Abstract
Exercise has been shown to improve postischemia perfusion of normal tissues; we investigated whether these effects extend to solid tumors. Estrogen receptor–negative (ER-, 4T1) and ER+ (E0771) tumor cells were implanted orthotopically into syngeneic mice (BALB/c, N = 11–12 per group) randomly assigned to exercise or sedentary control. Tumor growth, perfusion, hypoxia, and components of the angiogenic and apoptotic cascades were assessed by MRI, immunohistochemistry, western blotting, and quantitative polymerase chain reaction and analyzed with one-way and repeated measures analysis of variance and linear regression. All statistical tests were two-sided. Exercise statistically significantly reduced tumor growth and was associated with a 1.4-fold increase in apoptosis (sedentary vs exercise: 1544 cells/mm2, 95% CI = 1223 to 1865 vs 2168 cells/mm2, 95% CI = 1620 to 2717; P = .048), increased microvessel density (P = .004), vessel maturity (P = .006) and perfusion, and reduced intratumoral hypoxia (P = .012), compared with sedentary controls. We also tested whether exercise could improve chemotherapy (cyclophosphamide) efficacy. Exercise plus chemotherapy prolonged growth delay compared with chemotherapy alone (P < .001) in the orthotopic 4T1 model (n = 17 per group). Exercise is a potential novel adjuvant treatment of breast cancer.
Tumor blood vessels are structurally and functionally abnormal, resulting in heterogeneous intratumoral regions of hypoxia (1,2). Hypoxia and poor blood supply promote an aggressive phenotype and contribute to ineffective systemic anticancer therapy and treatment resistance (2–5). As such, several approaches to prevent and/or mitigate tumor hypoxia have been investigated (eg, hyperthermia, hypoxic cytotoxins) (6–10). Vascular normalization by vascular endothelial growth factor (VEGF) inhibition sensitizes tumors to systemic chemotherapy by improving oxygenation and delivery of chemotherapy (11–15). However, these effects are transient, lasting only days to weeks, which hinders long-term clinical use (16,17).
Intriguingly, emerging data indicate that both acute and chronic (repeated) aerobic exercise stimulates favorable improvements in intratumoral perfusion/vascularization and hypoxia in orthotopic models of human breast cancer and murine prostate cancer (18–20). Thus, exercise may promote a shift towards a more “normalized” tumor microenvironment (possibly via upregulation of regional and local physiologic angiogenesis) (21). However, whether exercise alters oxygen transport in tumors by modifying vascular density, oxygen consumption rate, and perfusion of existing microvessels has received little attention (22,23). Furthermore, because the abnormal solid tumor microenvironment is an important contributor to tumor progression and treatment resistance, exercise via its normalizing properties may inhibit tumor progression as well as act as a therapeutic sensitizer (21).
In previous work, our group studied the effects of exercise in an orthotopic model of human breast cancer (MDA-MB-231), requiring immunodeficient mice. However, the immune system is a key component of the tumor microenvironment and host defense against tumor progression (24). To our knowledge, no study has evaluated the physiologic and growth inhibitory effects of voluntary exercise in an immunocompetent mouse model of syngeneic tumor cells implanted in the orthotopic position (25). Furthermore, the question of whether exercise augments the efficacy of chemotherapy has been addressed in human tumor xenografts into immunodeficient mice, but has yet to be addressed in a preclinical model of immunocompetent mice (26).
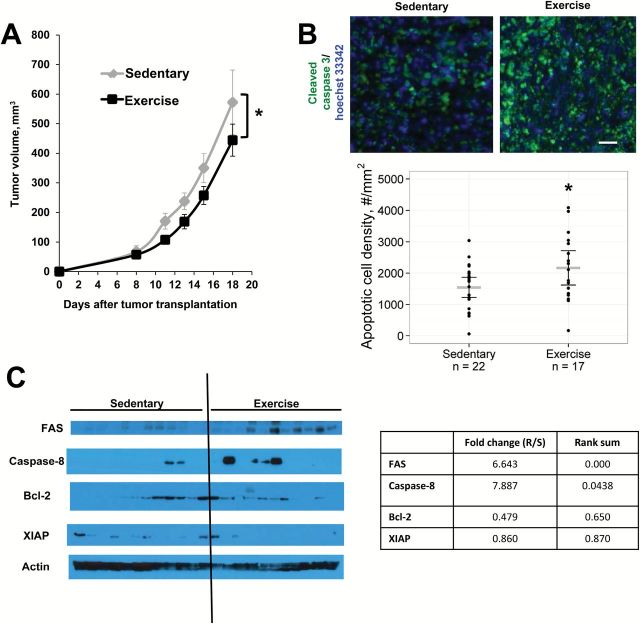
We implanted immunocompentent BALB/c female mice with syngeneic 4T1 murine breast cancer cells orthotopically in the dorsal mammary fat pad. Immediately after tumor cell implantation, mice were randomly assigned to voluntary wheel running or a sedentary control group (n = 11–12). Tumor volume, running distance, and body weights were recorded three times weekly for 18 days. The E0771 model in C57Bl/6 mice was used to confirm the effects of exercise on tumor growth. One-way analysis of variance (ANOVA) was used to compare differences in tumor variables between groups. Repeated measures ANOVA was used to compare differences in body weight and running distance between groups throughout the experiments. Tumor growth rates were determined by linear regression, and differences in growth rate between groups were assessed using Analysis of Covariance (ANCOVA). For all tests, a P value under .05 was considered statistically significant and all t tests were two-sided (see Supplementary Methods, available online, for further details).Exercise statistically significantly decreased tumor growth rate in both the 4T1 (P = .004) (Figure 1A) and E0771 models (P = .012) (Supplementary Figure 1, available online), compared with sedentary control. Body weight was similar between groups at the beginning and end of the study (starting weight: sedentary 23.9g [95% confidence interval {CI} = 23.1 to 24.73 g] vs exercise 24.3g [95% CI = 23.5 to 25.2 g], weight at necropsy: sedentary 24.5g [95% CI = 23.9 to 25.2 g] vs exercise 25.1g [95% CI = 24.2 to 25.9 g]) (Supplementary Figure 2, available online). Apoptosis (as measured by the downstream marker cleaved caspase-3) was 1.4-fold higher in exercise compared with control (sedentary 1544 cells/mm2 [95% CI = 1223 to 1865] vs exercise 2168 cells/mm2 [95% CI = 1620 to 2717], P = .048) (Figure 1B). Western blots of tumor extracts suggest that exercise may exert its proapoptotic effects via the extrinsic pathway by increasing Fas receptors (Exercise/Sedentary fold change = 6.6, rank sum < .001), which is associated with apoptosis mediated by caspase 8 (Exercise/Sedentary fold change = 7.9, rank sum = .044) (Figure 1C).
Figure 1.
Tumor response to voluntary aerobic exercise. BALB/c mice bearing 4T1 mammary tumors were randomly assigned to exercise or sedentary controls (n = 11–12/group) starting on day 0. Data are presented as means; error bars represent 95% confidence intervals (CIs). A) Tumor growth rate is statistically significantly slowed by exercise. * P = .004, ANCOVA. B) Representative color composites of immunostaining for apoptosis (cleaved caspase-3, green) in mice that exercised vs sedentary controls. Cellular nuclei are stained with Hoechst 33342 (blue). 5x objective, magnification 100%. Scale bar equals 33 µm. The density of apoptotic cells (cleaved caspase-3+ cells/mm2) is statistically significantly higher in the exercise group compared with sedentary controls. Gray lines = mean; black lines = 95% CI, P = .048, two-sided t test. C) Expression of apoptosis-related proteins in response to exercise. Protein was extracted from 4T1-luc tumors, and expression levels of components of the extrinsic (Fas, Caspase 8) and intrinsic (Bcl-2) pathways, as well as downstream mediators of apoptosis (XIAP, PARP), were visualized on western blots. Band intensity was quantified in comparison with actin loading controls. Both Fas (P < .001) and Caspase 8 (P = .044) expression were statistically significantly increased in mice that exercised, rank sum test. All statistical tests were two-sided.
Figure 2.
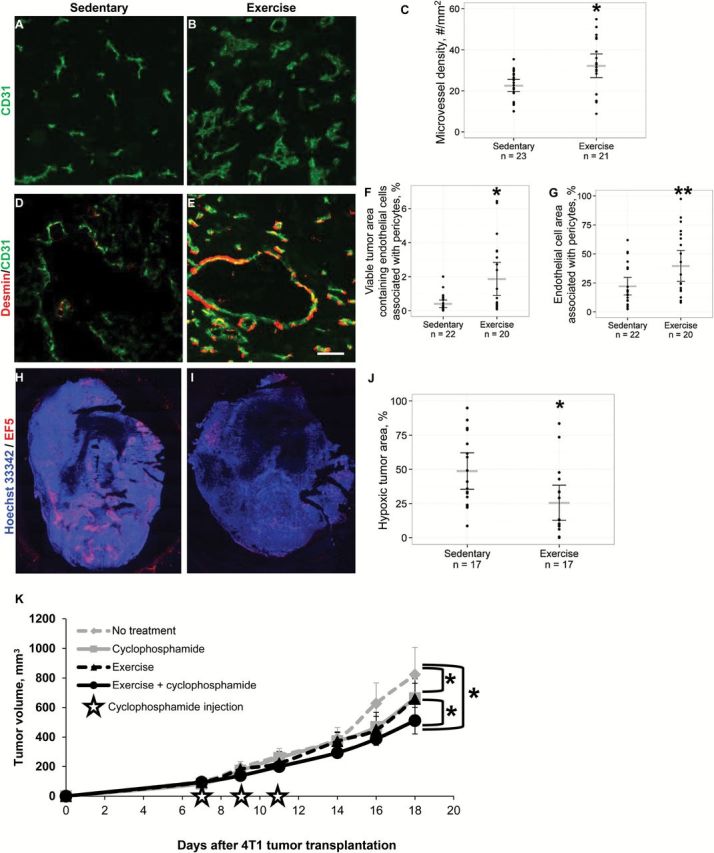
Effects of aerobic exercise on angiogenesis and vascular maturity in primary 4T1 breast tumors. Cellular nuclei were stained with Hoechst 33342 (blue). 5x objective, magnification 100%. A-C) Immunostaining for blood vessels shows that microvessel density (CD31, green) was statistically significantly higher in the tumors from exercised mice compared with sedentary controls. Scatter points = individual mice; gray lines = mean; black lines = 95% confidence intervals (CIs), P = .004, two-sided t test. D-E) Representative two-color composite images of vessel pericyte coverage determined by colocalization of CD31 and desmin (red) staining. Scale bar equals 40 µm. F) The percentage of tumor area containing pericyte-covered blood vessels (CD31-desmin colocalized pixels/total tumor area pixels x 100%) is significantly higher in the exercise group. Scatter points = individual mice; gray lines = mean; black lines = 95% CI, P = .006, two-sided t test. G) The percentage of vessel area covered by pericytes (CD31-desmin colocalized pixels/CD31+ pixels x 100%) was also significantly higher in tumors from running mice. Scatter points = individual mice; gray lines = mean; black lines = 95% CIs, P = .024, two-sided t test. H-J) Mice were injected with the hypoxia marker EF5 (red), which was detected by immunohistochemistry. Representative whole tumor images are shown. Quantification of hypoxic tumor percentage shows that exercise significantly reduces tumor hypoxia. Scatter points = individual mice; gray lines = mean; black lines = 95% CIs, P = .012, two-sided t test. K) Tumor response to voluntary aerobic exercise combined with cyclophosphamide chemotherapy. BALB/c mice bearing 4T1 mammary tumors were randomly assigned to voluntary wheel running or sedentary controls (n = 17 per group) starting on Day 0. Mice treated with chemotherapy received the maximal tolerated dose (100mg/kg) cyclophosphamide IP on days 7, 9, and 11. Data are presented as means; error bars are 95% CIs. Tumor growth rate is significantly slowed by exercise and cyclophosphamide monotherapies compared with sedentary controls (P < .001, ANCOVA). The combination of exercise and cyclophosphamide resulted in a statistically significant reduction in tumor growth rate relative to the groups that received no treatment, exercise alone, or cyclophosphamide alone (P < .01, ANCOVA). ANCOVA = Analysis of Covariance.
Microvessel density (assessed by CD31 immunohistochemistry) was statistically significantly higher in exercised mice compared with controls (sedentary, 22.5 vessels/mm2 [95% CI = 19.6 to 25.5] vs exercise, 32.2 vessels/mm2 [95% CI = 26.3 to 38.0], P = .004) (Figure 2, A-C). This was confirmed in the E0771 orthotopic breast cancer model (sedentary 30.3 vessels/mm2 [95% CI = 25.9 to 34.7] vs exercise 42.2 vessels/mm2 [95% CI = 34.2 to 50.2], P = .010) (Supplementary Figure 3, available online). Next, we evaluated tumor blood vessel maturity, because mature angiogenesis is more efficient for oxygen transport (27). Colocalization of CD31 with desmin, a marker of vascular maturity was 3- to 4-fold higher in exercised mice (Figure 2, D-G). Exercise statistically significantly increased the amount of viable tumor area containing pericyte-covered vessels compared with control (sedentary 0.4% of tumor area [95% CI = 0.2 to 0.6] vs exercise 1.9% [95% CI = 0.9 to 2.8], P = .006) and increased the vessel area covered by pericytes (sedentary 22.1% of vessel area [95% CI = 14.5 to 29.7] vs exercise 39.6% of vessel area [95% CI = 26.3 to 53.0], P = .024). These results were confirmed by staining for the pericyte marker NG2 (sedentary 0.8% of tumor area [95% CI = 0.2 to 1.4] vs exercise 3.3% of tumor area [95% CI = 2.1 to 4.5], P < .001; sedentary 16.5% of vessel area [95% CI = 9.1 to 23.9] vs exercise 46.6% (95% CI = 32.3 to 60.9], P < .001, data not shown). Together, these findings demonstrate that exercise increases tumor vessel density and improves vessel function and maturity.
We hypothesized that the improvements in vessel density and structure would lower intratumoral hypoxia; indeed, hypoxia was statistically significantly lower in exercised mice compared with controls, as assessed by the hypoxia marker EF5 (2-(2-nitro-1H-imidazole-1-yl)-N-(2,2,3,3,3-pentafluoropropyl acetamide)) (sedentary 48.8% of tumor area [95% CI = 35.5% to 62.1%] vs exercise 25.5% of tumor area [95% CI = 12.7 to 38.4], P = .012) (Figure 2, H-J). To provide mechanistic insight into how exercise modulates tumor vascularity and maturity, we evaluated the expression of key regulators of angiogenesis and normalization. Quantitative RT-PCR showed an approximately 1.5-fold increase in expression of VEGF, a key proangiogenic factor, in the exercised group compared with control (sedentary 0.83-fold [95% CI = 0.70 to 0.96] vs 1.14-fold [95% CI = 0.94 to 1.35], P = .011) (Supplementary Figure 4A, available online). In addition, exercise also downregulated PDGFR-β, which could account for the increased maturity of the vasculature in exercised mice by optimization of the PDGF-β pathway (Exercise/Sedentary fold change = 0.63, rank sum P = .013) (Supplementary Figure 4B, available online) (27). We next investigated whether exercise-induced improvements in tumor vascular structure altered tumor perfusion. MR perfusion imaging indicated that perfusion patterns of exercised 4T1 mice were more uniform and centralized relative to tumors from control mice; tumors from control mice, in general, displayed an outer ring-enhancement pattern of perfusion (Supplementary Figure 5, A-D, available online). This finding was confirmed in the E0771 model, which is consistent with our work in prostate cancer and preclinical data, showing that acute treadmill exercise increased tumor perfusion in rats (20,28) (Supplementary Figure 5E, available online).
Based on the observed effects on tumor physiology, we hypothesized that exercise would improve the efficacy of chemotherapy. BALB/c mice bearing 4T1-luc tumors were randomly assigned to one of four groups: no treatment, exercise alone, cyclophosphamide alone, or exercise plus cyclophosphamide (n = 17/group). The cyclophosphamide groups were injected with the maximally tolerated dose (100mg/kg) of cyclophosphamide intraperitoneally on days 7, 9, and 11, and tumor volume was measured three times weekly until death (Figure 2K). The rate of tumor growth was statistically significantly lower in the exercise + cyclophosphamide group compared with all other groups (P < .001). As expected, tumor growth delay was also statistically significantly increased in the exercise alone and cyclophosphamide alone groups, compared with control (P < .01). Intriguingly, there was no difference in tumor growth rate between cyclophosphamide alone and exercise alone.
Taken together, our data demonstrate a novel finding wherein aerobic exercise stimulates “productive” or “physiologic” angiogenesis and vascular normalization, leading to a substantial reduction in intratumoral hypoxia. This builds upon previous work from our group, which evaluated the effects of exercise on human breast cancer xenografts in immunodeficient mice (24). In those early experiments, we reported that exercise increases tumor perfusion, as measured by Hoechst-positive blood vessels. Here, we confirm that perfusion is indeed increased using advanced MRI techniques and demonstrate associated increases in vascular maturity in two strains of immunocompetent mice bearing orthotopic breast tumors, a more realistic model of the tumor microenvironment (25). Nevertheless, our findings need to be interpreted with caution and further experiments are needed to investigate whether exercise can be used to normalize tumor vasculature, decrease tumor hypoxia, and increase tumor sensitivity to chemotherapy in slower-growing tumor models that may be more clinically applicable. Additionally, the role of exercise in combination with other chemotherapy partner regimens with known effects on angiogenesis (eg, different agents including small molecule inhibitors, metronomic dosing) warrants further investigation (29).
The vascular normalizing effects of exercise were associated with inhibition of tumor growth, in association with activation of the proapoptotic Fas pathway. The increase in apoptosis resulted in decreased tumor cell mass, which would reduce oxygen consumption rate. Indeed, we found that exercise favorably modulates the three primary determinants of intratumoral hypoxia: tumor cell mass, apoptosis, and vessel density. These findings may provide initial mechanistic insights into observational data showing a strong inverse relationship between self-reported exercise and cancer-specific outcomes in women with early breast cancer (30,31). Finally, abnormal tumor vasculature and resulting hypoxia is a major source of therapeutic resistance. Our findings show that exercise, via its potent normalizing properties, may be an effective adjunct strategy that adds to the antitumor efficacy of systemic agents. Such a notion is supported by exploratory findings from two recent clinical trials. First, in women receiving neoadjuvant chemotherapy for early-stage breast cancer, we reported that addition of aerobic training was associated with substantial modulation of circulating proinflammatory cytokines as well as tumor gene expression pathways, including many that converge on NF-κß (32). This trial was not designed nor powered to examine changes in clinical response. However, in an exploratory subanalysis of a previously reported randomized controlled trial investigating the efficacy of aerobic or resistance training, relative to sedentary control patients, in 242 women receiving conventional adjuvant therapy for early breast cancer, Courneya et al. (33) found that when the exercise groups were combined there was a nonsignificant improvement in disease-free survival. Specifically, at a median of 89 months follow-up, the eight-year disease-free survival rates were 82.7% in exercise groups compared with 75.6% in the control group (hazard ratio =0.68, 95% CI=0.37 to 1.24, P=.21) (33). Overall, our findings shed new insights into the potential anticancer role of exercise as either adjuvant therapy or a combination therapeutic strategy in patients with solid tumors.
Funding
ASB was supported by DOD BC093532. LWJ is supported by grants from the National Cancer Institute. MWD is supported by NIH CA40355.
Supplementary Material
The authors have no conflicts of interest to report.
References
- 1. Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8(6):425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nature Rev Drug Discov. 2011;10(6):417–427. [DOI] [PubMed] [Google Scholar]
- 3. Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. [DOI] [PubMed] [Google Scholar]
- 4. Bottaro DP, Liotta LA. Cancer: Out of air is not out of action. Nature. 2003;423(6940):593–595. [DOI] [PubMed] [Google Scholar]
- 5. DeClerck K, Elble RC. The role of hypoxia and acidosis in promoting metastasis and resistance to chemotherapy. Fronti Biosci (Landmark Ed). 2010;15:213–225. [DOI] [PubMed] [Google Scholar]
- 6. Kelleher DK, Mattheinsen U, Thews O, et al. Blood flow, oxygenation, and bioenergetic status of tumors after erythropoietin treatment in normal and anemic rats. Cancer Res. 1996;56(20):4728–4734. [PubMed] [Google Scholar]
- 7. Brizel DM, Lin S, Johnson JL, et al. The mechanisms by which hyperbaric oxygen and carbogen improve tumour oxygenation. Br J Cancer. 1995;72(5):1120–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res. 2000;60(16):4440–4445. [PubMed] [Google Scholar]
- 9. Jones LW, Hornsby WE, Goetzinger A, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012;76(2):248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Overgaard J, Horsman MR. Modification of Hypoxia-Induced Radioresistance in Tumors by the Use of Oxygen and Sensitizers. Semin Radiat Oncol. 1996;6(1):10–21. [DOI] [PubMed] [Google Scholar]
- 11. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New Engl J Med. 2004;350(23):2335–2342. [DOI] [PubMed] [Google Scholar]
- 12. Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. [DOI] [PubMed] [Google Scholar]
- 13. Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–1234. [DOI] [PubMed] [Google Scholar]
- 14. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. New Engl J Med. 2006;355(24):2542–2550. [DOI] [PubMed] [Google Scholar]
- 15. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab vs paclitaxel alone for metastatic breast cancer. New Engl J Med. 2007;357(26):2666–2676. [DOI] [PubMed] [Google Scholar]
- 16. Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. [DOI] [PubMed] [Google Scholar]
- 17. Goel S, Duda DG, Xu L, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones LW, Viglianti BL, Tashjian JA, et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Applied Physiol. 2010;108(2):343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones LW, Antonelli J, Masko EM, et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Applied Physiol. 2012;113(2):263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCullough DJ, Stabley JN, Siemann DW, et al. Modulation of Blood Flow, Hypoxia, and Vascular Function in Orthotopic Prostate Tumors During Exercise. J Natl Cancer Inst. 2014;106(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones LW, Dewhirst MW. Therapeutic Properties of Aerobic Training After a Cancer Diagnosis: More Than a One-Trick Pony? J Natl Cancer Inst. 2014;106(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Secomb TW, Hsu R, Ong ET, et al. Analysis of the effects of oxygen supply and demand on hypoxic fraction in tumors. Acta Oncologica. 1995;34(3):313–316. [DOI] [PubMed] [Google Scholar]
- 23. Kimura H, Braun RD, Ong ET, et al. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res. 1996;56(23):5522–5528. [PubMed] [Google Scholar]
- 24. Teicher BA. Tumor models for efficacy determination. Mol Cancer Ther. 2006;5(10):2435–2443. [DOI] [PubMed] [Google Scholar]
- 25. Betof AS, Dewhirst MW, Jones LW. Effects and Potential Mechanisms of Exercise Training on Cancer Progression: A Translational Perspective. Brain Behav Immun. 2013;30(Supplement):S75-–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones LW, Eves ND, Courneya KS, et al. Effects of exercise training on antitumor efficacy of doxorubicin in MDA-MB-231 breast cancer xenografts. Clinical Cancer Res. 2005;11(18):6695–6698. [DOI] [PubMed] [Google Scholar]
- 27. Secomb TW, Hsu R, Dewhirst MW, et al. Analysis of oxygen transport to tumor tissue by microvascular networks. Int J Radiat Oncol Biol Phys. 1993;25(3):481–489. [DOI] [PubMed] [Google Scholar]
- 28. Jones LW, Viglianti BL, Tashjian JA, et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol. 2010;108(2):343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kerbel RS. Strategies for improving the clinical benefit of antiangiogenic drug based therapies for breast cancer. J Mammary Gland Biol Neoplasia. 2012;17(3–4):229–239. [DOI] [PubMed] [Google Scholar]
- 30. Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. [DOI] [PubMed] [Google Scholar]
- 31. Irwin ML, Smith AW, McTiernan A, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26(24):3958–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones LW, Fels DR, West M, et al. Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev Res (Phila). 2013;6(9):925–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Courneya KS, Segal RJ, McKenzie DC, et al. Effects of Exercise during Adjuvant Chemotherapy on Breast Cancer Outcomes. Med Sci Sports Exerc. 2014;46(9):1744–1751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.