Abstract
Background:
The aim of this study was to quantify the benefits and harms of mammography screening after age 74 years, focusing on the amount of overdiagnosis of invasive breast cancer and ductal carcinoma in situ (DCIS).
Methods:
Three well-established microsimulation models were used to simulate a cohort of American women born in 1960. All women received biennial screening starting at age 50 years with cessation ages varying from 74 up to 96 years. We estimated the number of life-years gained (LYG), quality-adjusted life-years, breast cancer deaths averted, false-positives, and overdiagnosed women per 1000 screens.
Results:
The models predicted that there were 7.8 to 11.4 LYG per 1000 screens at age 74 years (range across models), decreasing to 4.8 to 7.8 LYG per 1000 screens at age 80 years, and 1.4 to 2.4 LYG per 1000 screens at age 90 years. When adjusted for quality-of-life decrements, the LYG decreased by 5% to 13% at age 74 years and 11% to 22% at age 80 years. At age 90 to 92 years, all LYG were counterbalanced by a loss in quality-of-life, mainly because of the increasing number of overdiagnosed breast cancers per 1000 screens: 1.2 to 5.0 at age 74 years, 1.8 to 6.0 at age 80 years, and 3.7 to 7.5 at age 90 years. The age at which harms began to outweigh benefits shifted to a younger age when larger or longer utility losses because of a breast cancer diagnosis were assumed.
Conclusion:
The balance between screening benefits and harms becomes less favorable after age 74 years. At age 90 years, harms outweigh benefits, largely as a consequence of overdiagnosis. This age was the same across the three models, despite important model differences in assumptions on DCIS.
Mammography screening has been shown to be effective in reducing breast cancer mortality in randomized trials and nationwide screening programs in women age 50 to 74 years (1–4). Benefits and harms of screening mammography in women age 74 years and older are less well established and surrounded by uncertainty, because none of the randomized controlled trials designed to evaluate screening mammography included sufficient numbers of women age 74 years and older.
There are several factors that might influence the balance between benefits and harms of screening women older than 74 years. Because breast cancer incidence increases with age (5,6) and sensitivity is higher in the older age groups (7), the benefits of screening may be larger for older than for younger women. On the other hand, the benefits of screening might be limited because of the higher death rate from competing causes with advancing age. With regard to the harms of screening, the false-positive rates have been found to decrease or remain stable after age 74 years (7), while the amount of overdiagnosis may on average increase with age because of the shorter remaining life-expectancy.
The extent to which overdiagnosis occurs is uncertain and widely debated, reflected in the wide range of estimates of up to 54% that have been published (8–15). Overdiagnosis is generally defined as “the detection of tumors that would not have been detected in a woman’s lifetime in the absence of screening” (4,16). The difficulties associated with estimating the amount of overdiagnosis are reflected in this definition; once a screening program has been initiated it is impossible to know what would have happened in the absence of screening. The Independent UK Panel on Breast Cancer Screening stated that the results from observational studies support the occurrence of overdiagnosis, but estimates of its magnitude are unreliable (4). An alternative effective method to address this issue is to use microsimulation models, which represent tumor growth and/or transitions among cancer states, incorporating age-related difference in tumor biology, and evaluate screening effects based on the synthesis of detailed data.
Despite the uncertainty around the benefits and harms, many women age 75 years and older are being screened in the United States. A recent study found that 62% of women age 75 to 79 years and 50% of women over age 80 years reported receiving a mammogram in the past two years (17). A physician recommendation is a strong determinant of mammography screening (17–19), and 70% to 86% of primary care physicians would re-commend mammography for a healthy woman age 80 years (20).
There is, however, no consensus in the United States on whether or not to recommend screening for women beyond age 74 years. For example, the American Cancer Society recommends mammography screening as long as women are in good health (21), while the US Preventive Services Task Force (USPSTF) recommends screening from age 50 to 74 years and concluded that “the current evidence is insufficient to assess the additional benefits and harms of screening mammography in women 75 years or older” (22).
Previous modeling studies have assessed the benefits and harms of screening mammography, some of which have included older women. However, most of these studies focused on a subset of outcomes, without focusing on overdiagnosis explicitly (23), were based on the results of one model only (24,25), and/or looked at extending screening using large age ranges (eg, stopping at age 79 vs 70 years) (26).
In the present study, we therefore quantified the additional benefits (deaths averted and life-years gained) and harms (overdiagnosis and false-positives) of continuing mammography screening after age 74 years using three well-established microsimulation models. Furthermore, we aimed to provide information about the harms and benefits of screening in a meaningful way, as previous studies have found that women as well as primary care physicians may have difficulties in understanding cancer screening statistics (27,28). Therefore, we presented the outcomes in two ways, as previously recommended (29): Benefits and harms were presented on the same scale (ie, as absolute numbers per 1000 screens) and combined in a single metric, quality-adjusted life-years (QALYs). We also estimated the age at which mammography screening no longer resulted in a positive number of QALYs, as a proxy for the age at which the harms began to outweigh the benefits of screening.
Methods
Model Overview
We used three microsimulation models developed as part of the Cancer Intervention and Surveillance Modeling Network (CISNET): model E (Erasmus MC, Erasmus University Medical Center, Rotterdam, the Netherlands), model G-E (Georgetown University Medical Center, Washington, DC, and Albert Einstein College of Medicine, Bronx, NY), and model W (University of Wisconsin, Madison, WI, and Harvard Medical School, Boston, MA). The models have been described in detail elsewhere (30–32), and information about the models can be found online (http://cisnet.cancer.gov/). Briefly, the models simulated life histories for individual women. After estimating breast cancer incidence and mortality in the absence of screening and treatment, the models overlaid screening use and improvements in survival associated with treatment advances. A schematic representation of the influence of breast cancer screening on (simulated) life histories is shown in Figure 1.
Figure 1.
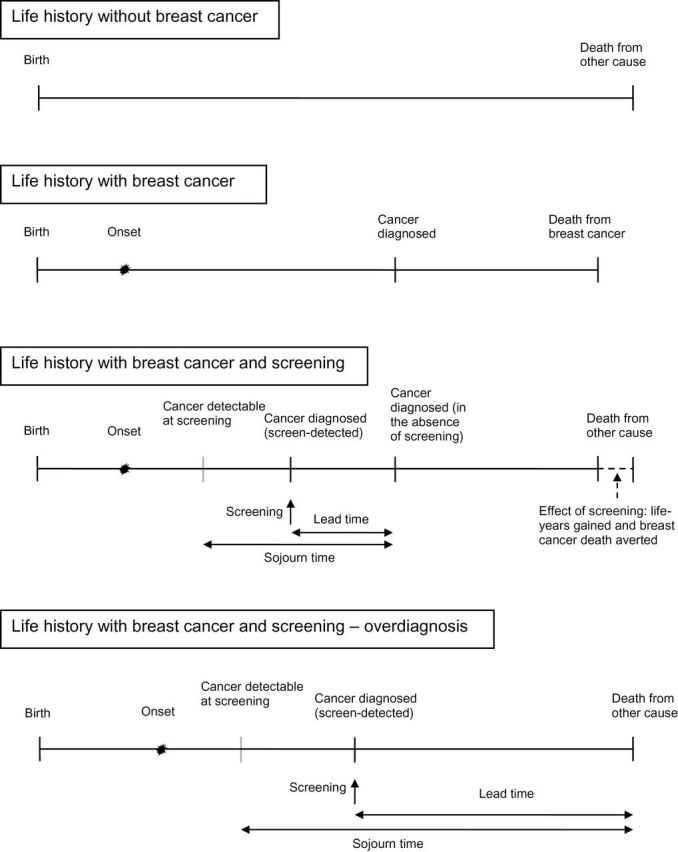
Schematic overview of simulated life histories and effect of screening. Sojourn time is the duration of the preclinical, screen-detectable phase of the tumor, and lead time is the interval from screen detection to the time of clinical diagnosis, when the tumor would have been diagnosed without screening. If the tumor is screen-detected without a clinical diagnosis in the absence of screening, the detection represents overdiagnosis. Lead time represents additional years that are lived with breast cancer because of screening.
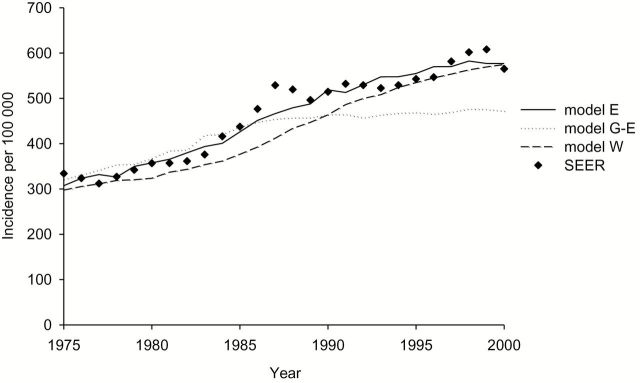
All three models incorporated age-related difference in tumor biology. Models E and W explicitly modeled tumor growth, and model G-E incorporated age-specific sojourn times and stage. At higher ages, the chance that a slow-growing tumor is detected increases. In addition, all three models included ductal carcinoma in situ (DCIS) with three different types of preclinical DCIS: regressive DCIS, DCIS that is diagnosed clinically, and DCIS that progresses to invasive disease. Model W also assumed that some cases of small invasive cancer are nonprogressive and have limited malignant potential (LMP). The models used incidence and mortality data by age and calendar year (1975–1999) from the Surveillance, Epidemiology, and End Results (SEER) Program to estimate natural history parameters, including the transition rates of DCIS becoming invasive or clinically diagnosed and DCIS regression rates (model E) and the proportion of DCIS with LMP (model G-E and model W). All models have been previously validated and adequately reproduced SEER breast cancer incidence in women age 70 to 79 years (Figure 2) and age-adjusted mortality rates over time (30–33).
Figure 2.
Age-adjusted breast cancer incidence rates from 1975 to 2000 predicted by the models vs reported to SEER for women age 70 to 79 years. SEER = Surveillance, Epidemiology, and End Results.
Model Parameters
We used a common set of age-specific model inputs (23) for the natural history of disease (incorporating age-related difference in tumor biology), breast cancer incidence (34), breast cancer–specific survival (35), and competing non–breast cancer causes of death (36). A cohort of women born in 1960 was simulated and followed throughout their entire lifetime. We assumed 100% adherence with screening and adjuvant treatment guidelines. In addition, we applied quality-of-life decrements by attaching weights to specific health states for women undergoing a mammogram and diagnostics (37) and life-years with breast cancer by stage of disease at diagnosis (38) (Table 1) to estimate QALYs.
Table 1.
Utility values and durations of different health states used in the simulation models of breast cancer
| State | Utility | 1-utility | Duration | Source |
|---|---|---|---|---|
| Screening attendance | 0.994 | 0.006 | 1 wk | de Haes (37) |
| Diagnostic phase | 0.895 | 0.105 | 5 wk | de Haes (37) |
| Breast cancer by stage of disease at diagnosis | ||||
| Local or DCIS* | 0.90 | 0.10 | 2 y | Stout (38) |
| Regional | 0.75 | 0.25 | 2 y | Stout (38) |
| Distant | 0.60 | 0.40 | Until death | Stout (38) |
*DCIS = ductal carcinoma in situ.
Analysis
Biennial screening with 100% adherence started at age 50 years with varying cessation ages of screening. First, we simulated the screening policy currently recommended by the USPSTF (biennial screening from age 50–74 years) and assessed the benefits and harms per 1000 women alive at age 50 years, followed until death. We then determined the benefits and harms of the last screen (at age 74 years), and the additional benefits and harms of adding one more screen after the last screening test were estimated for increasing stopping ages of up to 96 years. We estimated the number of life-years gained (LYG), QALYs, breast cancer deaths averted, false-positive exams, and overdiagnosed women for each screening scenario per 1000 screens. The number of overdiagnosed women was calculated as the difference in the predicted number of diagnosed women in the presence of screening and the predicted number of diagnosed women in the absence of screening.
Sensitivity Analysis
To evaluate how the results were influenced by certain assumptions and parameter values, we performed several sensitivity analyses. In particular, we assessed the effect of using different utility decrements associated with a breast cancer diagnosis for DCIS and local disease as used in previous studies (0.05, 0.15, and 0.20, instead of 0.10) (39,40). In addition, we assessed the effect of different durations for the utility decrements (5 years instead of 2 years for the effect of diagnosis in the DCIS, local or regional state).
Results
The models estimated that if 1000 women age 50 years underwent biennial screening from age 50 to 74 years (thus, undergoing a total of 11 117–11 337 mammograms), between 8.0 and 8.9 breast cancer deaths were prevented, depending on the model, and there were 132 to 142 LYG (Table 2). The models predicted that in the absence of screening there would be 137 to 154 breast cancers diagnosed. In the presence of screening this increased to 151 to 170 diagnoses. Thus, among these 1000 women, 5.9 to 33.0 women (0.5–3.0 per 1000 screens) were diagnosed in the presence of screening but would not have been diagnosed in the absence of screening, and were thus overdiagnosed.
Table 2.
Benefits and harms of biennial mammography screening age 50–74 y, assuming 100% attendance
| Outcomes per 1000 women alive at age 50 y | Model E | Model G-E | Model W |
|---|---|---|---|
| Number of mammograms | 11 151 | 11 337 | 11 117 |
| Number of breast cancers in the absence of screening | 139 | 154 | 137 |
| Number of breast cancers | 151 | 159 | 170 |
| Screen detected BCs (% DCIS) | 84 (26) | 81 (19) | 95 (30) |
| Life-years gained (per 1000 screens) | 136 (12.2) | 142 (12.6) | 132 (11.8) |
| QALYs (per 1000 screens)* | 132 (12) | 135 (12) | 119 (11) |
| Reduction in LYG after adjustment for QoL, % | 3 | 6 | 9 |
| BC deaths in the absence of screening | 31.3 | 36.0 | 35.3 |
| BC deaths | 22.4 | 28.0 | 27.2 |
| BC deaths averted (per 1000 screens) | 8.9 (0.8) | 8.0 (0.7) | 8.1 (0.7) |
| False-positives (per 1000 screens) | 865 (78) | 1030 (91) | 915 (82) |
| Overdiagnosis DCIS (per 1000 screens) | 9.2 (0.8) | 2.7 (0.2) | 11.4 (1.0) |
| Overdiagnosis invasive BCs (per 1000 screens) | 3.0 (0.3) | 3.2 (0.3) | 21.7 (2.0) |
| Overdiagnosis Total (per 1000 screens) | 12.2 (1.1) | 5.9 (0.5) | 33.0 (3.0) |
| % of all BCs detected at ages 50+† | 8 | 4 | 19 |
| % of BCs detected during screening‡ | 11 | 6 | 27 |
| % of screen-detected BCs§ | 14 | 7 | 35 |
* Example calculation of quality-adjusted life-years (QALYs): Model G-E predicts that screening biennially from age 50 to 74 years results in 142 life-years gained (LYG). These LYG are reduced when correcting for quality-of-life in different phases: undergoing screening results in a utility loss of 1.31 (11 337 screens x 0.006 [1-utility] x 7/365 [duration 1 week]) and undergoing testing because of additional positive tests results in a utility loss of 10.43 ([1030+6] x 0.105 [1-utility] x 35/365 [duration 5 weeks]). In addition, the number of life-years after breast cancer (BC) diagnosis in different stages is estimated in a situation with and without screening, which results in an increase in life-years after diagnosis in local stage and ductal carcinoma in situ (DCIS) of 44.47, resulting in a utility loss of 4.5 (44.47 x 0.10). Because of earlier diagnosis a reduction in life-years after diagnosis in the regional and distant stage is predicted, resulting in utility losses of -5.35 (-21.44 LY x 0.25 [1-utility]) and -2.97 (-7.44 x 0.40 [1-utility]), respectively. Taken together, this results in a total reduction in LYG of 7.8 (1.31+10.43+4.45-5.35-2.97), and thus 142.4 LYG - 7.8 = 134.6 QALYs. The corresponding reduction in LYG after adjustment for quality of life is 7.8/134.6 = 6%. BC = breast cancer; DCIS = ductal carcinoma in situ; LYG = life-years gained; QALY = quality-adjusted life-year; QoL = quality of life.
† Number of excess cancers as a proportion of cancers diagnosed from age 50 years to death.
‡ Number of excess cancers as a proportion of cancers diagnosed during the screening period (between age 50 and 74 years).
§ Number of excess cancers as a proportion of screen-detected cancers.
Extending screening beyond age 74 years resulted in a steep increase in the number of overdiagnosed women (Figure 3, A-C). The number of overdiagnosed breast cancers (DCIS + invasive) increased from 1.2 to 5.0 per 1000 screens at age 74 years, 1.8 to 6.0 at age 80 years, and 3.7 to 7.5 at age 90 years.
Figure 3.
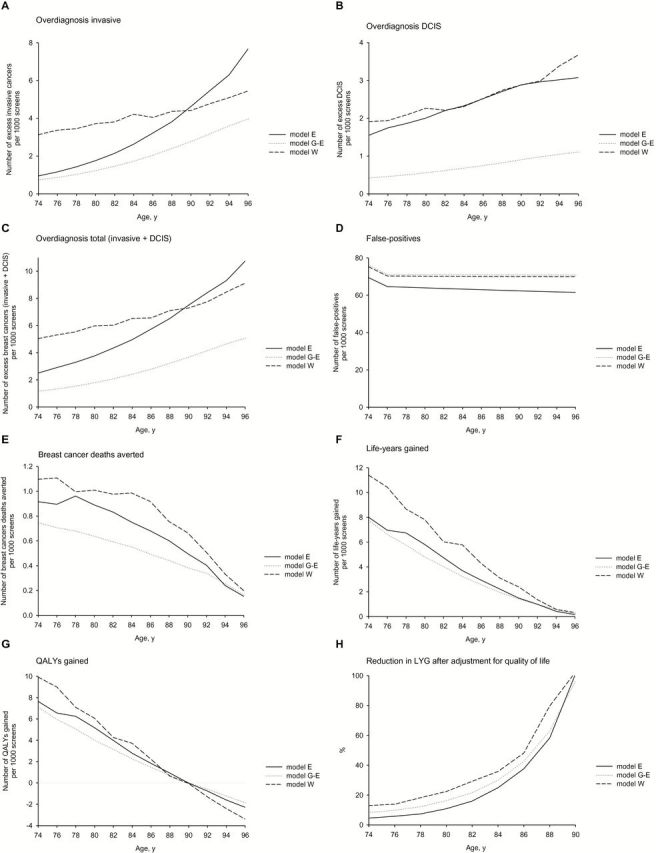
Benefits and harms of continuing screening after age 74 years (outcomes per 1000 screens at increasing ages). A) Number of excess invasive cancers per 1000 screens. B) Number of excess ductal carcinomas in situ (DCIS) per 1000 screens. C) Number of excess total breast cancers per 1000 screens (invasive + DCIS). D) Number of false-positives. E) Number of breast cancer deaths averted per 1000 screens. F) Number of life-years gained per 1000 screens. G) Number of quality-adjusted life-years gained per 1000 screens. H) Relative reduction in LYG after adjustment for quality of life (%). DCIS = ductal carcinoma in situ; LYG = life-years gained; QALY = quality-adjusted life-years.
If overdiagnosis is expressed as a percentage of screen-detected cancers, it also increased steeply in all three models (Table 3). Screening women from ages 50 to 74 results in 5% to 32% (range between the models) of the invasive breast cancers that were screen-detected being overdiagnosed, increasing to 14% to 36% for a screen at age 80 years and 28% to 41% for a screen at age 90 years (Table 3). For DCIS, the percentages were higher in all three models, and increased from 18% to 41% for screening from age 50 to 74 years to 35% to 72% for a screen at age 80 years and 53% to 91% for a screen at age 90 years.
Table 3.
Percentage of screen-detected breast cancers (invasive and DCIS) that are overdiagnosed* by screening age and model
| Stage at diagnosis | Screening age, y† | Model E, % | Model G-E, % | Model W, % |
|---|---|---|---|---|
| Invasive | 50–74 | 5 | 5 | 32 |
| 74 | 11 | 9 | 35 | |
| 80 | 17 | 14 | 36 | |
| 90 | 37 | 28 | 41 | |
| DCIS | 50–74 | 41 | 18 | 40 |
| 74 | 61 | 27 | 47 | |
| 80 | 72 | 35 | 52 | |
| 90 | 91 | 53 | 60 | |
| Total | 50–74 | 14 | 7 | 35 |
| 74 | 22 | 12 | 39 | |
| 80 | 29 | 17 | 41 | |
| 90 | 48 | 32 | 47 |
* Number of excess cancers as a proportion of cancers detected at screening. DCIS = ductal carcinoma in situ.
† All screens with 100% attendance. Screening age 50–74 years: biennial screening starting at age 50 years and ending at age 74 years. The percentage includes all excess breast cancers detected at screening between age 50 and 74 years divided by all screen-detected breast cancers between age 50 and 74 years. Screening age 74, 80, 90 years: all women have been screened biennially up to the screening age. The percentage includes all excess breast cancers detected at screening at age 74, 80, and 90 years divided by all screen-detected breast cancers at age 74, 80, and 90 years, respectively.
The models predicted that the numbers of false-positives were relatively stable after age 74 years (Figure 3D) and that screening beyond age 74 years resulted in benefits in terms of breast cancer deaths averted and LYG with no upper age limit, although the number of breast cancer deaths averted and LYG per 1000 screens steadily declined with increasing age (Figure 3, E and F); screening women at age 74 years results in 7.8 to 11.4 LYG per 1000 screens, which decreased to 4.8 to 7.8 for a screen at age 80 years and 1.4 to 2.4 for a screen at age 90 years. Thus, a breast cancer death averted at age 74 years saves 15.4 to 17.9 life-years, while at age 80 years this is 6.5 to 7.7 life-years, and the amount of LYG per death averted decreases to 3.0 to 3.7 at age 90 years.
The number of QALYs gained per 1000 screens decreased steadily with increasing age from 7.1 to 9.9 at age 74 years to 4.0 to 6.1 at age 80 years, and 2.4 to 3.7 at age 84 years. QALYs were still positive for screening up to age 90 years (Figure 3G). The number of QALYs gained became negative at age 90 years in models E and W and at age 92 years in model G-E. In other words, at age 90 to 92 years, all LYG were counterbalanced by a loss in quality of life because of undergoing a screening test and diagnostics and additional life-years with disease that would have been spent in a healthy state in the absence of screening. The percentages of LYG that were counterbalanced by losses in quality of life increased steeply with increasing age at screening: when adjusted for quality-of-life decrements, the LYG decreased by 5% to 13% at age 74 years and 11% to 22% at age 80 years (Figure 3H).
Sensitivity Analysis
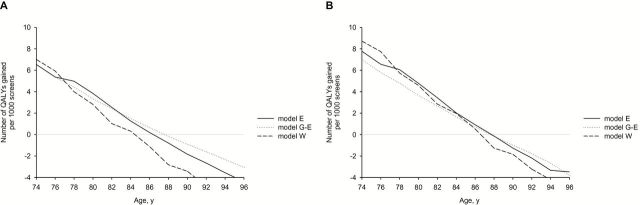
If a larger utility loss because of a breast cancer diagnosis was assumed for DCIS and local disease, the age at which QALYs became negative shifted to a younger age in all three models. For instance, when the utility loss is 0.2 instead of 0.1, the age at which the number of QALYs gained became negative shifted to age 86 years in model W and to age 88 years in models E and G-E (Figure 4A). If instead of a two-year duration, a five-year duration for the utility decrements was assumed, the age shifted to age 88 years in all three models (Figure 4B).
Figure 4.
The number of quality-adjusted life-years (QALYs) gained of continuing screening after age 74 years (outcomes per 1000 screens at increasing ages). A) QALYs gained per 1000 screens assuming a utility decrement of 0.2 instead of 0.1 for ductal carcinoma in situ (DCIS) and local disease. B) Assuming utility decrements for a breast cancer diagnosis of DCIS, local and regional disease for a duration of five years instead of two years. DCIS = ductal carcinoma in situ; LYG = life-years gained; QALY = quality-adjusted life-years.
Discussion
The model results were very consistent in estimating the age at which the harms began to outweigh the benefits of mammography screening. At age 90 to 92 years, all LYG were counterbalanced by a loss in quality of life, mainly because of the increasing amount of overdiagnosis. The consistency between models was remarkable, because the models included different assumptions on the natural history of DCIS.
Despite the consistency of our results, some limitations have to be considered. We estimated the benefits and harms for a cohort of women born in 1960, who on average have a remaining life expectancy at age 74 of 13 to 14 years. If life expectancy for older women continues to increase in the future, then we might have underestimated the benefits and overestimated the harms of screening. In addition, life expectancy varies by health status. Recently, two studies focused on more individualized decision-making about mammography in older women. A recently published review reported that observational studies favor extending screening mammography to older women who have a life expectancy of more than 10 years (41). However, tools to accurately estimate remaining life expectancy are still limited. Another recently published study showed that when screening at a certain age, for healthy women the balance between benefits and harms is more favorable than for women with severe comorbidity. For women with no, mild, moderate, and severe comorbid conditions, screening until ages 76, 74, 72, and 66 years, respectively, resulted in harms and benefits similar to screening average health persons until age 74 years (42).
To calculate QALYs we used utility values; ie, we attached weights to certain health states. We found that adjusting for quality of life (thus using QALYs instead of LYG) has only a small effect for ages 50 to 74 years, but an increasing effect at older ages because of the increasing amount of overdiagnosis, and hence additional life-years with disease. In addition, we found that the age at which harms began to outweigh benefits was sensitive to the utility values and shifted to a younger age when a larger disutility of the disease state or a longer duration for the utility decrements was assumed. This emphasizes the need for validated data on patient’s utilities and durations for specific breast cancer disease states and a better understanding of utilities for different breast cancer disease states among older women. The advantages and disadvantages of using utilities and QALYs have been widely discussed (43,44). For the present study, the most important drawback is that individual preferences might diverge from the assumed values. For those women, looking at the benefits and harms per 1000 screens might be more informative than looking at the number of QALYs.
Our results on QALYs gained are largely in line with what has been previously reported. For example, a previous study found no reduction in the number of QALYs gained, as the upper age of screening increases when optimistic assumptions about the preclinical durations were made. However, when pessimistic assumptions were made, the QALYs gained became negative when screening was continued beyond age 80 years (45). The estimates from the present study are in between those from the optimistic and pessimistic scenarios (45).
The amount of overdiagnosis has been the topic of intense debate, partly because of methodological issues. Overdiagnosis is overestimated when calculations are derived from the implementation period of a screening program and when there is insufficient follow-up to observe a reduction in breast cancer incidence (9). Similarly, the range of overdiagnosis estimates is considerably smaller and estimates are lower (1% to 10%) when only studies that adequately adjust for lead time and changes in breast cancer risk are included (46).
The models estimated that 4% to 19% of all breast cancers detected in women age 50 years and over who undergo biennial screening from age 50 to 74 years are overdiagnosed. This range is consistent with the overdiagnosis estimate of 11% of cancers in women invited for screening from the Independent UK Panel on Breast Cancer Screening. A recent study on overdiagnosis in the United States estimated that 31% of breast cancers diagnosed in 2008 were overdiagnosed (15). This estimate cannot be directly compared with the estimates presented here, as we estimated overdiagnosis for specific screening scenarios and not for the screening as observed in the United States. The estimates from the models will, however, likely be lower, mainly because the models incorporate an age-period-cohort model, which incorporates a stronger increase in background incidence over time (34).
The model results showed a large range in overdiagnosis estimates. For invasive disease, one model (model W) estimated markedly higher overdiagnosis than the other two models up to age 86 years. This difference between models is because of the fact that model W includes a subset of small invasive cancers with limited malignant potential, which are assumed to grow only to a limited size and then disappear.
There was also a large difference in the predicted amount of overdiagnosis of DCIS between models, which likely reflects the continued uncertainty about DCIS natural history (47). Little is known on the natural history of DCIS, because DCIS is usually removed as soon as it is detected. There is evidence for progression of DCIS from studies in women with low-grade DCIS that is initially mistakenly diagnosed as benign, reporting that 14% to 60% of those women develop invasive cancer within 10 to 20 years (48–50). There is, however, also evidence that not all DCIS become invasive, for example from autopsy studies that found a prevalence of DCIS of 0% to 15% in women not known to have had breast cancer (51). Our results do not provide additional information on the natural history of DCIS, because all three models adequately replicated incidence trends, despite differences in the assumed natural history of DCIS. This finding is in line with a previous modeling study that found that two alternative models with extreme assumptions on progression and regression rates of DCIS fit the observed breast cancer incidence in the Netherlands equally well (52).
The models estimated that at age 90 years, 53% to 91% of the screen-detected DCIS are overdiagnosed, indicating that for every 1000 screens performed at age 90 years, one to three women are overdiagnosed with DCIS. The majority of those women will undergo treatment for their disease; almost all women (97.5%) diagnosed with DCIS undergo a surgical procedure (53), 61% of women diagnosed with DCIS receive radiotherapy (54), and 47% receive adjuvant hormonal therapy (55). Although older women tend to receive less aggressive treatment than younger women (56), older women undergoing treatment may be exposed to more toxicity than younger women (57). Future research on the biological behavior of DCIS and predictors of risk for developing invasive disease is needed in order to prevent harm from treating nonprogressive disease (58,59).
In summary, the balance between benefits and harms of mammography becomes less favorable beyond age 74 years because of the increasing amount of overdiagnosis. For women with average life expectancy, beyond age 90 years screening harms outweigh benefits. An upper age limit of breast cancer screening, therefore, seems appropriate. The appropriate upper age for an individual woman depends on the weight she attaches to specific benefits and harms. From a societal perspective, the willingness to pay for a QALY may also need to be taken into account. If we were better able to distinguish between subtypes of DCIS that progress and those that do not, harm from treating nonprogressive disease could be prevented.
Funding
Data collection and sharing was supported by the National Cancer Institute–funded Breast Cancer Surveillance Consortium (BCSC) (U01CA63740, U01CA86076, U01CA86082, U01CA63736, U01CA70013, U01CA69976, U01CA63731, U01CA70040, HHSN261201100031C). A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are provided at: http://breastscreening.cancer.gov/.
This work was supported by the National Cancer Institute at the National Institutes of Health (grant numbers U01 CA152958, U01 CA88283). The funding sources had no role in the design and conduct of the study, the collection, management, analysis, or interpretation of the data, the preparation, review, or approval of the manuscript, nor the decision to submit this manuscript for publication.
References
- 1. Nystrom L, Andersson I, Bjurstam N, et al. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359(9310):909–919. [DOI] [PubMed] [Google Scholar]
- 2. Tabar L, Vitak B, Chen HH, et al. The Swedish Two-County Trial twenty years later. Updated mortality results and new insights from long-term follow-up. Radiol Clin North Am. 2000;38(4):625–651. [DOI] [PubMed] [Google Scholar]
- 3. Otto SJ, Fracheboud J, Looman CW, et al. Initiation of population-based mammography screening in Dutch municipalities and effect on breast-cancer mortality: a systematic review. Lancet. 2003;361(9367):1411–1417. [DOI] [PubMed] [Google Scholar]
- 4. Independent U. K. Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–1786. [DOI] [PubMed] [Google Scholar]
- 5. Ferlay J, Shin H, Bray F, et al. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. http://globocan.iarc.fr.
- 6. SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases. Linked To County Attributes - Total U.S., 1969–2010 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2012. Based on the November 2011 submission.
- 7. Sinclair N, Littenberg B, Geller B, et al. Accuracy of screening mammography in older women. AJR Am J Roentgenol. 2011;197(5):1268–1273. [DOI] [PubMed] [Google Scholar]
- 8. Biesheuvel C, Barratt A, Howard K, et al. Effects of study methods and biases on estimates of invasive breast cancer overdetection with mammography screening: a systematic review. Lancet Oncol. 2007;8(12):1129–1138. [DOI] [PubMed] [Google Scholar]
- 9. de Gelder R, Heijnsdijk EA, van Ravesteyn NT, et al. Interpreting overdiagnosis estimates in population-based mammography screening. Epidemiol Rev. 2011;33(1):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jorgensen KJ, Gotzsche PC. Overdiagnosis in publicly organised mammography screening programmes: systematic review of incidence trends. BMJ. 2009;339:b2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jorgensen KJ, Keen JD, Gotzsche PC. Is mammographic screening justifiable considering its substantial overdiagnosis rate and minor effect on mortality? Radiology. 2011;260(3):621–627. [DOI] [PubMed] [Google Scholar]
- 12. Kalager M, Adami HO, Bretthauer M, et al. Overdiagnosis of invasive breast cancer because of mammography screening: results from the norwegian screening program. Ann Intern Med. 2012;156(7):491–499. [DOI] [PubMed] [Google Scholar]
- 13. Kopans DB, Smith RA, Duffy SW. Mammographic screening and “overdiagnosis”. Radiology. 2011;260(3):616–620. [DOI] [PubMed] [Google Scholar]
- 14. Duffy SW, Tabar L, Olsen AH, et al. Absolute numbers of lives saved and overdiagnosis in breast cancer screening, from a randomized trial and from the Breast Screening Programme in England. J Med Screen. 2010;17(1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. [DOI] [PubMed] [Google Scholar]
- 16. Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Intern Med. 2013;158(11):831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bellizzi KM, Breslau ES, Burness A, et al. Prevalence of cancer screening in older, racially diverse adults: still screening after all these years. Arch Intern Med. 2011;171(22):2031–2037. [DOI] [PubMed] [Google Scholar]
- 18. Fox SA, Murata PJ, Stein JA. The impact of physician compliance on screening mammography for older women. Arch Intern Med. 1991;151(1):50–56. [PubMed] [Google Scholar]
- 19. Meissner HI, Breen N, Taubman ML, et al. Which women aren’t getting mammograms and why? (United States). Cancer Causes Control. 2007;18(1):61–70. [DOI] [PubMed] [Google Scholar]
- 20. Leach CR, Klabunde CN, Alfano CM, et al. Physician over-recommendation of mammography for terminally ill women. Cancer. 2012;118(1):27–37. [DOI] [PubMed] [Google Scholar]
- 21. Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2012: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2012;62:129–142. [DOI] [PubMed] [Google Scholar]
- 22. U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726, W-236. [DOI] [PubMed] [Google Scholar]
- 23. Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms Ann Int Med. 2009;151:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barratt A, Howard K, Irwig L, et al. Model of outcomes of screening mammography: information to support informed choices. BMJ. 2005;330(7497):936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duffy SW, Sasieni P, Olsen AH, et al. Modelling the likely effect of the increase of the upper age limit from 70 to 73 for breast screening in the UK National Programme. Stat Methods Med Res. 2010;19(5):547–555. [DOI] [PubMed] [Google Scholar]
- 26. Mandelblatt JS, Schechter CB, Yabroff KR, et al. Toward optimal screening strategies for older women. Costs, benefits, and harms of breast cancer screening by age, biology, and health status. J Gen Intern Med. 2005;20(6):487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwartz LM, Woloshin S, Sox HC, et al. US women’s attitudes to false-positive mammography results and detection of ductal carcinoma in situ: cross sectional survey. BMJ. 2000;320(7250):1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wegwarth O, Schwartz LM, Woloshin S, et al. Do physicians understand cancer screening statistics? A national survey of primary care physicians in the United States. Ann Intern Med. 2012;156(5):340–349. [DOI] [PubMed] [Google Scholar]
- 29. Harris R, Sawaya GF, Moyer VA, et al. Reconsidering the criteria for evaluating proposed screening programs: reflections from 4 current and former members of the U.S. Preventive services task force. Epidemiol Rev. 2011;33(1):20–35. [DOI] [PubMed] [Google Scholar]
- 30. Fryback DG, Stout NK, Rosenberg MA, et al. The Wisconsin Breast Cancer Epidemiology Simulation Model. J Natl Cancer Inst Monogr. 2006(36):37–47. [DOI] [PubMed] [Google Scholar]
- 31. Mandelblatt J, Schechter CB, Lawrence W, et al. The SPECTRUM population model of the impact of screening and treatment on U.S. breast cancer trends from 1975 to 2000: principles and practice of the model methods. J Natl Cancer Inst Monogr. 2006(36):47–55. [DOI] [PubMed] [Google Scholar]
- 32. Tan SY, van Oortmarssen GJ, de Koning HJ, et al. The MISCAN-Fadia continuous tumor growth model for breast cancer. J Natl Cancer Inst Monogr. 2006(36):56–65. [DOI] [PubMed] [Google Scholar]
- 33. Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. [DOI] [PubMed] [Google Scholar]
- 34. Holford TR, Cronin KA, Mariotto AB, et al. Changing patterns in breast cancer incidence trends. J Natl Cancer Inst Monogr. 2006(36):19–25. [DOI] [PubMed] [Google Scholar]
- 35. Cronin KA, Mariotto AB, Clarke LD, et al. Additional common inputs for analyzing impact of adjuvant therapy and mammography on U.S. mortality. J Natl Cancer Inst Monogr. 2006(36):26–29. [DOI] [PubMed] [Google Scholar]
- 36. Rosenberg MA. Competing risks to breast cancer mortality. J Natl Cancer Inst Monogr. 2006(36):15–19. [DOI] [PubMed] [Google Scholar]
- 37. de Haes JC, de Koning HJ, van Oortmarssen GJ, et al. The impact of a breast cancer screening programme on quality-adjusted life-years. Int J Cancer. 1991;49(4):538–544. [DOI] [PubMed] [Google Scholar]
- 38. Stout NK, Rosenberg MA, Trentham-Dietz A, et al. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98(11):774–782. [DOI] [PubMed] [Google Scholar]
- 39. Mandelblatt JS, Schechter CB, Yabroff KR, et al. Benefits and costs of interventions to improve breast cancer outcomes in African American women. J Clin Oncol. 2004;22(13):2554–2566. [DOI] [PubMed] [Google Scholar]
- 40. Wong IO, Kuntz KM, Cowling BJ, et al. Cost effectiveness of mammography screening for Chinese women. Cancer. 2007;110(4):885–895. [DOI] [PubMed] [Google Scholar]
- 41. Walter LC, Schonberg MA. Screening mammography in older women: a review. JAMA. 2014;311(13):1336–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lansdorp-Vogelaar I, Gulati R, Mariotto AB, et al. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med. 2014;161(2):104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96:5–21. [DOI] [PubMed] [Google Scholar]
- 44. McGregor M. Cost-utility analysis: use QALYs only with great caution. CMAJ. 2003;168(4):433–434. [PMC free article] [PubMed] [Google Scholar]
- 45. Boer R, de Koning HJ, van Oortmarssen GJ, et al. In search of the best upper age limit for breast cancer screening. Eur J Cancer. 1995;31A(12):2040–2043. [DOI] [PubMed] [Google Scholar]
- 46. Puliti D, Duffy SW, Miccinesi G, et al. Overdiagnosis in mammographic screening for breast cancer in Europe: a literature review. J Med Screen. 2012;19(Suppl 1):42–56. [DOI] [PubMed] [Google Scholar]
- 47. Morrow M. The certainties and the uncertainties of ductal carcinoma in situ. J Natl Cancer Inst. 2004;96(6):424–425. [DOI] [PubMed] [Google Scholar]
- 48. Eusebi V, Feudale E, Foschini MP, et al. Long-term follow-up of in situ carcinoma of the breast. Semin Diagn Pathol. 1994;11(3):223–235. [PubMed] [Google Scholar]
- 49. Feig SA. Ductal carcinoma in situ. Implications for screening mammography. Radiol Clin North Am. 2000;38(4):653–668, vii. [DOI] [PubMed] [Google Scholar]
- 50. Betsill WL, Jr, Rosen PP, Lieberman PH, et al. Intraductal carcinoma. Long-term follow-up after treatment by biopsy alone. JAMA. 1978;239(18):1863–1867. [DOI] [PubMed] [Google Scholar]
- 51. Welch HG, Black WC. Using autopsy series to estimate the disease “reservoir” for ductal carcinoma in situ of the breast: how much more breast cancer can we find? Ann Intern Med. 1997;127(11):1023–1028. [DOI] [PubMed] [Google Scholar]
- 52. de Gelder R, Fracheboud J, Heijnsdijk EA, et al. Digital mammography screening: weighing reduced mortality against increased overdiagnosis. Prev Med. 2011;53(3):134–140. [DOI] [PubMed] [Google Scholar]
- 53. Baxter NN, Virnig BA, Durham SB, et al. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96(6):443–448. [DOI] [PubMed] [Google Scholar]
- 54. Habel LA, Achacoso NS, Haque R, et al. Declining recurrence among ductal carcinoma in situ patients treated with breast-conserving surgery in the community setting. Breast Cancer Res. 2009;11(6):R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Livaudais JC, Hwang ES, Karliner L, et al. Adjuvant hormonal therapy use among women with ductal carcinoma in situ. J Womens Health (Larchmt). 2012;21(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schonberg MA, Marcantonio ER, Li D, et al. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28(12):2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Crivellari D, Bonetti M, Castiglione-Gertsch M, et al. Burdens and benefits of adjuvant cyclophosphamide, methotrexate, and fluorouracil and tamoxifen for elderly patients with breast cancer: the International Breast Cancer Study Group Trial VII. J Clin Oncol. 2000;18(7):1412–1422. [DOI] [PubMed] [Google Scholar]
- 58. Partridge AH, Elmore JG, Saslow D, et al. Challenges in ductal carcinoma in situ risk communication and decision-making: report from an American Cancer Society and National Cancer Institute workshop. CA Cancer J Clin. 2012;62(3):203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Marshall E. Breast cancer. Dare to do less. Science. 2014;343(6178):1454–1456. [DOI] [PubMed] [Google Scholar]