Abstract
Brief high intensity exercise induces peripheral leukocytosis possibly leading to a higher incidence of allergic symptoms in athletes undergoing excessive training. We studied the exercise-induced alternation of circulating Tregs and FoxP3+ Tregs due to acute intense swim exercise in elite swimmers (n = 22, 12 males, age = 15.4 yrs). Twelve had prior or current rhinitis or asthma and 10 had no current or prior allergy or asthma. Circulating Tregs increased significantly (p < .001) following exercise (pre = 133 ± 11.2, post = 196 ± 17.6) as did FoxP3+ cells (pre = 44, post = 64 cells/µl). Increases in Tregs and FoxP3+ Tregs occurred to the same extent in both groups of adolescent swimmers.
Mature T Helper lymphocytes can be identified using flow cytometry as those cells which express the surface marker protein CD4, and are thus appropriately called CD4+ lymphocytes. Active CD4+ cells which also express a receptor for the potent T cell growth factor, interleukin-2, can be further identified as CD4+CD25+ lymphocytes. It is believed that these CD4+CD25+ lymphocytes, also known as Regulatory T-cells (Tregs) play an important role in actively controlling or suppressing immune function, generally via inhibition (1,20). Fork-head box Protein 3 or (FoxP3), is a key transcription factor that governs maturation of Treg cells (14,15,18). There is mounting evidence that FoxP3 expression in CD4+CD25+ lymphocytes may play a role in attenuating the inflammatory cytokine activity of lymphocytes in the control of allergic disease.
The induction of allergy and asthma by exercise in child and adolescent populations remains a serious clinical problem (8). Paradoxically, exercise leads to an acute increase in the number of immune cells in the peripheral blood and lungs in all individuals and many of the cells are also known to be involved in the allergic response. The factors that prevent allergic responses such as rhinitis and bronchoconstriction from occurring in everyone upon exercise despite immune activation remain unknown. In this study, we began to test the hypothesis that immunosuppressive effects of Treg cells and/or FoxP3 might play a role in the attenuation of allergic symptoms associated with exercise.
Allergy and asthma appear to be more common in endurance-trained athletes than in nonathlete populations (20). Higher rates of airway inflammation, bronchial hyperresponsiveness and allergic rhinitis have been documented in highly trained swimmers as well (13,25). In the case of swimmers, there is evidence that immune system adaptation to high intensity exercise training and exposure to chlorine induce allergic disease, and increase airway sensitization and reactivity (2). The precise mechanism for the increased incidence of atopy in endurance-trained athletes is not known; however, several theories have been postulated. Possible mechanisms may involve depression in several aspects of both innate and adaptive immunity such as alterations in CD4:CD8 or T Helper to T Suppressor ratio, mitogen-stimulated lymphocyte proliferation, and antibody synthesis (4,9–11,16). The degree to which multiple acute exercise bouts contribute to any of the above mechanisms is not clear. To date, there are no studies on the effect of brief exercise bouts on Tregs or FoxP3+ cells in enduranced-trained athletes.
We hypothesized that: (1) a short bout of high-intensity swim exercise would lead to a substantial increase in both absolute cell counts and percentages of circulating Tregs in endurance-trained swimmers; and (2) that the alterations in circulating levels of Tregs in response to exercise would differ between swimmers with diagnosed allergic symptomology versus controls. Consequently, the purpose of this study was to characterize the influence of exercise on circulating patterns of regulatory T-cells (Tregs, CD4+CD25+) as well as those positive for FoxP3 in both swimmers with and without diagnosed chronic rhinitis or asthma.
The specific mechanisms by which Tregs cells function is yet to be determined, however it has been hypothesized that FoxP3 may be involved in feedback mechanisms to attenuate inflammation (23,24). Fewer FoxP3+ cells and decreased FoxP3 mRNA expression levels have been reported in patients with asthma and allergy compared with asymptomatic matched subjects. Surprisingly, FoxP3 expression levels have been shown to increase with severity of symptoms in patients with allergic rhinitis and asthma (17).
To begin to test these hypotheses, we focused on a group of highly-trained elite-level high school swimmers. As stated earlier, several studies have reported an increased prevalence of allergic rhinitis and asthma among elite-level swimmers (2,13,25). Therefore, we sought to indentify the acute affects of exposure to exercise in chlorinated water on circulating Tregs and FoxP3.
Although exercise-induced leukocytosis is well-recognized in both children and adults, it is only recently that methodologies exist to examine the effects of exercise on specific lymphocyte subtypes such as T Helper and T Suppressor lymphocytes, and, more to the point, the FoxP3 intracellular transcription factor. We demonstrated using flow cytometry that the pattern of the exercise response differed among various lymphocyte subtypes (22) suggesting that the regulation of the increase in lymphocyte subpopulations was varied as well.
Despite the clear relevance of this question, exercise-induced alterations in circulatory patterns of Tregs and FoxP3 cells have not been previously studied (6). In addition, though percentages of Tregs and FoxP3+ (% of PBMCs) in individuals with allergy and asthma versus controls have been reported, this is the first such study to quantify absolute cell counts based on leukocyte cell differential counts (CBCs).
This study consisted of three visits: (1) a health assessment and laboratory assessment of pulmonary function (PFT) using the American Thoracic Society’s (ATS) exercise-induced bronchoconstriction challenge (7,19); (2) a fitness assessment comprised of body composition testing via DEXA scan, and cardiovascular fitness on cycle ergometry (oxygen uptake (VO2max/lean body mass determined by gas exchange from a maximal graded exercise test); and (3) the field swim challenge with blood measurements before and after the swim. Details regarding exercise testing, blood collection and processing are provided in the methods section below.
Methods
Participants
Thirty-five high-school elite-level swimmers were recruited from the Southern California area for this study. Advertisement was conducted via circulation of fliers at USA Swimming invitational swim meets and contact with local swim clubs. All participants were competing at the high-school varsity level and/or training daily with a swim club. On average, all participants were training approximately 3 hr per day, 6 days per week. All 35 of the participants completed the laboratory testing including a fitness test (graded maximal exercise test on cycle ergometer), DEXA, medical history and exam, and pulmonary function testing.
All 35 swimmers were invited to participate in the swim visit. Twenty-two swimmers agreed to participate in the field exercise visit; 12 of which were male. The average age for the entire group was 15.4 ± 1.3 years. We categorized the subjects based on symptomology. Ten of the swimmers had no current or prior diagnosis of allergic rhinitis or asthma. These subjects are referred to as the “Control group.” The “Rhinitis/Asthma group” consisted of 12 swimmers with current or prior diagnosis of chronic rhinitis or mild persistent asthma by physician report. Medical histories revealed that the Rhinitis/Asthma participants were using commonly prescribed over-the-counter (OTC) or prescription medications as follows: three used antileukotrienes, two used inhaled corticosteroids, and two used combination drugs such as Advair. The five remaining Rhinitis/Asthma athletes used short-acting Beta-agonists and OTC antihistamines. Five swimmers in the Rhinitis/ Asthma group, had mild to moderate persistent asthma using current NHLBI standards (19) in addition to rhinitis. The University of California at Irvine Institutional Review Board approved this study. Informed written consent, as well as assent, were obtained from all participants and their parents or guardians.
Inclusion/Exclusion Criteria
Participating swimmers had to have achieved a minimum USA-Swimming Sectional or Invitational time standard within one year of consent. Exclusion criteria included having had an upper respiratory infection, severe persistent asthma or acute exacerbation within the previous 14 days. Also excluded were athletes that had used parenteral corticosteroids within the month before exercise challenge.
Field Protocol
All participants were instructed not to use any anti-inflammatory, prescription or OTC allergy medications for 72 hr before the swim visit. In addition asthmatics were told to withhold using short-acting beta-agonists within 12 hr of the study visit. Participants reported to the Anteater Recreation Center Aquatics Facility at approximately 9:00 a.m. on the Sunday following the completion of the last official Southern California high school swim competition, the California Interscholastic Federation (CIF) Master’s Meet. All of the participants had tapered for and competed in the CIF Master’s Meet on the Tuesday before our study. All participants returned to their regular workout schedules including practices on Thursday through Saturday, following the Master’s Meet and before our Sunday study.
The outdoor swim exercise protocol served to accomplish multiple aims. Specifically, the exercise type was appropriate for the group of athletes being tested, the duration and intensity has previously been shown to elicit approximately a 50% increase in circulating leukocytes in a laboratory settings (21), it included some of the psychosocial competitive atmosphere of a swim meet, and provided the appropriate environmental conditions of interest to this study, that of a cool outdoor morning in chlorinated water.
Upon arrival, and after written consent was verified, participants height, weight and body temperature were obtained. Participants were then seated in semirecumbent chairs where peripheral blood was collected from antecubital vein using standard phlebotomy procedures. Separate venupuncture was required since IV catheters could not be used during swim exercise. After blood collection, each swimmer performed baseline pulmonary function testing consisting of three separate maximal ventilatory maneuvers.
Swim Exercise Protocol
Each swimmer was informed of the stroke and distance they would be swimming only after pulmonary function testing was completed and that swim races would be conduced in a typical heat/race format. All swimmers performed a standard warm-up consisting of at least 15 min of moderate and performance-paced lap swimming. The work out was supervised by a USA Swimming certified swim coach. The exercise protocol consisted of swim races with three swimmers per heat. Males competed against other males. Each heat involved between six and seven minutes of race-pace swimming including 200 yards of their least favorite or lowest-ranked event, immediately followed by 200 yards of the best event and then freestyle laps to ensure a finish time of at least seven minutes for all swimmers. Swimmers did not exit the pool between strokes and were encouraged to swim as fast as they could. Timers kept track of swimmer’s heart rate via polar monitors and swim times via stop watch.
Post-Exercise Measurements
A separate stop watch was activated upon completion of the swim race and placed around each swimmer’s neck to track the amount of time between swim exercise, blood draw and pulmonary function testing. Swimmers were asked to exit the pool immediately upon finishing the swim race. They were then accompanied to the blood draw station where blood was collected via separate venupuncture after completion of between one minute and four minutes of swim exercise. Four phlebotomists were available to assure timely collection of blood from each of the three heat swimmers after they exited the pool. Postexercise pulmonary function tests (PFTs) were performed via spirometry no sooner then 10 min but nor more than 15 min after completion of the swim race. Spirometry was conducted using the Renaissance II, manufactured by Puritan-Bennett. Each swimmer performed three maximal respiratory maneuvers. The optimal valid effort of the three attempts for both baseline and post exercise spirometry was used for analysis.
Experimental Procedures
Blood Sampling and Flow Cytometric Analysis
Blood was collected using separate venupuncture from the antecubital vein before and following the swim race exercise. Peripheral blood mononuclear cells (PBMCs) were isolated by Lymphoprep density gradient separation from 20 mL of heparnized whole blood collected at each timepoint for flow cytometric analysis. A separate whole blood sample was used to quantify primary white cell populations (neutrophils, lymphocytes, monocytes, eosinophils, and basophils) using standard methods from the Clinical Hematology Laboratory at our institution. Blood lactate levels were measured in the fielding using a YSI Whole Blood Acutrend Lactate Analyzer. Noradrenaline was measured from plasma via ELISA using Bi-CAT EIA by ALPCO Diagnostics.
Flow cytometric analysis was performed according to manufacturer’s instructions. Briefly, after being washed for staining buffer, PBMC cell suspensions were labeled with the following antihuman mAbs in different combinations: fluorescein isothiocynate (FITC)-conjugated CD4, adenomatous polyposic coli (APC)-conjugated CD25, phycoerythrin (PE)-conjugated FoxP3 (all from eBioscience, San Diego, CA) For intracellular staining of FoxP3, cells were fixed and permeabilized using a fixation/permeabilzation buffer (eBioscience).
Acquisition and Analysis
Samples were acquired using a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA). A forward scatter threshold was used to acquire 100,000 events for each prepared sample. Lymphocyte subpopulations were identified by forward and side scatter and separated by antigenic expressions of CD3+/CD4+, CD4+/CD25+ and CD4+/FoxP3+ intracellular protein. Cell fluorescence was measured using a FACS Calibur flow cytometer, and data were analyzed using CellQuest software (BD Biosciences, San Diego, CA). Relative FoxP3 expression in the cells was quantified by determining the normalized mean fluorescent intensity (MFI).
Statistical Analysis
Unless otherwise noted, data are expressed as mean ± SEM (SEM). Statistical analysis was performed using the Version 15 SPSS statistical software package. Group differences were tested using Independent Sample T test. The exercise effect was tested using a Paired Sample T test. Results of analysis are for absolute cell counts (not percentage of cells). Variables not normally distributed were analyzed using nonparametric techniques. In the analysis of size or gender-dependent variables (FEV1, VO2max), linear regression was used to assess and control for the independent contribution of gender, group and height. Bivariate analysis (Pearson-product moment) was performed on all leukocyte subsets, lactate, noradrenaline and pulmonary and exercise parameters.
Results
Participants and Baseline Evaluation
Table 1 provides baseline descriptive measures for all participants. Swimmers in the Asymptomatic Group did not differ for those in the Rhinitis/Asthma Group on any of the anthropometric measures. Females were significantly smaller than the males and had more body fat (20.0% vs 12.5%). After adjusting for gender and lean body mass (lbm), the fitness level of the athletes did not differ across group.
Table 1.
Baseline Descriptive Statistics for Swimmers
| Control (n = 10) | Rhinitis/Asthma (n = 12) |
|
|---|---|---|
| Age (years) | 15.8 ± 0.4 | 15.1 ± 0.3 |
| Body Fat (%) | ||
| Male | 12.7 ± 1.2 | 12.3 ± 1.2 |
| Female | 19.5 ± 1.3* | 20.4 ± 2.2* |
| VO2max/lbm | ||
| Male | 63.9 ± 2.7 | 62.3 ± 2.0 |
| Female | 59.2 ± 1.8 | 57.3 ± 3.1 |
| Pulmonary Function (FEV1, FEV1/FVC)) | ||
| Male (l/min, %) | 4.8 ± 0.6 (84) | 4.6 ± 0.3 (81) |
| Female (l/min, %) | 4.2 ± 0.2 (83) | 3.8 ± 0.3 (80) |
indicates significant difference in body fat between males and females (p <.001). Control has no present or prior history of rhinitis/asthma diagnosis (5 males). Rhinitis/Asthma group includes five swimmers with mild persistent asthma and rhinitis and seven rhinitis only (7 males). Unless otherwise indicated, values represent baseline mean and standard errors for each variable. There were no differences between the Control and Rhinitis/Asthma group.
Average baseline Forced Expiratory Volume at one second (FEV1) and the FEV1 to FVC (forced vital capacity) ratio are also shown in Table 1. We observed no gender or group differences. Using the NHHANES prediction equations, all swimmers had normal or above predicted levels of lung function before the swim exercise (mean = 115% of predicted).
A summary of the exercise response is reported in Table 2. There was a significant increase in the average blood lactate levels (p < .001) approximately sevenfold increase over baseline line. The average heart rate after the swim exercise was 190 beats per minute. The post swim exercise heart rates were highly correlated with the maximal heart rates observed following the fitness assessments. Only one participant, had a significant drop in FEV1 following the swim exercise. The average FEV1 did not change during the swim exercise. After controlling for gender, height and age, we found no differences between asymptomatic and allergic swimmers in the FEV1, either at baseline or after the swim exercise (F = 0.39, p = .76).
Table 2.
Changes in Physiological and Immunological Parameters With Exercise
| Baseline (n = 22) | Post Exercise (n = 22) |
|
|---|---|---|
| Blood Lactate | 1.05 ± 0.1 | 7.35 ± 0.6** |
| End Exercise Heart Rate (bpm) | 189.7 | |
| Pulmonary Function (FEV1, (FEV1/FVC)) | ||
| Male (L/min, %) | 4.7 (83) | 4.6 (83) |
| Female (L/min, %) | 4.0 (81) | 4.0 (83) |
| Total Leukocytes (cells/µl) | 5959 ± 408 | 10186 ± 673** |
| Total Lymphocytes (cells/µl) | 1837 ± 87 | 4270 ± 244** |
| NK cells, CD16+CD56+CD45+ (cells/µl) | 233 ± 20 | 1103 ± 123** |
| B cells, CD3+CD19+CD45+ (cells/µl) | 263 ± 18 | 507 ± 35** |
| T cells, CD3+CD45+ (cells/µl) | 1314 ± 76 | 2581 ± 166** |
| T Helper cells CD4+CD45+ (cells/µl) | 718 ± 50 | 1152 ± 75** |
| T Suppressor cells CD8+CD45+ (cells/µl) | 482 ± 42 | 1082 ± 98** |
| T Regulatory cells, CD4+CD25+ (cells/µl) | 133 ± 11 | 196 ± 18** |
| Foxp3 positive T cells (cells/µl) | 44 ± 3 | 64 ± 6** |
| T Helper:T Suppressor Ratio | 1.72 | 1.25** |
Note. Unless otherwise noted, values indicate mean and standard errors before and after immediately after exercise. Leukocyte counts represent absolute cell counts expressed as the number of cells in the peripheral circulation per microliter of blood.
indicates significance at p < .001.
Exercise Effect
There was a robust acute exercise-induced leukocytosis (EIL) for all participants similar to what has been previously reported for short duration, high intensity exercise (4,20). Based on the total white blood cell counts, both the lymphocyte cell count in the peripheral circulation (pre = 1837, post = 4270 cells/µl, p < .001), and the percentage of lymphocytes in the circulation increased with exercise (pre =33%, post = 43% of total white blood cells). The percentage of T Helpers decreased with exercise (pre =55%, post = 47%) while the percentage of T Suppressors slightly increased (pre = 36%, post = 41%). Therefore, there was a significant reduction in the CD4:CD8 or T Helper to T Suppressor ratio with exercise (1.72 to 1.25, p < .001). Circulating levels of Tregs (CD4+CD25+) increased significantly following exercise (pre = 133 cells/µl, post = 196 cells/µl, p < .001). The number of T Helpers positive for FoxP3 intracellular protein (CD3+CD4+/FoxP3+) in the circulation also significantly increased following exercise (pre = 44 cells/µl, post = 64 cells/µl, p < .001).
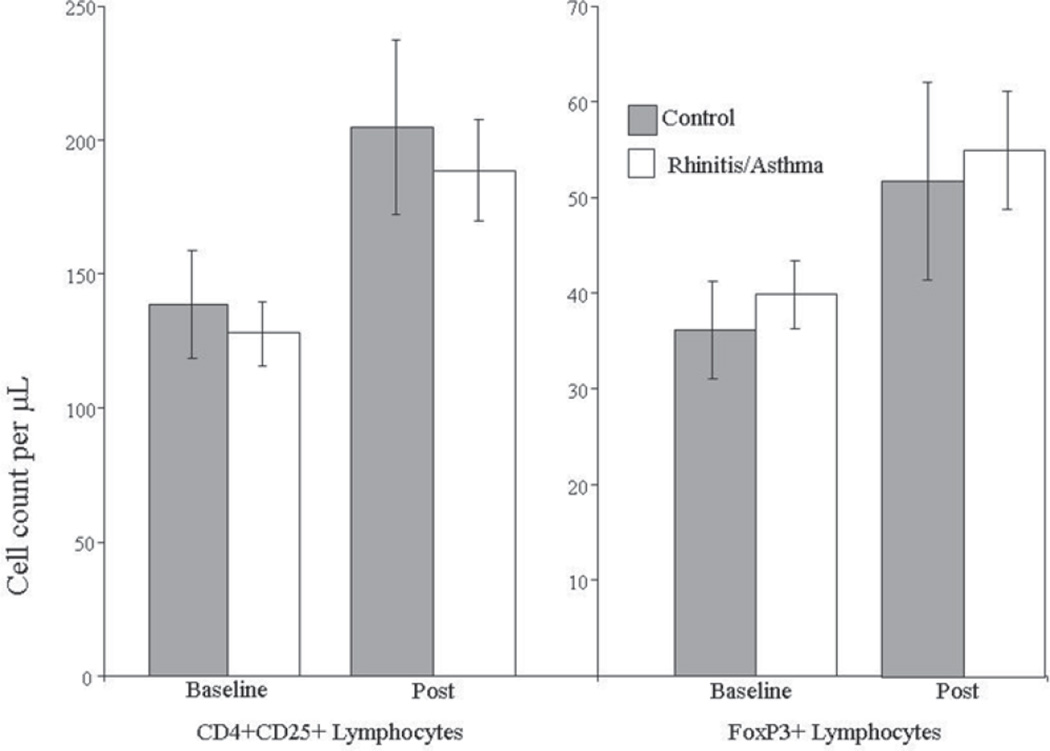
Changes from baseline for all of the physiological measures (lactate, lung function, maximal heart rate) and all leukocyte subsets did not differ between allergic or asymptomatic swimmers. Our initial hypothesis was not supported by this study since changes in Tregs or FoxP3+ cells did not differ between groups (see Figure 1).
Figure 1.
Comparison of baseline and post exercise absolute cell counts for Control and Rhinitis/Asthma Group. Note. Values indicate absolute cell counts per microliter of peripheral blood (mean and standard error). Changes from baseline were significant for both groups (p < .001). There was no difference in the exercise-induced increase in Tregs or FoxP3+ cells between swimmers with and without current or prior rhinitis/asthma.
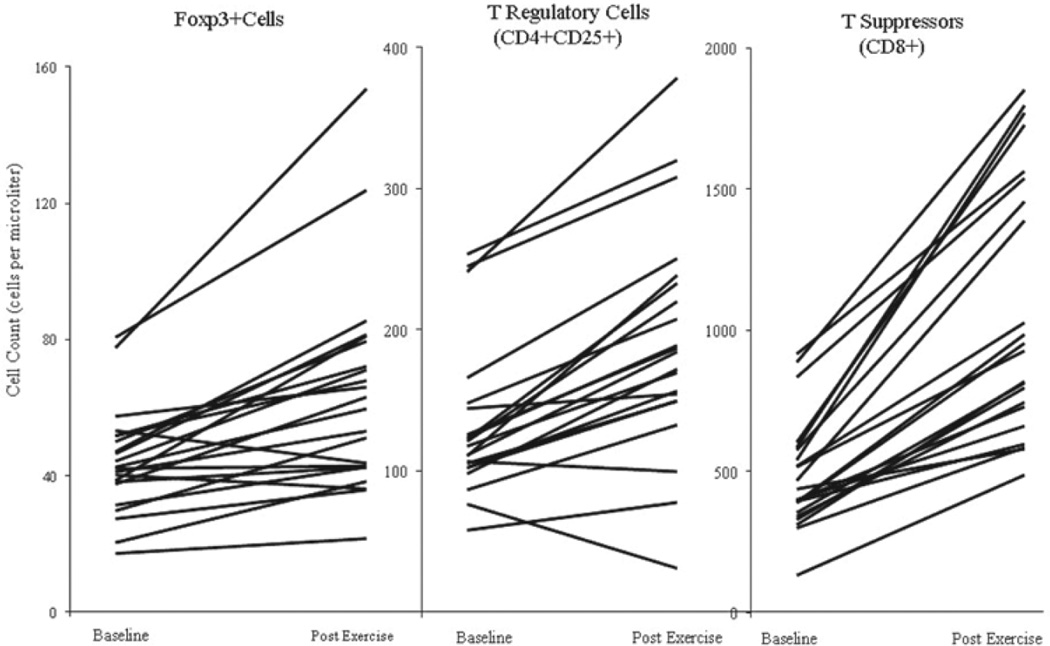
Figure 2 compares absolute cell counts of T Suppressors, T Regulatory and FoxP3 positive cells before and after exercise. While every participant had a robust and consistent increase in total leukocytes, total lymphocytes, and total T Helper and T Suppressors populations with exercise, there was a great deal of variability in the pattern of change for both Tregs and FoxP3+ cells.
Figure 2.
Exercise-induced changes by participant in lymphocyte subsets. Note. Exercise induced changes by participant in Tregs positive for FoxP3, total Tregs (CD4+CD25+), and T Suppressors (CD4+CD8+). Values indicated absolute cell counts per microliter of peripheral blood.
In an attempt to distinguish the causes of the observed variability, we calculated Pearson Product Moment correlations to look for concurrent association between changes in noradrenaline, lactate, and other leukocyte populations. The results of this analysis are contained in Table 3. It appeared that the increase in lactate was associated with increases in total lymphocytes, NK cells and to a lesser degree, T Suppressors, but not Tregs cells. As expected, exercise-induced changes in T Regulatory cells were weakly but significantly correlated with changes in FoxP3 (r = .55, p = .008).
Table 3.
Correlation Matrix for Exercise Associated Changes in Exercise Parameters and Leukocyte subsets
| Nor- Adrenaline |
Lactate | Total Neutrophils |
Total Lymphocytes |
NK Cells | T Suppressors |
T Regulatory |
|
|---|---|---|---|---|---|---|---|
| Lactate | r =.74** | ||||||
| p<.001 | |||||||
| Total Neutrophils | r =.14 | r = .05 | |||||
| p=.524 | p=.827 | ||||||
| Total Lymphocytes | r =.46* | r = .55* | r = .26 | ||||
| p=.029 | p=.008 | p = .238 | |||||
| NK cells | r = .40 | r = .56* | r = .04 | r = .87** | |||
| p=.065 | p = .007 | p = .862 | p <.001 | ||||
| T Suppressors | r = .40 | r = .37 | r = .28 | r = .75** | |||
| p=.069 | p=.091 | p=.216 | p <.001 | ||||
| T Regulatory | r = −.12 | r = −.07 | r = −.14 | r = .27 | r = .28 | r = .002 | |
| p=.589 | p=.750 | p=.532 | p=.230 | p = .214 | p=.994 | ||
| Foxp3+ | r = −.37 | r = −.38 | r = .26 | r = .14 | r = −.05 | r = .18 | r =.55* |
| p=.091 | p=.081 | p=.237 | p=.525 | p = .810 | p=.437 | p=.008 |
Values are Pearson product moment bivariate correlations. All variables indicate change with exercise or exercise-induced alterations.
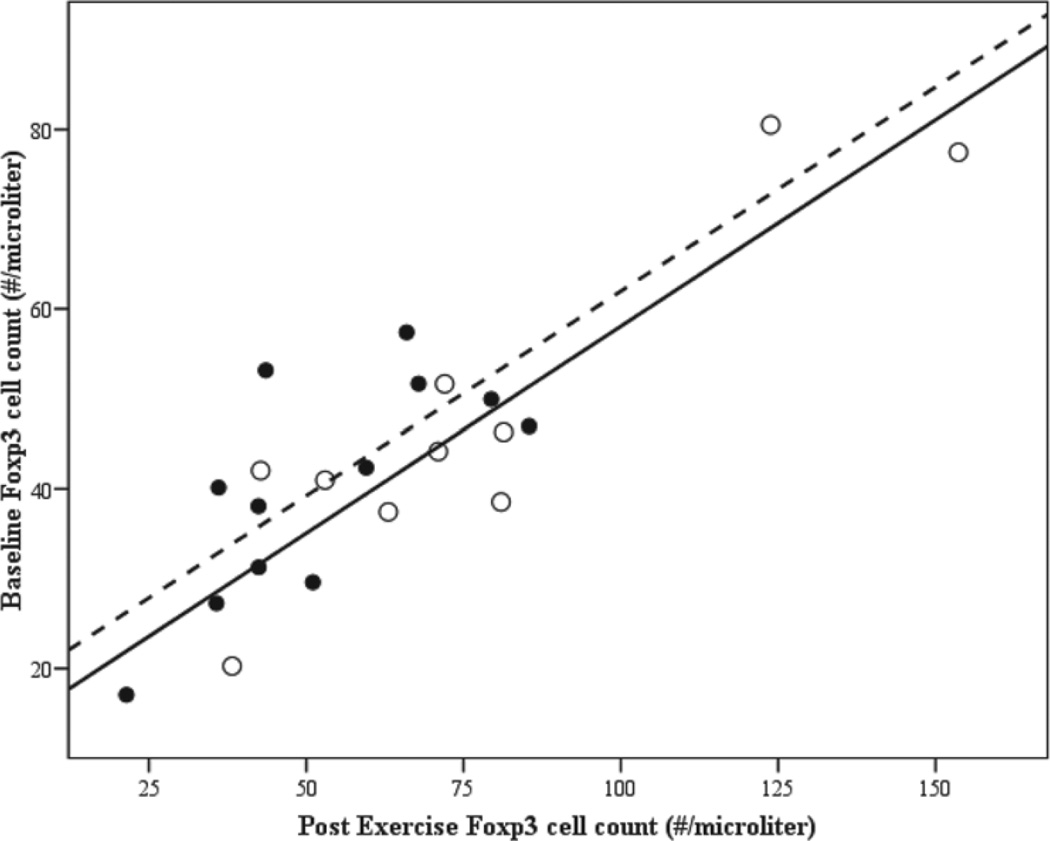
Linear regression was used to explore variability in the post exercise circulating level of T lymphocytes positive for Foxp3. It appeared that baseline FoxP3 level was highly predictive of the exercise effect (see Figure 3), in that individuals with higher baseline levels tended to have increases with exercise while individuals with lower baseline levels tended to decrease with exercise. Using linear regression to control for the baseline FoxP3 level, we found that females tended to have higher post exercise FoxP3 levels than males, after controlling for baseline levels (F = 30.5, R2 = .76, p < .001, gender p = .059). No other variables were found to be predictive.
Figure 3.
Scatterplot of Baseline versus Post Exercise FoxP3-positive cell counts by gender. Note. Individual association between baseline and post exercise level of FoxP3+ cells. Open circles indicated female swimmers (dotted regression line), close circles males (solid regression line).
Discussion
This is the first such study to investigate exercise-induced lymphocytosis within the T-regulatory cell population in adolescents. Consist with other exercise studies, all the other leukocyte subsets (granulocytes, total lymphocytes, T Helpers, T Suppressors, and Natural Killers) demonstrated similar and robust increases in the circulation following exercise. However, the exercise-induced alterations in peripheral Tregs and FoxP3+ cells were not in agreement with other leukocyte subsets. While the patterns of change did not significantly differ for controls and swimmers with rhinitis or asthma; unlike virtually every other cell type, some individuals showed lower levels of Tregs and FoxP3+ cells in the peripheral circulation after exercise than before.
The phenomenon of exercise-induced leukocytosis is well documented and easily reproducible; however, the cause for variability in exercise-induced changes across leukocyte cell types is less understood. Why, for example, within the lymphocyte population do we observe decreases in the percentage of T Helpers cells and an increase in the percentage of other T cell populations?
In his recent review, Gleeson (2006) suggests that the reduction in the CD4:CD8 or T Helper to T Suppressor ratio is associated with a decrease in the proliferative response of lymphocytes to mitogenic stimulants placing athletes at risk for upper respiratory infections following high intensity exercise.
One possible explanation could be related to alterations in peripheral circulation with exercise. At rest, the majority of arteriole sphincters in peripheral capillary beds are closed to the general circulation. During exercise, these arterial pathways are opened and/or dilate in response to catecholamine signals resulting in increased blood flow to otherwise closed capillary beds. Blum and Pabst (3) have reported that, at rest, lymphocytes in the peripheral circulation represent only about 2% of the total lymphocytes in the human body and the majority (approximately 85%) of the lymphocytes reside in the lymph nodes, spleen, gut, thymus and bone marrow. During exercise, blood flow is shunted away from these immune organs to exercising muscle and into the peripheral circulation, thus it is possible that while more lymphocyte rich blood is introduced into the circulation with exercise, blood rich in T Helper cells may remain in immune tissues, thereby mitigating possible deleterious activation of the immune system.
In a murine model, both CD4+CD25+ (Tregs) and FoxP3 levels are higher in both Bronchoalveolar Lavage (BAL) and lung tissue after both acute and chronic allergen exposure relative to naïve levels (4). Furthermore, it was determined that the level of Tregs present in the airway before sensitization influenced the severity of acute airway inflammation suggesting that Tregs are being selectively retained in the BAL during local inhalation tolerance. Bernard (2007) hypothesized that chronic chlorine exposure alters immunoregulatory function leading to allergic disease in swimmers (2). In his recent review, he discusses the role of hypochlorous acid, a biocide that ironically is both found in pool water and produced by immune cells, in the disruption in epithelial barriers in the airways and the development of allergic disease.
We observed that circulating levels of Tregs and FoxP3 cells increased following exercise, suggesting that translocation of these cells into the circulation is a general trend following exercise. The idea that Tregs function through a feedback mechanism to mitigate conventional antigen-specific immunity; and that persistent antigen stimulation and inflammation are responsible for increased FoxP3 expression (23,24) has been hypothesized.
This could explain some of the variability in Tregs and FoxP3 observed in our subjects; namely, FoxP3+ cells may have migrated to or remained in interstitial lung tissue to a greater degree in those individuals that showed attenuated or reduced levels of FoxP3 in the circulation following exercise. Unlike other leukocytes subsets which consistently increase in the circulation following exercise, movement of Tregs into the general circulation seem to be controlled by a separate signaling mechanism, which may serve to mitigate inflammation or allergic-responses during exercise. We again emphasize that we did not observe different post exercise trends for control and symptomatic swimmers. However, it is not unreasonable to assume, that control of these power immune cells is so tightly regulated that minor differences in prior exposure and health status could predict their acute exercise response. While it is generally recognized that Tregs and FoxP3 play some role in autoimmunity, it is difficult to distinguish a common theme based on the seemingly conflicting evidence in the literature. For example, several authors have reported no differences in the peripherally circulating percentage of FoxP3+ cells in controls versus individuals with asthma/allergy; however these same authors have shown a seemingly linear relationship between disease severity and FoxP3 level (1,12,21,22). A potential explanation for this inconsistency could be that most studies have only reported percentages of positive cells in the circulation without calculating absolute cell counts.
Tregs and FoxP3 represent between 1% and 10% of peripheral blood mononuclear cells in the circulation; a small proportion of the total leukocyte population. Like eosinophils and basophils, however, small changes in peripheral patterns may be highly predictive of disease (22). We note this because the normal range of lymphocyte counts can vary by more than 1000 cells per µL, and therefore, only looking at differences in percentages of these cells, (% of FoxP3+ or Tregs out of lymphocytes or peripheral blood mononuclear cells) may lead to erroneous conclusions.
Conclusion
This is the first study to show the effect of intense exercise on circulating Treg cells in highly-trained adolescents. The absolute numbers, but not percentage of this specialized set of T Helper lymphocytes increased suggesting that the mechanisms responsible for releasing Tregs into the circulation were distinct from the overall lymphocyte response to exercise. We could not identify specific patterns relating the changes in Tregs to a clinical history of allergy within this population. The acute response to exercise from this field study supports our previous comparisons of exercise-induced lymphocytosis associated with a brief bout of highintensity cycle ergometry exercise in nonathletic asthmatic and healthy children (22). In both studies, we found generally similar patterns of lymphocytosis following exercise for asthmatics and control. In our previous study, CD4+ lymphocyte levels did not differ between asthmatics and controls either at baseline or after exercise. The role of circulating Tregs in mitigating allergy remains enigmatic, requiring further study.
Acknowledgments
The authors wish to thank Dr. Szu-Yun Leu, Biostatistician for the University of California, Irvine Institute for Clinical and Translational Science for consultation in the statistical analysis. This work was supported in part by National Institutes of Health grants T32AR047752, RO1-HL080947, P01HD-048721 and the UCI Satellite ICTS:MO1 RR00827.
Contributor Information
Lori D. Wilson, Pediatric Exercise Research Center, Dept. of Pediatrics, University Children’s Hospital, University of California, Irvine, CA 92868
Frank P. Zaldivar, Pediatric Exercise Research Center, Dept. of Pediatrics, University Children’s Hospital, University of California, Irvine, CA 92868
Christina D. Schwindt, Pediatric Exercise Research Center, Dept. of Pediatrics, University Children’s Hospital, University of California, Irvine, CA 92868
Jessica Wang-Rodriguez, VA San Diego Healthcare Systems, University of California, San Diego, CA 92161.
Dan M. Cooper, Pediatric Exercise Research Center, Dept. of Pediatrics, University Children’s Hospital, University of California, Irvine, CA 92868
References
- 1.Akbari O, Stock P, DeKruyff RH, Umetsu DT. Role of regulatory T cells in allergy and asthma. Curr. Opin. Immunol. 2003;15:627–633. doi: 10.1016/j.coi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Bernard A. Chlorination products: Emerging links with allergic diseases. Curr. Med. Chem. 2007;14:1771–1782. doi: 10.2174/092986707781058940. [DOI] [PubMed] [Google Scholar]
- 3.Blum KS, Pabst R. Lymphocyte numbers and subsets in the human blood Do they mirror the situation in all organs? Immunol. Lett. 2007;1089:45–51. doi: 10.1016/j.imlet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Bruunsgaard H, Jensen MS, Schjerling P, et al. Exercise induces recruitment of lymphocytes with an activated phenotype and short telomeres in young and elderly humans. Life Sci. 1999;65:2623–2633. doi: 10.1016/s0024-3205(99)00531-7. [DOI] [PubMed] [Google Scholar]
- 5.Carson BD, Ziegler SF. Impaired T cell receptor signaling in FoxP3+ CD4 T cells. Ann. N Y Aced. Sc. 2008;1103:167–178. doi: 10.1196/annals.1394.022. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DM, Random-Aizik S, Schwindt CD, Zaldivar F. Dangerous Exercise—Lessons learned from dysregulated inflammatory responses to physical activity. J. Appl. Physiol. 2007;103:700–709. doi: 10.1152/japplphysiol.00225.2007. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DM, Springer C. Pulmonary function assessment in the laboratory during exercise. In: Chernick V, Boat TF, editors. Kendig’s Disorders of the Respiratory Tract in Children. 6th. Philadelphia: W. B. Saunders; 1998. pp. 214–237. [Google Scholar]
- 8.de Benedictis D, Bush A. The challenge of asthma in adolescence. Pediatr. Pulmonol. 2007;42:683–692. doi: 10.1002/ppul.20650. [DOI] [PubMed] [Google Scholar]
- 9.Fry RW, Morton AR, Grawford GPM, Keast D. Cell numbers and in vitro responses of leucocytes and lymphocyte subpopulations following maximal exercise and interval training session of different intensities. Eur. J. Appl. Physiol. 1991;64:218–227. doi: 10.1007/BF00626284. [DOI] [PubMed] [Google Scholar]
- 10.Gabriel H, Urhausen A, Kindermann W. Circulating leucocyte and lymphocyte subpopulations before and after endurance exercise to exhaustion. Eur. J. Appl. Physiol. 1991;63:449–557. doi: 10.1007/BF00868077. [DOI] [PubMed] [Google Scholar]
- 11.Gleeson M. Immune system adaptation in elite athletes. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:659–665. doi: 10.1097/01.mco.0000247476.02650.18. [DOI] [PubMed] [Google Scholar]
- 12.Hartl D, Koller B, Mehlhorn AT, et al. Quantitative and function impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J. Allergy Clin. Immunol. 2007;119:1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Helenius I, Rytila P, Sarna S, et al. Effect of continuing or finishing high-level sports on airway inflammation, bronchial hyperresponsiveness, and asthma: A 5-year prospective follow-up study of 42 highly trained swimmers. J. Allergy Clin. Immunol. 2002;109:962–968. doi: 10.1067/mai.2002.124769a. [DOI] [PubMed] [Google Scholar]
- 14.Host CR, Norton KI, Olds TS, Lowe ELA, Mulligan SP. The effects of altered exercise distribution on lymphocyte subpopulations. Eur. J. Appl. Physiol. 1995;72:157–164. doi: 10.1007/BF00964131. [DOI] [PubMed] [Google Scholar]
- 15.Karagiannidis C, Akdis M, Holopainen P. Glucocorticoids upregulate Foxp3 expression and regulatory T cells in asthma. J. Allergy Clin. Immunol. 2004;114:1425–1433. doi: 10.1016/j.jaci.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 16.LaPerriere A, Antoni MH, Ironson G, et al. Effects of aerobic exercise training on lymphocyte subpopulations. Int. J. Sports Med. 1994;15:S127–S130. doi: 10.1055/s-2007-1021127. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Yu HH, Wang LC, Yang YH, Lin YT, Chiang BI. The levels of CD4+CD25+ regulatory T cells in paediatric patients with allergic rhinitis and bronchial asthma. Clin. Exp. Immunol. 2007;148:53–63. doi: 10.1111/j.1365-2249.2007.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGee HS, Agrawal DK. TH2 cells in the pathogenesis of airway remodeling. Immunol. Res. 2007;35:219–231. doi: 10.1385/IR:35:3:219. [DOI] [PubMed] [Google Scholar]
- 19.NHLBI. Guidelines for the Diagnosis and Management of Asthma. NIH; 1997. [Google Scholar]
- 20.Parsons JP, Kaeding C, Phillips G, Jarjoura D, Wadley G, Mastronarde JG. Prevalence of exercise induced bronchospasm in a cohort of varsity college athletes. Med. Sci. Sports Exerc. 2007;39:1487–1492. doi: 10.1249/mss.0b013e3180986e45. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 22.Schwindt CD, Zaldivar F, Wilson L. Circulating leucocytes and lymphocyte subtypes increase in response to brief exercise in children with and without asthma? Br. J. of Sp. Med. 2007;41:34–40. doi: 10.1136/bjsm.2006.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi HZ, Li S, Xie ZF, Qin XJ, Qin X, Zhong XN. Regulatory CD4+CD25+ T lymphocytes in peripheral blood from patients with atopic asthma. Clin. Immunol. 2004;113:172–178. doi: 10.1016/j.clim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Xu G, Mou Z, Jiang H, et al. A possible role of CD4+CD25+ T cells as well as transcription factor Foxp3 in the dysregulation of allergic rhinitis. Laryngoscope. 2007;117:876–880. doi: 10.1097/MLG.0b013e318033f99a. [DOI] [PubMed] [Google Scholar]
- 25.Zwick H, Popp W, Budik G, Wanke T, Rauscher H. Increased sensitization to aeroallergens in competitive swimmers. Lung. 1990;168:111–115. doi: 10.1007/BF02719681. [DOI] [PubMed] [Google Scholar]