Abstract
Perception of time, in the seconds to minutes range, is not well characterized in autism. The required interval timing system (ITS) develops at the same stages during infancy as communication, social reciprocity, and other cognitive and behavioral functions. The authors used two versions of a temporal bisection procedure to study the perception of duration in individuals with autism and observed quantifiable differences and characteristic patterns in participants’ timing functions. Measures of timing performance correlated with certain autism diagnostic and intelligence scores, and parents described individuals with autism as having a poor sense of time. The authors modeled the data to provide a relative assessment of ITS function in these individuals. The implications of these results for the understanding of autism are discussed.
The ability to perceive the temporal properties of events and the temporal relations between them is fundamental for adaptive learning, cognition, and behavior. Individuals are continually timing events in their surrounding environment. The ability to estimate duration (which is shared by other species) and to use such temporal knowledge to mediate expectations and behavior is at the core of adaptive function. Research has revealed that distortions and perturbations in timing ability are present, to varying degrees, in many patient populations and may accompany differences in other aspects of sensory processing and cognitive and behavioral profiles. Recently research has suggested that differences in timing and time perception might directly contribute to severity and features of autistic disorder (and the triad of impairments; Allman & DeLeon, 2009; Boucher, 2001; Wimpory, 2002). Although to date there have been very few empirical studies examining timing ability in these individuals, there are not any studies (to our knowledge) that have attempted to examine any correspondence between timing performance and autism symptom severity. In the current study, we attempted to provide a preliminary assessment of the ability of individuals with autism to estimate duration (in the multiseconds range). We also took a “first look” to see whether timing ability was predictive of diagnostic features of autistic disorder. We used methods that are well established in the study of interval timing (e.g., see Allan & Gibbon, 1984).
Although time is not a stimulus, the experience of duration shares the same qualities as perception by other senses (e.g., vision, hearing) and is widely studied using a psychophysical approach, which can be defined as an attempt to quantify the sensory response to physical stimuli (see Gescheider, 1997). Accordingly, decades of basic interval timing research (in the seconds to minutes range) have revealed that the interval timing system (ITS) is likely to be made up of various components (Gibbon, Church, & Meck, 1984), with perceptual, memory, and decision systems being involved. The ITS subserves our sensitivity to relatively short time scales, and, in turn, adequate perception of duration is a key component of adaptive cognitive, behavioral, and social functions. Sensitivity to time is also a basic building block of higher order notions of past, present, and future (see Fraisse, 1982, 1984; Friedman, 1982), which form an intellectual structure for our everyday thoughts, intentions, and behavior.
Given the phylogenic generality of timing ability across species, it seems likely that the ability to estimate duration might emerge in early infancy. In fact, rhythmic changes in the seconds to minutes range are inherent in many biological systems and repetitive movement patterns (e.g., breathing, sucking, stereotypies, and early vocal development such as crying and babbling; see Wolff, 1991). These repetitive actions and behaviors allow the infant to adapt to the temporal contingencies of their physical and social environment (Pouthas, 1985). Throughout infancy, parent–infant interactions (including gaze and vocalizations) follow exquisite temporal patterns, with the parent often rearranging his/her behavior to temporally match the infant, in ways that are optimal for learning (e.g., see Jaffe, Beebe, Feldstein, Crown, & Jasnow, 2001). Furthermore, one of the so-called hallmark psychophysical properties of interval timing, the scalar property—the requirement that the standard deviation of time judgments is a constant fraction of the mean judgment (this varies with the interval being timed; Wearden & Lejeune, 2008)—was found in reciprocal parent–infant interactions (Stern, Beebe, Jaffe, & Bennett, 1977) and was observed in the brains of the youngest infants tested (6 months) during an interval timing task. In fact, a recent series of infant electroencephalography (EEG) studies (Brannon, Libertus, Meck, & Woldorff, 2008; Brannon, Roussel, Meck, & Woldorff, 2004) revealed that ability to time durations in the interval range further develops during infancy (i.e., there are quantifiable gains between 6 and 10 months of age). Beyond infancy, there are somewhat inconsistent, but quantifiable, differences in the perception of interval durations across childhood (e.g., between 3 and 5 year olds), but by 8 years of age timing performance is usually comparable with adults (temporal sensitivity improves between 3 and 8 years; e.g., Droit-Volet, Clément, & Wearden, 2001; Droit-Volet & Wearden, 2001). Behavioral studies on the typical development of time perception in childhood serve a useful frame of reference when comparing the performance of individuals with autism.
If the typical development of the interval timing system is disturbed in autistic disorder, several behavioral and cognitive profiles might be expected to occur (see Allman & DeLeon, 2009; Boucher, 2001). For example, if sensitivity to duration is reduced, infantile stereotyped behaviors (e.g., hand flapping, body rocking) may persist to facilitate the processing of duration and effectively “count” time (Killeen & Fetterman, 1988; Skinner, 1948). Moreover, problems with timing and time perception may correspond to difficulties in acquiring language and interacting with the social environment, as timed coordination with others is fundamental for joint attention, turn-taking, and social bonding (see Wimpory, 2002). Language itself has inherent references to time (e.g., past, present, and future), as do many executive functions (e.g., planning, episodic memory).
Two previous empirical reports that required individuals with autism to make a response or temporal judgment that was accurately timed are relevant to the current study. Szelag, Kowalska, Galkowski, and Poppel (2004) required participants to reproduce given durations (between approximately 1–5 s) and observed quantifiable and selective differences in the timed performance of individuals with autism. Unlike typically developing participants who revealed a close correspondence between the target and reproduced durations, those with autism displayed a tendency to reproduce all given durations around 3.0 to 3.5 s. In an equivalent study using adults with autistic disorder (Martin, Poirier, & Bowler, 2010), individuals with autism were less accurate in their temporal reproductions and more variable, particularly at longer durations (which tended to be under-reproduced).
In the current study, we used a time perception task that did not require a response or temporal judgment to be accurately timed, to examine the ability of individuals with autistic disorder to estimate duration. We also simulated our obtained data through a principled model similar to the one applied to data reported from a large group of typically developing children (Droit-Volet & Wearden, 2001), which used methods almost identical to our own. The best fit of the model produces various parameters that represent functioning of different aspects of ITS (i.e., clock, memory, and decision stages). Second, we administered a modified version of the It’s About Time questionnaire (Barkley, 1998, used with permission; Barkley, Koplowitz, Anderson, & McMurray, 1997), originally developed to assess sense of time in children with attention-deficit/hyperactivity disorder to parents of all individuals.
Method
Participants
We recruited the majority of participants with autism through existing research studies and the majority of those without autism via flyers posted around the pediatric facility and wider institution. The typically developing (comparison) group was a relative weakness of the current study because only a few of these participants contributed intelligence scores (due to the preliminary nature of the study, formal testing was not available), and, therefore, this group was particularly not well defined. Screening exclusion criteria for potential comparison participants included a diagnosis of developmental, childhood, or psychiatric disorder and educational difficulties that were determined by parent interview (all procedures were approved by the pertinent institutional review board). All participants were required to be free of any motor and/or visual impairment that would interfere with completion of task demands. All participants in the autism group contributed diagnostic and intelligence measures that had previously been administered by independent clinicians and researchers at the institution. All participants in the autism group met the stringent autism cutoff on both an observational assessment (Autism Diagnostic Observation Schedule [ADOS]; Lord et al., 2000) and parent interview (Autism Diagnostic Interview—Revised [ADI-R]; Lord, Rutter, & LeCouteur, 1994), with the exception of 2 participants who met the cutoff for autism spectrum disorder on the observational assessment (they also met criteria for autism on the ADI-R). Thirty-two children, 19 individuals with autism and 13 comparison participants, were recruited into the study. Of these, 2 children with autism did not successfully acquire the temporal discrimination (see Experimental Methods section) and were withdrawn from the study.
As is common to many studies of temporal bisection (e.g., Droit-Volet & Wearden, 2001; Wearden, Wearden, & Rabbitt, 1997), only those participants who produced orderly test data on both versions of the task were included in the final level of analysis (described later; 4 participants with autism and 1 without autism produced disorderly functions and were excluded from further analysis). Data from 13 children with autism and 12 comparison children who successfully completed both versions of the task are reported in the current study. The characteristics of individuals who constituted each group (autism or comparison) are presented in Table 1. Participants were not matched to each other by any factors, although they represented a similar size and distribution in age across a large range (7–16 years; M age = 10.3 years, Median = 9 years, per group). Although there were no statistical group differences for intelligence measures, only 3 (out of 12) comparison participants provided IQ data, and these few comparison IQ scores tended to be higher overall (see Table 1).
Table 1.
Participant Characteristics
| Autism group (n = 13; all males)
|
Comparison group (n = 12; 9 males)
|
|||||
|---|---|---|---|---|---|---|
| Variable | M | SD | Min.-max. | M | SD | Min.-max. |
| Age (years) | 10.3 | 2.4 | 7.3–15.2 | 10.3 | 3.1 | 7.3–16.8 |
| FSIQ | 92.31 | 17.13 | 72–118 | 109.80a | 18.14a | 78–122a |
| WISC IV only | ||||||
| WMIQ | 92.13b | 16.87b | 56–107b | |||
| VCIQ | 100.13b | 27.34b | 61–142b | |||
| PRI | 116.13b | 19.48b | 79–141b | |||
| PSI | 91.88b | 12.91b | 78–112b | |||
| ADOS (Mod 3) | ||||||
| Comm. | 4.3c | 2.17c | 1–8c | |||
| RSI | 8.69c | 2.25c | 4–13c | |||
| Comm.+ RSI | 12.92c | 3.81c | 8–21c | |||
| SB/RI | 2.54c | 2.22c | 0–7c | |||
| Total | 15.46c | 5.06c | 8.25c | |||
| ADI-R | ||||||
| A | 21.62 | 5.39 | 13–28 | |||
| B | 17 | 3.97 | 10–23 | |||
| C | 6.92 | 2.50 | 3–11 | |||
| D | 4.08 | .95 | 3–5 | |||
Note. FSIQ = Full scale intelligence quotient; WISC; Wechsler Intelligence Scale for Children, Version 4 (Wechsler, 2004); WMIQ = working memory IQ; VCIQ = verbal comprehension IQ; PRI = perceptual reasoning IQ; PSI = processing speed index; ADOS = Autism Diagnostic Observation Schedule (Lord et al., 2000); Mod = module; Comm = communication; RSI = reciprocal social interaction; SB/RI = stereotyped behavior/restricted interests; Total = (Comm. + RSI) + (SB/RI); ADI-R: = Autism Diagnostic Interview—Revised (Lord, Rutter, & LeCouteur, 1994); A = language and communication; B = social interaction; C = repetitive behaviors; D = onset of symptoms before 36 months.
n = 3.
n = 8.
n = 11 values denote the number of participants for whom scores were available (see text for details).
It is worth noting that the single previous study that had related temporal bisection performance to IQ (Wearden et al., 1997) used typical adults (rather than children) but did not find any aspect of bisection performance that was affected by IQ, even though performance measures on other tasks were IQ dependent. There is no evidence in the literature that bisection performance differs according to gender, and the comparison group included 3 females.
Experimental Methods
We used a basic procedure known as temporal bisection to examine the ability to estimate and discriminate durations in the seconds range. We adapted methods previously used in a study of temporal bisection in typically developing children (see Droit-Volet & Wearden, 2001, for additional procedural details). During the computerized procedure, individuals were initially shown, then trained to discriminate between, two anchor durations that were signaled by serial presentations of the same visual stimulus (a rabbit or clown, for each of the two versions of the task) that appeared for the two standard times (initially identified as short and long by the experimenter sitting next to them). Participants were required to classify a temporal judgment as either short or long by selecting between two different response options (e.g., two different buttons). The S and L keys on the laptop keyboard were each covered with a yellow, colored sticker with “S-” and “L—” printed on it (these keys are on the central row to the left and right, respectively) and were the short and long choice options. Whether the response was correct was signaled by computer feedback (and by the experimenter). After meeting an accuracy criterion (seven of eight correct responses in one block, up to six blocks) and a short break in the testing room, participants were presented with a new set of stimulus durations that were between the two anchors and were required to make a judgment about a given duration’s “similarity” to the short or long anchor (they were instructed, “You might find it a bit harder to decide and you won’t find out if you got it correct or wrong now, but you need to decide if you think it is it more like the short one, or more like the long one”). Five intermediate durations and the two anchors were each presented four times in an intermixed block (without feedback). Two test blocks were typically presented (with a short break in the testing room between each).
During experimental testing, the accompanying caregiver was required to complete the It’s About Time questionnaire (Barkley, 1998) in a separate area.
Arrangement of Timing Events
All participants completed two versions of the same task, in a counterbalanced order with respect to group allocation. A 20-min break with the accompanying caregiver was interleaved (in a playroom equipped with a television and toys and with the opportunity to leave and get a snack). The two versions differed with respect to the objective length of the pairs of anchor (and intermediate) durations used, with one being shorter and the other longer. In the shorter version of the task, the anchor durations were 1 and 4 s (intermediates durations = 1.5, 2, 2.5, 3, and 3.5 s), and in the longer version the anchors were 2 and 8 s (intermediates durations = 3, 4, 5, 6, and 7 s). We assumed these versions were equivalent in discrimination difficulty due to the 1:4 ratios between the respective anchor pairs. After the presentation of a given stimulus duration, the words Which Time? appeared on the screen, and a response (short or long) could be recorded. If no response was made within a 10-s period, the trial repeated (the duration was presented again). The interval between the end of one trial and the start of the next trial, denoted by a small fixation cross on the screen, was varied between 1 s and 5 s. Testing could be paused during the intertrial interval (ITI; e.g., to correct any off-task behavior).
Psychophysical Performance Measures
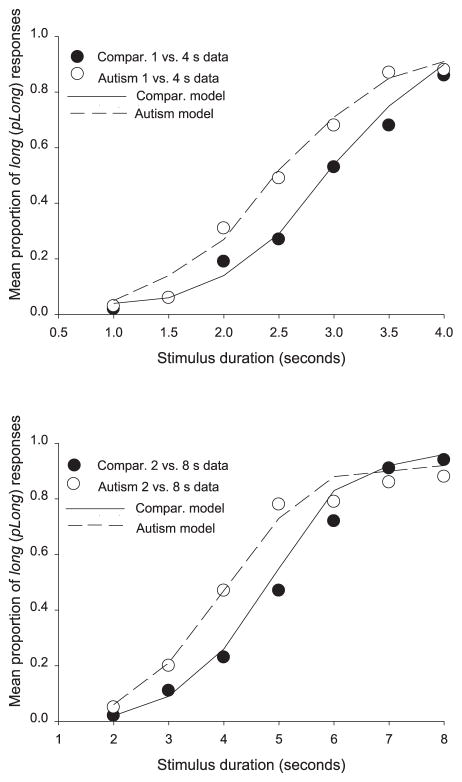
The proportion of long responses (denoted as pLong) produced at each test duration was calculated for each individual, on each separate version of the task. This was achieved for a given duration, by dividing the number of long responses to the sum of short and long responses. To be considered “orderly,” the pLong responses were required to generally increase with objective duration. These individual pLong values then contributed to the group mean functions (shown in Figure 1).
Figure 1.
Data points (empty and filled circles) and best fitting values from the model described in the text (dashed and solid lines) from participants with and without autism (respectively), in both versions of the task (upper panel: 1 vs. 4 s discrimination; lower panel: 2 vs. 8 s discrimination). Compar. = comparison group.
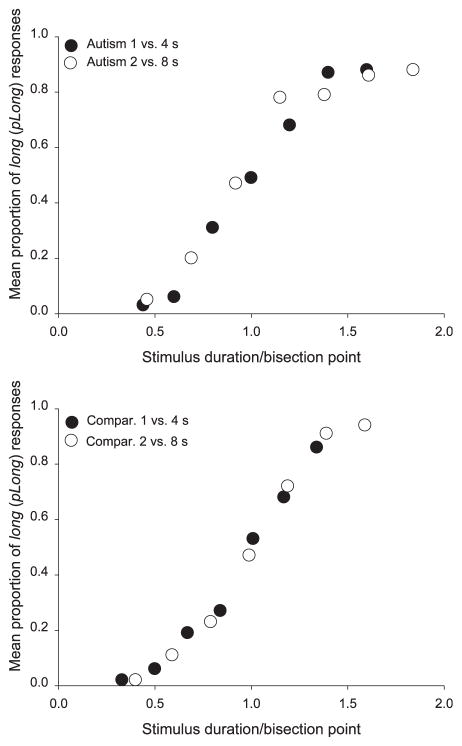
A given individual’s timing functions are referred to as psychophysical functions, because they allow extrapolation and quantification of indexes of timing sensitivity and ability: The duration that produces 50% pLong responses (when the individual is equally likely to classify the duration as short or long) is known as the bisection point (BP). In human timing, the BP is usually located around the arithmetic mean of the two anchor standards (Wearden, 1991); temporal variability is indexed by the difference limen (DL; half of the difference between 75% and 25% pLong divided by the BP); and temporal sensitivity is indexed by the Weber ratio (WR), which is also used to assess the hallmark scalar property of timing (DL divided by BP). As WR values are thus normalized, temporal sensitivity across a range of pair durations can be reliably compared. These measures were derived for all individuals, by three different methods (logistic curves, linear regression, interpolation by eye) that yielded very similar results; therefore, we averaged across them (these constitute the BP and WR data; see also Wearden & Ferrara, 1995). Data from participants with and without autism were then compared and averaged to produce the group mean (to examine any group BP and WR differences). The scalar property was further assessed by plotting group data obtained with two different duration ranges on the same relative scale, and we expected them to superimpose perfectly (so it looks as though only one function is present; this is often found in animal and human timing studies).
Computer Modeling
The same principled model (Wearden, 1991) that was applied to the data from typically developing children in a previous study (Droit-Volet & Wearden, 2001) was applied to our obtained group data. This treatment allowed us to make direct comparisons between the timing of individuals with autism with different age groups of typically developing children outside of the current study (which was particularly useful given the limitations with our comparison group). Because the model attempted to simulate timing performance, in doing so it made two assumptions: First, there was the assumption that the choice to respond (short or long) to any of the test durations was governed by the smaller of the two differences between a given test duration and the remembered short and long trained anchors (1 and 4 s, or 2 and 8 s). The second assumption was that the anchor durations may have been remembered incorrectly as shorter or longer than they really were (see Droit-Volet et al., 2001; Meck, 1983, for applications of this idea), and this “effective” value was used for the difference comparison during the test stage. The model derives various indexes of performance (K*, c), much like the ones derived from the psychophysical functions themselves, although these corresponded to temporal memory distortion (K*) and timing sensitivity (c). For example, if K* equals 1, then the anchors are stored as their real values; if K* is less than 1, the anchors are remembered as shorter; and if K* is greater than 1, they are remember as longer than they really were (e.g., if K* = .95, the anchors are remembered as 95% of the real values). For each version of the task, 1,000 trials were simulated at each of the seven test durations, and K* and c were varied to produce the best fit of the model, represented by the mean absolute deviation value (MAD; the sum of the absolute deviations divided by 7, the number of data points) between the model’s output and the data point for each condition. Small MAD values indicate a good fit, and the MAD values in this study could be classified as good in the wider literature. The lines in Figure 1 show the fit of the model to the obtained data.
Results
Training
The training performance of those participants with and without autism did not differ between groups, with respect to the number of training blocks required to successfully acquire the discrimination, in both versions of the task ( ps > .5). However, this does not preclude the possibility of differences in how participants in each group acquired the task. No participants demonstrated or subsequently reported counting during timing (see also Droit-Volet & Wearden, 2001). Two participants with autism displayed interesting features during timing, however. One individual put his fingers together and then pulled them apart during the training stage, particularly to longer durations, and continued to do this during a small proportion of test trials, and another individual (relatively higher functioning) reported that, “If my mind turns over once its short, and more than once its long” (he reported seeing a visual snake in his mind, which rotated). Unfortunately, a coding observer was not present and experimental sessions were not videotaped, so the possibility of conducting additional observational analyses of behaviors during timing was not possible in the current study.
Individuals who could not acquire the discrimination (i.e., did not complete the test phase) and those individuals who acquired the discrimination but produced disorderly timing functions (i.e., did not contribute data) tended to have autism: Two participants failed to acquire the initial discrimination, and both had autism. Five participants produced disorderly data and were excluded from final analysis; 4 had autism. Participants with autism who did not successfully acquire the discrimination were at the minimum of the group age range (~7 years), but those who produced disorderly functions spanned the full age range (up to 17 years; the typically developing participant who produced disorderly data was the youngest in the group). There were no obvious characteristics of these individuals with respect to diagnostic or intelligence scores, and, given our relatively small sample size, the current study was too limited in scope to speculate on what individual differences made it difficult for some individuals to complete the task.
Performance on the Time Perception Task
Across both versions of the task, individuals with autism produced a greater proportion of pLong responses compared with those without autism. In the 1- versus 4-s (shorter) version of the task, analysis of variance (ANOVA) with two between-subjects factors, group and task order, and a within-subject factor of stimulus duration revealed a significant effect of group, F(1, 21) = 4.62, p < .05; no effect of order, F(1, 21) = 1.64, p = .22; an effect of duration, F(6, 126) = 90.63, p < .001; and no significant interactions between these factors (largest F = 1.77). Planned comparisons revealed a significant group difference at the 3.5-s duration, F(1, 21) = 10.51, p = .003. In the 2- versus 8-s (longer) version of the task, an ANOVA with the same factors revealed no effect of group or order (ps > .1) and an effect of duration, F(6, 126) = 104.42, p < .001. There was also a significant interaction between group and duration, F(1, 21) = 3.93, p = .010, but no other significant interactions (largest F = 1.44, p = .206). Planned comparisons revealed group differences at the 4-s, F(1, 21) = 4.40, p < .05, and 5-s durations, F(1, 21) = 6.66, p = .02.
Figure 1 shows (empty and filled circles) that, in both the shorter and longer versions of the task (upper and lower panels, respectively), the obtained group timing functions from individuals with autism were shifted to the left, relative to the comparison group functions. Furthermore, in the 2- versus 8-s version of the task (lower panel), the autism group function is relatively flat to durations above about 5 s. (i.e., the empty circles are on a flatter plane). Across both versions, therefore, individuals with autism exhibited a greater proportion of pLong responses and produced somewhat leftward-shifted timing functions; they also began to show potentially interesting differences in the perception of durations over 3.5–5.0 s (relative to comparison individuals). The overall shape of the two timing functions produced by the comparison group was normal, especially in the longer version; although in the shorter version of the task, the function was slightly rightward shifted.
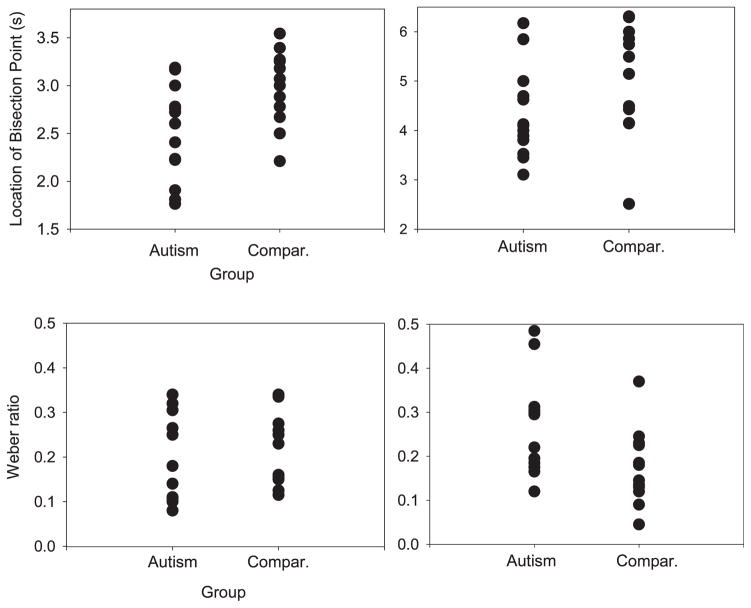
Psychophysical Measures
Figure 2 reveals the group distribution of the two derived psychophysical indexes on the shorter (left-hand panels) and longer (right-hand panels) versions of the task. As depicted in the upper row of panels, the autism group’s BP was located at a significantly shorter duration, on both versions of the task, compared with those without autism (autism group’s BP = 2.5; comparison group’s BP = 2.98; z = −2.34, p < .02; and autism group’s BP = 4.34, comparison group’s BP = 5.05; z = −2.01, p < .05, respectively). However, our comparison group produced a BP that was higher than would be normally expected in the 1- versus 4-s version of the task, and this contributed to the group difference (the comparison BP was typical in the 2- vs. 8-s version). In addition, for any given individual, we should expect to observe a reasonable difference between the two BPs for each version, as the two pairs of anchor durations we used differed. Close inspection of individual timing functions for all individuals showed that the majority of participants with autism did not have the extent of expected difference between the location of the BP across both versions of the task, but the majority of comparison participants displayed this expected difference (W. H. Meck, personal communication, April 2010).
Figure 2.
Derived individual values for participants with and without autism of the bisection point (upper panels) and Weber ratio (lower panels), on both versions of the task (left panels: 1 vs. 4 s; right panels: 2 vs. 8 s).
As depicted in the lower panels of Figure 2, there were no group differences in the sensitivity to duration (WR) when the anchors were 1 and 4 s (autism group’s WR = .18; comparison group’s WR = .21, p > .25). However, in the longer version of the task, the WR was significantly higher for the group with autism, indicating “worse” temporal sensitivity when the anchor durations were 2 and 8 s (autism group’s WR = .26, control group’s WR = .18, z = −1.96, p = .05). Both of the WR values for the comparison group (and the autism group WR on the shorter version of the task) were similar to those usually obtained with typically developing children (8 years old); however, the autism WR in the longer version of the task was comparable with that obtained with typically developing 3–5 year old children (see Droit-Volet & Wearden, 2001). The two timing functions produced by each group (one for each version of the task) were also subjected to the test of superimposition to assess the presence of the hallmark scalar property of interval timing (see Droit-Volet & Wearden, 2001, for details on how this is done). As shown in Figure 3, the two timing functions from the comparison group superimposed perfectly, and, for those with autism, the group functions superimposed to a lesser extent but still reasonably well (somewhat comparable with 3 year olds; Droit-Volet & Wearden, 2001). Our data were equivocal as to whether the scalar property was found in the timing of individuals with autism, and it would be of potential future interest to examine whether scalar timing patterns are found in aspects of communication, social reciprocity, and repetitive behaviors in individuals with autistic disorder.
Figure 3.
Obtained group data plotted on the same relative scale for participants with (upper panel) and without (lower panel) autism.
As an additional level of analysis, we examined relationships between the psychophysical timing indexes (BP and WR) and diagnostic and intelligence measures of our participants with autism (this type of analysis was not performed for comparison participants). Only those participants who were previously administered the Module 3 ADOS (Lord et al., 2000), and contributed Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler, 2004) intelligence scores were used in this level of analysis (see Table 1 note). We examined BP and WR (sensitivity) scores for each version of the task; diagnostic scores (ADOS and ADI-R [Lord et al., 2000, 1994]) for language and communication, social interaction, and restricted and repetitive behaviors; and total IQ and specific domain scores for processing speed, working memory, verbal comprehension, and perceptual reasoning. Pearson product-moment correlations revealed a correspondence between BP in the 1- versus 4-s (shorter) version of the task with the score for language and communication (ADOS) and score for working memory (BP with communication: r = −.641; p = .033; BP with working memory: r = .763, p = .028; communication and working memory: r = −.793; p = .019). That is, the shorter the location was of the BP, the “poorer” an individual’s language and communication and working memory was. (Total IQ score was not predictive of timing measures or diagnostic scores.) Close examination of the individual timing functions produced by the few individuals with autism who had the lowest scores for communication and working memory (and, hence, the shorter BP when the anchors were 1 s and 4 s) revealed that they tended to display a protracted flattening in their timing functions to durations over 2 s (in the 1- vs. 4-s version). At a more general level, it is striking that inspection of all individual timing functions produced by participants with autism when the anchor durations were 2 s and 8 s revealed that almost 70% (9 out of 13 participants) displayed characteristic flattening to durations over 5 s—of the 4 participants with autism who did not show this unusual pattern, 3 shared the highest group scores for working memory.
Parameters From Computer Modeling
The indexes derived from the modeling reveal that in both the shorter and longer versions of the task, individuals with autism had higher c values (.50 and .53, respectively) compared with those without autism (.42 and .37, respectively), suggesting more variable (i.e., “fuzzy”) temporal reference memories. The values obtained for the autism group were comparable with those previously reported using typically developing 5 year olds, and our comparison group values were consistent with those from older children (8 years old; see Droit-Volet & Wearden, 2001). The second parameter from the model that provides an index of memory distortion for the anchor pairs, revealed that both groups produced K* values (autism group = 0.97; comparison group =1.17) similar to those previously obtained with typically developing children (8 years old) when the anchor durations were 1 s and 4 s. However, although this was also true for the comparison group when the anchors were 2 s and 8 s (K* = 0.98), the autism group produced a lower value (K* = 0.83), suggesting a tendency to shorten the memory of the anchors on the longer version of the task to an extent that was comparable with typically developing 3 year olds (on a task of temporal generalization, Droit-Volet, Clément, & Wearden, 2001). Results from the modeling lend support to the idea that timing ability may be developmentally delayed in individuals with autistic disorder.
Findings From the Parental Time Questionnaire
Table 2 displays sample questions and scoring on this instrument (which was modified slightly from its original version). The maximum score on the adjusted questionnaire is 75 (best) and the minimum is 0 (worst). The mean score for participants with autism (18.08; range = 6–37) was significantly lower than for comparison participants (48.4; range = 31–73), t(21) = −6.12, p = .001. Parental responses to the final question (No. 25), “In general, compared to other children of their age, how well developed is your child’s sense of time?”, revealed a group difference, t(21) = −5.38, p = .001, with the modal answer for participants with autism being poor compared with above average for the comparison group (scale: poor [0], below average, average, above average, well above average [4]). Based on previous reports (Boucher, Pons, Lind, & Williams, 2007) of problems with diachronic thought (i.e., affected individuals are less likely to think about past or future stages of a current situation; have difficulty understanding that things can change or evolve over time but remain the same; and that successive states or events are part of a unitary whole) in autism, we also arbitrarily categorized the questions (with the exception of No. 25) according to past, managing current time, or future (see Table 2) and reported group differences for each type, smallest t(11) = −3.308, p < .004. When interpreting these findings, it is useful to note that this questionnaire is not tailored to compensate for known problems with communication and socialization in children with autism and cannot disentangle the assessment of time from other executive functions, nor can our rudimentary classification of managing current time, which included time frames that spanned many minutes and hours.
Table 2.
Example Questions as They Related to Past, Current, and Future Time Referencea
| Past | Managing current time | Future |
|---|---|---|
| How often does your child ask questions about their past? | When working on a task, how often does your child seem to get work done in time allotted? | How often does your child talk about or seem to think about what he/she will be doing tomorrow? |
| How often does your child seem to think about their past or use hindsight before responding to a situation? | How often does your child refer to a watch or clock in planning how much time he or she has left to do something? | How often does your child consider the future consequences of their actions for him/herself? |
Note. Scoring is typically as follows: rarely (0), sometimes (1), most of the time (2), and almost always (3).
Taken from the “It’s About Time” questionnaire. Copyright R. A. Barkley, 1998. Reprinted with permission.
Discussion
To our knowledge, the current study represents the first attempt to examine the perception of multisecond durations in individuals with autistic disorder. The study was preliminary in nature and has some notable strengths (the psychophysical method and modeling of data) and weaknesses (the inadequate characterization of the comparison group and small sample size). The extent of our different levels of analysis allows us to make a variety of claims about performance and the experience of duration for individuals with autistic disorder. Individuals with autism tended to produce a greater proportion of long responses and displayed a relatively robust flattening in their timing functions, particularly to durations exceeding about 5 s (that did not result from a failure of experimental control). Psychophysical measures for individuals with autism (BP and WR) revealed significant and potentially characteristic differences in bisection performance and suggest that individuals with autism experienced greater difficulty discriminating between longer durations.
BP values were correlated with language and communication, as well as working memory scores in the shorter version of the task. Computer modeling suggested that individuals with autism appeared to have more variable temporal memories (c) and were likely to truncate the anchor durations when they were 2 s and 8 s (K*). Additional comparisons with other childhood studies suggest the extent of these differences may be of some developmental significance. Last, parents of children with autism tended to classify their children as having a qualitatively poor sense of time, which attests to the many anecdotal reports that these individuals experience difficulties with time (see Allman & DeLeon, 2009; Boucher, 2001).
Some interesting features of the data are also consistent across various levels of analysis and strengthen the assertions that can be made, despite the limitations in scope. In particular, it appears that individuals with autism experience difficulties with durations exceeding about 3.5 s to 5.0 s and may be less able to discriminate between longer durations (likely related to poorer temporal sensitivity). The question then becomes: How might a problem experiencing longer durations influence test performance and produce the variety of measures obtained? There are at least two possibilities. First, it is possible that both the short and long anchor standard durations influenced bisection performance in the shorter, 1-versus 4-s version, but a different strategy was used in the longer, 2 versus 8-s version. When the anchors were 2 s and 8 s, the short anchor (2 s) may have had a stronger influence over test performance than the long anchor (8 s). This account requires some form of psychological explanation for why those with autism appear to be less influenced by the memory of the longest standard. Of course, this could have been due to difficulty maintaining attention, concentration, or (which leads us onto the second possible explanation for the two key findings [BP differences and the relative flattening]) there may have been some underlying difficulty with, or insensitivity to, longer durations (e.g., “time blindness”), particularly those over 3.5 s to 5.0 s in autistic disorder.
In fact, this 3.5–5.0-s period is not altogether arbitrary, as it is widely held to correspond to the specious or psychological present. The idea of such a putative mental platform has been posited since the dawn of psychology (James, 1890), and it is held to facilitate linking together sensory experiences close together in time. The temporal breadth of this platform (although not fixed) is believed to be around 3 s to 5 s (possibly up to 8 s; e.g., see Block, 1990; Fraisse, 1984; Michon, 1978; Poppel, 1997) and has been studied under a variety of experimental paradigms. These include the temporal segmentation of spontaneous speech, structuring a continuous stream of auditory input, programming anticipatory movement, and sensorimotor behavior (see Poppel, 1997). This platform can be considered intimately related to the perception of the durations used in both versions of our task. In accounting for their observation that individuals with autism tended to reproduce given durations that ranged between about 1 s and 5 s (around 3.0–3.5 s), Szelag et al. (2004) suggested that the processing of duration was dissociated from the temporal platform and that autism involved a unique form of timing disturbance. In the current study, we extended the generality of these findings by revealing that individuals with autism appear able to discriminate and perceive durations within the bounds of the psychological present (up to about 5 s), unless they have poor communication or working memory, in which case they may experience problems for durations within the bounds of the temporal platform (see also Mostofsky, Goldberg, Landa, & Denckla, 2000, who reported no differences in autism when discriminating millisecond intervals between stimuli). However, they may have difficulty with durations beyond the bounds of the psychological present. The weak central coherence hypothesis of autism (Frith, 1989; Happé, 1999) and reported deficits in the temporal binding of stimulus input in autistic disorder (e.g., Bebko, Weiss, Demark, & Gomez, 2006; Brock, Brown, Boucher, & Rippon, 2002) might be related to problems linking successive periods or platforms together. There is recent evidence that individuals with autism integrate sensory input over an extended temporal window (Foss-Feig et al., 2010) and experience difficulties grasping the concept of time and regulating and structuring their behavior and expectations within current and future imagined time frames (Boucher, 2001; Boucher et al., 2007).
A complication of the multiprocess nature of the ITS is that the development of psychological variables other than timing ability, such as differences in attention and memory and decision ability, may also play a role in timing performance (e.g., Lustig, Matell, & Meck, 2005). For example, the temporal limit of short-term (or immediate) memory is only a few seconds (Block, 1979), but temporal constraints are somewhat longer for working memory (several seconds; Baddeley & Hitch, 1974).
In many ways, the results of the current study create many more questions than they answer, and, given the preliminary nature of the study and its limitations, the findings should be treated with some caution. They will need to be replicated with at least double the number of participants and extended under a wide range of different temporal parameters before any reliable assessment of timing ability in autistic disorder can be made (ongoing research by the first author [M.A.]). In addition, the autism group likely included different subgroups of affected individuals (i.e., some of whom had phrase speech and, so, completed the Module 2 ADOS, whereas others had fluent speech), and this limits the generality of our findings. It may be particularly useful for future studies to examine timing ability in high-functioning individuals with autism, and those who are less verbal. Nevertheless, the strengths of our psychophysical approach allow us to make some intriguing observations about the timing functions of individuals with autism, and some meaningful comparisons with previous studies that used different age groups of typically developing children. At the very least, we have demonstrated the benefit of using this approach with children with autistic disorder. Undoubtedly, the potential benefit of continuing this relatively new line of investigation is to inform related avenues of cognitive and behavioral autism research (e.g., joint attention, social timing), both at the basic and applied levels, and to improve clinical outcomes. For example, temporal variables are often a core aspect of clinical and educational training and treatment programs (e.g., operant reinforcement schedules, timetables, and time-out), and these might be advanced by increased awareness of temporal experience in autistic disorder (see Critchfield & Kollins, 2001; Lalli, Casey, Goh, & Merlino, 1994; MacDuff, Krantz, & McClannahan, 1993). To the extent that the neurobiological basis of (typical) interval timing is beginning to be elucidated (e.g., Buhusi & Meck, 2005; Meck, 2003), an improved understanding of the neurobiological basis of timing in autistic disorder may be particularly informative with regard to potential pharmacological remediation.
Acknowledgments
This work was supported in part by a Eunice Kennedy Shriver National Institute of Child Health & Human Development career development award (Pathway to Independence) to Melissa J. Allman (Grant K99 HD058698).
Footnotes
A portion of these data were presented at the annual meeting of the Association for Behavior Analysis (2009) and Society for Neuroscience (2008).
Contributor Information
Melissa J. Allman, Johns Hopkins University School of Medicine and Kennedy Krieger Institute
Iser G. DeLeon, Johns Hopkins University School of Medicine and Kennedy Krieger Institute
John H. Wearden, Keele University, Staffordshire, United Kingdom
References
- Allman MJ, DeLeon . No time like the present: Time perception in autism. In: Giordano AC, Lombardi VA, editors. Causes and risks for autism. New York: Nova Science; 2009. pp. 65–76. [Google Scholar]
- Baddeley AD, Hitch G. Working memory. In: Bower GH, editor. The psychology of learning and motivation. Vol. 8. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Barkley RA. It’s About Time Questionnaire. Syracuse, NY: Author; 1998. [Google Scholar]
- Barkley RA, Koplowitz S, Anderson T, McMurray MB. Sense of time in children with ADHD: Effects of duration, distraction and stimulant medication. Journal of the International Neuropsychological Society. 1997;3:359–369. [PubMed] [Google Scholar]
- Bebko JM, Weiss JA, Demark JL, Gomez P. Discrimination of temporal synchrony in intermodal events by children with autism and children with developmental disabilities without autism. Journal of Child Psychology and Psychiatry. 2006;47:88–98. doi: 10.1111/j.1469-7610.2005.01443.x. [DOI] [PubMed] [Google Scholar]
- Block RA. Time and consciousness. In: Underwood G, Stevens R, editors. Aspects of consciousness: Vol. 1. Psychological issues. London: Academic Press; 1979. pp. 179–217. [Google Scholar]
- Block RA. Models of psychological time. In: Block RA, editor. Cognitive models of psychological time. Hillsdale, NJ: Erlbaum; 1990. pp. 1–35. [Google Scholar]
- Boucher J. “Lost in a sea of time”: Time-parsing and autism. In: Hoerl C, McCormack T, editors. Time and memory. Oxford, United Kingdom: Oxford University Press; 2001. pp. 111–135. [Google Scholar]
- Boucher J, Pons F, Lind S, Williams D. Temporal cognition in children with autistic spectrum disorders: Tests of diachronic thinking. Journal of Autism and Developmental Disorders. 2007;37:1413–1429. doi: 10.1007/s10803-006-0285-9. [DOI] [PubMed] [Google Scholar]
- Brannon EM, Libertus ME, Meck WH, Woldorff MG. Electropsychological measures of time processing in infant and adult brains: Weber’s law holds. Journal of Cognitive Neuroscience. 2008;20:193–203. doi: 10.1162/jocn.2008.20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon EM, Roussel LW, Meck WH, Woldorff M. Timing in the baby brain. Cognitive Brain Research. 2004;21:227–233. doi: 10.1016/j.cogbrainres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Development and Psychopathology. 2002;14:209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Critchfield TS, Kollins SH. Temporal discounting: Basic research and the analysis of socially important behavior. Journal of Applied Behavior Analysis. 2001;34:101–122. doi: 10.1901/jaba.2001.34-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droit-Volet S, Clément A, Wearden JH. Temporal generalization in 3- to 8-year-old children. Journal of Experimental Child Psychology. 2001;80:271–288. doi: 10.1006/jecp.2001.2629. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S, Wearden JH. Temporal bisection in children. Journal of Experimental Child Psychology. 2001;80:142–159. doi: 10.1006/jecp.2001.2631. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, Kwakye LD, Cascio CJ, Burnette CP, Kadivar H, Stone WL, Wallace MT. An extended multi-sensory temporal binding window in autism spectrum disorder. Experimental Brain Research. 2010;203:381–389. doi: 10.1007/s00221-010-2240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraisse P. The adaptation of the child to time. In: Friedman JW, editor. The developmental psychology of time. New York: Academic Press; 1982. pp. 113–140. [Google Scholar]
- Fraisse P. Perception and estimation of time. Annual Review of Psychology. 1984;35:1–36. doi: 10.1146/annurev.ps.35.020184.000245. [DOI] [PubMed] [Google Scholar]
- Friedman WJ. Introduction. In: Friedman WJ, editor. The developmental psychology of time. New York: Academic Press; 1982. pp. 1–11. [Google Scholar]
- Frith U. Autism: Explaining the enigma. Oxford, United Kingdom: Blackwell; 1989. [Google Scholar]
- Gescheider GA. Psychophysics: The fundamentals. Mahwah, NJ: Erlbaum; 1997. [Google Scholar]
- Gibbon J, Allan L, editors. Annals of the New York Academy of Sciences. Vol. 423. New York: New York Academy of Sciences; 1984. Timing and time perception. [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. Annals of the New York Academy of Sciences. In: Gibbon J, Allen L, editors. Timing and time perception. Vol. 423. New York: New York Academy of Sciences; 1984. pp. 52–77. [DOI] [PubMed] [Google Scholar]
- Happé FGE. Autism: Cognitive deficit or cognitive style? Trends in Cognitive Sciences. 1999;3:216–222. doi: 10.1016/s1364-6613(99)01318-2. [DOI] [PubMed] [Google Scholar]
- Jaffe J, Beebe B, Feldstein S, Crown C, Jasnow M. Rhythms of dialogue in infancy. Monographs of the Society for Research in Child Development. 2001;66 2 Serial No. 265. [PubMed] [Google Scholar]
- James W. The principles of psychology. Vol. 1. New York: Holt; 1890. pp. 605–642. [Google Scholar]
- Killeen PR, Fetterman JG. A behavioral theory of timing. Psychological Review. 1988;95:274–295. doi: 10.1037/0033-295x.95.2.274. [DOI] [PubMed] [Google Scholar]
- Lalli JS, Casey S, Goh H, Merlino J. Treatment of escape-maintained aberrant behavior with escape extinction and predictable routines. Journal of Applied Behavior Analysis. 1994;27:705–714. doi: 10.1901/jaba.1994.27-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview—Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lustig C, Matell MS, Meck WH. Not “just” a coincidence: Frontal-striatal interactions in working memory and interval timing. Memory. 2005;13:441–448. doi: 10.1080/09658210344000404. [DOI] [PubMed] [Google Scholar]
- MacDuff GS, Krantz PJ, McClannahan LE. Teaching children with autism to use photographic activity schedules: Maintenance and generalization of complex response chains. Journal of Applied Behavior Analysis. 1993;26:89–97. doi: 10.1901/jaba.1993.26-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JS, Poirier M, Bowler DM. Brief report: Impaired temporal reproduction performance in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009;40:640–646. doi: 10.1007/s10803-009-0904-3. [DOI] [PubMed] [Google Scholar]
- Meck WH. Selective adjustment of the internal clock and memory processes. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:171–201. [PubMed] [Google Scholar]
- Meck WH. Functional and neural mechanisms of interval timing. Boca Raton, FL: CRC Press; 2003. [Google Scholar]
- Michon JA. The making of the present. In: Requin J, editor. Attention and performance. Hillsdale, NJ: Erlbaum; 1978. pp. 90–111. [Google Scholar]
- Mostofsky SH, Goldberg MC, Landa RJ, Denckla MB. Evidence for a deficit in procedural learning in children and adolescents with autism: Implications for cerebellar contribution. Journal of International Neuropsychological Society. 2000;6:752–759. doi: 10.1017/s1355617700677020. [DOI] [PubMed] [Google Scholar]
- Poppel E. A hierarchical model of temporal perception. Trends in Cognitive Neuroscience. 1997;1:56–61. doi: 10.1016/S1364-6613(97)01008-5. [DOI] [PubMed] [Google Scholar]
- Pouthas V. Timing behavior in young children: A developmental approach to conditioned spaced responding. In: Michon JA, Jackson JL, editors. Time, mind and behavior. New York: Springer-Verlag; 1985. pp. 100–109. [Google Scholar]
- Skinner BF. “Superstition” in the pigeon. Journal of Experimental Psychology. 1948;38:168–172. doi: 10.1037/h0055873. [DOI] [PubMed] [Google Scholar]
- Stern DN, Beebe B, Jaffe J, Bennett SL. The infant’s stimulus world during social interaction: A study of caregiver behaviors with particular reference to repetition and timing. In: Schaffer HR, editor. Studies in mother-infant interaction. London: Academic Press; 1977. pp. 177–202. [Google Scholar]
- Szelag E, Kowalska J, Galkowski T, Poppel E. Temporal processing deficits in high-functioning children with autism. British Journal of Psychology. 2004;95:269–282. doi: 10.1348/0007126041528167. [DOI] [PubMed] [Google Scholar]
- Wearden JH. Human performance on an analogue of an interval bisection task. Quarterly Journal of Experimental Psychology. 1991;43B:59–81. [PubMed] [Google Scholar]
- Wearden JH. Internal clocks and the representation of time. In: Hoerl C, McCormack T, editors. Time and memory. Oxford, United Kingdom: Clarendon Press; 2001. pp. 37–58. [Google Scholar]
- Wearden JH, Ferrara A. Stimulus spacing effects in temporal bisection by humans. Quarterly Journal of Experimental Psychology. 1995;48B:289–310. [PubMed] [Google Scholar]
- Wearden JH, Lejeune H. Scalar properties in human timing: Conformity and violations. Quarterly Journal of Experimental Psychology. 2008;61:569–587. doi: 10.1080/17470210701282576. [DOI] [PubMed] [Google Scholar]
- Wearden JH, Wearden AJ, Rabbitt PMA. Age and IQ effects on stimulus response timing. Journal of Experimental Psychology: Human Perception and Performance. 1997;23:962–979. [Google Scholar]
- Wechsler D. The Wechsler Intelligence Scale for Children. 4. London: Pearson Assessment; 2004. [Google Scholar]
- Wimpory D. Social timing, clock genes and autism: A new hypothesis. Journal of Intellectual Disability Research. 2002;46:352–358. doi: 10.1046/j.1365-2788.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- Wolff PH. Endogenous motor rhythms in young infants. In: Fagard J, Wolff PH, editors. The development of timing control and temporal organization in coordinated action (advances in psychology) Vol. 81. Amsterdam: Elsevier; 1991. pp. 119–133. [Google Scholar]