Abstract
Background
The rebuilding of the connective tissue during wound healing requires the recruitment of fibroblasts to the wound area as well as reentry of quiescent fibroblasts to the proliferative cycle. Whether this process can be modulated by a small molecular weight thiol antioxidant N-acetyl-L-cysteine (NAC) was tested in normal human skin fibroblasts (NHFs) in this study.
Methods and Results
By using a uni-directional wound healing assay, NAC treated cells demonstrated a decreased migration rate but increased number of proliferating cells recruited into the wound area post wounding. Fifteen day quiescent control and NAC treated NHFs were re-plated at a lower density and cell numbers counted at different days post-plating. Interestingly, NAC treated cells exhibited increased cellular proliferation indicated by both decreased cell population doubling time and increased S phase cells. NAC treated cells demonstrated decreased steady state levels of reactive oxygen species as well as increased protein and activity levels of manganese superoxide dismutase (MnSOD). NAC treatment failed to induce proliferation in quiescent cells lacking MnSOD expression.
Conclusions
These results demonstrate that NAC enhanced the recruitment of quiescent NHFs into proliferation cycle during wound healing. Our results also suggest that the wound healing properties of NAC might be due to its ability to induce and enhance MnSOD expression and activity. Altogether, these findings suggest NAC might be potentially developed as a dietary intervention to improve tissue injury in animals and humans.
Keywords: NAC, MnSOD, quiescent, wound healing
Introduction
Wound healing abnormalities cause great physical and psychological stress to a large percentage of the population, such as aged individuals, diabetes, and cancer patients treated with immunosuppressive drugs, chemo- or radiotherapy. Refractory wounds in patients lead to many amputations each year despite advances in wound care. Reactive oxygen species (ROS) have been well recognized for playing a dual role as both deleterious and beneficial species in the wound healing process. Moderate amounts of ROS are required for efficient defense against invading pathogens and also for cellular signaling (e.g. angiogenesis). However, excessive production of ROS or impaired antioxidant defense system causes oxidative stress, which is one of the important factors that contribute to the pathogenesis of an impaired wound healing. Therefore, antioxidants especially naturally derived antioxidants are postulated to suppress wound oxidative stress and therefore help wound healing.
N-acetyl-cysteine (NAC) is an antioxidant that has been previously reported to improve different types of wound healing [1-4]. NAC is the acetylated precursor of both the amino acid L-cysteine and reduced glutathione (GSH) [5]. NAC is a naturally occurring compound, which can be commonly found in food (such as garlic and onion) and also synthesized by the body. There are three proposed mechanisms in which NAC is considered as an antioxidant. First, NAC has been shown to react directly with various ROS, including H2O2, O2•- and •OH [6]. Secondly, NAC is a cysteine pro-drug and may exert its antioxidant effects by enhancing tissue levels of GSH [7]. Finally, we have shown previously that NAC treatment in mouse fibroblasts induces MnSOD expression through a transit increase in superoxide measured by Electron Spin Resonance Spectroscopy (ESR) [8]. NAC has been in clinical use for more than 30 years, primarily as a mucolytic [9]. In addition to its mucolytic action, NAC is being studied and utilized in conditions characterized by decreased GSH or oxidative stress such as HIV infection, cancer, and heart disease [9]. Because of its hepato-protective activity, intravenous and oral administration of NAC have been used extensively in the management of acetaminophen poisoning [9].
Previous studies have shown that excessive ROS generation contributes to delayed wound healing through inhibition of nuclear factor-kappa B (NF-kB), which occupies a central role in the inflammatory process, essential for clearing the contaminating bacteria and creating an environment conducive to succeeding events involved in tissue repair and regeneration [4]. Therefore, dietary supplementation of NAC in protein malnutrition (PM) mice improved wound healing through restoring NF-kB-regulated signaling pathways [4]. Recently, we showed that NAC induced the mitochondrial antioxidant enzyme manganese superoxide dismutase (MnSOD) and regulated cell cycle progression [8]. In addition, we also shown that MnSOD protected the proliferative capacity of quiescent normal human fibroblast after a long time in quiescence through the regulation of two critical cyclin-dependent kinase (Cdk) inhibitors, p16 and p21 [10]. Recruitment of quiescent fibroblasts (usually around the wound area) to the proliferative cycle contributes to the rebuilding of the connective tissue during wound healing. Taken together, we hypothesized that antioxidants including NAC could have protective effects on the proliferative potential of quiescent cells, which facilitate both normal tissue renewal and wound healing. Therefore, the objective of this study is to investigate whether NAC promotes wound healing by modulating the cellular antioxidant systems and the cellular proliferation capability of quiescent cells.
Materials and Methods
Cell lines and cell culture conditions
Primary human normal skin (NHF, AG01522) fibroblasts were obtained from Coriell Cell Repositories. MnSOD wild type (MnSOD WT), heterozygous (+/-), and homozygous knock-out (-/-) mouse embryonic fibroblasts (MEFs) cells were generously provided by Dr. T. T. Huang (Stanford University). All cell lines were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, supplemented with penicillin/streptomycin antibiotics. Cells were grown in incubators with controlled temperature of 37°C, CO2 of 5% and humidity of 95%. MnSOD MEFs were grown in a 4% oxygen incubator. To minimize the differences in population doubling and the effects of replicative limit of cells, all experiments were performed using cells from the same passage number; human cells used were in passage 7-8. In addition, each single experiment was originated from the same cell population. Cells were harvested either by scraping or by trypsinization for further analysis.
Wound Healing assay for cell migration and cell density
A one-directional wound healing assay was used following a previously reported protocol with some modifications [11]. Cells were kept in the quiescent stage in tissue culture plastic dishes for at least 15 days before wounding. The wounding was done using a special razor blade in which the blade was used to cut and then dragged to remove the cell layer on one side of the plate. The cells were monitored for migration and cell density using high contrast bright field microscopy for up to 7 days. Pictures were taken for the same field each day. ImageJ analysis modules for spatial measurements and particle analysis were used to measure cell migration and density.
BrdU assay for S phase cells and confocal microscopy
For measurements of cellular S phase fraction in the wound area, cells in culture were pulsed with 10 μM Bromodeoxyuridine (BrdU) at day 3 after wounding for 1 hour. Cell then were fixed in 4% para-formaldehyde, washed with PBS, and incubated with 0.2% Tirton X-100, washed again with PBS and incubated with 2 N HCl for 30 min before they were washed again with PBS. Cells were incubated with 5% goat serum for 30 min and incubated with primary antibody of mouse anti-BrdU followed by incubation with a secondary antibody goat anti-Mouse Alexa 488 (green). Cells were counter stained with TO-PRO3 for nuclear staining (blue). Zeiss 510 confocal microscope was used for visualization and data storage (The University of Iowa Central Microscopy Research Core Facility). The number of BrdU positive cells was normalized to total number of cells in the wound area for the calculation of cellular S phase fraction using particle analysis module in ImageJ software (NIH).
Measurements of proliferative potential and proliferation cycle re-entry
One million cells per T-25 tissue culture flask were cultured in regular medium. Cells were allowed to reach quiescence (less than 5% S-phase were considered quiescent) before NAC treatments. Quiescent cultures were fed every 3 d with regular and NAC containing medium. Measurements of proliferative re-entry were performed by replating quiescent cell cultures at a lower density. Cells were collected 48 hours later and ethanol fixed. Ethanol-fixed cells were treated with RNase A for 30 min followed by staining with propidium iodide. Flow cytometry analysis of DNA content and percent cell cycle phase distributions were performed following our previously published methods [10,12]. Cells fractions in S and G2 phase were used as indicator for proliferation potential and re-entry.
ROS measurements using DHE and confocal microscopy
For dihydroethidium (DHE) measurements, cells at day 4 after wounding were incubated with Hanks buffer salt solution (HBSS) containing 2 μM Draq5 nuclear stain for 15 min and then with 10 μM DHE for another 20 min. Antimycin A (concentration 10 μM) was used as positive control for ROS production. Zeiss 510 confocal microscope was used for visualization and data collection (The University of Iowa Central Microscopy Research Core Facility). Measurements of DHE-fluorescence were performed using 488 nm excitation laser and 585/42 nm emission filter. Mean fluorescence intensity was analyzed using ImageJ software. Auto-fluorescence of unlabeled cells was used for background fluorescence correction, and nuclear stain was used to normalize the DHE mean fluorescent intensity to cell number.
Growth curves and population doubling times
Cell population doubling times were calculated from growth curves. Re-plated cells were continued in culture and cell numbers counted up to 4 days. Cell population doubling time (Td) values were calculated from the exponential portion of the growth curve using the following equation: Td=0.693t/ln(Nt/N0) where t is time in days, and Nt and N0 represent cell numbers at time t and the initial time 0, respectively.
Immunoblotting assay for protein levels and expression
Cells were harvested by either scraping or trypsinization, washed with PBS, and re-suspended in phosphate buffer (pH 7.8). Cells were sonicated on ice. Protein concentrations were determined with Bradford protein assay kit. Equal amounts of protein were separated by 12.5% SDS-PAGE, electrotransferred by semidry blotting onto a nitrocellulose membrane and probed with rabbit anti human polyclonal antibodies against MnSOD. MnSOD antibody was obtained from the antioxidant core facility at The University of Iowa. Immunoreactive bands were detected by chemiluminescence kit. The bands were visualized using X-ray film and imaged with computerized digital imaging system. Bands were quantified with ImageJ software (NIH). The integrated density value was obtained by integrating the entire pixel values in the area of one band after correction for background. All blots were re-probed with actin antibody for loading corrections.
MnSOD activity gel assay
Total cellular protein extracts were assayed for MnSOD activity following a previously reported assay [13]. Cells were harvested by scraping, washed with PBS, and re-suspended in phosphate buffer (pH 7.8). Cell suspensions were sonicated on ice with 5 bursts of 15 seconds. Protein concentrations were determined with Bradford protein assay kit. Equal amounts of protein were separated by a 12.5% non-dissociating (native) gel and a 5% stacking gel containing riboflavin and polymerized under white fluorescent light. Gels were stained for SOD activity with nitroblue tetrazolium (NBT) (2.43 mM) and riboflavin-TEMED (riboflavin 2.8 × 10-5 M and TEMED 28 mM) for 20 min at room temperature. Gels were kept in distilled water and illuminated under bright fluorescent light until SOD bands were visualized (1-4 h). Copper and zinc superoxide dismutase (CuZnSOD) and MnSOD can be differentiated in presence of soincubators with controlled temperature dium cyanide (0.75 mM) in the staining solution, which inhibits CuZnSOD activity. The bands were visualized and quantified with a computerized digital imaging system.
Statistical analysis
Statistical analysis was done using the one and two-way analysis of variance with Tukey's post-analysis. Homogeneity of variance was assumed with 95% confidence interval level. Results from at least n ≥ 3 with p < 0.05 were considered significant. All statistical analysis were done using SPSS computer software Standard version 10.0.5 (SPSS Inc., Chicago, IL).
Results
NAC decreases quiescent cell migration rates but increases their recruitment into the wound area
The effect of NAC on quiescent cell migration and their recruitment into the wound area during wounding was investigated using a one-directional wound healing assay. Representative bright light microscopy images of control and NAC treated (15 days) quiescent cells at day 2, 4, and 7 after wounding are shown in Fig. 1a. NAC treatments at the concentrations of 1 mM and 5 mM significantly decreased quiescent cell migration rates by 25%, when compared to control group (Fig. 1b). Interestingly, no difference in migration rates was seen between two doses of NAC (1mM vs. 5 mM). Cell density in wound area was also measured to investigate the effect of NAC on its ability to recruit cells to wound area. Effect of NAC on cell density in wound area was not significant until up to day 6 following wounding (Fig. 1c, left panel). NAC treatments significantly increased cell density in wound area at day 7 and this effect trended to be a dose-dependent response (Fig. 1c, right panel).
Fig. 1.
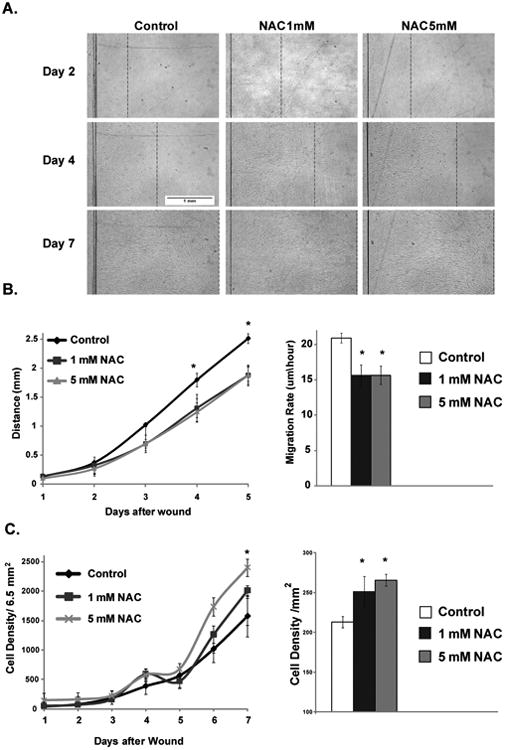
NAC effects on cellular migration and recruitment of cells during wound healing. A. Representative bright light microscopy images of control and NAC treated (15 days) quiescent cells at day 2, 4, and 7 after wounding (dotted line indicates the migration far edge). B. Left panel: migration progress of cells over 5 days, commutative average distance of migration across the field width was calculated each day for 5 days. Right panel: the migration rate of control and NAC treated cells. C. Left panel: Cells density of control and NAC treated measured up to 7 days in field area of 6.5 mm2. Right panel: Cells density were calculated at day 7 after wounding expressed per mm2 area. Values are means with their standard deviations (N=3). Asterisk indicates significant difference between control and NAC treated cells (P < 0.05).
NAC protects the proliferative capacity in quiescent cells and induces the recruitment of more proliferation-competent cells into the wound area
Fifteen day-quiescent control and NAC treated NHFs were re-plated at a lower density and cell numbers were counted at different days post-plating. While control cells showed cell population doubling of 48 h, 1 and 5 mM NAC treated cells exhibited 28 and 24 h doubling time, respectively (Fig. 2a). In addition, by using bright light microscopy, increased number of mitotic cells was observed in both 1 mM and 5 mM NAC treated groups at day 4 after wounding (highlighted in squares in Fig. 2b), when compared to control group. Cells treated by 1 and 5 mM NAC demonstrated a two-fold increase of the cell number in S phase indicated by BrdU staining (Fig. 2c and d). However, no difference in S phase cell number was seen between two doses of NAC (1mM vs. 5 mM).
Fig. 2.

NAC enhances the proliferative potential of quiescent cells and their recruitment during wound healing. A. Control and NAC treated quiescent cells for 15 days were sub-cultured at lower density and cell numbers were measured for up to 4 days post plating to calculate growth rate. B. Representative bright light microscopy images of control and NAC treated (15 days) quiescent cells at day 4 after wounding showing mitotic cells (squares), lower inserts show a higher magnification of the mitotic cells. C. Representative confocal microscopy images of control and NAC treated quiescent cells at day 3 after wounding pulsed with BrdU to label S phase cells and processed for immune staining as described in the material and methods. D. BrdU positive fraction representative of S phase cells in control and NAC treated cells. Values are means with their standard deviations (N=3). Asterisk indicates significant difference between control and NAC treated cells (P < 0.05).
NAC modulates cellular MnSOD levels and decreases ROS levels during wound healing
MnSOD has been previously shown to protect the proliferative capacity of confluent normal human fibroblasts [10]. Therefore, expression and activity levels of MnSOD were measured in control and NAC treated quiescent cells. NAC at concentration of 5 mM trended towards increasing MnSOD mRNA expression but without a statistical significance (Fig. 3a). By contrast, 1 mM and 5 mM NAC significantly induced both protein and activity levels of MnSOD (Fig. 3a). Consistently, 1 mM and 5 mM NAC also significantly decreased steady-state levels of ROS indicated by DHE mean fluorescent intensity in confocal microscopy. As a positive control for this assay, addition of antimycin A, which is an electron transport chain blocker for complex I, significantly increased DHE mean fluorescence (Fig. 3a). However, no difference in DHE mean fluorescent intensity was seen between two doses of NAC (1mM vs. 5 mM).
Fig. 3.
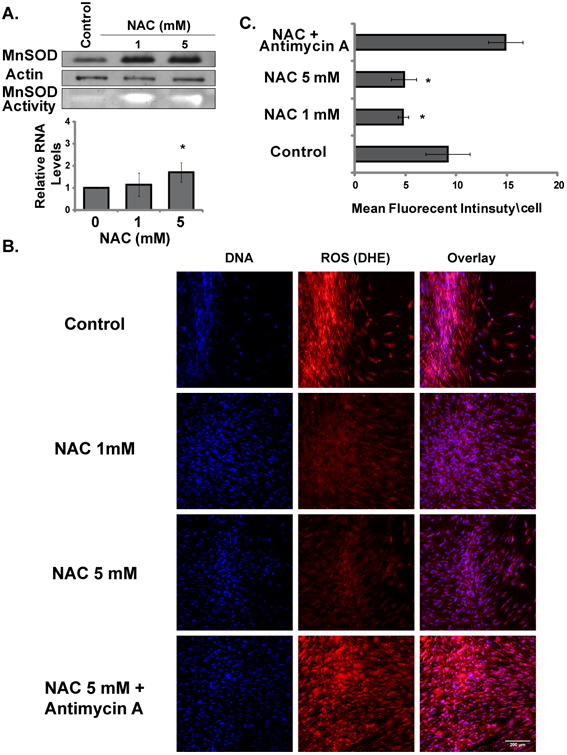
NAC induces MnSOD expression and activity and suppresses ROS levels in quiescent cells. A. MnSOD protein, activity and mRNA levels in control and NAC treated quiescent cells after 15 days. B. Representative confocal microscopy images of control and NAC treated quiescent cells at day 3 after wounding labeled with DHE for ROS levels. C. DHE mean fluorescent intensity in control and NAC treated quiescent cells normalized to total cells in the field of measurement. Values are means with their standard deviations (N=3). Asterisk indicates significant difference between control and NAC treated cells (P < 0.05).
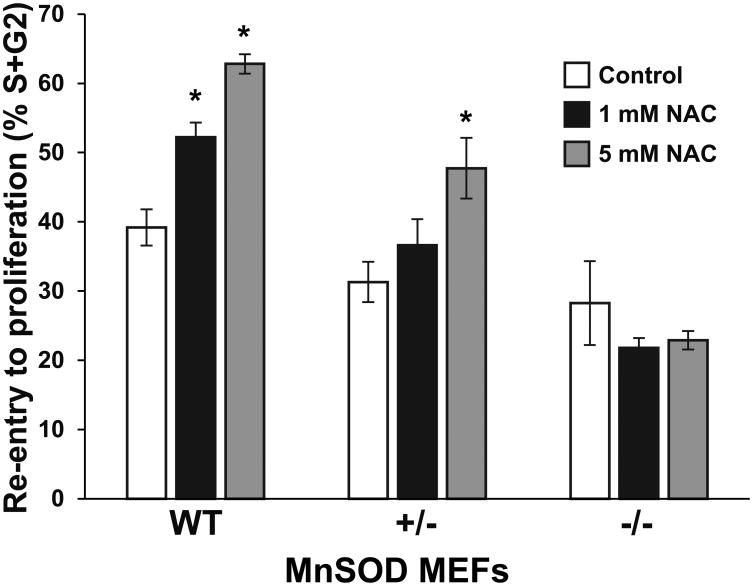
MnSOD regulates NAC induced increase in fibroblasts' recruitment into the proliferation cycle
To further investigate the role of MnSOD in regulating the NAC effect on fibroblasts' proliferation during wound healing, we examined the effects of NAC on quiescent MnSOD (WT), heterozygous (+/-), knock-out (-/-) MEFs during re-entry into the proliferation cycle. Control and NAC treated quiescent MEFs were sub-cultured at lower density post treatment for 48 hours and then collected and evaluated for cell cycle distribution using PI DNA content assay and flow cytometry. The cell proliferation potential was calculated from the fractions of cells entering the S and G2 phases of the cell cycle form the quiescent phase (G0/G1). Higher percentages of cells in the S and G2 phase indicate increased number of cells entering the proliferation cycle and better proliferation potential. Results showed NAC treatment enhanced MnSOD WT and +/- quiescent cells re-entry into the proliferation cycle (Fig. 4). MnSOD WT showed a significant increase in S+G2 fraction in both 1mM and 5 mM NAC treatment (52% and 63% respectively). MnSOD +/- showed significate increase only at 5 mM NAC treatment (Fig 4). In contrast, NAC treatments did not induce any changes in the S+G2 fraction in MnSOD knockouts -/- (Fig 4). These results indicate that MnSOD expression and activity are required for NAC induced enhancement of fibroblasts proliferation potential.
Fig. 4.
MnSOD regulates a NAC induced increase in fibroblasts' recruitment into the proliferation cycle. Control and NAC treated quiescent MnSOD WT, +/-, and -/- knockout MEFs (15 days) were re-plated at lower cell density, 48 hours later, cells were harvested and ethanol-fixed. Samples were then processed for PI DNA content assay to measure cell cycle phase distribution (G0/G1, S and G2). Data was analyzed with computer software ModFit (Verity Software House,USA) and expressed as percentage of the total population. S+G2 fractions were used as indicator for proliferation potential and cell cycle re-entry. Values are means with their standard deviations (N=3). Asterisk indicates significant difference between control and NAC treated cells (P < 0.05).
Discussion
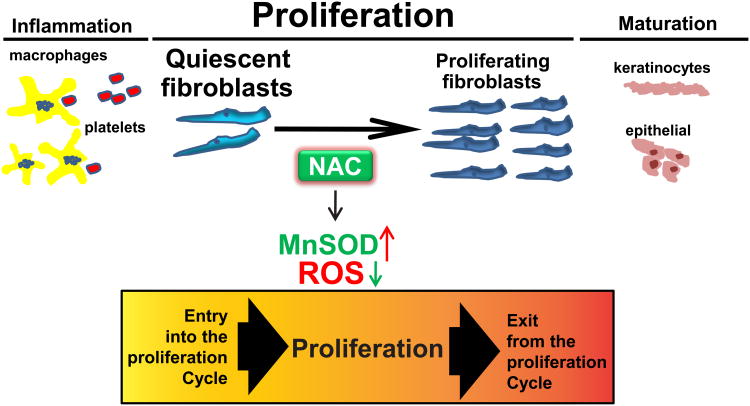
Wound healing is an integrative and well-coordinated regenerative response to tissue injury that involves a complex process consisting of three stages including inflammation, proliferation, and maturation and remodeling, with different types of cells involved in each stage (Fig. 4). Effect of NAC on inflammation during wound healing has been previously studied. Inflammation helps clear the contaminating bacteria and facilitate wound healing process. Dietary supplementation of NAC has been shown to improve cutaneous wound healing in protein malnourished mice through enhancing early inflammatory responses including interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) expression [4], which have been demonstrated to elicit dramatic elevations of both the mRNA and protein levels of MnSOD [14,15]. In addition, the effect of NAC on extracellular matrix (ECM) remodeling during wound healing has also been reported. Topical NAC administration has been demonstrated to accelerate burn wound healing through induction of collagenous expression of matrixmetalloproteinase-1 (MMP-1) via protein kinase C (PKC) signaling pathway, which has been implicated in pathologies involving ECM remodeling [1].
The current study suggests that during the second stage of wound healing, NAC decreased levels of ROS, which enhanced the recruitment and proliferation of quiescent human fibroblasts, indicating a redox control of quiescent cells entry into the proliferative cycle (Fig. 5). Consistent with our observation, NAC has also been observed to promote cellular proliferation in adipose-derived stem cells, which hold great promise for wound repair and regeneration [16]. In addition, a recent study has also reported that NAC enhanced cellular proliferation as well as scratch wounding healing in CCD966SK cells (human skin fibroblast cell line) [1]. We also observed that increased cellular proliferation induced by NAC during wound healing was paralleled with increased levels of MnSOD protein expression and activity. MnSOD is a nuclear-encoded and mitochondria-matrix-localized oxidation-reduction (redox) enzyme that catalyzes the dismutation of superoxide radicals to hydrogen peroxide and therefore regulates cellular redox homeostasis. The observation above supports the concept we have put forth that cellular redox processes can regulate proliferative and quiescent growth states [17]. Even though the direct reaction of NAC with superoxide radicals is fairly slow [6], we and others have demonstrated that NAC can execute its “antioxidant” property in different experimental models by increasing MnSOD expression or activity [8,18,2,19]. However, the molecular mechanisms by which NAC regulates MnSOD expression and activity are still elusive. Many studies and reports including ours [20,21], have shown that MnSOD expression is regulated at different levels from transcriptional, post transcriptional to post translational levels. On the transcriptional level, NF-kB is known in the literature as an upstream regulator of MnSOD transcription [22,23] and has also been reported to be re-activated in NAC-mediated enhanced wound healing [4] which may indicate a potential mechanism for effect of NAC on MnSOD expression. MnSOD mRNA has two poly(A) sites resulting in two transcripts: 1.5 and 4.2 kb which we recently showed preferential selection during quiescence and under oxidative stress by radiation with the more stable 1.5 kb transcript resulting in higher MnSOD protein and activity levels [20]. Furthermore, in our recent report [21] we have shown that MnSOD activity is regulated by post translational mechanism. We have shown that methylation of the lysine 89 in the active site of MnSOD increases its activity during quiescence [21]. These studies may provide another proposed mechanisms in which NAC may affect MnSOD expression and activity in quiescent NHF. NAC could contribute to selection of stable 1.5k transcript resulting in high protein levels or may affect the methylation pattern on the MnSOD protein during quiescence to enhance its activity. Future research is warranted to investigate the specific mechanisms by which NAC regulates MnSOD levels.
Fig. 5.
Redox control of quiescent cells entry into the proliferative cycle during wound healing. Wound healing involves a complex process consisting of three important stages including inflammation, proliferation, and maturation and remodeling. In the first stage, cells such as platelets and macrophages are involved in inflammation, which helps clear the contaminating bacteria and facilitate wound healing process. In the second stage, fibroblasts begin to enter the wounding site and initiate the phase of cellular proliferation. Our working model suggests that NAC treatment induces increased MnSOD expression and activity and leads to low levels of ROS, which facilitate quiescent cells entry into the proliferation cycle and improve wound healing. In the final stage, cells such as epithelial cells and keratinocytes which can migrate over the wound site are responsible for the epithelialization, which contributes to the maturation during wound healing.
Superoxide is known to be a critical signaling molecule that regulates wound healing through its reaction with nitric oxide (NO). NO is a pleiotropic mediator of inflammation, proliferation, and angiogenesis, all of which are central to wound healing. Increased levels of superoxide inactivate NO and delay wound healing. In diabetic wounds from a mouse model of type 1 diabetes, decreased levels of endothelial NO synthase (NOS) protein and activity were correlated with increased levels of superoxide [24]. The same study further demonstrated that gene therapy of endothelial NOS and MnSOD restored delayed wound healing, accompanied with a concomitant suppression of wound superoxide levels and restoration of NOS protein and activity levels [24]. Furthermore, the same group reported that decreased expression of MnSOD in endothelia progenitor cells (EPCs) contributes to impaired wound healing in a mouse model of type 2 diabetes [25]. This conclusion was supported by the observation that transplantation of diabetic EPCs which have decreased levels of MnSOD after MnSOD gene therapy restored their ability to mediate angiogenesis and wound repair, whereas siRNA-mediated knockdown of MnSOD in normal EPCs reduced their ability in wound healing [25]. Interestingly, cytosolic superoxide dismutase CuZnSOD has failed to restore wound repair [25]. Our results are consistent with these previous studies where we demonstrate that loss of MnSOD diminishes the effect of NAC on the recruitment of quiescent cells into proliferation cycle during wound healing. Altogether, these suggest a specific mitochondria-derived superoxide signaling pathway regulates the wound healing in this diabetic model and probably also other wound repair models.
Taken together, we demonstrated in this study that NAC suppresses ROS levels through upregulating MnSOD and enhances cellular recruitment to proliferation of quiescent cells during the wound healing process (Fig. 5). The protective effect of NAC on quiescent cells that is usually found in the peri wound area is of great importance for tissue repair and renewal under normal and pathological conditions. These protective effects of NAC could result in better wound healing prognosis and better wound closure. NAC has the advantages of being safe, inexpensive, and well-tolerated and therefore merits additional research to develop NAC as a potential dietary or topical application intervention to improve wound healing.
Acknowledgments
We thank Mr. Jian Shao from the Central Microscopy facility for assisting in the light and confocal imaging. We also thank Mr. John Lafin for critical reading of the manuscript. This work was supported by NIH 2R01 CA111365 and McCord research foundation. The authors' contributions are as follows: G. M. and E. H. S. drafted the manuscript; G. M., M. G., A. L. K., and E. H. S. performed the experiments; P. C. G. and E. H. S. provided advice on experimental design and supervised the study. All authors read and approved the final version of the manuscript.
List of Abbreviations
- BrdU
Bromodeoxyuridine
- Cdk
Cyclin-dependent kinase
- CuZnSOD
Copper and zinc superoxide dismutase
- DHE
Dihydroethidium
- ECM
Extracellular matrix
- EPCs
Endothelia progenitor cells
- GSH
Reduced glutathione
- HBSS
Hanks buffer salt solution
- IL-1β
Interleukin-1β
- MMP-1
Matrixmetalloproteinase-1
- MnSOD
Manganese superoxide dismutase
- NAC
N-acetyl-L-cysteine
- NHFs
Normal human skin fibroblasts
- NO
Nitric oxide
- NOS
Endothelial NO synthase
- PI
Propidium iodide
- PKC
Protein kinase C
- PM
Protein malnutrition
- ROS
Reactive oxygen species
- TNF-α
Tumor necrosis factor-α
Footnotes
The authors have no financial or personal conflicts of interest to declare.
References
- 1.Tsai ML, Huang HP, Hsu JD, Lai YR, Hsiao YP, Lu FJ, Chang HR. Topical N-Acetylcysteine Accelerates Wound Healing in Vitro and in Vivo via the PKC/Stat3 Pathway. Int J Mol Sci. 2014;15(5):7563–7578. doi: 10.3390/ijms15057563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demir EO, Cakmak GK, Bakkal H, Turkcu UO, Kandemir N, Demir AS, Tascilar O. N-acetyl-cysteine improves anastomotic wound healing after radiotherapy in rats. J Invest Surg. 2011;24(4):151–158. doi: 10.3109/08941939.2011.560237. [DOI] [PubMed] [Google Scholar]
- 3.Aktunc E, Ozacmak VH, Ozacmak HS, Barut F, Buyukates M, Kandemir O, Demircan N. N-acetyl cysteine promotes angiogenesis and clearance of free oxygen radicals, thus improving wound healing in an alloxan-induced diabetic mouse model of incisional wound. Clin Exp Dermatol. 2010;35(8):902–909. doi: 10.1111/j.1365-2230.2010.03823.x. [DOI] [PubMed] [Google Scholar]
- 4.Lim Y, Levy MA, Bray TM. Dietary supplementation of N-acetylcysteine enhances early inflammatory responses during cutaneous wound healing in protein malnourished mice. J Nutr Biochem. 2006;17(5):328–336. doi: 10.1016/j.jnutbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Millea PJ. N-acetylcysteine: multiple clinical applications. Am Fam Physician. 2009;80(3):265–269. [PubMed] [Google Scholar]
- 6.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6(6):593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 7.Fabiani R, De Bartolomeo A, Rosignoli P, Servili M, Montedoro GF, Morozzi G. Cancer chemoprevention by hydroxytyrosol isolated from virgin olive oil through G1 cell cycle arrest and apoptosis. Eur J Cancer Prev. 2002;11(4):351–358. doi: 10.1097/00008469-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Menon SG, Sarsour EH, Kalen AL, Venkataraman S, Hitchler MJ, Domann FE, Oberley LW, Goswami PC. Superoxide signaling mediates N-acetyl-L-cysteine-induced G1 arrest: regulatory role of cyclin D1 and manganese superoxide dismutase. Cancer Res. 2007;67(13):6392–6399. doi: 10.1158/0008-5472.CAN-07-0225. [DOI] [PubMed] [Google Scholar]
- 9.Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998;3(2):114–127. [PubMed] [Google Scholar]
- 10.Sarsour EH, Agarwal M, Pandita TK, Oberley LW, Goswami PC. Manganese superoxide dismutase protects the proliferative capacity of confluent normal human fibroblasts. J Biol Chem. 2005;280(18):18033–18041. doi: 10.1074/jbc.M501939200. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez LG, Wu X, Guan JL. Wound-healing assay. Methods Mol Biol. 2005;294:23–29. doi: 10.1385/1-59259-860-9:023. [DOI] [PubMed] [Google Scholar]
- 12.Sarsour EH, Kumar MG, Kalen AL, Goswami M, Buettner GR, Goswami PC. MnSOD activity regulates hydroxytyrosol-induced extension of chronological lifespan. Age (Dordr) 2012;34(1):95–109. doi: 10.1007/s11357-011-9223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 14.Visner GA, Dougall WC, Wilson JM, Burr IA, Nick HS. Regulation of manganese superoxide dismutase by lipopolysaccharide, interleukin-1, and tumor necrosis factor. Role in the acute inflammatory response. The Journal of biological chemistry. 1990;265(5):2856–2864. [PubMed] [Google Scholar]
- 15.Eastgate J, Moreb J, Nick HS, Suzuki K, Taniguchi N, Zucali JR. A role for manganese superoxide dismutase in radioprotection of hematopoietic stem cells by interleukin-1. Blood. 1993;81(3):639–646. [PubMed] [Google Scholar]
- 16.Xiong L, Sun J, Hirche C, Yang J, Yang Y, Xia Y, Lehnhardt M, Wang R, Fu X. In vitro N-acetyl-L-cysteine promotes proliferation and suppresses interleukin-8 expression in adipose-derived stem cells. Aesthetic Plast Surg. 2012;36(5):1260–1265. doi: 10.1007/s00266-012-9960-8. [DOI] [PubMed] [Google Scholar]
- 17.Sarsour EH, Kalen AL, Goswami PC. Manganese superoxide dismutase regulates a redox cycle within the cell cycle. Antioxid Redox Signal. 2014;20(10):1618–1627. doi: 10.1089/ars.2013.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata K, Iwasaki Y, Yamada T, Yuba T, Kono K, Hosogi S, Ohsugi S, Kuwahara H, Marunaka Y. Overexpression of manganese superoxide dismutase by N-acetylcysteine in hyperoxic lung injury. Respir Med. 2007;101(4):800–807. doi: 10.1016/j.rmed.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Yang YY, Lee KC, Huang YT, Wang YW, Hou MC, Lee FY, Lin HC, Lee SD. Effects of N-acetylcysteine administration in hepatic microcirculation of rats with biliary cirrhosis. J Hepatol. 2008;49(1):25–33. doi: 10.1016/j.jhep.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhuri L, Nicholson AM, Kalen AL, Goswami PC. Preferential selection of MnSOD transcripts in proliferating normal and cancer cells. Oncogene. 2012;31(10):1207–1216. doi: 10.1038/onc.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarsour EH, Kalen AL, Xiao Z, Veenstra TD, Chaudhuri L, Venkataraman S, Reigan P, Buettner GR, Goswami PC. Manganese superoxide dismutase regulates a metabolic switch during the mammalian cell cycle. Cancer Res. 2012;72(15):3807–3816. doi: 10.1158/0008-5472.CAN-11-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Kiningham KK, Devalaraja MN, Yeh CC, Majima H, Kasarskis EJ, St Clair DK. An intronic NF-kappaB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-alpha and interleukin-1beta. DNA Cell Biol. 1999;18(9):709–722. doi: 10.1089/104454999314999. [DOI] [PubMed] [Google Scholar]
- 23.Dhar SK, Xu Y, St Clair DK. Nuclear factor kappaB- and specificity protein 1-dependent p53-mediated bi-directional regulation of the human manganese superoxide dismutase gene. J Biol Chem. 2010;285(13):9835–9846. doi: 10.1074/jbc.M109.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo JD, Wang YY, Fu WL, Wu J, Chen AF. Gene therapy of endothelial nitric oxide synthase and manganese superoxide dismutase restores delayed wound healing in type 1 diabetic mice. Circulation. 2004;110(16):2484–2493. doi: 10.1161/01.CIR.0000137969.87365.05. [DOI] [PubMed] [Google Scholar]
- 25.Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest. 2010;120(12):4207–4219. doi: 10.1172/JCI36858. [DOI] [PMC free article] [PubMed] [Google Scholar]