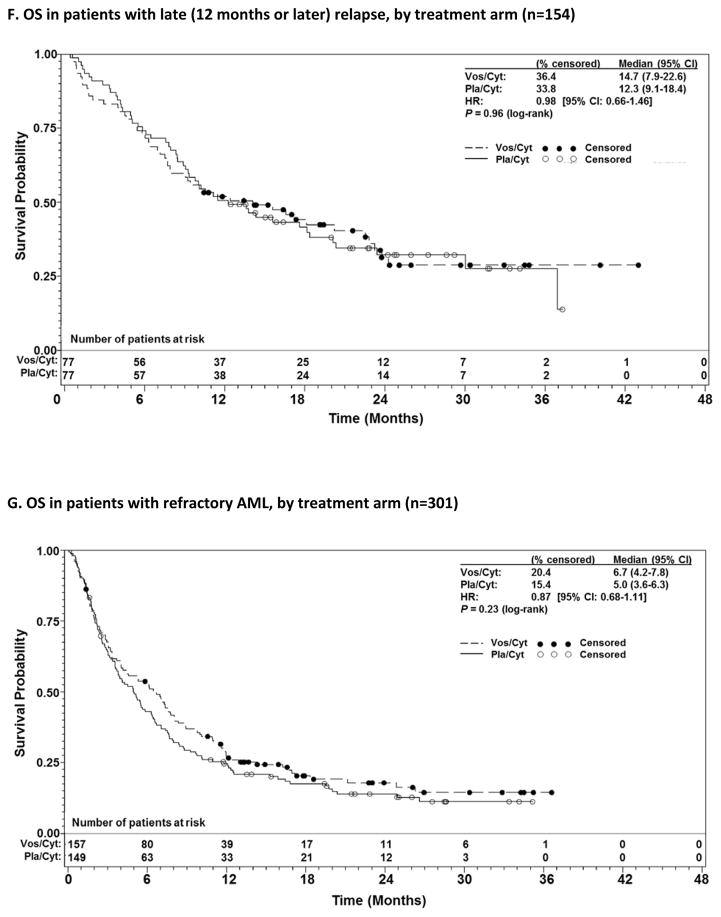
Figure 1.
Overall survival (OS) in patients with relapsed and refractory acute myeloid leukaemia treated with vosaroxin or placebo in combination with cytarabine, for all patients (A), censored for allogeneic stem cell transplant (alloSCT) (B), patients aged ≥60 years vs <60 years (C, D); and early relapsed vs late relapsed vs refractory patients (E, F, G).
CI= confidence interval; HR=hazard ratio; Pla/Cyt=placebo+cytarabine; Vos/Cyt=vosaroxin+cytarabine.
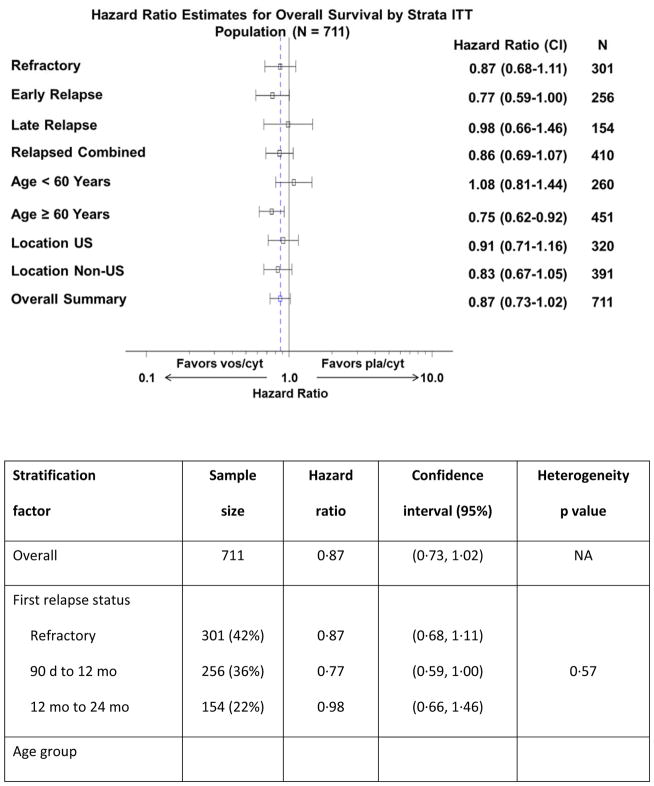
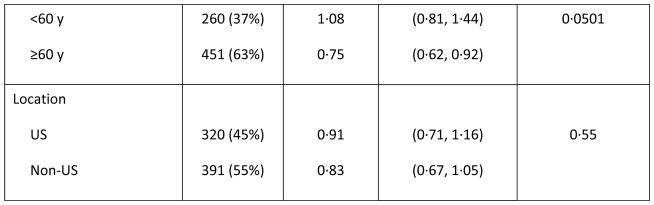
Prespecified subgroup analyses according to randomisation strata demonstrated that OS benefit with vosaroxin was greatest in patients age ≥60 years (7·1 months vs 5·0 months with pla/cyt; HR 0·75; p=0·0030) (Figure 1C) and those with early relapse (6·7 months vs 5·2 months with pla/cyt; HR 0·77; p=0·0388) (Figure 1E). Median OS was not significantly different between treatment arms in patients age <60 years (HR 1·08; p=0·60) (Figure 1D), nor in patients with late relapse (HR 0·98; p=0·96) (Figure 1F) or refractory disease (HR 0·87; p=0·23) (Figure 1G). HRs for OS by subgroup are presented in Figure 2. The heterogeneity p value for treatment by subgroup interaction approached significance for the age group stratum (p=0·0501). In post-hoc, exploratory analyses, OS benefit was observed in patients with unfavorable cytogenetic risk (Supplementary Table S2) and FLT3 mutations (Supplementary Table S3).
Figure 2.
Forest plot of overall survival in subgroups of patients treated with vosaroxin or placebo in combination with cytarabine.
The vertical dashed line indicates the hazard ratio in the overall population. CI= confidence interval; ITT=intention-to-treat.
CR was achieved in 30·1% of patients treated with vos/cyt vs 16·3% treated with pla/cyt (p<0·0001) (Table 2). ORR was 37·9% and 18·9% for the vos/cyt and pla/cyt treatment arms, respectively (a difference of 19.0% [95% CI: 12.6, 25.5; p<0·0001). Prespecified subgroup analyses demonstrated significantly higher response rates for vos/cyt-treated patients across all randomisation strata except for those less than 60 years of age (Table 2), with the most pronounced improvement in patients aged ≥60 years (CR: 31·9% for vos/cyt vs 13·8% for vos/pla; p<0·0001). A higher proportion of patients in the vos/cyt arm achieved CR with study drug prior to transplant (48% [51/107] vos/cyt; 32% [33/103] pla/cyt). In patients with CR, median LFS was 11·0 months with vos/cyt vs 8·7 months with pla/cyt (HR 0·89 [95% CI: 0·57–1·40]; p=0·63). Median EFS was significantly prolonged in vos/cyt-treated patients: 1·9 months vs 1·3 months in patients receiving pla/cyt (HR 0·67 [95% CI: 0·57–0·79]; p<0·001).
Table 2.
Response in all patients and pre-defined subgroups by treatment arm (N=711)
| CR, % (95% CI) | Combined CR (CR/CRp/CRi), % (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| vos/cyt | pla/cyt | difference | p value | vos/cyt | pla/cyt | difference | p value | |
| Overall | 30·1 (25·3, 35·1) | 16·3 (12·6, 20·6) | 13.7 (7.6, 19.8) | <0·0001 | 37·1 (32·0, 42·3) | 18·6 (14·7, 23·0) | 18.5 (12.0, 24.9) | <0·0001 |
| By age, years | ||||||||
| <60 | 26·9 (19·5, 35·4) | 20·8 (14·2, 28·8) | 6.2 (−4.2, 16.5) | 0·24 | 34·6 (26·5, 43·5) | 23·1 (16·1, 31·3) | 11.5 (0.6, 22.5) | 0·0400 |
| ≥60 | 31·9 (25·8, 38·4) | 13·8 (9·6, 19·0) | 18.1 (10.5, 25.6) | <0·0001 | 38·5 (32·1, 45·2) | 16·0 (11·5, 21·5) | 22.5 (14.5, 30.4) | <0·0001 |
| By disease status | ||||||||
| Early relapse* | 27·6 (20·0, 36·2) | 12·4 (7·3, 19·4) | 15.2 (5.5, 24.8) | 0·0024 | 34·6 (26·4, 43·6) | 15·5 (9·7, 22·9) | 19.1 (8.8, 29.5) | 0·0004 |
| Late relapse† | 53·2 (41·5, 64·7) | 33·8 (23·4, 45·4) | 19.5 (4.1, 34.8) | 0·0148 | 59·7 (47·9, 70·8) | 36·4 (25·7, 48·1) | 23.4 (8.0, 38.7) | 0·0037 |
| Refractory | 20·4 (14·3, 27·7) | 10·7 (6·3, 16·9) | 9.7 (1.5, 17.8) | 0·0210 | 27·6 (20·7, 35·5) | 12·1 (7·3, 18·4) | 15.6 (6.7, 24.4) | 0·0007 |
| By region | ||||||||
| US | 28·0 (21·2, 35·6) | 14·5 (9·4, 20·9) | 13.5 (4.7, 22.3) | 0·0032 | 35·4 (28·0, 43·3) | 17·0 (11·5, 23·7) | 18.4 (9.0, 27.8) | 0·0002 |
| Outside US | 31·8 (25·3, 38·8) | 17·9 (12·8, 23·9) | 13.9 (5.5, 22.4) | 0·0014 | 38·5 (31·6, 45·7) | 19·9 (14·5, 26·2) | 18.6 (9.7, 27.4) | <0·0001 |
First complete remission duration of 90 days to 12 months.
First complete remission duration of 12 months to 24 months.
CR=complete remission; CRi=CR with incomplete recovery of platelets or neutrophils; CRp=CR with incomplete recovery of platelets; cyt=cytarabine; pla=placebo; vos=vosaroxin
Thirty-day and 60-day all-cause mortality was similar in the two treatment arms (30-day: 7·9% vs 6·6%; 60-day: 19·7% vs 19·4% for vos/cyt vs pla/cyt, respectively). Grade 3 and higher AEs were primarily related to myelosuppression, infection, and gastrointestinal events (Table 3). Granulocyte colony-stimulating factors use was more frequent in the vos/cyt arm (40.8% vs 22.0% of patients in the pla/cyt arm). Importantly, there was no increase in the incidence of organ-specific toxicity (cardiac, renal, hepatic, or pulmonary) in the vos/cyt arm compared to the pla/cyt arm. Serious AEs attributed to study drug were more frequent in the vos/cyt arm (Supplementary Table S3), including febrile neutropenia, infections, and gastrointestinal mucosal toxicity.
Table 3.
Grade 3, 4, and 5 treatment-emergent adverse events occurring in >5% of patients treated with vosaroxin or placebo in combination with cytarabine
| n (%) | Grade 3 | Grade 4 | Grade 5 | |||
|---|---|---|---|---|---|---|
| vos/cyt (n=355) | pla/cyt (n=350) | vos/cyt (n=355) | pla/cyt (n=350) | vos/cyt (n=355) | pla/cyt (n=350) | |
| Any AE | 133 (37·5) | 140 (40) | 151 (42·5) | 129 (36·9) | 50 (14·1) | 26 (7·4) |
| Haematologic events | ||||||
| Febrile neutropenia | 163 (45·9) | 115 (32·9) | 4 (1·1) | 2 (0·6) | 0 | 0 |
| Thrombocytopenia | 6 (1·7) | 8 (2·3) | 78 (22) | 79 (22·6) | 0 | 0 |
| Anaemia | 72 (20·3) | 76 (21·7) | 6 (1·7) | 5 (1·4) | 0 | 0 |
| Neutropenia | 9 (2·5) | 5 (1·4) | 57 (16·1) | 44 (12·6) | 0 | 0 |
| Nonhaematologic events | ||||||
| Stomatitis | 48 (13·5) | 10 (2·9) | 6 (1·7) | 0 | 0 | 0 |
| Diarrhoea | 19 (5·4) | 7 (2·0) | 1 (0·3) | 0 | 0 | 0 |
| Pneumonia | 23 (6·5) | 16 (4·6) | 3 (0·8) | 1 (0·3) | 13 (3·7) | 9 (2·6) |
| Sepsis | 8 (2·3) | 5 (1·4) | 20 (5·6) | 7 (2·0) | 14 (3·9) | 6 (1·7) |
| Bacteraemia | 39 (11·0) | 14 (4·0) | 4 (1·1) | 1 (0·3) | 0 | 1 (0·3) |
| Hypokalaemia | 41 (11·5) | 19 (5·4) | 11 (3·1) | 2 (0·6) | 0 | 0 |
| Hypophosphataemia | 25 (7·0) | 11 (3·1) | 3 (0·8) | 0 | 0 | 0 |
| Hyperglycaemia | 18 (5·1) | 15 (4·3) | 1(0·3) | 0 | 0 | 0 |
| Decreased appetite | 20 (5·6) | 7 (2·0) | 0 | 0 | 0 | 0 |
| Hypertension | 21 (5·9) | 12 (3·4) | 0 | 0 | 0 | 0 |
AE=adverse event; ALT=alanine aminotransferase; AST=aspartate aminotransferase; cyt=cytarabine; pla=placebo; vos=vosaroxin.
At the time of last follow-up, 273 (76·9%) and 288 (82·3%) deaths were recorded in the vos/cyt and pla/cyt arms, respectively. Progressive disease was the primary cause of death (65·9% and 79·5% for vos/cyt and pla/cyt arms, respectively). Deaths attributed to serious AEs occurred in 14% (n=50) and 7% (n=26) of patients (considered related to therapy by the investigator in 5·1% and 2·3%) in the vos/cyt and pla/cyt treatment arms, respectively. In both arms, most deaths attributed to serious AEs occurred within 60 days (80% with vos/cyt and 77% with pla/cyt).
Discussion
In the past 40 years, little progress has been achieved in prolonging survival in patients with relapsed/refractory AML. Median survival with current salvage options, including high-dose cytarabine or anthracycline- or anthracenedione-based combination regimens, is less than 1 year, ranging from 3.3 to 9.0 months in recent phase 2/3 clinical trial reports.3, 4, 23, 28 Many investigational agents have failed to improve survival in randomised trials in this setting, despite promising early clinical data.3,4,22–25 Two of these agents, laromustine and clofarabine, improved response rates (producing CR rates of 20% and 35%, respectively, vs 16% and 18% in the control arms); however, improvements in response did not translate into improved survival, in part due to higher early mortality than in the control arms.4,22,23
In contrast, the VALOR trial demonstrated that the addition of vosaroxin to cytarabine prolonged survival in patients with relapsed/refractory AML. Considering the rapidity of progression and death in patients with relapsed/refractory AML, a one month improvement in median survival should be considered clinically significant. In the primary analysis, the statistical significance of OS was borderline according to the standard criteria of p<0·05 (unstratified, log-rank p=0·0610). An OS benefit was supported, however, by two additional, preplanned analyses: a stratified log-rank test, and a sensitivity analysis in which OS was censored for subsequent alloSCT due to the potential to confound survival analyses.26 In both of these analyses, statistical significance was observed (p=0·0241 and 0.0243). The near significance of the primary endpoint together with the additional analyses support the conclusion that the addition of vosaroxin to cytarabine provides clinical benefit. The survival benefit was particularly compelling in patients older than 60 years, one of the most treatment-resistant groups. In these patients, a 2 month improvement in median survival was observed, corresponding to a 25% reduction in the risk of death. There are a number of factors that could underlie the difference in the treatment effect between younger and older patients, including differences in disease biology and chemosenstivity. Among patients <60 years of age, a survival benefit with vosaroxin may not have been detected due to higher transplant rates and the ability to effectively salvage younger patients with more aggressive subsequent therapies.
The CR rate was nearly doubled in the vos/cyt arm compared with the pla/cyt arm (30·1% vs 16·3%, p<0·0001). The CR rate was significantly higher in all subgroups analysed except patients <60 years of age, and the combined CR rate was significantly improved in all subgroups, including younger patients. Similar to survival, the improvement in response was most compelling in patients ≥60 years. Responses in both arms were durable, as indicated by median LFS of 11·0 months with vos/cyt and 8·7 months with pla/cyt.
Despite a significantly higher CR rate in the vosaroxin arm, rates of transplantation were similar between treatment arms. Among transplanted patients, a higher proportion achieved CR with vos/cyt prior to transplant than with pla/cyt; however, a considerable number of patients were transplanted either without remission, or after achieving remission with subsequent non-protocol therapy.
Importantly, the combination of vosaroxin and cytarabine increased OS and CR rates without an appreciable increase in 30- or 60-day mortality, supporting the conclusion that the benefit associated with vosaroxin in combination with cytarabine outweighs the toxicity. AEs observed in this study are consistent with those in prior studies of vosaroxin, including myelosuppression and gastrointestinal toxicities. Rates of neutropenia, neutropenic fever, and infection were higher in the vosaroxin arm as would be expected with the addition of a second cytotoxic agent. Although the number of deaths within 60 days was not increased, the number of deaths within 60 days due to AEs, mainly infection, was higher in the vos/cyt arm. Careful management of infections is critical. Stomatitis was also more common in the vosaroxin arm, as expected from phase 1/2 trials. Oral mucositis typically resolves within a few weeks of therapy and is thus unlikely to delay transplantation; however, it should also be managed attentively. Significantly increased cardiac, renal, neurologic, and hepatic AEs with vosaroxin were not observed.
The VALOR study represents one of the largest datasets available in the relapsed/refractory AML setting. Vosaroxin plus cytarabine is the first new regimen to show an improvement in survival in patients with relapsed/refractory AML. The toxicity observed with the addition of vosaroxin is acceptable in light of the benefit conferred. VALOR data support the use of this combination as a new option for salvage therapy in patients with relapsed/refractory AML and as a standard of care in patients older than 60 years of age.
Panel: Research in context
Evidence before this study
There is no generally accepted standard of care for patients with relapsed/refractory AML. The benefit of current treatment options is limited, and enrolment in a clinical trial is recommended when possible.2,27 We searched PubMed for randomised trials in patients with relapsed/refractory AML in the decade prior to the initiation of the VALOR trial (January 1, 2000, through December 31, 2010) using the following search string: (‘acute myeloid leukemia’ or ‘acute myeloid leukaemia’ or ‘AML’) AND (‘relaps*’ OR ‘refractory’ OR ‘second-line’ OR ‘salvage’) AND (‘randomized’ or ‘randomised’). Dose-finding trials, trials evaluating transplant or maintenance therapy, and trials in populations other than patients with relapsed and/or refractory AML were excluded. In the previous decade, none of the novel agents or regimens studied in a randomised trial of relapsed/refractory AML patients demonstrated an overall survival benefit.22–24,28–30 Since the initiation of the VALOR study, three additional randomized studies conducted in this population have demonstrated no improvement in survival over controls.3,4,25
Added value of this study
VALOR is a phase 3 trial assessing vosaroxin, a first-in-class anticancer quinolone derivative that represents a new therapy for patients with relapsed or refractory AML. VALOR is one of the largest studies to be conducted in the relapsed/refractory AML setting. Vosaroxin plus cytarabine is the first regimen to show a survival benefit in relapsed/refractory AML, with the greatest benefit observed in patients older than 60 years, a population with limited treatment options.
Implications of all the available evidence
VALOR data support the use of vosaroxin plus cytarabine as a treatment option for patients with relapsed/refractory AML and as a new standard of care in patients 60 years of age or older.
Supplementary Material
Acknowledgments
Medical writing assistance was provided by Janis Leonoudakis, PhD, of Powered 4 Significance LLC, and was funded by Sunesis Pharmaceuticals, Inc.
Footnotes
Contributors
FR, NV, GJS, HPE, ARC, JAF, RW, JAS, GA, CM, RKS, and HMK contributed to the study design. FR, EKR, HS, JEL, MEC, NV, SAS, GJS, EJ, HPE, AP, H-AH, CR, VMK, JC, GJR, OO, XT, VH, JM, H-GD, MH, LD, BLP, GG, A-MC, AW, DH, RKS, and HMK contributed to data collection. ARC, JAF, RW, JAS, GA, and CM contributed to data analysis. All authors contributed to the interpretation of the data and all authors critically reviewed the manuscript and approved the final version for submission.
Declaration of interests
FR reports grants and advisory fees from Sunesis during the conduct of the study, and grants from Sunesis outside the submitted work. NV reports advisory fees from Sunesis during the conduct of the study. SAS and AW report advisory fees from Sunesis outside the submitted work. HPE reports grants and consulting fees from Sunesis during the conduct of the study, and reports fees from Novartis, Incyte, Celgene, Ariad, and Seattle Genetics and grants from Millennium, Seattle Genetics, Celator, Amgen, and Astellas, outside the submitted work. CR reports advisory fees from Sunesis during the conduct of the study, and reports grants from Amgen, Chugai, and Celgene and advisory fees from Celgene outside the submitted work. VMK and GJR report clinical trial support from Sunesis during the conduct of the study and advisory fees from Sunesis outside the submitted work. JC reports grants from Ambit, Arog, Astellas, Celator, Novartis and consulting fees from Novartis and Astellas, outside the submitted work. OO reports grants from Sunesis during the conduct of the study, and reports fees from Sunesis, Algeta, and Spectrum Pharmaceuticals and grants from Astex, MEI-Pharma, Topotarget, Lilly, and Celgene outside the submitted work. MH reports grants and consulting fees from Sunesis during the conduct of the study. LD reports research funds from Sunesis during the conduct of the study, and reports consulting fees from Maxygen and research funds from Celator outside the submitted work. GG reports fees from Amgen, Celgene, GlaxoSmithKline, Janssen, Novartis, and Roche, outside the submitted work. RKS reports grants and advisory fees from Sunesis during the conduct of the study. CM is an employee of Cytel Inc. and receives no remuneration other than his Cytel salary; he has nothing to disclose. ARC, JAF, RW, JAS, and GA were employed by Sunesis Pharmaceuticals during the conduct of the study. EKR, HS, JEL, MEC, GJS, EJ, AP, H-AH, XT, VH, JM, H-GD, BLP, A-MC, and DH have nothing to disclose.
References
- 1.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23:1969–78. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. [accessed April 6, 2015];NCCN Clinical Practice Guidelines in Oncology: acute myeloid leukemia. Version 1.2015. http://www.nccn.org/professionals/physician_gls/pdf/aml.pdf.
- 3.Roboz GJ, Rosenblat T, Arellano M, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2014;32:1919–26. doi: 10.1200/JCO.2013.52.8562. [DOI] [PubMed] [Google Scholar]
- 4.Faderl S, Wetzler M, Rizzieri D, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol. 2012;30:2492–9. doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara F, Palmieri S, Mele G. Prognostic factors and therapeutic options for relapsed or refractory acute myeloid leukemia. Haematologica. 2004;89:998–1008. [PubMed] [Google Scholar]
- 6.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–87. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 7.Hawtin RE, Stockett DE, Byl JA, et al. Voreloxin is an anticancer quinolone derivative that intercalates DNA and poisons topoisomerase II. PLoS One. 2010;5:e10186. doi: 10.1371/journal.pone.0010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evanchik MJ, Allen D, Yoburn JC, Silverman JA, Hoch U. Metabolism of (+)-1,4-dihydro-7-(trans-3-methoxy-4-methylamino-1-pyrrolidinyl)-4-oxo-1-(2-thiaz olyl)-1,8-naphthyridine-3-carboxylic acid (voreloxin; formerly SNS-595), a novel replication-dependent DNA-damaging agent. Drug Metab Dispos. 2009;37:594–601. doi: 10.1124/dmd.108.023432. [DOI] [PubMed] [Google Scholar]
- 9.Mjos KD, Cawthray JF, Jamieson G, Fox JA, Orvig C. Iron(III)-binding of the anticancer agents doxorubicin and vosaroxin. Dalton Trans. 2015;44:2348–58. doi: 10.1039/c4dt02934h. [DOI] [PubMed] [Google Scholar]
- 10.Walsby EJ, Coles SJ, Knapper S, Burnett AK. The topoisomerase II inhibitor voreloxin causes cell cycle arrest and apoptosis in myeloid leukemia cells and acts in synergy with cytarabine. Haematologica. 2011;96:393–9. doi: 10.3324/haematol.2010.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scatena CD, Kumer JL, Arbitrario JP, et al. Voreloxin, a first-in-class anticancer quinolone derivative, acts synergistically with cytarabine in vitro and induces bone marrow aplasia in vivo. Cancer Chemother Pharmacol. 2010;66:881–8. doi: 10.1007/s00280-009-1234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lancet JE, Roboz GJ, Cripe LD, et al. A phase 1b/2 study of vosaroxin in combination with cytarabine in patients with relapsed or refractory acute myeloid leukemia. Haematologica. 2015;100:231–7. doi: 10.3324/haematol.2014.114769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plunkett W, Liliemark JO, Adams TM, et al. Saturation of 1-beta-D-arabinofuranosylcytosine 5'-triphosphate accumulation in leukemia cells during high-dose 1-beta-D-arabinofuranosylcytosine therapy. Cancer Res. 1987;47:3005–11. [PubMed] [Google Scholar]
- 14.Estey EH, Plunkett W, Kantarjian H, Rios MB, Keating MJ. Treatment of relapsed or refractory AML with intermediate-dose arabinosylcytosine (ara-C): confirmation of the importance of ara-C triphosphate formation in mediating response to ara-C. Leuk Lymphoma. 1993;10(Suppl):115–21. doi: 10.3109/10428199309149123. [DOI] [PubMed] [Google Scholar]
- 15.Kern W, Aul C, Maschmeyer G, et al. Superiority of high-dose over intermediate-dose cytosine arabinoside in the treatment of patients with high-risk acute myeloid leukemia: results of an age-adjusted prospective randomized comparison. Leukemia. 1998;12:1049–55. doi: 10.1038/sj.leu.2401066. [DOI] [PubMed] [Google Scholar]
- 16.Hiddemann W, Aul C, Maschmeyer G, et al. High-dose versus intermediate dose cytosine arabinoside combined with mitoxantrone for the treatment of relapsed and refractory acute myeloid leukemia: results of an age adjusted randomized comparison. Leuk Lymphoma. 1993;10(Suppl):133–7. doi: 10.3109/10428199309149125. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian HM, Estey EH, Plunkett W, et al. Phase I-II clinical and pharmacologic studies of high-dose cytosine arabinoside in refractory leukemia. Am J Med. 1986;81:387–94. doi: 10.1016/0002-9343(86)90287-1. [DOI] [PubMed] [Google Scholar]
- 18.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Cui L, Hung HM, Wang SJ. Modification of sample size in group sequential clinical trials. Biometrics. 1999;55:853–7. doi: 10.1111/j.0006-341x.1999.00853.x. [DOI] [PubMed] [Google Scholar]
- 21.Lehmacher W, Wassmer G. Adaptive sample size calculations in group sequential trials. Biometrics. 1999;55:1286–90. doi: 10.1111/j.0006-341x.1999.01286.x. [DOI] [PubMed] [Google Scholar]
- 22.Feldman EJ, Brandwein J, Stone R, et al. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol. 2005;23:4110–6. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 23.Giles F, Vey N, DeAngelo D, et al. Phase 3 randomized, placebo-controlled, double-blind study of high-dose continuous infusion cytarabine alone or with laromustine (VNP40101M) in patients with acute myeloid leukemia in first relapse. Blood. 2009;114:4027–33. doi: 10.1182/blood-2009-06-229351. [DOI] [PubMed] [Google Scholar]
- 24.Litzow MR, Othus M, Cripe LD, et al. Failure of three novel regimens to improve outcome for patients with relapsed or refractory acute myeloid leukaemia: a report from the Eastern Cooperative Oncology Group. Br J Haematol. 2010;148:217–25. doi: 10.1111/j.1365-2141.2009.07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levis M, Ravandi F, Wang ES, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117:3294–301. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist. 2008;13(Suppl 2):19–21. doi: 10.1634/theoncologist.13-S2-19. [DOI] [PubMed] [Google Scholar]
- 27.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg PL, Lee SJ, Advani R, et al. Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: a phase III trial (E2995) J Clin Oncol. 2004;22:1078–86. doi: 10.1200/JCO.2004.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Yin JA, Wheatley K, Rees JK, Burnett AK, Party UMALW. Comparison of 'sequential' versus 'standard' chemotherapy as re-induction treatment, with or without cyclosporine, in refractory/relapsed acute myeloid leukaemia (AML): results of the UK Medical Research Council AML-R trial. Br J Haematol. 2001;113:713–26. doi: 10.1046/j.1365-2141.2001.02785.x. [DOI] [PubMed] [Google Scholar]
- 30.Milligan DW, Wheatley K, Littlewood T, Craig JI, Burnett AK NCRI Haematological Oncology Clinical Studies Group. Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood. 2006;107:4614–22. doi: 10.1182/blood-2005-10-4202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.