abstract
We recently revealed that glucose is a critical activator of sterol regulatory element-binding proteins (SREBPs) by promoting trafficking of SREBP-cleavage activating protein (SCAP)/SREBP complexes from the ER to the Golgi and subsequent SREBP activation via N-glycosylation of SCAP. Our study also demonstrated that SCAP plays a critical role in tumor growth.
KEYWORDS: Glioblastoma, glucose, lipid metabolism, N-glycosylation, SCAP, SREBP, tumor growth
Metabolic reprogramming has emerged as a new hallmark of cancer. Growing evidence shows that alteration of metabolic pathways contributes to the rapid generation of bioenergetics and macromolecular building blocks that support the rapid growth and proliferation of tumor cells.1 It is critical to understand the underlying metabolic regulation of cancer cells as this may lead to the identification of promising strategies to treat malignancies.
In recent years, many talented scientists and physicians have turned their attention to the field of metabolism, aiming to identify the Achilles' heel to fight cancer. While glucose and glutamine metabolism in cancer cells has been largely investigated, lipid metabolism is still vastly underexplored. Although lipid regulation is actively studied in cardiovascular diseases and obesity, there is little crosstalk between the fields of metabolic syndromes and cancer.
Our previous studies revealed that lipid metabolism is altered in glioblastoma (GBM), a common and deadly brain tumor.2-4 We found that oncogenic epidermal growth factor receptor (EGFR) signaling upregulates sterol regulatory element-binding protein-1 (SREBP-1), a membrane-bound transcription factor with a central role in lipid metabolism.5-7 These studies demonstrated that oncogenic signaling hijacks the intrinsic machinery of lipid metabolism, enhancing lipid synthesis and uptake to promote tumor growth. However, the detailed molecular mechanisms by which cancer cells regulate lipid metabolism remain largely elusive.
Intriguingly, increased glucose consumption and enhanced lipogenesis often occur in concert in tumorigenesis.1,2 However, the existence of an intrinsic molecular link between glucose and lipids had not been demonstrated. This important question triggered our interest and the ensuing investigations in our laboratory.
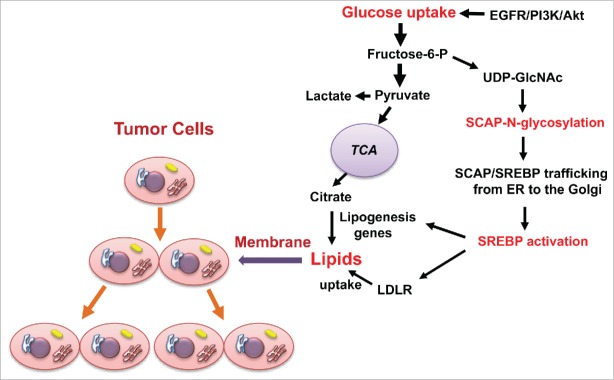
Over the last 20 years, the Nobel laureates Brown and Goldstein developed an elegant model for SREBP regulation in which sterols serve as the main regulator of SREBP activation via a negative feedback regulation loop.8 When sterol levels decrease, SREBP-cleavage activating protein (SCAP) transports SREBPs from the endoplasmic reticulum (ER) to the Golgi for subsequent proteolytic activation of SREBP.8 Surprisingly, when we started our investigations our results were completely unexpected and not in line with those published by Brown and Goldstein, as we found that removal of sterols was unable to activate SREBPs when cells were cultured in the absence of glucose.9 Addition of glucose upregulated SCAP, and activated both SREBP-1 and -2.9 These data demonstrated that glucose functions as a critical activator of SREBP function (Fig. 1).
Figure 1.
Glucose promotes tumor growth by activating lipid synthesis and uptake upon N-glycosylation of SCAP. Glucose-mediated N-glycosylation of SCAP promotes the trafficking of SCAP/SREBP from the ER to the Golgi, leading to subsequent SREBP activation. SREBP acts as a transcription factor to enhance the expression of lipogenesis genes and LDLR, thereby increasing levels of cellular lipids to support tumor growth. Oncogenic EGFR/PI3K/Akt signaling upregulates the glucose uptake-SCAP N-glycosylation-SREBP activation pathway to promote tumor growth. EGFR, epidermal growth factor receptor; ER, endoplasmic reticulum; LDLR, low-density lipoprotein receptor; PI3K, phosphoinositide 3-kinase; UDP-GlcNAc, uridine diphosphate (UDP)-N-acetylglucosamine (GlcNAc); TCA, tricarboxylic acid.
We next explored how glucose activates SREBPs. After uptake by cells, glucose feeds into distinct metabolic pathways, i.e., glycolysis, oxidative phosphorylation, and hexosamine synthesis for glycosylation modification. We first examined the effects of the intermediate metabolites in each pathway on SCAP protein levels and SREBP-1 activation in cells cultured in the absence of glucose. We found that only N-acetlyglucosamine (GlcNAc), the intermediate product of the hexosamine pathway serving as a precursor for N-glycosylation, enhanced SCAP protein levels and activated SREBP-1 similarly to glucose stimulation. We then used inhibitors to block the N-glycosylation or O-glycosylation pathways and found that inhibition of the N-glycosylation pathway abolished glucose-mediated SCAP upregulation and SREBP-1 activation. Together, these data demonstrated that glucose-mediated N-glycosylation is critical for SCAP stability and promotes SCAP/SREBP trafficking to the Golgi, leading to SRBEP activation (Fig. 1).
We then investigated how glucose-mediated N-glycosylation regulates SCAP/SREBP activation. In 1998, the Brown and Goldstein laboratory had reported that SCAP is modified by N-glycosylation on 3 asparagine residues.10 Nohturfft and coworkers demonstrated that mutation of 1 or 2 N-glycosylation sites on SCAP had no noticeable effect on SREBP-2 activation.10 However, they were not able to examine the function of the mutated SCAP protein with all 3 asparagines (NNN) replaced with glutamines (QQQ) because of its instability.10 As we reasoned that glucose-stimulated SREBP activation might be mediated by N-glycosylation of SCAP, we decided to revisit this issue. We were very fortunate that Drs. Brown, Goldstein, and Nohturfft kindly provided us with all the SCAP mutant constructs, including QQQ-SCAP. We subcloned the SCAP mutant fragment into a new plasmid with a GFP-tag driven by the CMV promoter, and thus were able to enrich the mutated QQQ-SCAP protein in sufficient amounts to proceed with our study. Our data showed that loss of all N-glycosylation sites resulted in the inability of SCAP to move to the Golgi and activate SREBP-1 and -2 under sterol deprivation.
We then demonstrated that N-glycosylation of SCAP reduced its association with insulin-induced gene 1 (Insig-1), an ER-resident protein that blocks exit of SCAP/SREBP from the ER, and directed trafficking of the SCAP/SREBP complex to the Golgi. Finally, we demonstrated that EGFR signaling activates SREBP-1 by enhancing glucose uptake and SCAP N-glycosylation (Fig. 1).
In summary, our study has expanded our understanding of tumor metabolism and identified the intrinsic link between glucose and lipid metabolism. These data suggest that key players in lipid metabolism may serve as effective therapeutic targets for cancer.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH/NINDS under Awards RO1 NS079701 and R21 NS072838 (DG), and an American Cancer Society Research Scholar Grant RSG-14-228-01–CSM (DG).
References
- 1.Ru P, Williams TM, Chakravarti A, Guo D. Tumor metabolism of malignant gliomas. Cancers (Basel) 2013; 5:1469-84; PMID:24217114; http://dx.doi.org/ 10.3390/cancers5041469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo D, Bell EH, Chakravarti A. Lipid metabolism emerges as a promising target for malignant glioma therapy. CNS Oncol 2013; 2:289-99; PMID:24159371; http://dx.doi.org/ 10.2217/cns.13.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo D, Hildebrandt IJ, Prins RM, Soto H, Mazzotta MM, Dang J, Czernin J, Shyy JY, Watson AD, Phelps M, et al.. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc Natl Acad Sci U S A 2009; 106:12932-7; PMID:19625624; http://dx.doi.org/ 10.1073/pnas.0906606106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo D, Cloughesy TF, Radu CG, Mischel PS. AMPK: a metabolic checkpoint that regulates the growth of EGFR activated glioblastomas. Cell Cycle 2010; 9:211-2; PMID:20023392; http://dx.doi.org/ 10.4161/cc.9.2.10540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo D, Bell EH, Mischel P, Chakravarti A. Targeting SREBP-1-driven lipid metabolism to treat cancer. Curr Pharm Des 2014; 20:2619-26; PMID:23859617; http://dx.doi.org/ 10.2174/13816128113199990486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo D, Prins RM, Dang J, Kuga D, Iwanami A, Soto H, Lin KY, Huang TT, Akhavan D, Hock MB, et al.. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci Signal 2009; 2:ra82; PMID:20009104; http://dx.doi.org/ 10.1126/scisignal.2000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, Kuga D, Amzajerdi AN, Soto H, Zhu S, et al.. An LXR agonist promotes GBM cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov 2011; 1:442-56; PMID:22059152; http://dx.doi.org/ 10.1158/2159-8290.CD-11-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell 2006; 124:35-46; PMID:16413480; http://dx.doi.org/ 10.1016/j.cell.2005.12.022 [DOI] [PubMed] [Google Scholar]
- 9.Cheng C, Ru P, Geng F, Liu J, Yoo JY, Wu X, Cheng X, Euthine V, Hu P, Guo JY, et al.. Glucose-mediated N-glycosylation of SCAP is essential for SREBP-1 activation and tumor growth. Cancer Cell 2015; 28:569-81; PMID:26555173; http://dx.doi.org/ 10.1016/j.ccell.2015.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nohturfft A, Brown MS, Goldstein JL. Topology of SREBP cleavage-activating protein, a polytopic membrane protein with a sterol-sensing domain. J Biol Chem 1998; 273:17243-50; PMID:9642295; http://dx.doi.org/ 10.1074/jbc.273.27.17243 [DOI] [PubMed] [Google Scholar]