Abstract
Mitochondrial translation is important for the synthesis of proteins involved in oxidative phosphorylation, which yields the bulk of the ATP made in cells. During evolution most mitochondria-containing organisms have lost tRNA genes from their mitochondrial genomes. Thus, to support the essential process of nuanced mitochondrial translation, mechanisms to actively transport tRNAs from the cytoplasm across the mitochondrial membranes into the mitochondrion have evolved. Here, we review the currently known tRNA import mechanisms, comment on recent discoveries of various import factors, and suggest a rationale for forces that lie behind the evolution of mitochondrial tRNA import.
Keywords: import, localization, mitochondria, protein complex, tRNA
Introduction
Eukaryotic cells contain extensive intracellular compartmentalization that allows sub-cellular organelles to keep distinct metabolic processes separate, often as a means to prevent unwanted reactions. In kinetoplastid flagellates (Trypanosoma and Leishmania) for example, recent studies have shown that compartmentalization of glycolytic enzymes prevents accumulation of toxic glycolytic intermediates in the cytoplasm (Haanstra et al., 2008). Likewise, in the case of tRNAs, given that the mitochondrial translation system is bacteria-like in nature, compartmentalization could prevent unwanted side reactions due to miscoding, during protein synthesis, caused by differences in ribosome specificity. What leads to compartmentalization is an extensive network of membrane systems, which are the foundations of intracellular architecture. Mitochondria and various types of plastids are unique membrane-bound intracellular compartments that usually contain their own genome, which encodes a number of protein-coding genes, ribosomal RNA genes, and a variable number of tRNAs (Gray et al., 2004).
Mitochondrial genomes, owing to their bacterial ancestry, still maintain characteristics of their α-proteobacterial progenitor. Mitochondrial protein synthesis utilizes a set of tRNAs encoded in the mitochondrial genome that bear resemblance to their bacterial counterparts. For example, as in Mycoplasma, the genetic code in most mitochondria (with the exception of plants) employs UGA as a tryptophan codon which is read by a mitochondria-encoded tryptophan tRNA (tRNATrp) containing a UCA anticodon. As in bacteria, tRNAs that contain G34 in the first anticodon position are used to decode the C-ending codons for the amino acids Ala, Arg, Ile, Leu, Pro, Ser, Thr, and Val. In fact, neither inosine 34-containing tRNAs nor the enzymes necessary to generate inosine are found in mitochondria. Inosine, however, is still widely used for cytoplasmic translation. Despite evolutionary conservation, mitochondrial genomes are dynamic and throughout evolution have relegated a number of functions to the nucleus via gene transfer and gene loss. To date, a wide majority of mitochondrial proteins are indeed encoded in the nucleus, synthesized in the cytoplasm and subsequently imported into the organelle where together with a few mitochondria-encoded subunits constitute the mature, fully functional respiratory complexes, which in turn yield the lion's share of the ATP made in mitochondria-containing eukaryotes.
Remarkably, compartmentalization and the loss of mitochondrial genetic material have also led to the disappearance of tRNA genes from mitochondrial genomes. This has in turn driven the evolution of mechanisms for the import of tRNAs from the cytoplasm, to enable translation of the few mRNAs still encoded in mitochondria (Salinas et al., 2008). However, the factors and/or mechanisms that control tRNA import are not fully understood, but recent advances in the field have started to shed light on the nuances of the various import pathways. In the present review, we will focus on our current knowledge of the diverse import systems, examine some experimental discrepancies, and highlight future challenges.
A number of studies have recently highlighted differences between import systems leading to the claim of the existence of various different import mechanisms. However in our view, the mitochondrial tRNA import field is still too young to support such claims; more rigorous biochemical and genetic data are needed. For the sake of clarity we will refrain from using the term mechanism too loosely in this review, and will strictly divide import systems into broadly defined types: (i) those utilizing the known protein import pathway and (ii) those in which import is shown to occur independent of the protein import machinery. The present review will describe (1) the prevalence of tRNA import systems among various organisms, (2) possible factors, key determinants, and bioenergetics involved in tRNA import, and (3) possible driving forces that led to the evolution of tRNA import systems. There are certain similarities between tRNA import systems and the protein import pathways; yet protein import will not be the subject of this review.
Many organisms… not so many mechanisms
Although mitochondrial protein synthesis is invariably essential, many mitochondrial genomes retained only an incomplete set of tRNAs, which despite being fully functional are insufficient for translation (Salinas et al., 2008). Since the original description of mitochondrial tRNA import in 1967 (Suyama, 1967), examples of tRNA import have increased steadily. To date, tRNA import systems have been described in diverse organisms including the ciliate Tetrahymena (Suyama, 1967, 1986; Rusconi and Cech, 1996a,b), the apicomplexan Toxoplasma (Esseiva et al., 2004), the kinetoplastid flagellates Trypanosoma and Leishmania (Simpson et al., 1989; Mottram et al., 1991; Hancock et al., 1992), plants (Marechal-Drouard et al., 1988), yeast (Martin et al., 1979; Rinehart et al., 2005), and most recently mammals (Rubio et al., 2008). The number of imported tRNAs varies greatly from as few as two tRNAs in the case of yeast to a full set of tRNAs in kinetoplastids and apicomplexans (Salinas et al., 2008).
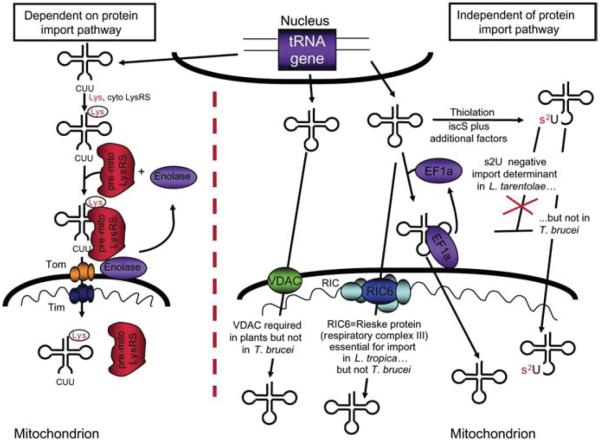
Two main general mechanisms have been described for tRNA import. The first mechanism was originally described for import of yeast tRNALys and is similar to protein import (Tarassov et al., 1995a). This system requires an electrochemical potential across the mitochondrial inner membrane and with the exception of the mitochondrial outer membrane protein MOM72, all other protein import components play a role in tRNALys import (Martin et al., 1979; Tarassov et al., 1995a; Tarassov and Martin, 1996). Import also requires the in vivo association of the tRNA with the precursor mitochondrial tRNA synthetase and aminoacylation of the tRNA by the cognate cytosolic synthetase (Schmitz and Lonsdale, 1989; Tarassov et al., 1995b). In addition, the metabolic enzyme enolase functions as a shuttle helping the delivery of the imported tRNA/synthetase complex to the import machinery on the mitochondrial surface (Entelis et al., 2006; see Figure 1).
Figure 1.
Two major pathways for tRNA import into mitochondria.
One pathway utilizes the protein import machinery including the translocases of the outer membrane (TOMs) and inner membrane (TIMs). This pathway has thus far only been described in the import of tRNALysCUU in yeast and is depicted in the left side of the Figure. In this pathway, the imported tRNA is first aminoacylated by its cognate cytoplasmic synthetase (cyto-LysRS), bound by the pre-mitochondrial lysyl tRNA synthetase (pre-mitoLysRS) and with the aid of enolase is delivered to the mitochondrial surface. The complex is then translocated via the protein import machinery, while enolase remains in the cytoplasm where it can start a new import cycle. The second and perhaps phylogenetically the most widely distributed pathway does not require cytoplasmic factors and it is not directly dependent on the protein import machinery. However, various factors may play a role in various organisms including the VDAC (plants), tRNA thiolation (Leishmania but not in T. brucei), etc. In the specific case of T. brucei, the translation elongation factor eEF1a plays a role in delivering the tRNA to the mitochondrial import machinery. This role is similar to that of enolase in yeast, but has yet to be demonstrated in other organisms. In L. tropica, the RNA import complex (RIC) was recently described and is formed by six essential subunits, all are identical to subunits of the respiratory complex. In T. brucei, however, at least one of the essential RIC subunits is not involved in tRNA import; therefore, the pathway may differ between the two organisms despite being evolutionarily closely related.
The second, and possibly the most common tRNA import mechanism, was first described for Leishmania but has now been found in many other organisms ranging from kinetoplastids to humans (Schneider and Marechal-Drouard, 2000; Salinas et al., 2008). This mechanism is independent of the protein import pathway and can be efficiently reproduced in vitro in the absence of cytoplasmic factors (Mahapatra et al., 1994; Yermovsky-Kammerer and Hajduk, 1999; Rubio et al., 2000, 2008); however, it may still be influenced by their presence. For example, in Trypanosoma brucei, cytosolic translation elongation factor 1a (eEF1a) plays a key role as a specificity determinant for some imported tRNAs (Bouzaidi-Tiali et al., 2007). Recently, the import of tRNAGln into yeast mitochondria was shown to occur in vitro in the absence of added cytosolic factors (Rinehart et al., 2005). Therefore, Saccharomyces cerevisiae contains two pathways for tRNA import: one utilizes the protein import machinery, the second is independent of protein import, a feature that is thus far unique to this organism (Rinehart et al., 2005).
Factors and determinants of tRNA import
To delineate different import mechanisms, one has to first define what specifically constitutes a conserved tRNA determinant or recognition element for organellar import. The presence of universal import determinants has been under much debate. Among the most studied tRNA import pathways are those of kinetoplastids, where a number of in vitro and/or in vivo determinants for import have been described (Salinas et al., 2008). In L. tarentolae, we have adopted an operational definition for tRNA distribution, where tRNAs can be divided into three groups: mostly cytoplasmic, mostly mitochondrial and equally partitioning between the two compartments, where assessment of distribution is based on comparisons of normalized levels of a specific tRNA to a non-imported control (like ribosomal RNA) and quantitation of this signal between mitochondrial vs. cytoplasmic RNA fractions as determined by Northern blots (Kapushoc et al., 2000, 2002). Earlier studies in L. tarentolae showed that generating chimeric tRNAs where the D-arm of a mostly mitochondrial tRNA was placed in the context of a mostly cytoplasmic one could in fact reverse its import distribution in vivo (Lima and Simpson, 1996). Nonetheless, different tRNA sequence determinants for import have since been reported even within similar systems. For example, specific sequences in the D-arm and/or the TΨC-arm of tRNAs in Leishmania tropica represent motifs determining in vivo localization (Mahapatra et al., 1994). However, the presence of these sequences and their evolutionary conservation do not correlate with tRNA localization in Leishmania tarentolae (Suyama et al., 1998). In addition, in L. tarentolae, mature tRNAs are the in vivo substrate for import (Kapushoc et al., 2000) and a full-length tRNA is also the preferred substrate in vitro (Rubio et al., 2000). However, smaller substrates representing regions of the full-length tRNA could be imported albeit at much lower efficiencies. Contrary to this, in T. brucei, evidence was provided for pre-tRNAs as the substrate for in vivo import, in a mechanism akin to pre-protein import (LeBlanc et al., 1999). However, experiments in which tRNA-containing cassettes were integrated at various locations in the T. brucei genome, effectively generating different precursor sequences, suggested that in vivo import occurs independent of genomic context and thus the pre-sequence cannot contain specific import information (Tan et al., 2002).
Recently, the involvement of modifications as import determinants has been suggested. It was reported that thiolation of tRNAs in the cytoplasm of L. tarentolae acted as a negative determinant for import of tRNAGlu (Kaneko et al., 2003); however, the degree of phylogenetic conservation of this mechanism has not been explored further. In their system, only the unmodified tRNA (not containing s2U) was the substrate for import in vivo and in vitro, while the modified version was retained in the cytoplasm (Kaneko et al., 2003). We have investigated the possibility of s2U as a more general determinant for tRNA distribution. In T. brucei we knocked down the expression of iscS, the conserved master desulfurase essential for thiolation. We found that, in T. brucei, a close relative of Leishmania, s2U formation at the wobble position of tRNAGlu is not a negative determinant for import in vivo and/or in vitro (Paris et al., 2009), again suggesting that at least this modification is not universally conserved as an import determinant. This of course does not rule out the possibility that other modifications may have a conserved role in import, but most likely RNA modifications will play roles as identity elements in a similar manner as they do for tRNA aminoacylation, where their general role can be more system specific.
One feature common to all tRNA import systems described so far is the requirement for ATP, which has led to a foray into the bioenergetic requirements of import in various organisms (Salinas et al., 2008). Yet different groups have reported different bioenergetic requirements. While an absolute requirement of mitochondrial membrane potential for tRNA import has been postulated in kinetoplastids (Yermovsky-Kammerer and Hajduk, 1999; Bhattacharyya and Adhya, 2004), such a requirement has been questioned (Rubio et al., 2000). Membrane potential is definitely essential for import of tRNALys into the yeast mitochondria, where several features of the protein import pathway are required (Tarassov et al., 1995a). Yet membrane potential is dispensable in vitro for a tRNA import pathway described in yeast for tRNAGln (Rinehart et al., 2005). Likewise, in the mammalian system, a membrane potential does not play a major role in tRNAGln import. Coincidentally, inhibition of iscS activity in T. brucei also leads to ablation of the membrane potential, but no effects on tRNA import were observed (Paris et al., 2009). Therefore, we can conclude that lack of requirement for a membrane potential is a conserved feature of all organisms that import tRNAs by a protein-import independent mechanism. The sole outlier being the case of L. tropica where we argue that a requirement for membrane potential may really reflect more on technical nuances of the experimental system rather than on actual realities of in vivo import requirements. In recent years, numerous protein factors have been implicated as additional requirements for import of tRNAs into mitochondria. In plants, for instance, the voltage-dependent anion channel (VDAC) in the outer membrane was shown to play a crucial role for import in vitro (Salinas et al., 2006). However, the homologous protein is dispensable for import in T. brucei (Pusnik et al., 2008).
To date, one of the most studied import systems is that of Leishmania, where a putative RNA import complex (RIC) in L. tropica has been identified (Mukherjee et al., 2007). This complex contains a number of proteins known to serve alternative functions in respiration, introducing the concept of a `moonlighting' function for these proteins. The core import complex contains six essential factors required for both in vivo and in vitro import: α subunit of the F1-ATP synthase, subunit 6b and the Rieske protein of the respiratory complex III, two proteins with no similarities to any other proteins in the database and lastly subunit 6 of the respiratory complex IV (Mukherjee et al., 2007). As part of our studies we have also explored the possibility of the RIC as a conserved feature of import in T. brucei. Similar to the case of iscS, we knocked down expression of the Rieske protein, an essential subunit of the RIC. We again found that this protein plays no role in tRNA import distribution in these cells in vivo or in vitro (Lukes and Alfonzo, unpublished data), bringing into question the universal conservation of this complex. Taken together, recent data illuminated various facets of tRNA import in different organisms, but much more information is needed to define the universal rules, and truly answer the question of how many different mechanisms exist for tRNA import into mitochondria.
Evolutionary basis for the appearance of tRNA import mechanisms
Mammalian and yeast mitochondria encode many tRNA species; according to our current understanding of decoding by the wobble rules, their nature and number should suffice for normal protein synthesis (Agris, 2004; Agris et al., 2007; Gustilo et al., 2008). Furthermore, recent studies solved the last question of mitochondrial aminoacyl-tRNA synthesis by demonstrating that mammalian and yeast mitochondria use the indirect amino acid transformation route for Gln-tRNA formation (T. Suzuki, personal communication). This then raises the question of why tRNA import – and sometimes aminoacyl-tRNA synthetase import – exists, how general it is, and what purpose it serves.
Considering the facts discussed above, it is reasonable to believe that protein import into mitochondria is evolutionarily older than tRNA import. Once systems to transport proteins developed, then these systems were exploited to also import tRNAs. A case in point is yeast tRNALys whose import is coupled to that of the mitochondrial lysyl-tRNA synthetase (LysRS). Alternatively, pre-existing proteins can be recruited to transport RNAs in a manner that is independent of the protein import pathway. In this realm, the system may have to modify pre-existing proteins with the ability to make channels or gates in the membrane, such as the case of the plant VDAC (Salinas et al., 2006) to adapt them to tRNA transport. The system may have also recruited other factors that utilize ATP hydrolysis as the energy fuel for import, while bypassing any requirements for other membrane bioenergetics that play key roles in protein transport. Uncoupling mitochondrial translation from protein import may in the long term effectively add one more layer of regulation of mitochondrial functions at the level of tRNA availability. Supporting this view is a recent report establishing a correlation between tRNA partitioning and codon usage in Chlamydomonas (Vinogradova et al., 2009). Clearly, recruitment of additional factors for import does not necessarily imply that the components of modern import machines need resemble pre-existing proteins in sequence, such as the case of the Leishmania RIC, many years of evolution could have led to significant sequence divergence.
Despite an ever-increasing number of tRNA import examples, we posit that the most conserved system for tRNA import is that of tRNAGln. We have suggested that this tRNA (specifically tRNAGlnCUG) is universally imported into mitochondria, especially since a tRNAGlnCUG gene is absent from any sequenced mitochondrial genome. Furthermore, in terms of bioenergetics, what is true of tRNAGln import in yeast is also true for import of this tRNA species into Leishmania, T. brucei, rat and human mitochondria, where import requires ATP but not a membrane potential (Alfonzo et al., 1999; Nabholz et al., 1999; Rubio et al., 2000, 2008; Rinehart et al., 2005).
A final question concerns the role of the imported cytoplasmic tRNAs in the mitochondrion. An unusual feature of aminoacyl-tRNA synthesis is the fact that Gln-tRNA formation is least conserved and is generated by kingdom-specific pathways that guarantee proper glutamine incorporation during protein synthesis (Tumbula et al., 2000). What roles are then played by the imported cytoplasmic tRNAGlnCUG (cyto-tRNAGlnCUG) and tRNAGlnUUG? The yeast and mammalian mitochondrial genomes already encode a mitochondrial tRNAGln with anticodon UUG (mt-tRNAGlnUUG). Two mitochondrial tRNA species, t-RNAGlnUUG and tRNALysUUU, were shown to contain 5-carboxymethylaminomethyl-2-thiouridine (mcm5s2U) in position 34, the first anticodon nucleotide (Nakai et al., 2004). Wobble base pairing would be restricted by mcm5s2U at position 34; thus, mt-tRNAGlnUUG would only decode CAA codons (Okumura et al., 1995). This should necessitate the import of a cyto-tRNAGlnCUG isoacceptor to decode the CAG codons in mitochondrial mRNA. Similarly, mcm5s2U34 in mito-tRNALysUUU would also explain the need for the imported cyto-tRNALysCUU (Kolesnikova et al., 2000). The function of the imported cyto-tRNAGlnUUG is less clear. The S. cerevisiae and mammalian nuclear genomes encode multiple different tRNAGlnUUG species, and the redundancy in this tRNA population may have some important and as of yet unknown function common to both the cytoplasm and the mitochondria. Clearly, the mammalian or yeast cytoplasm contains a much more diverse tRNA population than do the corresponding mitochondria. However, the biosynthesis of certain – possibly non-housekeeping – proteins under changing environmental conditions may demand a more varied codon complement than that found in the mitochondrially encoded tRNAs. In such a case, tRNA import from the cytoplasm may be the solution for increasing decoding diversity. As the cytoplasmic tRNAs are eukaryotic and therefore not ideal substrates for the mitochondrial aminoacyl-tRNA synthetases, import of the cognate cytoplasmic enzyme may also be beneficial. Thus, a driving force for the evolution of tRNA import may have been the requirement of expanding decoding ability by increasing the diversity of the pool of decoding tRNAs far and beyond those species already present in mitochondria. This in turn could support synthesis of a more varied protein population by altering codon recognition under certain conditions, e.g., environmental stress (Kamenski et al., 2007).
Future prospects
Despite differences in the number and types of factors shown to be associated with tRNA import in various organisms, tRNAs can mechanistically be imported in two different ways: either via the protein import pathway (e.g., co-import with a cytoplasmic aminoacyl-tRNA synthetase) (Tarassov et al., 1995b) or via a pathway independent from protein import that does not require cytosolic factors (Rubio et al., 2000). Future studies may reveal additional pathways. However, the disparate nature of the import mechanisms suggests that tRNA import evolved independently in different systems (Schneider and Marechal-Drouard, 2000). Differences among various systems may also suggest import as a dynamic process that has adapted to intracellular environmental changes provided by a particular organism.
Our discovery that tRNAGln import may be present in all mitochondria-containing eukaryotes raises the possibility that a thorough understanding of this import system will unveil some truly evolutionarily conserved mechanistic features. Clearly, this would not rule out organism-specific nuances of different import pathways; it may just be a reflection of the long evolutionary road of different eukaryotes from the original appearance of multiple import systems. The challenge to answer many of these standing questions will still require rigorous genetic, biochemical, and structural approaches.
Acknowledgments
We wish to thank members of the Alfonzo and Söll groups for useful comments. Work presented was supported by grants from the National Institute of General Medical Sciences to D.S. and to J.D.A. and from the National Science Foundation (to J.D.A.).
References
- Agris PF. Decoding the genome: a modified view. Nucleic Acids Res. 2004;32:223–238. doi: 10.1093/nar/gkh185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agris PF, Vendeix FA, Graham WD. tRNA's wobble decoding of the genome: 40 years of modification. J. Mol. Biol. 2007;366:1–13. doi: 10.1016/j.jmb.2006.11.046. [DOI] [PubMed] [Google Scholar]
- Alfonzo JD, Blanc V, Estevez AM, Rubio MA, Simpson L. C to U editing of the anticodon of imported mitochondrial tRNA(Trp) allows decoding of the UGA stop codon in Leishmania tarentolae. EMBO J. 1999;18:7056–7062. doi: 10.1093/emboj/18.24.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya SN, Adhya S. tRNA-triggered ATP hydrolysis and generation of membrane potential by the Leishmania mitochondrial tRNA import complex. J. Biol. Chem. 2004;279:11259–11263. doi: 10.1074/jbc.C300540200. [DOI] [PubMed] [Google Scholar]
- Bouzaidi-Tiali N, Aeby E, Charriere F, Pusnik M, Schneider A. Elongation factor 1a mediates the specificity of mitochondrial tRNA import in T. brucei. EMBO J. 2007;26:4302–4312. doi: 10.1038/sj.emboj.7601857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entelis N, Brandina I, Kamenski P, Krasheninnikov IA, Martin RP, Tarassov I. A glycolytic enzyme, enolase, is recruited as a cofactor of tRNA targeting toward mitochondria in Saccharomyces cerevisiae. Genes Dev. 2006;20:1609–1620. doi: 10.1101/gad.385706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esseiva AC, Naguleswaran A, Hemphill A, Schneider A. Mitochondrial tRNA import in Toxoplasma gondii. J. Biol. Chem. 2004;279:42363–42368. doi: 10.1074/jbc.M404519200. [DOI] [PubMed] [Google Scholar]
- Gray MW, Lang BF, Burger G. Mitochondria of protists. Annu. Rev. Genet. 2004;38:477–524. doi: 10.1146/annurev.genet.37.110801.142526. [DOI] [PubMed] [Google Scholar]
- Gustilo EM, Vendeix FA, Agris PF. tRNA's modifications bring order to gene expression. Curr. Opin. Microbiol. 2008;11:134–140. doi: 10.1016/j.mib.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haanstra JR, van Tuijl A, Kessler P, Reijnders W, Michels PA, Westerhoff HV, Parsons M, Bakker BM. Compartmentation prevents a lethal turbo-explosion of glycolysis in trypanosomes. Proc. Natl. Acad. Sci. USA. 2008;105:17718–17723. doi: 10.1073/pnas.0806664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock K, LeBlanc AJ, Donze D, Hajduk SL. Identification of nuclear encoded precursor tRNAs within the mitochondrion of Trypanosoma brucei. J. Biol. Chem. 1992;267:23963–23971. [PubMed] [Google Scholar]
- Kamenski P, Kolesnikova O, Jubenot V, Entelis N, Krasheninnikov IA, Martin RP, Tarassov I. Evidence for an adaptation mechanism of mitochondrial translation via tRNA import from the cytosol. Mol. Cell. 2007;26:625–637. doi: 10.1016/j.molcel.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Suzuki T, Kapushoc ST, Rubio MA, Ghazvini J, Watanabe K, Simpson L. Wobble modification differences and subcellular localization of tRNAs in Leishmania tarentolae: implication for tRNA sorting mechanism. EMBO J. 2003;22:657–667. doi: 10.1093/emboj/cdg066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapushoc ST, Alfonzo JD, Rubio MA, Simpson L. End processing precedes mitochondrial importation and editing of tRNAs in Leishmania tarentolae. J. Biol. Chem. 2000;275:37907–37914. doi: 10.1074/jbc.M007838200. [DOI] [PubMed] [Google Scholar]
- Kapushoc ST, Alfonzo JD, Simpson L. Differential localization of nuclear-encoded tRNAs between the cytosol and mitochondrion in Leishmania tarentolae. RNA. 2002;8:57–68. doi: 10.1017/s1355838202012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikova OA, Entelis NS, Mireau H, Fox TD, Martin RP, Tarassov IA. Suppression of mutations in mitochondrial DNA by tRNAs imported from the cytoplasm. Science. 2000;289:1931–1933. doi: 10.1126/science.289.5486.1931. [DOI] [PubMed] [Google Scholar]
- LeBlanc AJ, Yermovsky-Kammerer AE, Hajduk SL. A nuclear encoded and mitochondrial imported dicistronic tRNA precursor in Trypanosoma brucei. J. Biol. Chem. 1999;274:21071–21077. doi: 10.1074/jbc.274.30.21071. [DOI] [PubMed] [Google Scholar]
- Lima BD, Simpson L. Sequence-dependent in vivo importation of tRNAs into the mitochondrion of Leishmania tarentolae. RNA. 1996;2:429–440. [PMC free article] [PubMed] [Google Scholar]
- Mahapatra S, Ghosh T, Adhya S. Import of small RNAs into Leishmania mitochondria in vitro. Nucleic Acids Res. 1994;22:3381–3386. doi: 10.1093/nar/22.16.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal-Drouard L, Weil JH, Guillemaut P. Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res. 1988;16:4777–4788. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RP, Schneller JM, Stahl AJ, Dirheimer G. Import of nuclear deoxyribonucleic acid coded lysine-accepting transfer ribonucleic acid (anticodon C-U-U) into yeast mitochondria. Biochemistry. 1979;18:4600–4605. doi: 10.1021/bi00588a021. [DOI] [PubMed] [Google Scholar]
- Mottram JC, Bell SD, Nelson RG, Barry JD. tRNAs of Trypanosoma brucei. Unusual gene organization and mitochondrial importation. J. Biol. Chem. 1991;266:18313–18317. [PubMed] [Google Scholar]
- Mukherjee S, Basu S, Home P, Dhar G, Adhya S. Necessary and sufficient factors for the import of transfer RNA into the kinetoplast mitochondrion. EMBO Rep. 2007;8:589–595. doi: 10.1038/sj.embor.7400979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabholz CE, Horn EK, Schneider A. tRNAs and proteins are imported into mitochondria of Trypanosoma brucei by two distinct mechanisms. Mol. Biol. Cell. 1999;10:2547–2557. doi: 10.1091/mbc.10.8.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y, Umeda N, Suzuki T, Nakai M, Hayashi H, Watanabe K, Kagamiyama H. Yeast Nfs1p is involved in thio-modification of both mitochondrial and cytoplasmic tRNAs. J. Biol. Chem. 2004;279:12363–12368. doi: 10.1074/jbc.M312448200. [DOI] [PubMed] [Google Scholar]
- Okumura S, Takai K, Yokoyama S, Takaku H. Codon recognition by tRNA molecules with a modified or unmodified uridine at the first position of the anticodon. Nucleic Acids Symp. Ser. 1995;34:203–204. [PubMed] [Google Scholar]
- Paris Z, Rubio MAT, Lukeš J, Alfonzo JD. Mitochondrial tRNA import in Trypanosoma brucei is independent of thiolation and the Rieske protein. RNA. 2009;15 doi: 10.1261/rna.1589109. epub ahead of print; DOI: 10.1261/rna.1589109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusnik M, Charriere F, Maser P, Waller R, Dagley MJ, Lithgow T, Schneider A. The single mitochondrial porin of Trypanosoma brucei is the main metabolite transporter in the outer mitochondrial membrane. Mol. Biol. Evol. 2008;3:671–680. doi: 10.1093/molbev/msn288. [DOI] [PubMed] [Google Scholar]
- Rinehart J, Krett B, Rubio MA, Alfonzo JD, Soll D. Saccharomyces cerevisiae imports the cytosolic pathway for Gln-tRNA synthesis into the mitochondrion. Genes Dev. 2005;19:583–592. doi: 10.1101/gad.1269305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio MA, Liu X, Yuzawa H, Alfonzo JD, Simpson L. Selective importation of RNA into isolated mitochondria from Leishmania tarentolae. RNA. 2000;6:988–1003. doi: 10.1017/s1355838200991519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio MA, Rinehart JJ, Krett B, Duvezin-Caubet S, Reichert AS, Soll D, Alfonzo JD. Mammalian mitochondria have the innate ability to import tRNAs by a mechanism distinct from protein import. Proc. Natl. Acad. Sci. USA. 2008;105:9186–9191. doi: 10.1073/pnas.0804283105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi CP, Cech TR. Mitochondrial import of only one of three nuclear-encoded glutamine tRNAs in Tetrahymena thermophila. EMBO J. 1996a;15:3286–3295. [PMC free article] [PubMed] [Google Scholar]
- Rusconi CP, Cech TR. The anticodon is the signal sequence for mitochondrial import of glutamine tRNA in Tetrahymena. Genes Dev. 1996b;10:2870–2880. doi: 10.1101/gad.10.22.2870. [DOI] [PubMed] [Google Scholar]
- Salinas T, Duchene AM, Delage L, Nilsson S, Glaser E, Zaepfel M, Marechal-Drouard L. The voltage-dependent anion channel, a major component of the tRNA import machinery in plant mitochondria. Proc. Natl. Acad. Sci. USA. 2006;103:18362–18367. doi: 10.1073/pnas.0606449103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas T, Duchene AM, Marechal-Drouard L. Recent advances in tRNA mitochondrial import. Trends Biochem. Sci. 2008;33:320–329. doi: 10.1016/j.tibs.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Schmitz UK, Lonsdale DM. A yeast mitochondrial presequence functions as a signal for targeting to plant mitochondria in vivo. Plant Cell. 1989;1:783–791. doi: 10.1105/tpc.1.8.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Marechal-Drouard L. Mitochondrial tRNA import: are there distinct mechanisms? Trends Cell Biol. 2000;10:509–513. doi: 10.1016/s0962-8924(00)01854-7. [DOI] [PubMed] [Google Scholar]
- Simpson AM, Suyama Y, Dewes H, Campbell DA, Simpson L. Kinetoplastid mitochondria contain functional tRNAs which are encoded in nuclear DNA and also contain small minicircle and maxicircle transcripts of unknown function. Nucleic Acids Res. 1989;17:5427–5445. doi: 10.1093/nar/17.14.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama Y. The origins of mitochondrial ribonucleic acids in Tetrahymena pyriformis. Biochemistry. 1967;6:2829–2839. doi: 10.1021/bi00861a025. [DOI] [PubMed] [Google Scholar]
- Suyama Y. Two dimensional polyacrylamide gel electrophoresis analysis of Tetrahymena mitochondrial tRNA. Curr. Genet. 1986;10:411–420. doi: 10.1007/BF00418415. [DOI] [PubMed] [Google Scholar]
- Suyama Y, Wong S, Campbell DA. Regulated tRNA import in Leishmania mitochondria. Biochim. Biophys. Acta. 1998;1396:138–142. doi: 10.1016/s0167-4781(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Tan TH, Pach R, Crausaz A, Ivens A, Schneider A. tRNAs in Trypanosoma brucei: genomic organization, expression, and mitochondrial import. Mol. Cell. Biol. 2002;22:3707–3717. doi: 10.1128/MCB.22.11.3707-3716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassov I, Entelis N, Martin RP. An intact protein translocating machinery is required for mitochondrial import of a yeast cytoplasmic tRNA. J. Mol. Biol. 1995a;245:315–323. doi: 10.1006/jmbi.1994.0026. [DOI] [PubMed] [Google Scholar]
- Tarassov I, Entelis N, Martin RP. Mitochondrial import of a cytoplasmic lysine-tRNA in yeast is mediated by cooperation of cytoplasmic and mitochondrial lysyl-tRNA synthetases. EMBO J. 1995b;14:3461–3471. doi: 10.1002/j.1460-2075.1995.tb07352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassov IA, Martin RP. Mechanisms of tRNA import into yeast mitochondria: an overview. Biochimie. 1996;78:502–510. doi: 10.1016/0300-9084(96)84756-0. [DOI] [PubMed] [Google Scholar]
- Tumbula DL, Becker HD, Chang WZ, Soll D. Domain-specific recruitment of amide amino acids for protein synthesis. Nature. 2000;407:106–110. doi: 10.1038/35024120. [DOI] [PubMed] [Google Scholar]
- Vinogradova E, Salinas T, Cognat V, Remacle C, Marechal-Drouard L. Steady-state levels of imported tRNAs in Chlamydomonas mitochondria are correlated with both cytosolic and mitochondrial codon usages. Nucleic Acids Res. 2009;37:1521–1528. doi: 10.1093/nar/gkn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermovsky-Kammerer AE, Hajduk SL. In vitro import of a nuclearly encoded tRNA into the mitochondrion of Trypanosoma brucei. Mol. Cell. Biol. 1999;19:6253–6259. doi: 10.1128/mcb.19.9.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]