Abstract
Background:
The nonvitamin K antagonist oral anticoagulants pivotal clinical trials for stroke prevention in atrial fibrillation have important differences in trial designs and baseline patient characteristics.
Objective:
We sought to evaluate the relative efficacy and safety of edoxaban versus other nonvitamin K antagonist oral anticoagulants in the management of stroke prevention in atrial fibrillation by adjusting for differences in baseline stroke risk and the length of follow-up among the four phase 3 randomized controlled trials.
Methods:
We conducted a systematic literature review of randomized controlled trials evaluating the nonvitamin K antagonist oral anticoagulants for stroke prevention in atrial fibrillation and performed a network meta-analysis using data from ENGAGE AF-TIMI 48, RE-LY, ROCKET-AF, and ARISTOTLE, with warfarin as a common comparator. To adjust for between-trial differences in CHADS2 score and length of follow-up, annualized event rates among patients with CHADS2 score ⩾ 2 were analyzed using a mixed Poisson’s regression model.
Results:
Once-daily high-dose edoxaban was associated with significant lower major bleeding episodes compared with once-daily rivaroxaban (risk ratio, 0.76; 95% confidence interval, 0.66–0.89), twice-daily dabigatran 150 mg (risk ratio, 0.78; 95% confidence interval, 0.61–0.84), and twice-daily dabigatran 110 mg (risk ratio, 0.83; 95% confidence interval, 0.71–0.98) and similar bleeding risk compared with twice-daily apixaban (risk ratio, 1.08; 95% confidence interval, 0.91–1.28). Risk of stroke and systemic embolism was similar for the high-dose edoxaban and other nonvitamin K antagonist oral anticoagulant regimens. The low-dose edoxaban regimen was associated with a significant lower risk of major bleeding than other nonvitamin K antagonist oral anticoagulants and a significant higher risk of stroke and systemic embolism compared with apixaban and dabigatran 150 mg.
Conclusion:
Among patients with atrial fibrillation and CHADS2 score ⩾ 2, the high-dose edoxaban regimen may offer similar efficacy to the other nonvitamin K antagonist oral anticoagulants but with a significant major bleeding benefit over rivaroxaban and dabigatran.
Keywords: Cardiovascular, anticoagulants, atrial fibrillation, meta-analysis
Introduction
Atrial fibrillation (AF), the most common age-related arrhythmia, is associated with substantial morbidity and mortality from stroke and thromboembolism.1 Specifically, AF is an important independent risk factor for ischemic stroke. At least 15%–20% of all ischemic strokes occur in patients with AF, and this risk of ischemic stroke increases with age.2 In patients with AF aged 75 years or older, the risk of stroke is higher according to the stroke risk stratification schemes CHADS23 and CHA2DS2-VASc.4 Oral anticoagulation therapy is a well-established treatment for preventing strokes in patients with AF who are at moderate to high risk of stroke, and the nonvitamin K antagonist oral anticoagulants (NOACs) have been recommended as alternatives to warfarin for stroke prevention in patients with nonvalvular AF in various treatment guidelines.5,6
The efficacy and safety of currently marketed NOACs versus warfarin have been evaluated in four phase 3 clinical trials: RE-LY (dabigatran),7 ROCKET-AF (rivaroxaban),8 ARISTOTLE (apixaban),9 and ENGAGE AF-TIMI 48 (edoxaban).10 Several published meta-analyses have evaluated the efficacy and safety of the NOACs. Ruff et al.11 conducted a meta-analysis of data from all patients included in the four pivotal trials and found that NOACs as a class significantly reduced stroke or systemic embolism events, all-cause mortality, and intracranial hemorrhage, with a significant increase in gastrointestinal bleeding relative to warfarin. Similar results were obtained by other meta-analyses evaluating the NOACs as a class versus warfarin.12–16
The relative efficacy and safety of one NOAC to another is an important topic of interest for clinicians and other health-care stakeholders. Although this research question is best addressed by head-to-head clinical trials, researchers have resorted in the absence of such data to performing meta-analyses to indirectly compare one treatment versus another utilizing data from pivotal trials.17–24 While these studies have acknowledged the potential biases caused by the significant differences in study design and study population characteristics among the NOAC pivotal trials, most did not control for the differences. For example, ARISTOTLE, ROCKET-AF, and ENGAGE AF-TIMI 48 were double-blind, double-dummy trials, whereas RE-LY administered warfarin as an open-label treatment. Randomized, open-label, blinded trials have been shown to be associated with an enhanced treatment effect compared with double-blind trials for hemorrhagic stroke.25 In addition, ROCKET-AF and ENGAGE AF-TIMI 48 enrolled a higher stroke risk population (CHADS2 score ⩾ 2) compared with ARISTOTLE and RE-LY (CHADS2 score ⩾ 1). Time in therapeutic range (TTR) for warfarin also varied widely across the trials. Differences in baseline underlying stroke risk and TTR will affect the effectiveness of warfarin control, favoring treatment that has been studied against a less vigorous warfarin reference. Failure to adjust for these differences could result in significant bias in the results of indirect treatment comparisons assessing the relative efficacy of one NOAC versus another.
The objective of this study was to surpass previous indirect treatment comparisons of NOACs and to evaluate the relative efficacy and safety of edoxaban versus other NOACs in the management of stroke prevention in atrial fibrillaton (SPAF) by adjusting for differences in baseline stroke risk and the length of follow-up among the four phase 3 randomized controlled trials.
Methods
Data sources and search strategy
A systematic literature search using PubMed, Embase, and the Cochrane Library in accordance with the standards set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was conducted in March 2013 and updated in December 2013.26 Table 1 presents the search strategy developed for PubMed using a combination of Medical Subject Heading (MeSH) terms and keywords for the studies and the treatments of interest. The PubMed search strategy was then translated for searching the Embase and the Cochrane Library databases. (Additional details are provided in the “Methods” section of the online Appendix.) In addition, the bibliographies of existing literature reviews and meta-analyses, study register websites, submission dossiers presented to the National Institute for Health and Care Excellence, and abstracts presented in 2012 and 2013 at various conference were searched.
Table 1.
PubMed literature search strategy.
| Search number | Search terms | No. of records |
|---|---|---|
| Population | ||
| 1 | “Atrial Fibrillation”[Mesh] OR atrial fibrillation*[Title/Abstract] | 42,823 |
| Intervention and comparators | ||
| 2 | “Anticoagulants”[Majr] OR “Coumarins”[Majr] OR “warfarin”[MeSH] OR “warfarin”[Title/Abstract] OR “vitamin k antagonists”[Title/Abstract] OR “DU 176b”[Title/Abstract] OR “edoxaban”[Title/Abstract] OR “BIBR 1048”[Title/Abstract] OR “dabigatran”[Title/Abstract] OR “pradaxa”[Title/Abstract] OR “pradax”[Title/Abstract] OR “BAY 59 7939”[Title/Abstract] OR “rivaroxaban”[Title/Abstract] OR “xarelto”[Title/Abstract] OR “BMS 562247”[Title/Abstract] OR “apixaban”[Title/Abstract] OR ((“aspirin”[MeSH] OR “aspirin”[Title/Abstract]) AND (“Anticoagulants”[Majr] OR “Coumarins”[Majr] OR “warfarin”[MeSH] OR “warfarin”[Title/Abstract] OR “vitamin k antagonists”[Title/Abstract])) | 58,891 |
| Study type | ||
| 3 | “Clinical Trial, Phase III”[Publication Type] OR “Clinical Trials, Phase III as Topic”[Mesh] OR “Clinical Trial, Phase IV”[Publication Type] OR “Clinical Trials, Phase IV as Topic”[Mesh] OR “Clinical Trial, Phase II “[Publication Type] OR “Clinical Trials, Phase II as Topic”[Mesh] OR “Controlled Clinical Trial”[Publication Type] OR “Controlled Clinical Trials as Topic”[Mesh] OR “controlled clinical trial” OR “controlled clinical study” OR “Randomized Controlled Trial”[Publication Type] OR “Randomized Controlled Trials as Topic”[Mesh] OR “randomized controlled trial” OR “randomized controlled study” OR randomised controlled trial*[Title/Abstract] OR randomised controlled stud*[Title/Abstract] OR systematic[sb] OR “Meta-Analysis”[Publication Type] OR “Meta-Analysis as Topic”[Mesh] | 708,018 |
| Exclusions | ||
| Population | ||
| 4 | “Animals”[Mesh] NOT “Humans”[Mesh] | 3,759,657 |
| Study type | ||
| 5 | “Clinical Trial, Phase I”[Publication Type] OR “Clinical Trials, Phase I as Topic”[Mesh] OR “Comment”[Publication Type] OR “Editorial”[Publication Type] OR “Letter”[Publication Type] OR “Case Reports”[Publication Type] OR “Review”[ptyp] OR “Practice Guideline”[ptyp] | 4,317,784 |
| Total | ||
| 6 | 4 OR 5 | 7,882,801 |
| Totals | ||
| 7 | (1 AND 2 AND 3) NOT 6 | 507 |
Conducted on 5 March 2013. No limits regarding date of publication or language of publication were applied for this literature search.
Inclusion criteria and selection of studies
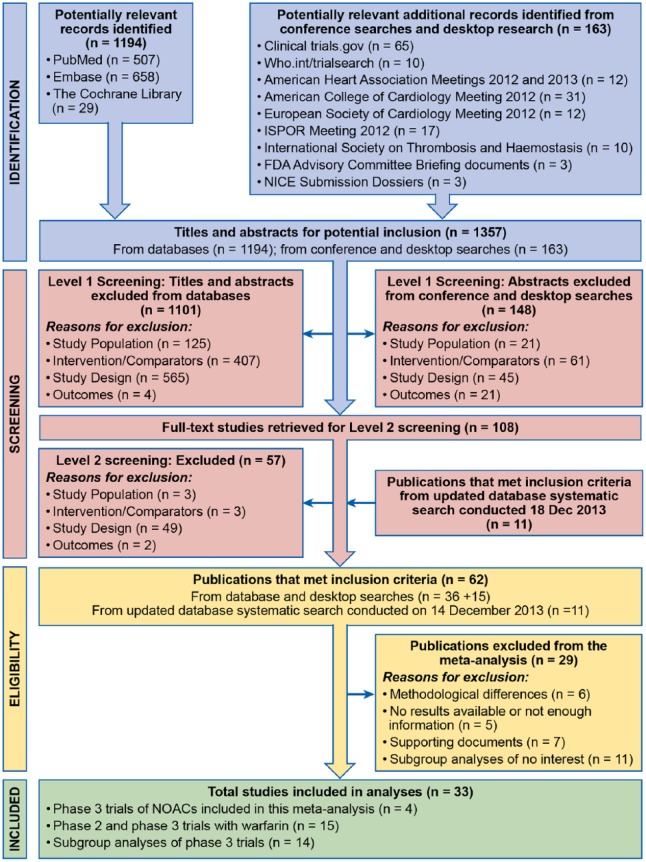
Using predefined inclusion and exclusion criteria, two reviewers independently reviewed titles and abstracts to identify published clinical studies that were potentially relevant to this study. Randomized controlled trials of patients with nonvalvular AF requiring anticoagulation were selected if the trial compared the efficacy and safety of warfarin with a marketed NOAC. Full-text articles of potentially relevant clinical studies were reviewed to confirm eligibility for inclusion. Any discrepancies were reconciled between the two reviewers or by a third reviewer if necessary. A total of 33 publications were identified as relevant. The PRISMA diagram is presented in Figure 1.
Figure 1.
Flow Diagram of Study Selection.
FDA, United States Food and Drug Administration; NICE, National Institute for Health and Care Excellence.
The update of the database systematic search was conducted from March 1, 2013, to December 18, 2013, with the objective of capturing additional published subgroup data from dabigatran, rivaroxaban, and apixaban.
Four phase 3 pivotal trials for stroke prevention in AF (RE-LY, ROCKET-AF, ARISTOTLE, and ENGAGE AF-TIMI 48) were included in this meta-analysis. Publications of subgroup analyses from RE-LY, ROCKET-AF, and ARISTOTLE were also identified and reviewed.
Data extraction and assessment of risk of bias
Data on study design information, baseline patient characteristics, interventions, and treatment outcomes were extracted from the original study publications using a standardized abstraction form. Data were checked for accuracy by two reviewers independently; the risk of bias of individual studies was assessed at the study and outcome levels using the quality criteria recommended by the Institute for Quality and Efficiency in Health Care.27 Overall, the phase 3 studies included in this meta-analysis were high quality and had a low risk of bias at the study level and at each study endpoint level.
Table 2 summarizes the main differences across the trials in terms of the trial designs, length of follow-up, and patient characteristics. (Additional details are presented in the online Appendix.) At baseline, more patients in ROCKET-AF and ENGAGE AF-TIMI 48 than in RE-LY and ARISTOTLE had a previous stroke or transient ischemic attack, as well as other comorbidities such as diabetes, hypertension, and heart failure, resulting in a higher mean CHADS2 score in these trial populations. ENGAGE AF-TIMI 48 was the longest trial, with a median follow-up of 2.8 years, compared with 1.8 years in ARISTOTLE, 1.9 years in ROCKET-AF, and 2.0 years in RE-LY. Mean TTR for the warfarin comparator was higher in ENGAGE AF-TIMI 48 (64.9%), ARISTOTLE (62.2%), and RE-LY (64.0%) than for ROCKET-AF (55.0%). The proportion of patients with baseline aspirin use also varied from 29% for ENGAGE AF-TIMI 48 to 40% for RE-LY.
Table 2.
Summary of randomized controlled trials of NOACs versus warfarin.
| RE-LY (dabigatran) | ROCKET-AF (rivaroxaban) | ARISTOTLE (apixaban) | ENGAGE AF-TIMI 48 (edoxaban) | |
|---|---|---|---|---|
| Total patients in phase 3 trial | 18,113 | 14,264 | 18,201 | 21,105 |
| Trial design | Open-label | Double-blinded | Double-blinded | Double-blinded |
| Years of follow-up (median) | 2.0 | 1.9 | 1.8 | 2.8 |
| Male (%) | 63.2% (D), 63.3% (W) | 60.3% (R), 60.3% (W) | 64.5% (A), 65.0% (W) | 62.1% (E), 62.4% (W) |
| Mean CHADS2 score | 2.2 (D), 2.1 (W) | 3.5 (R), 3.5 (W) | 2.1 (A), 2.1 (W) | 2.8 (E), 2.8 (W) |
| Patients with CHADS2 score ⩾ 2 | 67.8% (D), 69.1% (W) | 100% | 66% | 100% |
| Patients with CHADS2 score ⩾ 3 | 32.6% (D), 32.1% (W) | 87% | 30.2% | 53.4% (E); 52.6% (W) |
| Patients with previous stroke or transient ischemic attack | 20.3% (D), 19.8% (W) | 54.9% (R), 54.6% (W) | 19.2% (A), 19.7%(W) | 28.1% (E), 28% (W) |
| Patients with diabetes | 23% (D), 23% (W) | 40% (R), 40% (W) | 25% (A), 25% (W) | 36% (E), 36% (W) |
| Patients with hypertension | 79% (D), 79% (W) | 90% (R), 91% (W) | 87% (A), 88% (W) | 94% (E), 94% (W) |
| Patients with heart failure | 31.8% (D), 31.9% (W) | 62.6% (R), 62.3% (W) | 35.5% (A), 35.4% (W) | 58.2% (E), 58% (W) |
| Patients who used aspirin at baseline | 38.7% (D), 40.6% (W) | 36.3% (R), 36.7% (W) | 31.3% (A), 30.5% (W) | 29.4% (E), 29.7% (W) |
| Mean TTR | 64.0% | 55.0% | 62.2% | 64.9% |
A: apixaban; AF: atrial fibrillation; CHADS2: stroke risk factor scoring system in which 1 point is given for history of congestive heart failure, hypertension, age ⩾ 75 years, and diabetes and 2 points are given for history of stroke or transient ischemic attack; D: dabigatran; E: edoxaban; NOAC: nonvitamin K antagonist oral anticoagulant; R: rivaroxaban; TTR: time in therapeutic range; W: warfarin.
Outcome measures
Consistent with study endpoints in the pivotal trials, the composite endpoint of stroke and systemic embolism was defined as the primary efficacy endpoint, and major bleeding was defined as the primary safety endpoint in the network meta-analyses (NMAs). Across the trials, major bleeding was defined using the International Society on Thrombosis and Haemostasis (ISTH) criteria28 (see Table A-1 in supplemental appendix). In addition, the following secondary endpoints were evaluated, depending on data availability: composite of major bleeding and clinically relevant nonmajor bleeding, ischemic stroke, hemorrhagic stroke, systemic embolism, all-cause mortality, cardiovascular mortality, myocardial infarction, intracranial hemorrhage, gastrointestinal bleeding, clinically relevant nonmajor bleeding, and fatal bleeding. For consistency across trials, efficacy endpoints in general were based on intention-to-treat (ITT) population in the overall study period, and safety endpoints were based on safety population on-treatment period (with a few exceptions due to data availability). No hierarchical testing was performed for the meta-analyses.
Statistical methods
The NMA included the four large clinical trials that compared a NOAC with warfarin. To minimize potential biases resulting from differences in patient clinical characteristics between the trials, only data from patients with CHADS2 score ⩾ 2 in RE-LY and ARISTOTLE were used in the NMA. Previous studies have shown that the net clinical benefit of warfarin varied by CHADS2 score, where warfarin has essentially no clinical benefit in patients with CHADS2 score of 0 and 1, and net clinical benefit increased to 2.22% in patients with CHADS2 score ⩾ 4.29 Therefore, it is important to adjust for differences in underlying stroke risk when comparing the relative efficacy and safety of one NOAC versus another when warfarin is used as the reference comparator. In addition, to minimize potential biases resulting from differences in the duration of study follow-up, annualized event rates were analyzed.
A mixed Poisson’s regression model with treatment as a fixed effect and study as a random effect was developed separately for each outcome to provide treatment-effect estimates of the relative efficacy and safety of edoxaban in comparison with apixaban, dabigatran, and rivaroxaban. In this analysis, we derived total person-years of exposure for each specific event in each treatment arm based on the event rate (percentage of patients per year) and the number of patients experiencing an event as reported in the pivotal trial publications. Risk ratios (RRs) comparing the annualized event rate (percentage of patients with event per year) for the high- and low-dose edoxaban regimens versus other NOACs along with the 95% confidence intervals (CIs) were reported. Assessment of the consistency of effects across studies is an essential part of meta-analysis. However, we were unable to perform heterogeneity test and report the I2 statistic that is typically included with other meta-analyses due to the lack of repeated pair of treatment data in our network. We only have data from one pivotal trial for each of the NOAC evaluated in this study. To confirm model validity, estimated RRs of the primary efficacy and primary safety endpoints for each treatment versus warfarin derived from the NMA were compared with results of direct comparison from the pivotal trials. All analyses were performed using SAS software version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Safety endpoints
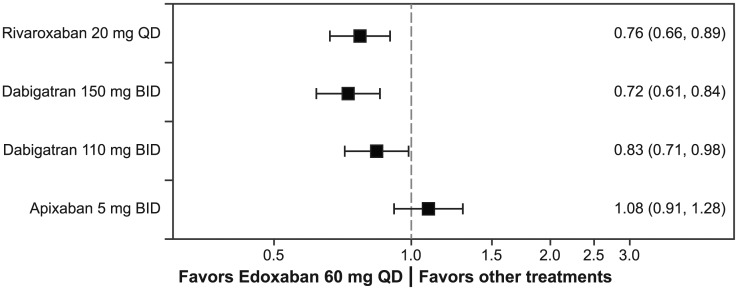
For the primary safety endpoint, major bleeding, the high-dose edoxaban regimen (60 mg/30 mg dose reduced) had a significantly lower risk of major bleeding than rivaroxaban (RR, 0.76; 95% CI, 0.66–0.89), dabigatran 150 mg (RR, 0.72; 95% CI, 0.61–0.84), and dabigatran 110 mg (RR, 0.83; 95% CI, 0.71–0.98; Figure 2). The risk of major bleeding was similar between the high-dose edoxaban regimen and apixaban (RR, 1.08; 95% CI, 0.91–1.28). The risk of intracranial hemorrhage for the high-dose edoxaban regimen was similar to that for apixaban, dabigatran 150 mg, dabigatran 110 mg, and rivaroxaban (Table 3). Notably, the high-dose edoxaban regimen also had significantly lower rates of the composite of major bleeding and clinically relevant bleeding (RR, 0.81; 95% CI, 0.72–0.90), major gastrointestinal bleeding (RR, 0.75; 95% CI, 0.63–0.91), and clinically relevant nonmajor bleeding (RR, 0.80; 95% CI, 0.71–0.90) compared with those for rivaroxaban. Data for composite major and clinically relevant nonmajor bleeding and major gastrointestinal bleeding in patients with CHADS2 score ⩾ 2 were not available for dabigatran and apixaban.
Figure 2.
Major bleeding ratios: Risk ratios and 95% confidence intervals are represented for major bleeding for the high-dose edoxaban regimen versus other NOACs. Ratios are estimated only for patients with CHADS2 score ≥ 2 at baseline.
BID, twice daily; CHADS2, stroke risk factor scoring system in which 1 point is given for history of congestive heart failure, hypertension, age ≥ 75 years, and diabetes, and 2 points are given for history of stroke or transient ischemic attack; NOAC, non -vitamin K antagonist oral anticoagulant; QD, once daily.
Table 3.
Key secondary endpoints from network meta-analysis: risk ratio (95% confidence interval) for high- and low-dose edoxaban versus other NOACs in patients with CHADS2 score ⩾ 2 at baseline.
| Secondary endpoints | High-dose edoxaban regimen versus rivaroxaban | High-dose edoxaban regimen versus apixaban | High-dose edoxaban regimen versus dabigatran 110 mg | High-dose edoxaban regimen versus dabigatran 150 mg |
|---|---|---|---|---|
| Safety endpointsa | ||||
| Intracranial hemorrhage | 0.76 (0.52–1.10) | 1.06 (0.69–1.62) | 1.63 (0.96–2.76)b | 1.02 (0.65–1.59)b |
| Major gastrointestinal bleed | 0.75 (0.63–0.91) | N/A | N/A | N/A |
| CRNM bleeding | 0.80 (0.71–0.90) | N/A | N/A | N/A |
| Fatal bleeding | 1.22 (0.68–2.17) | N/A | N/A | N/A |
| Efficacy endpointsc | ||||
| Ischemic stroke | 0.95 (0.73–1.24) | 1.09 (0.81–1.48) | N/A | N/A |
| Hemorrhagic stroke | 0.87 (0.56–1.35) | N/A | N/A | N/A |
| Systemic embolism | 0.57 (0.25–1.27) | N/A | N/A | N/A |
| All-cause mortality | 0.95 (0.83–1.08)d | 1.01 (0.87–1.16) | 0.95 (0.81–1.10) | 0.94 (0.81–1.09) |
| Cardiovascular mortality | 0.99 (0.78–1.25)d | N/A | 0.93 (0.80–1.08) | 0.96 (0.82–1.12) |
| Myocardial infarction | 1.05 (0.72–1.51)d | 1.15 (0.86–1.55) | N/A | N/A |
| Secondary endpoints | Low-dose edoxaban regimen versus rivaroxaban | Low-dose edoxaban regimen versus apixaban | Low-dose edoxaban regimen versus dabigatran 110 mg | Low-dose edoxaban regimen versus dabigatran 150 mg |
| Safety endpointsa | ||||
| Intracranial hemorrhage | 0.50 (0.33–0.77) | 0.71 (0.45–1.12) | 1.09 (0.62–1.90)b | 0.68 (0.42–1.10)b |
| Major gastrointestinal bleed | 0.41 (0.33–0.51) | N/A | N/A | N/A |
| CRNM bleeding | 0.61 (0.54–0.69) | N/A | N/A | N/A |
| Fatal bleeding | 0.75 (0.40–1.41) | N/A | N/A | N/A |
| Efficacy endpointsc | ||||
| Ischemic stroke | 1.34 (1.04–1.74) | 1.55 (1.15–2.09) | N/A | N/A |
| Hemorrhagic stroke | 0.53 (0.33–0.87) | N/A | N/A | N/A |
| Systemic embolism | 1.07 (0.51–2.23) | N/A | N/A | N/A |
| All-cause mortality | 0.90 (0.79–1.03)d | 0.96 (0.83–1.10) | 0.90 (0.77–1.05) | 0.90 (0.77. 1.04) |
| Cardiovascular mortality | 0.97 (0.77–1.24)d | N/A | 0.92 (0.79–1.07) | 0.95 (0.81–1.10) |
| Myocardial Infarction | 1.35 (0.94–1.93)d | 1.47 (1.10–1.95) | N/A | N/A |
CHADS2: stroke risk factor scoring system in which 1 point is given for history of congestive heart failure, hypertension, age ⩾ 75 years, and diabetes and 2 points are given for history of stroke or transient ischemic attack; CRNM: clinically relevant nonmajor; N/A: not available; NOAC: nonvitamin K antagonist oral anticoagulant; ITT: intention-to-treat.
Data from safety on-treatment period analyses with exceptions noted.
Data from ITT overall period population were used for dabigatran due to data availability.
Data from ITT population in the overall study period, with exceptions noted.
Data from safety on-treatment population were used for rivaroxaban due to data availability.
The low-dose edoxaban regimen (30 mg/15 mg dose reduced) had a significantly lower rate of major bleeding than all other NOACs, with a RR of 0.63 (95% CI, 0.52–0.76) versus apixaban, 0.42 (95% CI, 0.35–0.50) versus dabigatran 150 mg, 0.49 (95% CI, 0.41–0.59) versus dabigatran 110 mg, and 0.45 (95% CI, 0.38–0.53) versus rivaroxaban, respectively. The risk of intracranial hemorrhage for the low-dose edoxaban regimen was similar to apixaban and both dabigatran doses. Compared with rivaroxaban, the low-dose edoxaban regimen had significantly lower rates of the composite of major bleeding and clinically relevant nonmajor bleeding, intracranial hemorrhage, major gastrointestinal bleeding, and clinically relevant nonmajor bleeding.
Efficacy endpoints
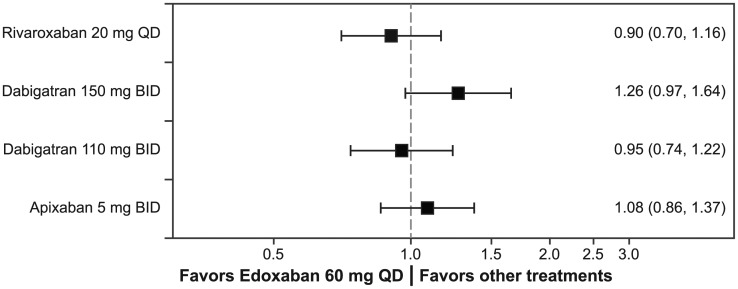
Among the patients with CHADS2 score ⩾ 2, the risk of the primary efficacy endpoint, the composite of stroke and systemic embolism, for the high-dose edoxaban regimen was similar to that for apixaban, dabigatran 150 mg, dabigatran 110 mg, and rivaroxaban (all RRs vs the high-dose edoxaban regimen, P > 0.05; Figure 3). There were no significant differences in ischemic stroke risk among the high-dose edoxaban regimen, apixaban, and rivaroxaban treatment groups (dabigatran data for patients with CHADS2 score ⩾ 2 were not available). The risk of hemorrhagic stroke was similar between the high-dose edoxaban regimen and rivaroxaban. Comparison of the risk of hemorrhagic stroke was not possible for edoxaban versus apixaban and dabigatran because apixaban and dabigatran data were not available for patients with CHADS2 score ⩾ 2 (Table 3).
Figure 3.
Stroke and systemic embolism ratios: Risk ratios and 95% confidence intervals are represented for the composite of stroke and systemic embolism for the high-dose edoxaban regimen versus other NOACs. Ratios are estimated only for patients with CHADS2 score ≥ 2 at baseline.
BID, twice daily; CHADS2, stroke risk factor scoring system in which 1 point is given for history of congestive heart failure, hypertension, age ≥ 75 years, and diabetes, and 2 points are given for history of stroke or transient ischemic attack; NOAC, non-vitamin K antagonist oral anticoagulant; QD, once daily.
The high-dose edoxaban regimen also had a risk of all-cause mortality similar to those for apixaban, dabigatran 150 mg, dabigatran 110 mg, and rivaroxaban (all RRs vs the high-dose edoxaban regimen, P > 0.05). Cardiovascular mortality was also similar between the high-dose edoxaban regimen, rivaroxaban, and dabigatran (P > 0.05). Cardiovascular mortality data among patients with CHADS2 score ⩾ 2 were not available for apixaban; thus, a comparison between the high-dose edoxaban regimen and apixaban for this endpoint was not made. No significant differences were found among the high-dose edoxaban regimen, rivaroxaban, and apixaban for the myocardial infarction endpoint (P > 0.05; Table 3).
The low-dose edoxaban regimen had a significantly higher risk of stroke and systemic embolism than apixaban (RR, 1.41; 95% CI, 1.12–1.77) and dabigatran 150 mg (RR, 1.64; 95% CI, 1.26–2.13) but a similar risk of rivaroxaban (RR, 1.17; 95% CI, 0.92–1.50). The rate of ischemic stroke was significantly higher with the low-dose edoxaban regimen than with apixaban, rivaroxaban, and the high-dose edoxaban regimen. The low-dose edoxaban regimen was associated with a lower risk of hemorrhagic stroke (RR, 0.53; 95% CI, 0.33–0.87) than rivaroxaban. Compared with apixaban, the risk of myocardial infarction was higher for the low-dose edoxaban regimen (RR, 1.47; 95% CI, 1.10–1.95). There were no significant differences in the risk of all-cause mortality and cardiovascular mortality for the low-dose edoxaban regimen versus other NOACs (comparison vs apixaban for cardiovascular mortality was not performed due to the lack of available apixaban data).
Other analyses
Table 4 presents the results of each NOAC versus warfarin. Hazard ratios (HRs) for apixaban and dabigatran versus warfarin among patients with CHADS2 score ⩾ 2 were not directly reported in published literature. Nevertheless, RR estimates comparing NOACs versus warfarin among patients with CHADS2 score ⩾ 2 from the NMA were generally similar to the HR results based on direct comparison from the individual pivotal trials, confirming the validity of the model results. The significantly lower risk of the primary efficacy endpoint in the high-dose edoxaban regimen versus warfarin observed in the analysis could be due to the inclusion of less-well-controlled warfarin from ROCKET-AF in the pooled warfarin arm. The treatment effect of warfarin depends on TTR, and the mean TTR below 60% indicates that warfarin may be inefficient.30 The TTR level varied across the NOAC pivotal trials. In particular, mean TTR was only 55% of the time (median, 58%; interquartile range, 43%–71%) among patients in the warfarin group in ROCKET-AF study. As the result, mixing warfarin data from studies with lower TTR may result in a better relative treatment effect compared with warfarin for treatments that have been studied under a more rigorous TTR.
Table 4.
Annualized events and risk ratios of NOACs versus warfarin in patients with CHADS2 score ⩾ 2 at baseline.
| Pivotal trials (CHADS2 score ⩾ 2) |
Network meta-analysis |
|||
|---|---|---|---|---|
| Warfarin (%/year) | NOAC (%/year) | HR (95% CI) | RR versus warfarin (95% CI) | |
| Major bleeding (safety, on-treatment period) | ||||
| Apixaban versus warfarin | 3.52 | 2.56 | Not reported | 0.73 (0.63–0.84) |
| Dabigatran 150 mg versus warfarin | 3.90 | 3.91 | Not reported | 1.09 (0.96–1.24) |
| Dabigatran 110 mg versus warfarin | 3.90 | 3.38 | Not reported | 0.94 (0.82–1.08) |
| Rivaroxaban versus warfarin | 3.4 | 3.6 | 1.04 (0.90–1.20) | 1.03 (0.91–1.15) |
| Edoxaban high-dose regimen versus Warfarin | 3.43 | 2.75 | 0.80 (0.71–0.91) | 0.78 (0.70–0.88) |
| Edoxaban low-dose regimen versus warfarin | 3.43 | 1.61 | 0.47 (0.41–0.55) | 0.46 (0.40–0.53) |
| Major bleeding–CRNM bleeding (safety, on-treatment period) | ||||
| Apixaban versus warfarin | Not reported | Not reported | Not reported | Not reported |
| Dabigatran 150 mg versus warfarin | Not reported | Not reported | Not reported | Not reported |
| Dabigatran 110 mg versus warfarin | Not reported | Not reported | Not reported | Not reported |
| Rivaroxaban versus warfarin | 14.5 | 14.9 | 1.03 (0.96–1.11) | 1.04 (0.97–1.13) |
| Edoxaban high-dose regimen versus Warfarin | 13.02 | 11.10 | 0.86 (0.80–0.92) | 0.84 (0.78–0.90) |
| Edoxaban low-dose regimen versus warfarin | 13.02 | 7.97 | 0.60 (0.57–0.67) | 0.60 (0.56–0.65) |
| Stroke and systemic embolic events (ITT, overall study period) | ||||
| Apixaban versus warfarin | 2.00 | 1.56 | Not reported | 0.77 (0.64–0.93) |
| Dabigatran 150 mg versus warfarin | 2.00 | 1.34 | Not reported | 0.66 (0.53–0.83) |
| Dabigatran 110 mg versus warfarin | 2.00 | 1.78 | Not reported | 0.88 (0.72–1.08) |
| Rivaroxaban versus warfarin | 2.4 | 2.1 | 0.88 (0.75–1.03) | 0.93 (0.78–1.11) |
| Edoxaban high-dose regimen versus warfarin | 1.80 | 1.57 | 0.87 (0.73–1.04) | 0.84 (0.72–0.98) |
| Edoxaban low-dose regimen versus warfarin | 1.80 | 2.04 | 1.13 (0.96–1.34) | 1.09 (0.94–1.26) |
CI: confidence interval; CRNM: clinically relevant nonmajor; HR: hazard ratio; ITT: intention to treat; NOAC: nonvitamin K antagonist oral anticoagulant; RR: risk ratio; CHADS2: stroke risk factor scoring system in which 1 point is given for history of congestive heart failure, hypertension, age ⩾ 75 years, and diabetes and 2 points are given for history of stroke or transient ischemic attack.
Discussion
In an era of evidence-based health-care decision making, health-care stakeholders are very interested in comparative research data. In the absence of head-to-head clinical trials, indirect treatment comparison or NMA is often used as an alternative to provide data on the relative efficacy and safety of one treatment versus another. However, the validity of NMA requires the studies included in the network to be “clinically and methodologically” similar,31,32 and appropriate adjustment must be made for differences. The differences in study design and clinical characteristics of patients in the four pivotal trials comparing apixaban, dabigatran, edoxaban, and rivaroxaban versus warfarin have been widely discussed in the literature.11,17,18 However, adjustment for these differences is challenging in the absence of patient-level data from each of these trials.
We attempted to adjust for differences among the pivotal trials by adjusting for length of follow-up and limiting the analysis to patients with CHADS2 score ⩾ 2. Because there were no informative priors to use for a Bayesian approach, we opted for a frequentist approach in which we generated the likelihood function using a mixed Poisson’s regression model. Poisson’s analysis of annualized event rates assumed the risk of events of interest to be constant over the follow-up period. This assumption would be violated when the chance of events is varied during the exposure time. This concern was somewhat abated by the fact that the relative efficacy and safety of NOAC versus warfarin estimated by Poisson’s models were consistent with the direct evidence from the original pivotal trials, supporting the validity of our methodology.
Our results on the relative efficacy of edoxaban versus other NOACs were generally consistent with previous indirect treatment comparisons that did not control for these differences11,17,18 with a few exceptions. Similar to previous studies,11,17,18 we found no significant differences in the efficacy of the high-dose edoxaban regimen in reducing the risk of stroke and systemic embolism when compared with other NOACs, and we found that the risk of major bleeding for the high-dose edoxaban regimen was similar to that of apixaban and lower than that of rivaroxaban. In contrast to two other NMAs incorporating data from all patients enrolled in RE-LY (patients with CHADS2 score ⩾ 1), which found that the high-dose edoxaban regimen had rates of major bleeding similar to those of the dabigatran 150 mg and 110 mg regimens,17,18 our results showed a lower risk of major bleeding with the high-dose edoxaban regimen relative to both dabigatran doses in patients with CHADS2 score ⩾ 2. Because of the need to limit the analysis to include data for patients with CHADS2 score ⩾ 2, we were unable to compare and draw conclusions about many secondary efficacy and safety endpoints, as other previous studies have done. However, the noted differences in study results further underscore the importance of adjusting for baseline patient characteristics when conducting NMA to minimize biases.
This study has several limitations. Foremost, data for patients with CHADS2 score ⩾ 2 were not available for many endpoints for the RE-LY and ARISTOTLE trials. Therefore, we were not able to fully evaluate the relative efficacy and safety of edoxaban relative to dabigatran and apixaban. Similar to many meta-analyses,17–24 heterogeneity across the individual studies included in the analysis is a potential limitation. Although limiting study populations to patients with CHADS2 score ⩾ 2 across individual studies may somewhat mitigate heterogeneity bias, there were still other characteristics unique to each study that we were unable to adjust for. For example, in the absence of patient-level data from each of the pivotal trials and published subgroup data among patients with CHADS2 score ⩾ 2, we were unable to control for two important treatment-effect modifiers: TTR and concomitant aspirin use that can significantly affect efficacy and safety outcomes. While concomitant aspirin use may increase the risk of bleeding, poor international normalized ratio (INR) control or decrease (TTR) has been shown to increase the risk of mortality, thromboembolic events, and major bleeding risk in patients treated with warfarin.33,34 TTR subgroup data for the overall trial population is only available for RE-LY, ARISTOTLE, and ENGAGE AF-TIMI 48 trials in the published literature, but TTR and aspirin use subgroup data for CHADS2 ⩾ 2 patients are not available. Adjustment for aspirin use is also difficult in the absence of patient-level data to allow for appropriate adjustment of aspirin dose on an individual basis. For example, the ENGAGE AF-TIMI 48 and RE-LY trials allowed concomitant aspirin up to 100 mg/day in the study; no information on concomitant aspirin dosages is available for the ARISTOTLE and ROCKET-AF trials, except that in ARISTOTLE, patients needed aspirin at a dose of >165 mg/day were excluded. Furthermore, the percentages of patients who were taking concomitant aspirin during the ARISTOTLE trial are not available from published sources. Hence, the inability to adjust for differences in TTR across pivotal trials will bias results in favor of NOAC treatment with the lowest TTR in the pivotal trial. In addition, the small number of available studies (i.e. four pivotal trials) had prevented us from using meta-regression to control for multiple treatment-effect modifiers at the same time. Furthermore, we were unable to statistically adjust for differences between open-label administration of warfarin in RE-LY and double-blind administration of warfarin in other studies. Because data for each NOAC were only available from a single pivotal trial, and the four clinical trials included in our NMA did not form a single closed loop in the network, and heterogeneity test for results consistency could not be performed. Finally, recent treatment guidelines have adopted the CHA2-DS2-VASc score as stroke risk stratification scheme for the recommendation of oral anticoagulation therapy.35 The generalizability of our study findings to clinical practice could be limited by the use of CHADS2 scores as stroke risk stratification in the pivotal studies. Therefore, future comparative effectiveness research is warranted to confirm our study findings.
Conclusion
Significant differences in patient characteristics and trial design exist among the four pivotal phase 3 trials comparing an individual NOAC with warfarin. The results of comparing NOACs versus warfarin from our NMA were generally consistent with the results of direct comparison of the NOACs versus warfarin in the individual pivotal studies. Our study showed that the high-dose edoxaban regimen had a significantly lower risk of major bleeding compared with the risks of rivaroxaban, dabigatran 150 mg, and dabigatran 110 mg, and a risk similar to that of apixaban. The high-dose edoxaban regimen was also similarly efficacious in reducing the risk of stroke and systemic embolism compared with the other NOACs. Low-dose edoxaban had similar efficacy and reduced bleeding compared with rivaroxaban and dabigatran 110 mg and had higher rates of stroke and systemic embolic events and reduced bleeding rates compared with apixaban and dabigatran 150 mg. Notwithstanding the study’s limitations, high-dose edoxaban regimen may be a favorable once-daily alternative to other NOACs for stroke prevention among patients with nonvalvular AF who have a CHADS2 score of ⩾2. Study findings need to be confirmed by future head-to-head comparison studies.
Supplementary Material
Acknowledgments
The authors would like to thank Qiaoyi Zhang, MD, PhD, for her constructive review and input and Kate Lothman for her careful and helpful edits.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Xin Ye and Jackie Kwong are employees of Daiichi Sankyo, Inc., Parsippany, NJ, USA, the manufacturer of edoxaban. Maria M. Fernandez, Jianmin Wang, Bintu Sherif, Susan Hogue, and Beth Sherrill are employees of RTI Health Solutions, an independent scientific research organization that received consulting fees from Daiichi Sankyo, Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article This study was supported by Daiichi Sankyo, Inc., Parsippany, NJ, USA.
References
- 1. Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol 2005; 14(2): 56–61. [DOI] [PubMed] [Google Scholar]
- 2. Sanoski CA. Clinical, economic, and quality of life impact of atrial fibrillation. J Manag Care Pharm 2009; 15(6 Suppl. B): S4–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001; 285(22): 2864–2870. [DOI] [PubMed] [Google Scholar]
- 4. Lip GY, Halperin JL. Improving stroke risk stratification in atrial fibrillation. Am J Med 2010; 123(6): 484–488. [DOI] [PubMed] [Google Scholar]
- 5. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64(21): 2246–2280. [DOI] [PubMed] [Google Scholar]
- 6. Camm AJ, Lip GY, De Caterina R, et al. ; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33(21): 2719–2747. [DOI] [PubMed] [Google Scholar]
- 7. Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361(12): 1139–1151. [DOI] [PubMed] [Google Scholar]
- 8. Patel MR, Mahaffey KW, Garg J, et al. ; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365(10): 883–891. [DOI] [PubMed] [Google Scholar]
- 9. Granger CB, Alexander JH, McMurray JJ, et al. ; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365(11): 981–992. [DOI] [PubMed] [Google Scholar]
- 10. Giugliano RP, Ruff CT, Braunwald E, et al. ; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369(22): 2093–2104. [DOI] [PubMed] [Google Scholar]
- 11. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383(9921): 955–962. [DOI] [PubMed] [Google Scholar]
- 12. Jia B, Lynn HS, Rong F, et al. Meta-analysis of efficacy and safety of the new anticoagulants versus warfarin in patients with atrial fibrillation. J Cardiovasc Pharmacol 2014; 64(4): 368–374. [DOI] [PubMed] [Google Scholar]
- 13. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation 2012; 126(20): 2381–2391. [DOI] [PubMed] [Google Scholar]
- 14. Dogliotti A, Paolasso E, Giugliano RP. Novel oral anticoagulants in atrial fibrillation: a meta-analysis of large, randomized, controlled trials vs warfarin. Clin Cardiol 2013; 36(2): 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller CS, Grandi SM, Shimony A, et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol 2012; 110(3): 453–460. [DOI] [PubMed] [Google Scholar]
- 16. Gómez-Outes A, Terleira-Fernández AI, Calvo-Rojas G, et al. Dabigatran, rivaroxaban, or apixaban versus warfarin in patients with nonvalvular atrial fibrillation: a systematic review and meta-analysis of subgroups. Thrombosis 2013; 2013: 640723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fu W, Guo H, Guo J, et al. Relative efficacy and safety of direct oral anticoagulants in patients with atrial fibrillation by network meta-analysis. J Cardiovasc Med 2014; 15(12): 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skjøth F, Larsen TB, Rasmussen LH, et al. Efficacy and safety of edoxaban in comparison with dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation. An indirect comparison analysis. Thromb Haemost 2014; 111(5): 981–988. [DOI] [PubMed] [Google Scholar]
- 19. Baker WL, Phung OJ. Systematic review and adjusted indirect comparison meta-analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes 2012; 5(5): 711–719. [DOI] [PubMed] [Google Scholar]
- 20. Lip GY, Larsen TB, Skjøth F, et al. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2012; 60(8): 738–746. [DOI] [PubMed] [Google Scholar]
- 21. Mantha S, Ansell J. An indirect comparison of dabigatran, rivaroxaban and apixaban for atrial fibrillation. Thromb Haemost 2012; 108(3): 476–484. [DOI] [PubMed] [Google Scholar]
- 22. Testa L, Agnifili M, Latini RA, et al. Adjusted indirect comparison of new oral anticoagulants for stroke prevention in atrial fibrillation. QJM 2012; 105(10): 949–957. [DOI] [PubMed] [Google Scholar]
- 23. Harenberg J, Marx S, Diener HC, et al. Comparison of efficacy and safety of dabigatran, rivaroxaban and apixaban in patients with atrial fibrillation using network meta-analysis. Int Angiol 2012; 31(4): 330–339. [PubMed] [Google Scholar]
- 24. Schneeweiss S, Gagne JJ, Patrick AR, et al. Comparative efficacy and safety of new oral anticoagulants in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2012; 5(4): 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lega JC, Mismetti P, Cucherat M, et al. Impact of double-blind vs. open study design on the observed treatment effects of new oral anticoagulants in atrial fibrillation: a meta-analysis. J Thromb Haemost 2013; 11(7): 1240–1250. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Institute for Quality and Efficiency in Health Care. General methods, version 4.0 (English version), https://www.iqwig.de/download/General_Methods_4-0.pdf [PubMed]
- 28. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3(4): 692–694. [DOI] [PubMed] [Google Scholar]
- 29. Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151(5): 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Asarcıklı LD, Taner Şen T, İpek EG, et al. Time in therapeutic range (TTR) value of patients who use warfarin and factors which influence TTR [abstract]. J Am Coll Cardiol 2013; 62(18 Suppl. 2): C127–C128. [Google Scholar]
- 31. Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health 2011; 14(4): 429–437. [DOI] [PubMed] [Google Scholar]
- 32. Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol 2011; 64(11): 1187–1197. [DOI] [PubMed] [Google Scholar]
- 33. White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulation control. Arch Intern Med 2007; 167: 239–245. [DOI] [PubMed] [Google Scholar]
- 34. Jones M, McEwan P, Morgan CL, et al. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non-valvar atrial fibrillation: a record linkage study in a large British population. Heart 2005; 91: 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. January CT, Wann LS, Alpert JS, et al. ; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130(23): 2071–2104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.