Significance
We established a new genetic method for rapidly activating Cre recombinase that is based on suppression of a nonsense codon within Cre by a new generation of aminoglycosides. We applied this strategy to successfully silence both enzymes responsible for GABA biosynthesis in hypothalamic agouti-related peptide (AgRP) neurons that control appetite and other behaviors. Inactivation of GABA signaling in AgRP neurons of young adult mice resulted in severe loss of body weight and abnormal glucose metabolism, whereas older mice only manifested transient appetite deficiency. This genetic system adds another robust tool that can be used to understand complex neurological processes.
Keywords: inducible gene knockout, feeding behavior, eating disorders, GABA, AgRP neurons
Abstract
Currently available inducible Cre/loxP systems, despite their considerable utility in gene manipulation, have pitfalls in certain scenarios, such as unsatisfactory recombination rates and deleterious effects on physiology and behavior. To overcome these limitations, we designed a new, inducible gene-targeting system by introducing an in-frame nonsense mutation into the coding sequence of Cre recombinase (nsCre). Mutant mRNAs transcribed from nsCre transgene can be efficiently translated into full-length, functional Cre recombinase in the presence of nonsense suppressors such as aminoglycosides. In a proof-of-concept model, GABA signaling from hypothalamic neurons expressing agouti-related peptide (AgRP) was genetically inactivated within 4 d after treatment with a synthetic aminoglycoside. Disruption of GABA synthesis in AgRP neurons in young adult mice led to a dramatic loss of body weight due to reduced food intake and elevated energy expenditure; they also manifested glucose intolerance. In contrast, older mice with genetic inactivation of GABA signaling by AgRP neurons had only transient reduction of feeding and body weight; their energy expenditure and glucose tolerance were unaffected. These results indicate that GABAergic signaling from AgRP neurons plays a key role in the control of feeding and metabolism through an age-dependent mechanism. This new genetic technique will augment current tools used to elucidate mechanisms underlying many physiological and neurological processes.
In experimental systems, there are many instances in which one would like to inactivate (or activate) a gene in adult animals to ascertain its function and avoid early developmental influences. A common strategy involves making conditional alleles of the gene of interest by flanking critical exons with loxP sites and then breeding those mice with mice expressing an inducible Cre recombinase. The tamoxifen-based CreER and RU486-based CrePR systems are two popular tools that are used to achieve inducible control of gene expression (1, 2). However, these systems possess some caveats in addressing fundamental biological issues, such as silencing effects, as well as Cre- and inducer-mediated cytotoxicity (3–6). We and others have found that, in many cases, the inducibility of CreER and CrePR systems was partially or even completely silenced, suggesting that estrogen receptor (ER)-binding or progesterone receptor (PR)-binding domains may negatively affect the expression of these Cre fusion proteins (5–8). We generated an agouti-related peptide Agrp–CreER line of mice by gene targeting, but the gene was completely silenced and could not be activated with tamoxifen or the active metabolite 4-OH-tamoxifen, whereas when Cre alone was targeted to the same location within the Agrp gene, it functioned as expected (9, 10). However, BAC transgenic mice with a functional Agrp–CreER have been described suggesting that chromosomal location influences silencing (11).
In-frame, nonsense mutations that generate premature termination codons (PTCs) in mRNA coding regions account for a sizable portion of human genetic diseases, such as cystic fibrosis (CF) and Duchenne muscular dystrophy (DMD) (12). In the absence of any intervention, the majority of PTC-containing mRNAs is rapidly degraded by the process of nonsense-mediated decay (NMD), whereas only a small fraction of the transcripts can be translated into truncated, nonfunctional peptides (13). Aminoglycosides, such as gentamycin and geneticin (G418), protect mutant transcripts from NMD and promote read-through of PTCs (14–16). This process, termed nonsense suppression, has been shown to restore synthesis of full-length, functional proteins in numerous cell culture assays, animal models of human diseases, and even in patients with DMD or CF (17–19).
To better understand the genetic mechanisms underlying complex brain functions, we designed a novel inducible gene-targeting system by introducing an in-frame nonsense mutation into the coding sequence of the Cre recombinase gene. Mutant mRNAs transcribed from nsCre transgene can be efficiently translated into full-length, functional Cre recombinase in the presence of nonsense suppressors. In this work, we applied this new technique to acutely inactivate GABA signaling by neurons in the hypothalamus that are important regulators of feeding and metabolism.
Results
We conceived a new, inducible Cre-expression system by engineering an in-frame nonsense mutation (TGA) into the coding region of the Cre recombinase gene (nsCre) with the expectation that treatment with an aminoglycoside would promote read-through of this nonsense codon, thereby allowing synthesis of functional Cre recombinase that could mediate recombination of loxP-flanked genes to inactivate their expression. Aminoglycosides lead to mispairing of a near-cognate aminoacyl-tRNA with a PTC, so that one of a specific set of amino acids can be incorporated, thus preventing termination (Fig. 1A) (16). Hence, to produce a functional Cre recombinase, the protein must tolerate the random incorporation of an exogenous amino acid at the PTC site. Evidence from a large-scale, random transposon insertion/deletion analysis revealed that regions preceding α-helix A and between α-helices J and K in the Cre coding sequence confer significant tolerance to insertional mutagenesis (20). Based upon these data, we designed a cell-culture recombination assay to validate candidate nsCre transgenes in which a TGA stop codon was inserted into either one of these two regions (Fig. 1B). We chose the TGA codon over the other two stop codons because evidence from previous studies indicated that UGA in mRNA exhibits the highest rate of read-through (15–17, 21). Neo-resistant HEK293 cells were cotransfected with CMV-loxP-Neo-loxP-DsRed2, along with Pgk-nsCre plasmids with stop codons inserted into one of the two positions. The next day, the cells were treated with increasing doses of the aminoglycoside G418 (Fig. 1C). Four days after drug treatment, DsRed2 fluorescence indicated that nonsense suppression had occurred in a dose-dependent manner when PTC was inserted into the region between α-helices J and K (nsCre2), but not ahead of α-helix A (nsCre1) (Fig. 1 D–F). In the case of nsCre1, functional Cre recombinase was produced without G418 treatment, presumably because translation started at a downstream, in-frame initiation codon. These results were confirmed with an assay in which an HEK293 clone with stable expression of CMV-loxP-Neo-loxP-DsRed2 was transfected with the Pgk-nsCre2 plasmid and treated with various doses of G418. Short exposure to G418 (2 mg/mL) followed by a recovery period without the aminoglycoside resulted in almost as many fluorescent cells as the transfection with the wild-type Pgk-Cre plasmid.
Fig. 1.
Designing and validation of a novel inducible nsCre transgene. (A) A schematic diagram showing the aminoglycoside (AG)-mediated nonsense suppression of the PTC. Without treatment of AG, the majority of PTC-bearing mRNAs are rapidly degraded by the process of NMD, whereas only a small fraction of the transcripts can be translated into truncated, nonfunctional peptides. In contrast, AG, such as geneticin (G418), promotes read-through of PTCs during the translation, thereby restoring full-length functional proteins. Of note, a random amino acid (blue spheres) is incorporated into the PTC locus through AG-mediated nonsense suppression. (B) A PTC, TGA, was inserted into position 9 or 837 of the wild-type Cre gene. (C) A diagram illustrating the functional validation of nsCre transgene in a cell culture-based assay. Neo-resistant HEK293 cells were transfected with CMV-flox-Neo-dsRed2 and Pgk-myc-nls-nsCre plasmids. (D–F) Representative images showing DsRed fluorescence in nsCre2-expressing HEK293 cells 4 d after treatment of vehicle (D) or G418 at a concentration of either 1 mg/mL (E) or 2 mg/mL (F). These results indicated that nsCre-mediated homologous recombination is restored by G418 in a dose-dependent manner. (Scale bar, 200 µm.)
To establish a proof-of-concept mouse model, an nsCre2-containing transgene was targeted to the Agrp gene locus to generate AgrpnsCre mice, and these mice were subsequently bred with a Cre-dependent fluorescence reporter line (Rosa26tdTomato) (22), in which removal of a loxP-flanked Neo gene allows expression of tdTomato (Fig. 2A). After injection of saline into the third ventricle of AgrpnsCre/+::Rosa26tdTomato mice, tdTomato fluorescence was undetectable throughout the whole brain, indicating that nsCre2 was completely silent and that no recombination of the reporter gene had occurred (Fig. 2B). In contrast, administration of G418 into the third ventricle led to robust, dose-dependent activation of tdTomato in the arcuate nucleus (ARC), where AgRP neurons are located (Fig. S1 A and E). These results suggested that G418 allows translational read-through of PTC in nsCre2 transcripts, resulting in functional Cre recombinase activity in vivo. However, the toxicity of G418 to eukaryotic cells limits its application to proof-of-concept experiments, as shown in several genetic disease models (17, 21). Based upon an idea that the structural elements in aminoglycosides that induce PTC read-through are separable from those that affect toxicity (20), we designed and synthesized a series of G418 derivatives, including NB124, NB127, and NB128 (Fig. S2). These lead compounds displayed significantly reduced cytotoxicity while retaining equal or better read-through capacity compared with G418 (23). Based upon toxicological assays, NB124, NB127, and NB128 displayed ∼30% of the cytotoxicity compared with G148, while maintaining comparable potency in cell-culture assays. We tested the read-through capacity in vivo by injection of each of the compounds into the third ventricle of AgrpnsCre/+::Rosa26tdTomato mice. Among these compounds, NB124 displayed superior read-through capacity in vivo as indicated by the number of tdTomato-expressing neurons in the ARC 4 d after drug treatment (Fig. S1 B–D and F–H). More importantly, a series of dose–response experiments resulted in a regimen in which treatment with NB124 resulted in tdTomato fluorescence in ∼95% of AgRP neurons, suggesting that AgrpnsCre transgene could be expressed in almost all AgRP neurons (Fig. 2 C–F). The best regimen involved injecting 80 μg of NB124 twice (2 d apart); higher single doses revealed some toxic effects, including lethargy and dyspnea, and a single 80-μg dose did not result in complete recombination. These results demonstrate that this inducible gene targeting system can exhibit high efficiency and fidelity, but careful titration of the drug is necessary.
Fig. 2.
Engineering an inducible AgrpnsCre transgenic mouse line. (A) A schematic diagram showing the targeting construct for generation of AgrpnsCre mice. Specifically, the nsCre2-containing cassette was cloned into Agrp gene locus immediately 5′ of the translation start codon. (B) Fluorescence image shows no leaky expression of tdTomato (red) in AgrpnsCre/+::Rosa26tdTomato mice. Dotted circles indicate the ARC region of the hypothalamus where AgRP-expressing neurons are located. (C) Fluorescence image shows tdTomato expression profile (red) in the AgrpnsCre/+::Rosa26tdTomato mice 4 d after injection of NB124 into the third ventricle. (D) Immunostaining image shows expression profile of AgRP (green) in the same mice as described in C. (E) Merged image of C and D illustrates the colocalization of tdTomato marker and AgRP peptides. (F) Quantification of AgRP neurons expressing tdTomato under vehicle or NB124 treatment. Asterisks in B and C indicate the third ventricle. (Scale bar: B–E, 300 µm.)
Fig. S1.
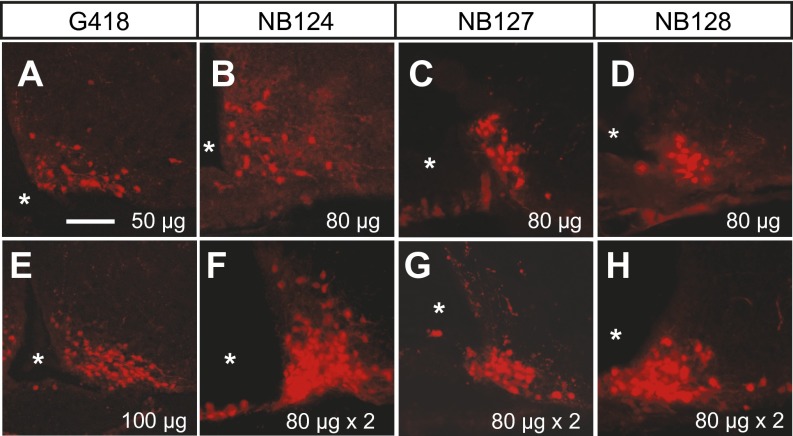
Screening of the lead compounds that mediate nonsense suppression of nsCre transgene in vivo. Fluorescence image shows tdTomato expression profile (red) in the ARC region of AgrpnsCre/+::Rosa26tdTomato mice 4 d after injection of either G418 (A and E), NB124 (B and F), NB127 (C and G), or NB128 (D and H) into the third ventricle. Each compound was delivered either once (A–E) or twice 48 h apart (F–H). Asterisks indicate the third ventricle. (Scale bar: 150 µm.)
Fig. S2.
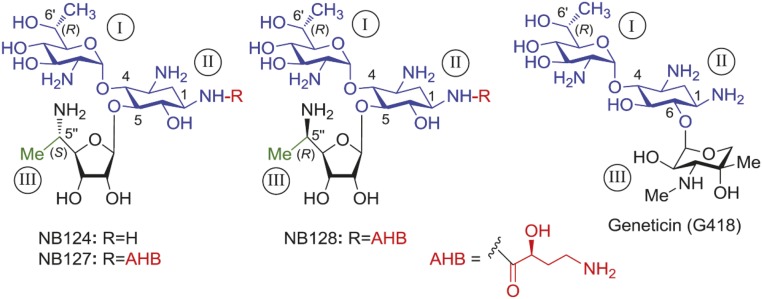
Chemical structures of the aminoglycoside G418 and its synthetic derivatives NB124, NB127, and NB128 that were investigated in this study. The common part of all of the structures is highlighted in blue color. The ring numbers are in capital roman numerals (circled). The identity of a particular pharmacophore and its attachment site in the synthetic derivatives are highlighted: (S)-4-amino-2-hydroxybutanoyl (AHB; red), (S)-5″-Me (green), and (R)-5″-Me (green).
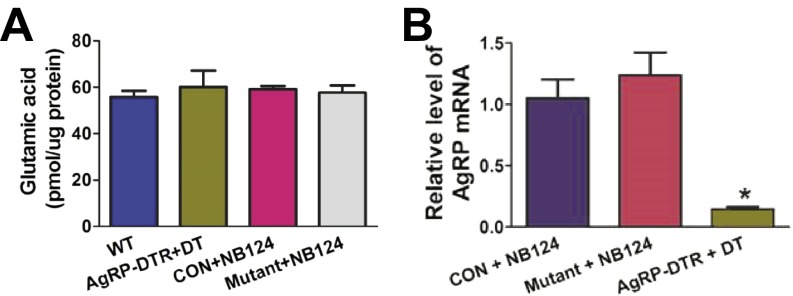
To demonstrate the usefulness of this new genetic approach in controlling an important physiological process, we set out to disrupt GABA synthesis by AgRP neurons, a small population of neurons in the ARC that is important for the control of feeding and energy metabolism (9, 24–27). We generated two different lines of mice for the behavioral assays: AgrpnsCre/+::Gad1lox/lox::Gad2lox/lox::Rosa26tdTomato mice, termed the mutant group, and AgrpnsCre/+::Gad1lox/+::Gad2lox/+::Rosa26tdTomato mice, termed the control group. These two groups of mice share identical genetic backgrounds, but carry either heterozygous or homozygous alleles of floxed Gad1 or Gad2, genes encoding the two essential enzymes (GAD67 and GAD65) for GABA biosynthesis. Injection of NB124 into the third ventricle of the control group led to the expression of tdTomato in AgRP neurons, and GABA staining was retained (Fig. 3 A–D) (25, 28). In contrast, treatment with NB124 in the mutant group abolished GABA immunological detection exclusively in the tdTomato-labeled AgRP neurons (Fig. 3 E–H). To further demonstrate the efficacy of genetic inactivation, we quantitatively examined the expression level of Gad1 and Gad2 mRNA from AgRP neurons that were isolated by fluorescence-activated cell sorting (FACS). Real-time PCR results indicated that Gad1 and Gad2 mRNA in AgRP neurons were almost completely depleted in NB124-treated mutant mice compared to NB124-treated control or the AgrpCre::Rosa26tdTomato groups (Fig. 3 I and J). Furthermore, HPLC analysis revealed that the abundance of GABA in the ARC region of the NB124-treated mutant group was reduced to the same extent as displayed by diphtheria toxin (DT)-treated AgrpDTR/+ mice, in which nearly all AgRP neurons were ablated (Fig. 3K) (29). The levels of glutamic acid and Agrp mRNA were unchanged in the ARC of the NB124-treated mutant group compared to wild-type or NB124-treated control mice (Fig. S3 A and B). In addition, strong Fos induction was observed in brain regions that receive afferents from AgRP neurons, including the bed nucleus of the stria terminalis, the paraventricular nucleus of the hypothalamus, and the parabrachial nucleus (PBN; Fig. S4 A–F) (25, 30). Deletion of Gad1 and Gad2 from AgRP neurons did not affect GABA levels in other brain regions—including the lateral hypothalamic nucleus (LH), dorsal medial hypothalamic nucleus (DMH), and central amygdala (CeA)—suggesting high selectivity of the NB124-mediated, gene-knockout strategy (Fig. S5). Our results suggest that treatment with NB124 in the mutant mice leads to sufficient production of functional Cre recombinase to inactivate Gad1 and Gad2 specifically in the AgRP neurons within 4 d, thereby disinhibiting postsynaptic neurons resulting from the loss of GABA signaling from AgRP neurons. Note that during those 4 d, the nsCre gene had to be transcribed and translated; the Gad1 and Gad2 alleles needed to recombine; Gad1 and Gad2 mRNAs and the two biosynthetic enzymes needed to decay; and GABA uptake from neighboring neurons had to be insufficient to maintain normal GABA signaling.
Fig. 3.
Deletion of GABA neurotransmitter from the AgRP neurons upon NB124 treatment. (A and B) Immunostaining images show anti-GABA (A; green) and tdTomato (B; red) in the ARC of control group mice 4 d after NB124 treatment. (C) Merged image of A and B shows colocalization of GABA and tdTomato in the ARC. (D) High-magnification image of the dotted area in C shows that almost all AgRP neurons coexpress GABA neurotransmitter (yellow) in control mice. (E and F) Immunostaining images show anti-GABA (E) and tdTomato (F) in the ARC of mutant-group mice 4 d after NB124 treatment. (G) Merged image of E and F shows GABA and tdTomato in the ARC. (H) Higher-magnification image of the dotted area in G shows that almost all AgRP neurons are depleted of GABA in mutant mice. Asterisks in A and E indicate the third ventricle. (I and J) Real-time qPCR analysis of transcript levels of Gad1 (I) and Gad2 (J) expressed in AgRP neurons that were isolated by a flow cytometry approach from AgrpCre::Rosa26tdTomato mice (WT group) and control and mutant group mice treated with NB124. (K) Abundance of GABA in the ARC was measured by HPLC in wild-type mice, DT-treated AgrpDTR/+ mice, and NB124-treated control and mutant groups. Values represent group means and SEM; n = 5 or 6 mice per group. *P < 0.05 [analysis of variance (ANOVA) with SNK post hoc]. Control group, AgrpnsCre::Rosa26tdTomato mice; mutant group, AgrpnsCre/+::Gad1lox/lox::Gad2lox/lox::Rosa26tdTomato mice. (Scale bars: A–C and E–G, 100 µm; D and H, 60 µm.)
Fig. S3.
Measurement of glutamic acid and Agrp mRNA. (A) Abundance of glutamic acid in the ARC was measured by HPLC in wild-type mice, DT-treated AgrpDTR/+ mice, and NB124-treated control and mutant groups. (B) qPCR results showed relative abundance of Agrp transcripts from the ARC in the NB124-treated control and mutant groups as well as DT-treated AgrpDTR/+ mice. Control group, AgrpnsCre::Rosa26tdTomato mice; mutant group, AgrpnsCre/+::Gad1lox/lox::Gad2lox/lox::Rosa26tdTomato mice. Values represent group means and SEM; n = 5 or 6 mice per group. *P < 0.05 (ANOVA with SNK post hoc).
Fig. S4.
Fos activation in postsynaptic neurons after rapid deletion of GABA signaling from AgRP neurons at different age. (A–C) Representative immunostaining pictures of Fos in postsynaptic regions of AgRP neurons, including the BNST (A), the PVN (B), and the PBN (C), 5 d after injection of NB124 into the third ventricle of 3-mo-old control group AgrpnsCre/+::Gad1lox/+::Gad2lox/+::Rosa26tdTomato mice. (D–F), Fos in postsynaptic regions of AgRP neurons, including the BNST (D), the PVN (E), and the PBN (F), 5 d after injection of NB124 (80 µg × 2, i.c.v.) into 3-mo-old mutant group AgrpnsCre/+::Gad1lox/lox::Gad2lox/lox::Rosa26tdTomato mice. (G–I) Fos in postsynaptic regions of AgRP neurons, including the BNST (G), the PVN (H), and the PBN (I), 5 d after injection of NB124 (80 µg × 2, i.c.v.) into 8-mo-old mutant group AgrpnsCre/+::Gad1lox/lox::Gad2lox/lox::Rosa26tdTomato mice. BNST, the bed nucleus of the stria terminalis; PVN, the paraventricular nucleus of hypothalamus. (Scale bar: 250 µm.)
Fig. S5.
GABA level in other brain regions after deletion of GABA from AgRP neurons. (A–C) Immunostaining images show anti-GABA in the LH (A), DMH (B), and CeA (C) of control group mice 4 d after NB124 treatment. (D–F) Immunostaining images show anti-GABA in the LH (D), DMH (E), and CeA (F) of mutant group mice 4 d after NB124 treatment. Control group, AgrpnsCre::Rosa26tdTomato mice; mutant group, AgrpnsCre/+::Gad1lox/lox::Gad2lox/lox::Rosa26tdTomato mice. (Scale bar: 250 µm.)
We characterized the effects on energy balance of NB124-mediated conditional deletion of GABA from AgRP neurons. The behavioral results revealed that injections of NB124 into the 3-mo-old mutant mice reduced food intake and resulted in significant loss of body weight (Fig. 4 A and B). Six days after initial treatment with NB124, these moribund mice had to be removed from the experiment because they had lost ∼20% of their initial body weight, and their daily food intake had almost ceased. In contrast, three groups of controls—including the control mice treated with NB124, control mice treated with vehicle, and mutant mice treated with vehicle—showed normal daily food intake and body weight throughout the testing period (Fig. 4 A and B). To assess the potential role of GABA in mediating energy expenditure, we measured metabolic responses in Comprehensive Lab Animal Monitoring System chambers (Columbus Instruments). In comparison with the control groups, the mutant group showed significant increases in O2 consumption, CO2 production, and locomotor activity between 2 and 4 d after the first treatment with NB124 (Fig. 4 C–E). Furthermore, loss of GABA from AgRP neurons resulted in glucose intolerance, compared with both the NB124-treated control mice and vehicle-treated mutant mice that were pair-fed to the same degree of weight loss (Fig. 4F). In line with a recent study, these results provide direct evidence that GABA signaling by AgRP neurons normally promotes food intake while simultaneously inhibiting energy expenditure (31). More surprisingly, this experiment revealed that GABAergic signaling by AgRP neurons also plays a role in maintaining normal glucose metabolism.
Fig. 4.
Acute inactivation of GABA signaling from AgRP neurons in 3-mo-old adult mice leads to reduction of feeding, severe weight loss, enhanced energy expenditure, and glucose intolerance. (A and B) Body weight (A) and daily calorie intake (B) of the following four groups of mice with drug or vehicle delivered into the third ventricle: control mice treated with vehicle, control mice treated with NB124 (80 µg × 2, i.c.v.), mutant mice treated with vehicle, and mutant mice treated with NB124 (80 µg × 2, i.c.v.). (C–E) Immediately after the second treatment of NB124, O2 consumption (C), CO2 production (D), and locomotor activity (E) of the mice as described in A and B. *P < 0.05 (between mutant+NB124 group and the control groups; ANOVA with SNK post hoc). (F) Glucose tolerance test (GTT) was performed in control mice treated with NB124, mutant mice pair-fed to the mutant group treated with NB124, and mutant group treated with NB124. *P < 0.05 (between mutant+NB124 group and control+NB124 group; Student t test); #P < 0.05 (between mutant+NB124 group and mutant+pair-fed group; Student t test). Values represent group means and SEM; n = 8–10 mice per group. Control group, AgrpnsCre/+::Gad1lox/+::Gad2lox/+::Rosa26tdTomato mice; mutant group, AgrpnsCre/+::Gad1lox/lox::Gad2lox/lox::Rosa26tdTomato mice.
We also tested the role of GABA signaling from AgRP neurons in the control of feeding and metabolism in 8-mo-old mice because of reports of synaptic remodeling by aging AgRP neurons (32). Compared with the age-matched control group, i.c.v. delivery of NB124 into 8-mo-old mutant mice activated Cre-dependent tdTomato reporter gene and reduced Gad1 and Gad2 transcript levels by ∼90% in AgRP neurons within 6 d (Fig. S6). Surprisingly, deletion of GABA synthesis from AgRP neurons in older mice mediated a moderate, but only transient, reduction of food intake and body weight (Fig. 5 A and B). Furthermore, no significant changes were observed in energy metabolism, locomotion, or glucose metabolism (Fig. 5 C–F). Notably, deletion of GABA from AgRP neurons in 8-mo-old mice resulted in Fos induction in postsynaptic neurons, but to a lesser extent compared with young mice (Fig. S4). These data suggest that AgRP neurons in older mice are more resistant to the loss of GABA signaling.
Fig. S6.
Treatment of NB124 leads to acute activation of Cre recombinase and subsequent removal of GABA signaling from AgRP neurons in 8-mo-old transgenic mice. (A) Immunostaining image showed tdTomato expression profile in the ARC of 8-mo-old mutant AgrpnsCre/+::Gad1lox/lox::Gad2lox/lox::Rosa26tdTomato mice 4 d after i.c.v. injection of NB124. (B and C) qPCR analysis of transcripts level of Gad1 (B) and Gad2 (C) expressed in tdTomato-positive AgRP neurons that were isolated through a flow cytometry approach 6 d after i.c.v. injection of NB124 or vehicle into 8-mo-old mutant group AgrpnsCre/+::Gad1lox/lox::Gad2lox/lox::Rosa26tdTomato mice. Values represent means and SEM; n = 5 or 6 mice per group. *P < 0.05 (Student t test). (Scale bar, 300 µm.)
Fig. 5.
Metabolic characterization of 8-mo-old mice upon acute disruption of GABA signaling from AgRP neurons. (A and B) Body weight (A) and daily calorie intake (B) of the following two groups of mice with drug or vehicle delivered into the third ventricle: control mice treated with NB124 and mutant mice treated with NB124 (80 µg × 2, i.c.v.). (C–E) Immediately after the second treatment of NB124, O2 consumption (C), CO2 production (D), and locomotor activity (E) of the mice as described in A and B. (F) GTT was performed in control mice treated with NB124, mutant mice treated with vehicle, and mutant group treated with NB124. *P < 0.05 (between mutant+NB124 group and control+NB124 group; Student t test). Values represent group means and SEM; n = 8–10 mice per group. Control group, AgrpnsCre/+::Gad1lox/+::Gad2lox/+::Rosa26tdTomato mice; mutant group, AgrpnsCre/+::Gad1lox/lox::Gad2lox/lox::Rosa26tdTomato mice.
Discussion
In this study, we established a new method for inducible Cre-dependent inactivation of loxP-flanked genes and demonstrated its effectiveness by eliminating GABA signaling from AgRP neurons and monitoring biological consequences on behavior and metabolism. Abrupt inactivation of GABA biosynthesis genes in adult mice permitted us to interrogate the pathophysiological roles exhibited by this key inhibitory neurotransmitter in AgRP neurons at various ages. We chose this model system because constitutive inactivation of GABA signaling starting when Agrp genes become active during late embryonic development results in a compensatory response, such that there is minimal effect on food intake or body weight of adult mice, a response that is similar to that observed when AgRP neuron are ablated in newborn mice (9, 29). In contrast, ablation of AgRP neurons or disruption of GABA signaling in young adult mice leads to anorexia and severe weight loss (29, 33, 34). Considerable evidence indicates that this starvation phenotype is mediated by hyperactivity of neurons that express calcitonin gene-related protein in the PBN due to loss of GABA signaling from AgRP neurons (35). We discovered that chronic infusion of a partial GABAA agonist for 2 wk can prevent starvation after ablation of AgRP neurons, and, importantly, food intake and body weight were maintained even after termination of GABAA agonist infusion (27), suggesting that mice had adapted to loss of AgRP neurons (28). Mice can also adapt if the ablation is performed in moderately obese, leptin-deficient mice (36), presumably because they have sufficient energy reserves to sustain them while adaptation occurs. We suggest that loss of GABA signaling by AgRP neurons in young mice triggers a similar compensatory mechanism that can supersede the physiological function of these neurons. Apparently, NPY and AgRP alone are unable to sustain adequate food intake when only GABA is acutely eliminated from AgRP neurons in young mice. Conversely, we suggest that ectopic enhancement of GABA input and/or GABAA receptor signaling at postsynaptic targets of AgRP neurons could be critically involved in the compensatory mechanism.
Previously, AgRP neuron ablation experiments were performed in relatively young mice. Thus, we were surprised that the 8-mo-old mice in this study were resistant to the severe consequences of inactivating GABA signaling, suggesting that there is an age-related adaptive mechanism that allows adequate feeding in the absence of AgRP neurons. We suggest that long-term nutritional or environmental conditions favor desensitization of homeostatic feeding mediated by AgRP neurons, while promoting reliance on the brainstem feeding circuit and/or midbrain reward circuit (37). These ancillary feeding pathways may progressively overtake the physiological role of AgRP neurons under an emergent or age-dependent mechanism. The reduced Fos induction in postsynaptic targets of the older mice further suggests that an age-dependent mechanism promotes the establishment of a novel balance between excitatory and inhibitory inputs onto these brain regions, where GABA from AgRP neurons has less impact upon the excitability of postsynaptic neurons, such as the calcitonin gene-related peptide neurons in the PBN (28, 35).
In contrast to the CreER and CrePR systems, we avoided the problem of tethering Cre recombinase with a bulky addition by introducing a single amino acid into a particular locus within Cre recombinase—a strategy that prevents gene silencing, at least at the Agrp locus. Aminoglycoside derivatives, unlike lipid-soluble tamoxifen and RU486, cannot penetrate the blood–brain barrier or diffuse far from the site of injection (38). Thus, the nsCre system is particularly useful to distinguish between central and peripheral contributions when the gene of interest is expressed in both periphery and brain, such as the Rip–Cre case (3, 4). Furthermore, one might be able to target one brain region with an aminoglycoside without affecting genes in others regions. Some studies indicated that tamoxifen and RU486 interfere with reproductive and heart physiology, which could affect interpretation of acquired phenotypes (39, 40). Moreover, emerging evidence indicates that constitutive expression of full-length functional Cre or CreER protein exhibits serious metabolic side effects, such as Nestin–Cre on pituitary functions as well as Rip–Cre or CreER on β-cell functions (3, 41). In contrast, PTC promotes NMD, a conserved pathway that destroys the majority of nsCre transcripts, thereby leaving only a few mutant mRNAs that are translated into truncated peptides (13). Furthermore, aminoglycosides have not been cited for causing adverse effects on fertility, heart physiology, digestive systems, or central nervous systems (14, 16). Although aminoglycosides generally elicit nonspecific cytotoxicity, the dosage of a new class of G418 derivatives, including NB124, that we used did not reveal noticeable adverse effects on feeding, body weight, locomotion, or energy metabolism in the control group. We used aminoglycoside activation of nsCre to both activate a reporter gene (Rosa26tdTomato) and inactivate Gad1 and Gad2 genes, thus demonstrating two useful applications. This nonsense suppression-based genetic system provides an ideal alternative strategy for regulating expression of Cre-dependent genes in diverse scenarios where other inducible Cre strategies are either nonapplicable or problematic.
Materials and Methods
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committees at Baylor College of Medicine, the University of Iowa, and the University of Washington. To generate nsCre transgene, a nonsense codon, UGA, was introduced into P1 phage Cre recombinase coding sequence by a PCR-based replacement method. The inducible AgrpnsCre and the conditional Gad2lox/+ transgenic lines were generated by standard cloning and ES cell-based gene targeting protocols. Other mouse strains have been reported (22, 25, 29, 42). Mice were microinjected with 0.8 µL of G418, NB compounds, or saline vehicle into the third ventricle either once or twice (2 d apart). Measurements of GABA and glutamate concentrations, immunohistochemistry, quantitative PCR, and FACS techniques were performed by standard procedures (36, 43, 44). Food intake, body weight, energy expenditure, and glucose tolerance test (GTT) were measured as described (28, 36, 45). All statistical analyses were performed by Microsoft Excel and GraphPad Prism. Refer to SI Materials and Methods for experimental details.
SI Materials and Methods
Generation and Maintenance of Transgenic Mice.
Mice were housed in a temperature- and humidity-controlled facility with a 12-h light/dark cycle. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committees at Baylor College of Medicine, the University of Iowa, and the University of Washington. In compliance with our approved protocol, all experiments were terminated if the body weight of mice fell to 80% of their original body weight. Mice were group-housed with standard chow diet and water provided ad libitum.
AgrpDTR and Gad1lox/lox mice were generated and genetically identified by PCR of tail DNA as described (22, 25, 29, 42). The Rosa26tdTomato mice [Gt(ROSA)26Sortm14(CAG-tdTomato)Hze; also known as Ai14] were obtained from the Jackson Laboratory (stock no. 007914). The conditional Gad2 targeting construct (Gad2lox/+) was prepared by flanking the first exon with loxP sites along with an frt-flanked SV-Neo gene in the first intron. The construct was electroporated in G4 ES cells, and correctly targeted clones were identified by Southern blotting. After removal of the SV-Neo gene by breeding with a mouse expressing FLP recombinase, heterozygotes were bred to generate Gad2lox/lox mice that were used for generation of conditional GABA knockout mice. Similarly, AgrpnsCre targeting construct were generated by insertion of a fragment containing myc-nls-nsCre2 transgene and frt-flanked SV-Neo gene just 5′ of the initiation codon of the Agrp gene. All strains of mice were back-crossed onto the C57BL/6 background for at least eight generations. The experimental AgrpnsCre/+::Gad1lox/lox::Gad2lox/lox::Rosa26tdTomato mice and the littermate controls AgrpnsCre/+::Gad1lox/+::Gad2lox/+::Rosa26tdTomato were generated by several generations of cross-breeding and inbreeding among the four transgenic lines.
The following pair of primers were used to genotype Gad2lox/+ mice:
Forward: 5′-AGCACTGGTTTGGAGGAAGGWT-3′
Reverse: 5′-CAGGGAGACCTTGGCAAGAT-3′
WT band size: 130 bp; loxP band size: 190 and 200 bp.
The following group of primers were used to genotype AgrpnsCre/+ mice:
Forward: 5′-GTCTCTCCTCATCCTTAGGGTAC-3′
Reverse-1: 5′-TTTCCAGGGAGGCACCTCATGC-3′
Reverse-2: 5′-GGCAAATTTTGGTGTACGGTCAG-3′
WT band size: 200 bp; nsCre band size: 170 bp.
Generation and Validation of nsCre Transgene in Vitro.
To generate nsCre transgene, a nonsense codon, UGA, was introduced to one of the desired loci of wild-type P1 phage Cre coding sequence in a Pgk-myc-nls-Cre plasmid by a PCR-based replacement method. Insertion site of nonsense codon was verified by the DNA sequencing (Genewiz). HEK293 cells were cultured in DMEM (Invitrogen), containing 10% (wt/vol) FBS (Atlanta Biologicals), 100 units/mL penicillin G (Corning), and 100 μg/mL streptomycin (Corning). Cells were cultured at 37 °C in a 5% (vol/vol) CO2-humidified incubator (Thermo Fisher). When at ∼50% confluency, HEK293 cells were cotransfected with three plasmids, including PGK-myc-nls-nsCre, CMV-loxP-Neo-loxP-DsRed2, and Pgk-NeoR. The transfection was performed by Lipofectamine 2000 (Invitrogen) following the manufacturer’s suggested protocol. Various doses of G418 (Calbiochem) were added to the culture medium 1 d after the transfection. After another 4 d, fixed cells were stained by DAPI (Sigma) and visualized under an Axio Observer microscope (Zeiss) to assess the number of DsRed fluorescent cells. We also generated a stable clone of HEK293 cells expressing CMV-loxP-Neo-loxP-DsRed2 that responds to Cre recombinase. When these cells were at ∼20% confluency, they were transfected with Pgk-nsCre2 or wild-type Pgk-Cre by using the calcium phosphate method. G418 was added at varying concentrations for 2 d, and then the cells were split and incubated without G418 for several days before counting the number of fluorescent cells.
Surgery and Drug Administration.
G418 and the NB series compounds were dissolved in normal saline at a concentration of 100 mg/mL. Under anesthesia, mice were microinjected with 0.8 µL of G418, NB compounds, or saline vehicle into the third ventricle either once or twice (2 d apart) by using the coordinates 0 mm (x axis), −1.6 mm (y axis), and −5.7 mm (z axis). Brain samples from all groups of mice were collected at the end of each behavioral experiment and were further processed for fluorescent immunohistological analysis. To ablate AgRP neurons, two intramuscular injections of DT (50 μg/kg, 2 d apart; List Biological Laboratories) were performed in adult AgrpDTR/+ mice (29).
Measurement of GABA and Glutamate.
Brains were dissected and cut to 1-mm thick slices by using the brain matrix (Zivic Instruments). The slices were moved onto an ice-cold metal pad, and the arcuate nuclei were collected under a dissecting microscope (M60; Leica). The tissues were rapidly frozen in the liquid nitrogen and stored at −80 °C. The concentrations of GABA and glutamate were measured by a standard HPLC procedure performed at Neurochemistry Core of Vanderbilt University. Data were normalized to total protein level.
Immunohistochemistry.
Immunostaining was performed as described with modification (43). Mice were killed and perfused transcardially with ice-cold PBS buffer (pH 7.4) containing 3% (wt/vol) paraformaldehyde (Alfa Aesar) and 1% glutaraldehyde (Sigma). Brains were collected and postfixed overnight under 4 °C in a fixation buffer containing 3% paraformaldehyde. Free-floating sections (20 µm) were cut by a microtome (Thermo Fisher) and then blocked with 5% (wt/vol) normal donkey serum in 0.1% Triton X-100 (TBST buffer, pH 7.2) for overnight. For each different assay, either rabbit anti-AgRP (1:500 dilution; Phoenix Pharmaceuticals), rabbit anti-GABA (1:500 dilution; Thermo Fisher), or rabbit anti-Fos (1:1,500 dilution; EMD Millipore) was applied to the sections for overnight incubation under 4 °C, followed by 4 × 15-min rinses in the TBST buffer. Finally, sections were incubated with Alex Fluor 488-conjugated secondary antibody (1:1,000 dilution; Jackson Immunolab) for 2 h at room temperature, followed by 4 × 15-min rinses in TBST buffer. For mounted sections, fluorescent images were captured by a digital camera mounted on a DMI6000B microscope (Leica).
FACS.
TdTomato-positive AgRP neurons were isolated by a standard flow cytometry method as described (44). Briefly, brain tissues containing the ARC were collected into 0.5 mL of ice-cold HBSS buffer, then transferred to 37 °C warmed dispase buffer (BD Biosciences), and incubated for 15 min. During the incubation period, tissues were gently pipetted up and down until completely dissociated. Next, 0.5 mL ice-cold FACS buffer [10% (wt/vol) glucose, 0.5 mM EDTA, and 3 mg/mL BSA] was added to stop the dispase-mediated dissociation process. Cells were pelleted by centrifuging for 2 min at 3,000 × g at 4 °C. The pellets were resuspended in 0.5 mL of ice-cold FACS buffer and then filtered into polypropylene tubes (Falcon) before the FACS procedure.
Real-Time qPCR.
Sorted cells were immediately lysed by RLT buffer from the RNeasy Plus Micro kit (Qiagen). Total mRNA was subsequently extracted and purified based on the manufacturer’s suggested protocol (Qiagen). For some experiments, total RNA was extracted from fresh arcuate nucleus by TRIzol (Invitrogen) per the manufacturer’s instructions. The purified RNA was quantified by One-drop spectrophotometer (Thermo Fisher), and mRNA was reverse-transcribed by using the SuperScript II kit (Invitrogen) per the manufacturer’s suggested protocol. qPCR was performed in the Bio-Rad CFX96 Real-Time PCR system with a set of Taqman probes (IDT). Relative abundance of Agrp transcripts was determined by using the 2−ΔΔCt method and normalized to Gapdh, a housekeeping gene.
Food Intake and Body Weight.
Mice were individually housed and allowed to acclimate for 3 d before experiments were initiated. Consumption of chow pellets and body weight were measured at 1600 hours on a daily basis throughout the experimental period (28, 36, 45).
Energy Expenditure.
Mice were acclimatized in the Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments) for 3 d before initiating each experiment. Immediately after the second drug injection, energy expenditure parameters, including oxygen consumption (VO2), carbon dioxide production (VCO2), locomotor activity (in x axis), and respiratory exchange ratio were recorded by indirect calorimetry methods in the CLAMS chambers while mice were supplied with standard chow diet and ad libitum water. Values of VO2 and VCO2 were normalized to lean body mass, which was obtained from TD-NMR Whole Body Composition Analyzer (Bruker).
GTT.
The GTT was performed as described (36). Briefly, mice were fasted overnight for 16 h, a blood sample was taken, and then the mice were injected with d-glucose (1 g/kg, i.p.), and blood was drawn from the tail vein at 0, 15, 30, 60, and 120 min later. Blood glucose levels were determined with a FreeStyle Lite glucometer (Abbott Laboratories).
Statistical Analysis.
Data are presented as means ± SEM and taken as statistically significant at P < 0.05. Differences between two groups were analyzed by one ANOVA followed by the Student–Newman–Keuls method for statistical significance. All statistical analyses were performed by Microsoft Excel and GraphPad Prism.
Acknowledgments
We thank Kathy Kafer (University of Washington) for help making the new lines of gene-targeted mice; Sicong Dong (Baylor College of Medicine) for help breeding and maintaining the transgenic mice; and Drs. Qingchun Tong, Makoto Fukuda, Yuxiang Sun, and Zheng Sun for helpful comments on the manuscript. This work was supported by the Pew Charitable Trust (Q.W.); American Diabetes Association Junior Faculty Award 7-13-JF-61 (to Q.W.); Baylor Collaborative Faculty Research Investment Program grants (to Q.W.); US Department of Agriculture/Agricultural Research Service Current Research Information System grants (to Q.W.); Baylor College of Medicine and University of Iowa New Faculty Start-Up grants (to Q.W.); NIH Grants R01DK093587 and R01DK101379 (to Y.X.); and NIH Grant R01-DA24908 (to R.D.P.). R.D.P. is an Howard Hughes Medical Institute Investigator; Q.W. is the Pew Scholar of Biomedical Sciences and the Kavli Scholar.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602049113/-/DCSupplemental.
References
- 1.Feil R, et al. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci USA. 1996;93(20):10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kellendonk C, et al. Regulation of Cre recombinase activity by the synthetic steroid RU 486. Nucleic Acids Res. 1996;24(8):1404–1411. doi: 10.1093/nar/24.8.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harno E, Cottrell EC, White A. Metabolic pitfalls of CNS Cre-based technology. Cell Metab. 2013;18(1):21–28. doi: 10.1016/j.cmet.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Magnuson MA, Osipovich AB. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab. 2013;18(1):9–20. doi: 10.1016/j.cmet.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8(24):1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 6.Birling MC, Gofflot F, Warot X. Site-specific recombinases for manipulation of the mouse genome. Methods Mol Biol. 2009;561:245–263. doi: 10.1007/978-1-60327-019-9_16. [DOI] [PubMed] [Google Scholar]
- 7.Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: Genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA. 1999;96(15):8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwenk F, Kuhn R, Angrand PO, Rajewsky K, Stewart AF. Temporally and spatially regulated somatic mutagenesis in mice. Nucleic Acids Res. 1998;26(6):1427–1432. doi: 10.1093/nar/26.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11(9):998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaelin CB, Xu AW, Lu XY, Barsh GS. Transcriptional regulation of agouti-related protein (Agrp) in transgenic mice. Endocrinology. 2004;145(12):5798–5806. doi: 10.1210/en.2004-0956. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, et al. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab. 2014;3(1):64–72. doi: 10.1016/j.molmet.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mort M, Ivanov D, Cooper DN, Chuzhanova NA. A meta-analysis of nonsense mutations causing human genetic disease. Hum Mutat. 2008;29(8):1037–1047. doi: 10.1002/humu.20763. [DOI] [PubMed] [Google Scholar]
- 13.Kervestin S, Jacobson A. NMD: A multifaceted response to premature translational termination. Nat Rev Mol Cell Biol. 2012;13(11):700–712. doi: 10.1038/nrm3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bidou L, Allamand V, Rousset JP, Namy O. Sense from nonsense: Therapies for premature stop codon diseases. Trends Mol Med. 2012;18(11):679–688. doi: 10.1016/j.molmed.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Shalev M, Baasov T. When proteins start to make sense: Fine-tuning aminoglycosides for PTC suppression therapy. MedChemComm. 2014;5(8):1092–1105. doi: 10.1039/C4MD00081A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keeling KM, Xue X, Gunn G, Bedwell DM. Therapeutics based on stop codon readthrough. Annu Rev Genomics Hum Genet. 2014;15:371–394. doi: 10.1146/annurev-genom-091212-153527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noensie EN, Dietz HC. A strategy for disease gene identification through nonsense-mediated mRNA decay inhibition. Nat Biotechnol. 2001;19(5):434–439. doi: 10.1038/88099. [DOI] [PubMed] [Google Scholar]
- 18.Wilschanski M, et al. Gentamicin-induced correction of CFTR function in patients with cystic fibrosis and CFTR stop mutations. N Engl J Med. 2003;349(15):1433–1441. doi: 10.1056/NEJMoa022170. [DOI] [PubMed] [Google Scholar]
- 19.Malik V, Rodino-Klapac LR, Viollet L, Mendell JR. Aminoglycoside-induced mutation suppression (stop codon readthrough) as a therapeutic strategy for Duchenne muscular dystrophy. Ther Adv Neurol Disorder. 2010;3(6):379–389. doi: 10.1177/1756285610388693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petyuk V, McDermott J, Cook M, Sauer B. Functional mapping of Cre recombinase by pentapeptide insertional mutagenesis. J Biol Chem. 2004;279(35):37040–37048. doi: 10.1074/jbc.M406042200. [DOI] [PubMed] [Google Scholar]
- 21.Nudelman I, et al. Repairing faulty genes by aminoglycosides: Development of new derivatives of geneticin (G418) with enhanced suppression of diseases-causing nonsense mutations. Bioorg Med Chem. 2010;18(11):3735–3746. doi: 10.1016/j.bmc.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 22.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandasamy J, et al. Increased selectivity toward cytoplasmic versus mitochondrial ribosome confers improved efficiency of synthetic aminoglycosides in fixing damaged genes: A strategy for treatment of genetic diseases caused by nonsense mutations. J Med Chem. 2012;55(23):10630–10643. doi: 10.1021/jm3012992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sohn JW, Elmquist JK, Williams KW. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 2013;36(9):504–512. doi: 10.1016/j.tins.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137(7):1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krashes MJ, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483(7391):594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 30.Wu Q, Howell MP, Palmiter RD. Ablation of neurons expressing agouti-related protein activates fos and gliosis in postsynaptic target regions. J Neurosci. 2008;28(37):9218–9226. doi: 10.1523/JNEUROSCI.2449-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruan HB, et al. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell. 2014;159(2):306–317. doi: 10.1016/j.cell.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kmiec Z. Aging and peptide control of food intake. Curr Protein Pept Sci. 2011;12(4):271–279. doi: 10.2174/138920311795906718. [DOI] [PubMed] [Google Scholar]
- 33.Wu Q, Palmiter RD. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. Eur J Pharmacol. 2011;660(1):21–27. doi: 10.1016/j.ejphar.2010.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima K, et al. Gs-coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake. Nat Commun. 2016;7:10268. doi: 10.1038/ncomms10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503(7474):111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc Natl Acad Sci USA. 2012;109(8):3155–3160. doi: 10.1073/pnas.1120501109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denis RG, et al. Palatability can drive feeding independent of AgRP neurons. Cell Metab. 2015;22(4):646–657. doi: 10.1016/j.cmet.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lexow J, Poggioli T, Sarathchandra P, Santini MP, Rosenthal N. Cardiac fibrosis in mice expressing an inducible myocardial-specific Cre driver. Dis Model Mech. 2013;6(6):1470–1476. doi: 10.1242/dmm.010470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huh WJ, Mysorekar IU, Mills JC. Inducible activation of Cre recombinase in adult mice causes gastric epithelial atrophy, metaplasia, and regenerative changes in the absence of “floxed” alleles. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G368–G380. doi: 10.1152/ajpgi.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teitelman G, Kedees M. Mouse insulin cells expressing an inducible RIPCre transgene are functionally impaired. J Biol Chem. 2015;290(6):3647–3653. doi: 10.1074/jbc.M114.615484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chattopadhyaya B, et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54(6):889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Q, Howell MP, Cowley MA, Palmiter RD. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc Natl Acad Sci USA. 2008;105(7):2687–2692. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panchision DM, et al. Optimized flow cytometric analysis of central nervous system tissue reveals novel functional relationships among cells expressing CD133, CD15, and CD24. Stem Cells. 2007;25(6):1560–1570. doi: 10.1634/stemcells.2006-0260. [DOI] [PubMed] [Google Scholar]
- 45.Wu Q, Zheng R, Srisai D, McKnight GS, Palmiter RD. NR2B subunit of the NMDA glutamate receptor regulates appetite in the parabrachial nucleus. Proc Natl Acad Sci USA. 2013;110(36):14765–14770. doi: 10.1073/pnas.1314137110. [DOI] [PMC free article] [PubMed] [Google Scholar]