Significance
Here, we show how anatomical and functional data recorded from patients undergoing stereo-EEG can be combined to generate highly resolved four-dimensional maps of human cortical processing. We used this technique, which provides spatial maps of the active cortical nodes at a millisecond scale, to depict the somatosensory processing following electrical stimulation of the median nerve in nearly 100 patients. The results showed that human somatosensory system encompasses a widespread cortical network including a phasic component, centered on primary somatosensory cortex and neighboring motor, premotor, and inferior parietal regions, as well as a tonic component, centered on the opercular and insular areas, lasting more than 200 ms.
Keywords: intracranial recordings, stereo-EEG, median nerve, cerebral cortex, temporal dynamics
Abstract
A fine-grained description of the spatiotemporal dynamics of human brain activity is a major goal of neuroscientific research. Limitations in spatial and temporal resolution of available noninvasive recording and imaging techniques have hindered so far the acquisition of precise, comprehensive four-dimensional maps of human neural activity. The present study combines anatomical and functional data from intracerebral recordings of nearly 100 patients, to generate highly resolved four-dimensional maps of human cortical processing of nonpainful somatosensory stimuli. These maps indicate that the human somatosensory system devoted to the hand encompasses a widespread network covering more than 10% of the cortical surface of both hemispheres. This network includes phasic components, centered on primary somatosensory cortex and neighboring motor, premotor, and inferior parietal regions, and tonic components, centered on opercular and insular areas, and involving human parietal rostroventral area and ventral medial-superior-temporal area. The technique described opens new avenues for investigating the neural basis of all levels of cortical processing in humans.
A detailed description of the spatiotemporal dynamics of human brain activity is a major goal of neuroscientific research. However, it has been impossible so far to attain both high spatial and temporal resolution using the available noninvasive recording and imaging techniques. Hence, a precise and comprehensive four-dimensional cartography of human neural activity has not yet been obtained. High spatial resolution, provided by neuroimaging techniques such as functional magnetic resonance imaging (fMRI), is crucial for highlighting the topographical organization of specific areas (e.g., somatotopy of sensorimotor areas) as well as identifying the nodes of brain networks endowed with specific functional properties (1). It is not sufficient, however, to know which nodes are active; information is also needed about the local dynamics of the nodes, as well as the relative timing of their activity, to fully understand human brain functions (2, 3). Even if the temporal resolution of electroencephalography (EEG) and magnetoencephalography (MEG) allowed one to observe the intra- and interareal dynamics, to date such recordings remain too poor in localization power (1–2 cm) (3, 4). Combining EEG and fMRI has been suggested as a solution, using EEG to determine the temporal dynamics within and between the areas identified with fMRI (5). However, the disparate nature of the two signals recorded (hemodynamic for fMRI, electrical for EEG) creates discrepancies in the results that prevent precise matching of these methods (3).
Invasive intracranial EEG offers a unique opportunity to observe human brain activity with an unparalleled combination of spatial and temporal resolution. Depending on the electrodes used, two kinds of recordings can be made: (i) intraparenchymal recordings, also called stereo-EEG (sEEG) (6), obtained using stereotactically inserted needle-like electrodes with multiple recording leads; and (ii) the electrocorticogram (ECoG), obtained from subdural electrode grids, covering regions of cortex. The latter technique suffers from three disadvantages: (i) the technique only samples from cortical gyri, missing cortical regions buried in sulci (7); (ii) the technique is affected by volume conduction (8); and (iii) the technique does not record directly from gray matter, because pia and arachnoid mater lie in between the electrodes and the cortex, reducing the amplitude and making it more difficult to extract high-frequency activity from the recorded signal. Both approaches suffer from the so-called sparse-sampling problem (i.e., the limited and uneven coverage of the patients’ brain because the positioning of the electrodes in any given patient is dictated by clinical criteria), leading to difficulties in carrying out analyses at the population level.
The primary aim of the present study is to show how anatomical and functional data recorded from a large number of patients could be combined to generate highly resolved four-dimensional maps of human cortical processing. To demonstrate the feasibility of computing such maps, indicating consistently active cortical nodes and the nodes’ time course at a millisecond scale, we leveraged the advantages of sEEG to investigate somatosensory processing following electrical stimulation of the median nerve in nearly 100 patients. fMRI studies have consistently reported activation of the primary somatosensory complex (SI), secondary somatosensory complex (SII), and insula in response to transient nonpainful stimuli (9) but less so of supplementary motor area (SMA) (10), anterior cingulate cortex (11), superior parietal lobule (SPL) and inferior parietal lobule (IPL) (12), and premotor cortex (13). In addition to providing sensitive detection of responsive regions, sEEG responses also reveal time courses, which could be relevant to debates concerning cortical dynamics, such as the parallel vs. serial operation of SII relative to SI (14, 15). Here, we show for the first time, to our knowledge, that comprehensive four-dimensional maps of human cortical somatosensory processing can be computed from high-frequency broadband gamma activity, thus surpassing previous subdural and EEG/MEG maps in spatiotemporal precision.
Results
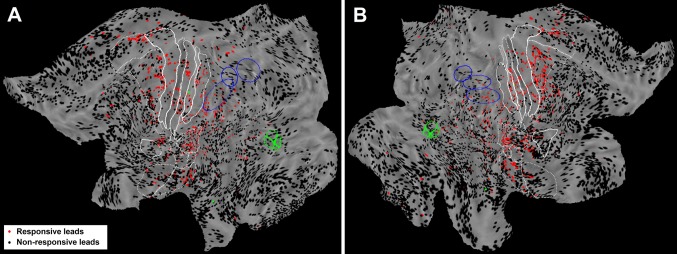
Recordings were obtained from 17,009 leads in 99 patients, of which 11,983 (5,678 in the left hemisphere and 6,305 in the right) were localized in the cortical gray matter according to the anatomical reconstruction procedure (Fig. 1 and Figs. S1–S3). Of note, 48.8% of cortical leads (2,733 in the left hemisphere and 3,112 in the right) were localized in sulcal areas [i.e., cortical regions characterized by a negative folding index (Materials and Methods)], many of which are not accessible to ECoG recordings. The sampling density maps computed for the two hemispheres (Fig. 1D and Figs. S2 and S3) demonstrate the extensive coverage of cortical sheet, with peaks bilaterally in the middle temporal gyrus and in the mesial temporal region (32 leads/cm2 in the left, 48 leads/cm2 in the right hemisphere). Only the frontal and occipital tips of the hemispheres as well as the cortical crowns were poorly represented because of the obligatory orthogonal insertion of electrodes and to the anatomical and vascular constraints (presence of frontal bone sinus and superior sagittal sinus, respectively).
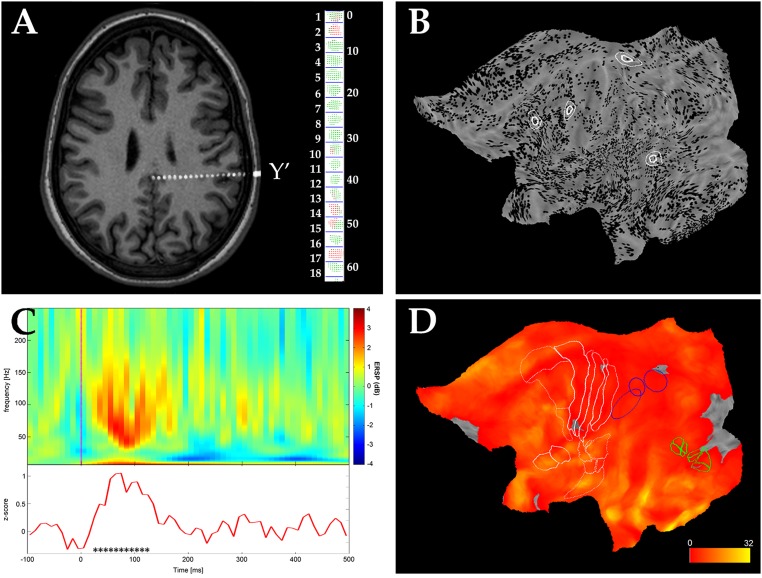
Fig. 1.
Anatomical and functional data analysis. (A) Axial MR slice of a patient, after the coregistration with the postimplantation CT, on which the entire Y′ electrode implanted in the left hemisphere is visible. The reconstruction of the Y′ electrode is shown on the right side, with the leads numbered 1–18 from the tip, for a total distance of 50 mm. Leads are represented by sets of voxels, colored red if located in cortical gray matter and green otherwise. (B) Midthickness surface of the fs_LR brain template with all leads located in gray matter of the left hemisphere (from 49 patients) indicated as black dots. For four nodes, circles with 0.5 cm (thick line) and 1 cm (thin line) of radius represent the masks. (C, Upper) Average time frequency plot (100 trials) in response to median nerve stimulation in one lead of one patient. (C, Lower) Time course of the average gamma power (50–150 Hz) for the same channel, reported as z scores based on the prestimulus interval. Black asterisks indicate time bins with gamma power significantly exceeding baseline (P < 0.001). (D) Sampling density of the left hemisphere computed from data in B. The color scale is expressed in the number of recording leads per cm2. Cytoarchitectonic areas (1–4, 6, OP1–4, and 44) and anatomically defined areas (long and short gyri of insula) are indicated by white lines, and functionally defined regions are indicated in blue [confidence ellipses of phAIP, DIPSA, and DIPSM from Jastorff et al. (78)] or green [MT cluster from Abdollahi et al. (72)].
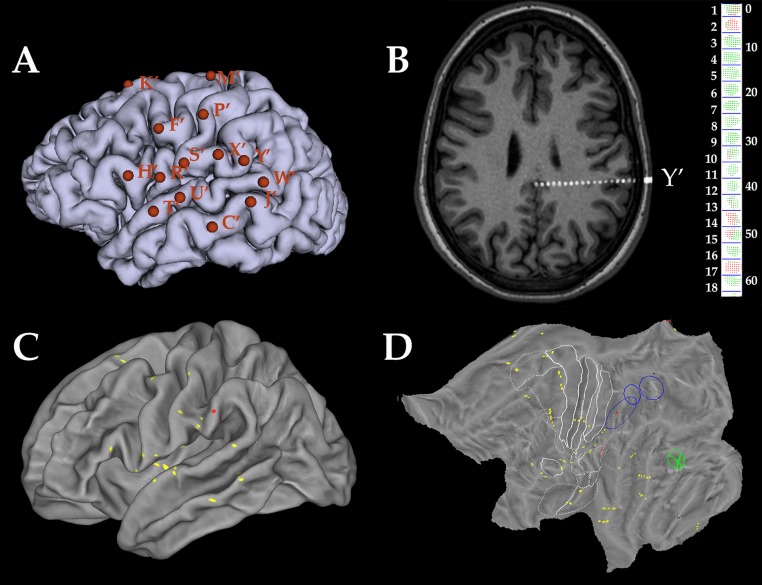
Fig. S1.
Single-patient anatomical data processing. (A) Lateral view of the left hemisphere of a patient with the entry points of 14 implanted electrodes marked by orange letters (with asterisks to indicate left side). (B) Axial MR slice from the same patient, after the coregistration with the cone-beam CT, on which the entire Y′ electrode is visible. The reconstruction of the Y′ electrode is shown on the right side, with the leads numbered from the tip of the electrode. Leads are represented by sets of voxels colored in red if located in gray matter and green otherwise. (C) Midthickness surface of the same patient warped to the fs_LR brain template with all leads exploring gray matter projected onto the surface and indicated in yellow. The leads of electrode Y′ are indicated in red. (D) Same data as C shown on a flat map of the left hemisphere. Note that whereas in C, only the three outermost leads of electrode Y′ are visible, flat map visualizes all leads located in cortical gray matter.
Fig. S3.
Inflated view of sampling density of the left (A) and right (B) hemispheres.
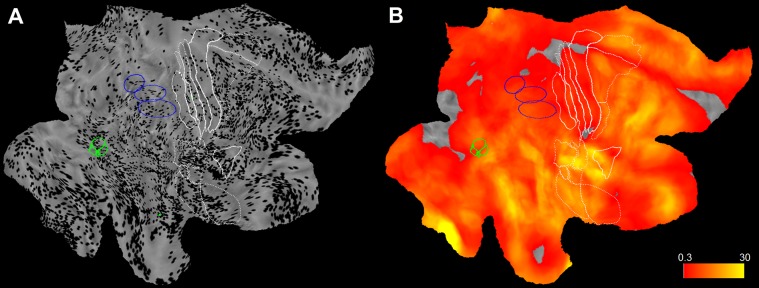
Fig. S2.
Sampling in right hemisphere. (A) Midthickness surface of the fs_LR brain template with all leads located in gray matter of the right hemisphere (from 58 patients) indicated as black dots. Anatomical and functional borders are defined as in Fig. 2. (B) Sampling density of the right hemisphere computed from data in A.
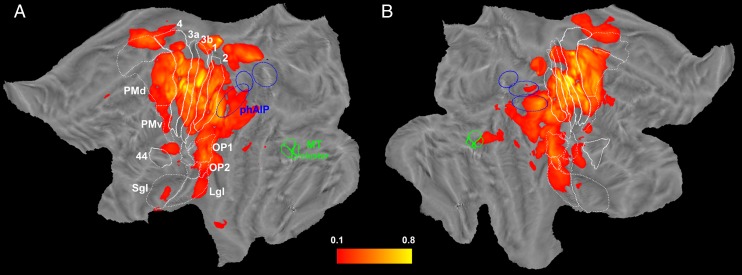
Statistical analysis revealed that 1,146 of the leads exploring gray matter presented a significant broadband gamma power (50- to 150-Hz) increase (see Fig. 1C for an example of single lead analysis on gamma-band time course) in response to the median nerve stimulation (489 in the left hemisphere, 657 in the right; Fig. S4). By localizing each lead, we computed the overall responsiveness (in % of responsive leads) maps for both hemispheres (Fig. 2), in principle comparable with neuroimaging data in that timing is not considered. High responsiveness values were found in SI. In particular, the highest proportion of responsive leads was present bilaterally in areas 3a (left 81%, right 94%) and 3b (left 95%, right 87%). Lower values characterized areas 1 (left 60%, right 60%) and 2 (left 55%, right 64%). These results are in agreement with previous MEG results (16). Opercular (OP) and insular regions were active in both hemispheres, peaking at 50% in OP1 (17). Responsive regions included mainly OP1, OP2, and the long gyri of insular cortex (LgI), with both LgI active in the right hemisphere and the posterior LgI active in the left hemisphere. OP3, OP4, as well as short gyri of the insula (SgI) were poorly responsive (Fig. 2 and Fig. S4). OP1 and OP4 [corresponding to areas S2 (secondary somatosensory area) and PV (parietal ventral area), as defined by Disbrow et al. (18)] are the main parts of classically defined SII.
Fig. S4.
Responsive leads. (A and B) Midthickness surface of the fs_LR brain template with all leads located in gray matter of left (49 patients) (A) and right (58 patients) (B) hemisphere. Red dots indicate responsive leads, and black dots nonresponsive ones. The same conventions as in Fig. 1 are used.
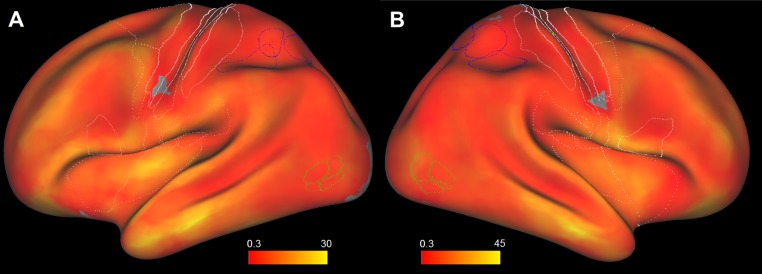
Fig. 2.
Overall responsiveness maps. Overall responsiveness (responsive leads as a percentage of total explored leads per disk) maps for the left (A) and right (B) hemispheres. Only surface nodes with values exceeding 10% were shown. The same conventions as in Fig. 1 are used.
High proportions of responsive leads were observed in the motor system, including the primary motor area (maximum values around 85%), large sectors of dorsal and ventral premotor cortex, and SMA. Anterior intraparietal sulcus was reliably responsive in both hemispheres, approaching 60% in its most rostral extent. Further posterior parietal cortex activations were biased in favor of the left hemisphere in supramarginal gyrus and in a region located between OP4 and area 44 resembling parietal rostroventral (PR) area, described by Disbrow et al. (19) in the monkey. This area may correspond to OP6 of Amunts and coworkers (20, 21). Interestingly, a weak but reliable responsiveness (around 20%) was found in right middle temporal (MT) cluster (22), extending dorsally into middle temporal gyrus (MTG). This activation partially overlaps with hOc5 (23).
To rule out the strong responsiveness of motor and premotor areas being due to the intensity of stimulation, just above motor threshold, we computed overall responsiveness for a second set of recordings, obtained in 70 patients, in which the intensity was 20% below the motor threshold. No difference in the proportions of active leads (overall responsiveness >10%) of sensory (BA2) and motor (BA4) areas were found between the supra- and subthreshold data in the left hemisphere (χ2 = 3.97; not significant). A significant difference (after correction for two comparisons) was found in the right hemisphere (χ2 = 14.75; P < 0.01), but the proportion of active leads decreased more in BA2 than in BA4 (Table S1) with subthreshold stimulation.
Table S1.
Reduction in responsiveness of different areas of left and right hemisphere for submotor threshold compared with supramotor threshold electrical stimulation
| Area | Left hemisphere | Right hemisphere | ||||||
| Area size, no. of nodes | Responsive Supra, % | Responsive Sub, % | Difference, % | Area size, no. of nodes | Responsive Supra, % | Responsive Sub, % | Difference, % | |
| 1 | 1,211 | 91.4 | 77.4 | −14.0 | 1,260 | 86.7 | 89.4 | 2.6 |
| 2 | 3,020 | 94.3 | 41.8 | −52.5 | 2,674 | 84.6 | 47.7 | −36.9 |
| 3a | 1,481 | 64.3 | 36.7 | −27.5 | 1,505 | 68.1 | 54.3 | −13.8 |
| 3b | 2,385 | 66.8 | 46.7 | −20.0 | 2,003 | 76.3 | 49.6 | −26.8 |
| 4 | 4,200 | 64.5 | 31.5 | −33.0 | 4,131 | 63.8 | 43.0 | −20.7 |
| LgI | 1,491 | 30.9 | 1.2 | −29.7 | 1,690 | 67.2 | 20.8 | −46.4 |
| MST | 328 | 0.0 | 0.0 | 0.0 | 279 | 57.3 | 0.0 | −57.3 |
| MT | 222 | 0.0 | 0.0 | 0.0 | 372 | 0.0 | 0.0 | 0.0 |
| OP1 | 2,394 | 90.1 | 70.0 | −20.1 | 2,397 | 97.5 | 34.0 | −63.5 |
| OP2 | 324 | 99.4 | 75.0 | −24.4 | 326 | 100.0 | 99.1 | −0.9 |
| OP3 | 551 | 37.7 | 3.1 | −34.7 | 515 | 72.8 | 26.0 | −46.8 |
| OP4 | 865 | 1.5 | 0.2 | −1.3 | 868 | 48.5 | 4.4 | −44.1 |
| PMd | 3,941 | 65.1 | 34.6 | −30.4 | 4,414 | 55.8 | 31.3 | −24.5 |
| PMm | 2,693 | 29.3 | 14.2 | −15.0 | 2,291 | 35.6 | 22.9 | −12.7 |
| PMv | 961 | 71.3 | 2.8 | −68.5 | 851 | 75.0 | 59.6 | −15.4 |
| phAIP | 1,707 | 82.2 | 6.3 | −76.0 | 1,476 | 71.1 | 0.0 | −71.1 |
Area size is indicated as no. of nodes. PMm, medial premotor area; Sub, submotor threshold; Supra, supramotor threshold.
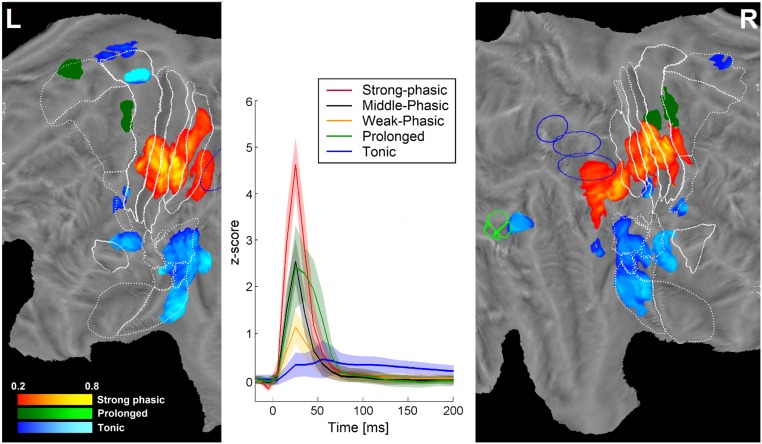
Clustering (k means) the time course of gamma band activity (Table S2) over all responsive leads indicated five to be the optimal number of clusters (average silhouette value: 0.448). The centroids obtained for each cluster (Fig. 3B) depicted three synchronous patterns (strong-, middle-, and weak-phasic), characterized by a steep rise after stimulus delivery, a peak in the third time bin (20–30 ms) and a return to baseline level within 50 ms from stimulation. A prolonged cluster (green line) exhibited a similar time course in the rising phase, but persisted two time bins (20 ms) longer than phasic clusters. A completely different pattern was shown by a tonic cluster (blue line) lasting more than 200 ms, whose peak was consistently lower and later than all other clusters. Quality of the clustering was indicated by the small percentage of negative silhouette values (less than 5% of leads, most of them belonging to middle-phasic and prolonged clusters, whose peak amplitudes were very similar). These leads were excluded from subsequent cluster-related analyses.
Table S2.
Characteristics of the five clusters
| Cluster | No. of leads | Average silhouette | SD silhouette | Negative silhouette | Negative, % | Gamma power peak | Peak timing, ms |
| Strong-phasic | 163 | 0.537 | 0.245 | 7 | 4.3 | 4.41 | 20–30 |
| Middle-phasic | 235 | 0.463 | 0.223 | 12 | 5.1 | 2.43 | 20–30 |
| Weak-phasic | 359 | 0.496 | 0.160 | 0 | 0.0 | 1.06 | 20–30 |
| Prolonged | 83 | 0.073 | 0.269 | 16 | 19.3 | 2.26 | 20–30 |
| Tonic | 306 | 0.435 | 0.221 | 16 | 5.2 | 0.31 | 50–60 |
Fig. 3.
Clustering of time courses. (Center) Time courses (centroid ± SD) of the five clusters, as indicated (Inset). (Left and Right) Relative responsiveness (leads belonging to one cluster as a percentage of total number of responsive leads per disk) maps of left (L) and right (R) hemispheres (middle portion) for strong-phasic (yellow red), delayed-phasic (green), and tonic (blue) clusters. Only nodes with values exceeding 20% are shown. The same conventions as in Fig. 1 are used.
The relative responsiveness (percentage of responsive leads belonging to a cluster) maps showed a remarkable topographical distribution across the two hemispheres. As shown in Fig. 3 A–C, the strong phasic cluster (see red palette) covered the middle strip of primary somatosensory areas (peaks of 81% in left 3b and 70% in right 3a), primary motor area and anterior part of inferior parietal cortex, corresponding to the caudal sectors of PF, PFt, and PFm (24, 25). The prolonged cluster (green palette) was restricted to a small sector of dorsal premotor area bilaterally (both reaching 33%), area 4 in the right hemisphere and SMA in the left hemisphere. The tonic cluster (see blue palette) was highly specific for secondary somatosensory areas (OP1 and OP2) and long gyri of insular cortex (peaks around 80% and 90%). Also, activity in human PR, ventral premotor cortex (bilaterally), and MT cluster (right hemisphere) exhibited a tonic time course. Middle- and weak-phasic clusters (Fig. S5) showed less focal distributions, instead spreading centrifugally from the primary sensory areas with the middle-phasic cluster confined to closer areas and the weak-phasic cluster to more distant ones. Of note, these two clusters largely avoided the regions belonging to the three focal clusters.
Fig. S5.
Relative responsiveness maps of middle (A and B) and weak-phasic (C and D) clusters on flat maps of the left (A and C) and right (B and D) hemispheres. Color coding is as in Fig. 3. The yellow frame indicates weak-phasic, and the black frame indicates middle-phasic.
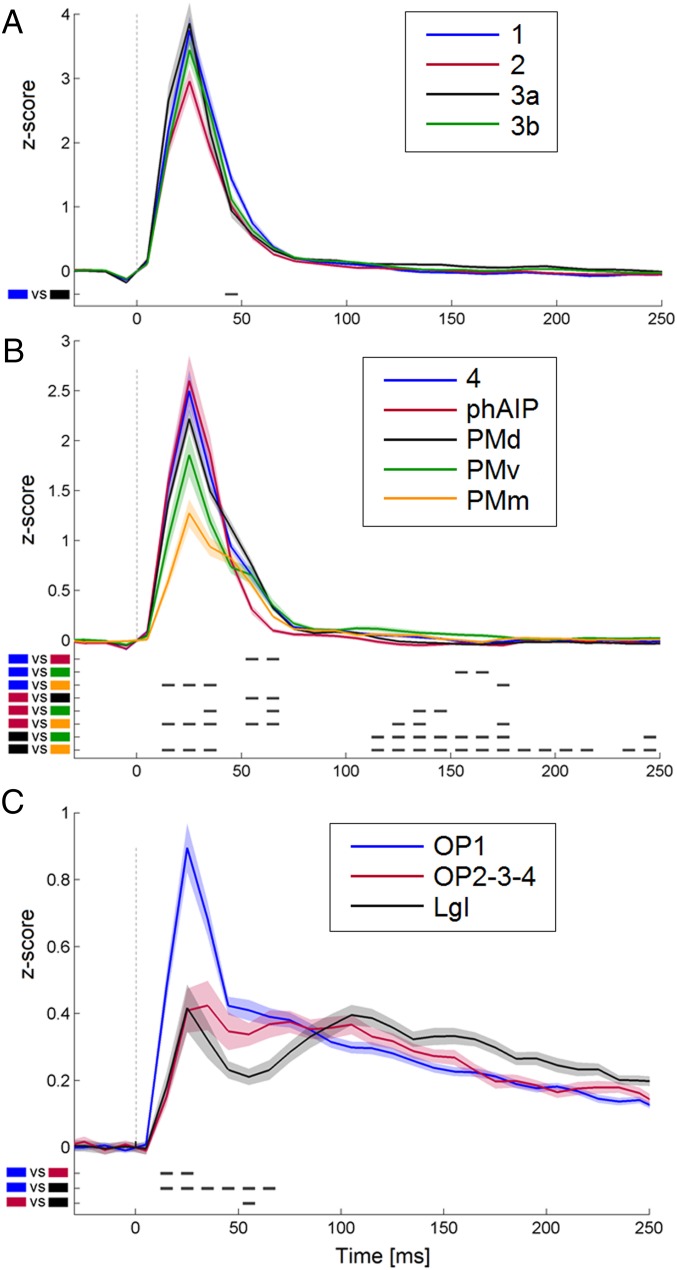
The region of interest (ROI) analysis confirmed that all four primary somatosensory areas are characterized by a strong-phasic time course, with areas 3a, 3b, and 1 similar in terms of peak amplitude (Fig. 4A). A repeated-measures ANOVA returned significant main effects for both time and ROIs, and a significant ROI-by-time interaction [F(3,177) = 2.787; P < 0.0001]. However, post hoc comparisons did not reveal any consistent difference across ROIs. The same analysis conducted on areas of the motor system (Fig. 4B) showed significant main effects for both time and ROIs, and a significant ROI-by-time interaction [F(4,236) = 6.545; P < 0.0001]. Interestingly, post hoc analysis identified three time intervals differentiating the motor ROIs: (i) around the peak response (synchronous for all ROIs), the medial premotor area shows significantly lower power values than area 4, putative human anterior intraparietal area (phAIP), and dorsal premotor area (PMd); (ii) in the 50- to 70-ms interval, phAIP shows no sustained power, in contrast to all other ROIs, with PMd presenting the highest values; (iii) between 100 and 200 ms, power in the ventral premotor area (PMv) and the medial premotor area significantly exceeded that of PMd, probably reflecting the presence of tonic cluster activity (Fig. 3). A repeated-measures ANOVA run on secondary somatosensory areas returned a significant ROI-by-time interaction [F(2,118) = 4.367; P < 0.0001]. Post hoc analysis showed a significant difference between OP1 and long gyri of insula, and between OP1 and other OPs combined in the 20- to 30-ms period, with OP1 presenting a stronger phasic response relative to other ROIs. In a late interval (100–200 ms), however, gamma power recorded from LgI exceeded that in opercular areas.
Fig. 4.
ROI analysis. Average (±SE) time course of leads in somatosensory areas (A), motor regions (B), and opercular/insular areas (C). Areas are listed (Insets). Black marks under the curves indicate significant post hoc comparisons (P < 0.002). Number of responsive leads for each graph: 34 in area 1, 54 in area 2, 21 in area 3a, 50 in area 3b (A); 68 in area 4, 157 in PMd, 45 in medial premotor areas, 42 in PMv, and 28 in phAIP (B); and 133 in OP1, 54 in other OPs, and 38 in LgI (C).
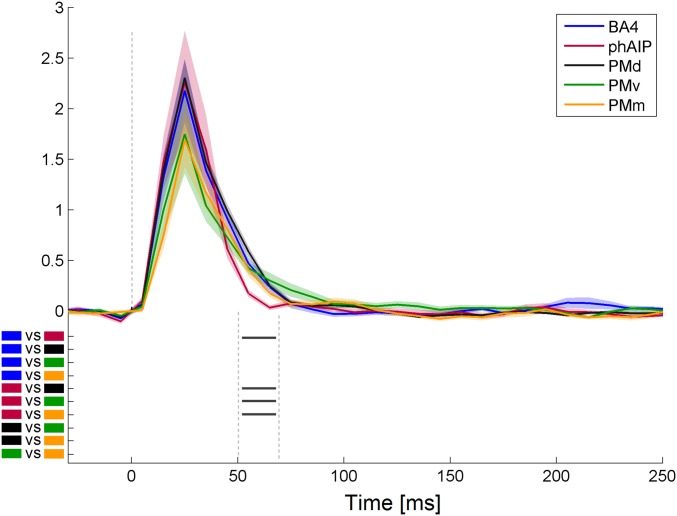
The motor temporal pattern was tested also for the subthreshold dataset, with the specific aim of validating that the sustained behavior of PMd between 50 and 70 ms was unrelated to thumb twitch induced by the median nerve stimulation. The ROI-by-time interaction again reached significance [F(4,236) = 1.294; P < 0.005] with subthreshold stimulation. Post hoc analysis, comparing mean gamma power across ROIs in the 50- to 70-ms time window confirmed the prolonged activation in motor and premotor areas relative to phAIP (Fig. S6).
Fig. S6.
Subthreshold results. Average (±SE) time course of leads in motor areas (Inset) following submotor threshold stimulation of median nerve. Black marks under the curves indicate significant post hoc comparisons (P < 0.02) for the average gamma power between 50 and 70 ms.
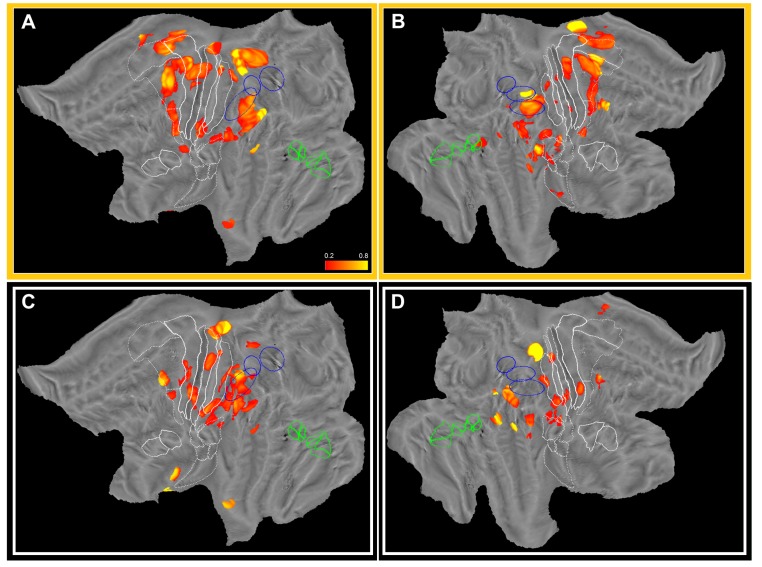
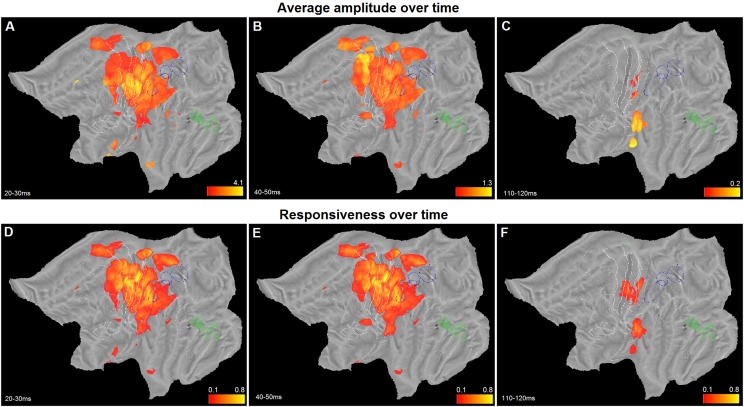
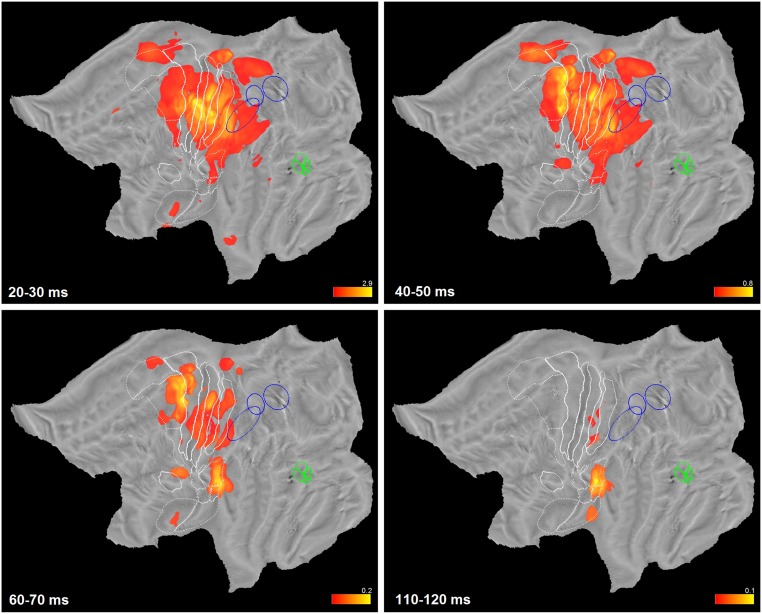
Clustering and ROI analyses have provided valuable insights into the dynamics of hand somatosensory processing. Both analyses have limitations because the first lumps large numbers of leads together, and the second blends leads selected on a priori anatomical criteria. To provide data-driven evidence, we applied map computation directly to gamma power amplitude at each time bin. In this way, we constructed a temporal sequence of maps, one every 10 ms, depicting the distribution of average amplitude (considering only responsive leads, see Fig. S7 A–C) over cortex. To complement this information, we time-resolved the overall responsiveness maps (Fig. 2) to achieve time dependent responsiveness maps (Fig. S7 D–F). Both datasets contain distinct and independent information, related to variations in time of strength and reliability of response. Hence, we combined them by means of a pointwise multiplication, thus obtaining weighted amplitude maps over time. Because this operation is performed for each time-bin, a movie that links together the entire sequence visualizes the 4-dimensional dynamics of the cortical activity in left and right hemispheres (Movies S1–S4). By inspecting four frames of such movie (Fig. 5) one can easily see: (i) the prevalence of the strong phasic response in area 3b, 3a and 1 at 20–30 ms (Fig. 5A); (ii) the marked involvement of posterior part of dorsal premotor cortex visible around 40–50 ms after stimulation (Fig. 5B); (iii) the dominance of premotor cortex and OP1 at 60–70 ms after stimulation, when primary sensory areas are already on the decrease (Fig. 5C); and, finally, (iv) the restriction of late activity (more than 100 ms following the onset of the electrical stimulation) to OP1, OP2 and LgI.
Fig. S7.
Average amplitude and responsiveness over time. Three average amplitude (A–C) and responsiveness (D–F) maps of the left hemisphere at time bins indicated. The color range for average amplitude is dynamically adjusted from frame to frame. The same conventions as in Fig. 1 are used.
Fig. 5.
Weighted amplitude maps over time. Four weighted amplitude maps of the left hemisphere at time bins indicated. The color range is dynamically adjusted from frame to frame. Note how the activity peaks in primary sensory areas in first frame, spreads to dorsal premotor area (second frame), remains active longer (third frame), whereas residual activity after 100 ms is recorded mostly from parietal operculum (OP1) and long gyri of insula (last frame). The same conventions as in Fig. 1 are used.
Discussion
In the present study, by localizing intracerebral electrodes in individual hemispheres, assessing the gamma broadband activity of all recording leads, and mapping the results to a common template, we obtained a comprehensive four-dimensional cartography of the cortical processing of median nerve stimulation. Beyond its neuroscientific relevance, this knowledge may allow a more careful interpretation of the sEEG activity and a better design of the sEEG implantations based on the ictal clinical semiology.
This result was achieved thanks to the group analysis of intracranial recordings from a large number of patients. Similar attempts to solve the sparse-sampling problem have been recently made using ECoG (26–31). However, although useful for investigating activity from areas near the crowns of gyri, such recordings are blind to neuronal activity from deep regions (7). It is worth noting that half of the leads recorded in the present study by sEEG were localized in sulci, including major sulci for somatosensory processing like the sylvian and the rolandic fissures. Previous sEEG studies (32–35) reported maps derived from population analyses, but the limited number of patients prevented them from obtaining the full picture of cortical processing.
High-frequency broadband gamma activity (50–150 Hz) was extracted from sEEG as an index of cortical activity, being spatially and functionally more specific than other frequency bands and presumably reflecting population spiking activity (36, 37), and thus allowing networks to be investigated at millisecond time-scales (33). A comparison between gamma time course and ERP responses from the same leads is presented in Figs. S8 and S9. To obtain a precise spatial localization for continuous maps, we minimized the circular kernel size by using the geodesic distance (38) and logistic weighting function. Of note, masks centered at 1.5-cm geodesic distance were completely independent, having no shared nodes.
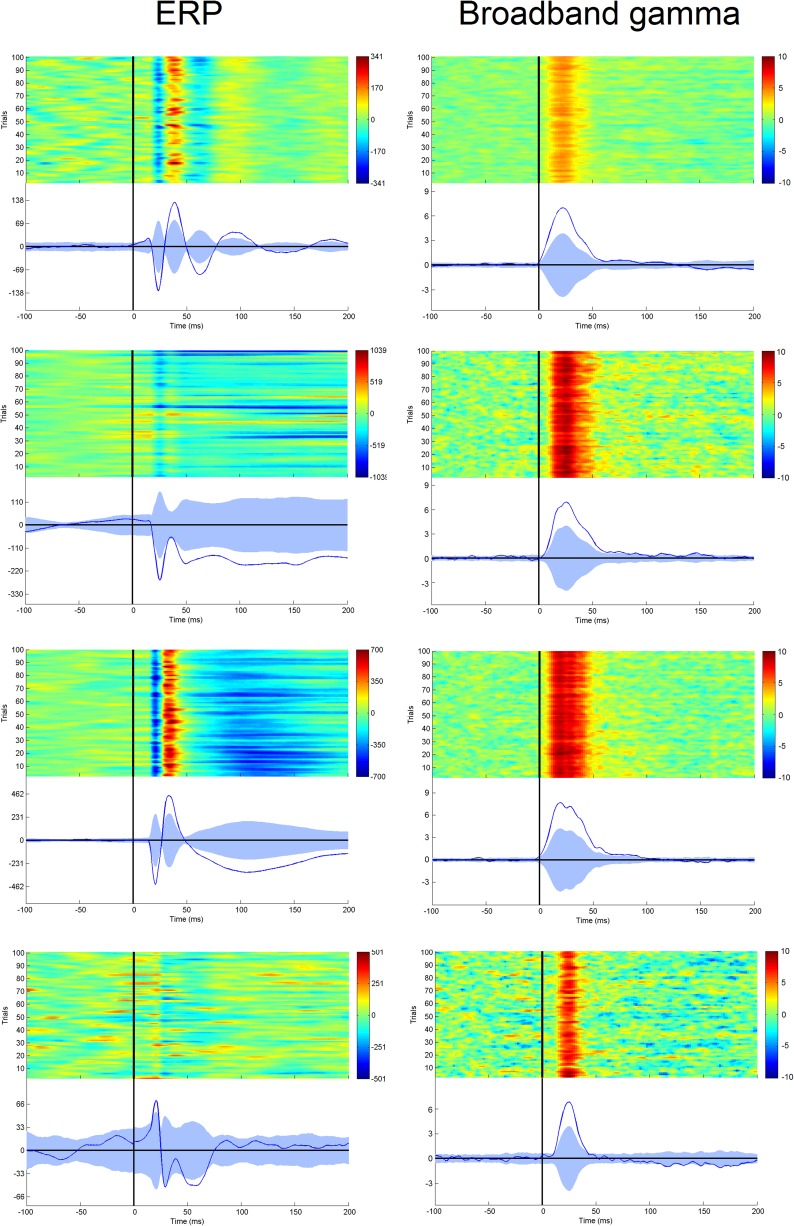
Fig. S8.
Comparison of intracranial ERP (left column) and broadband gamma (right column). Responses in four leads (four different patients) recording from area 3b. The color scale indicates voltage and z scores, respectively.
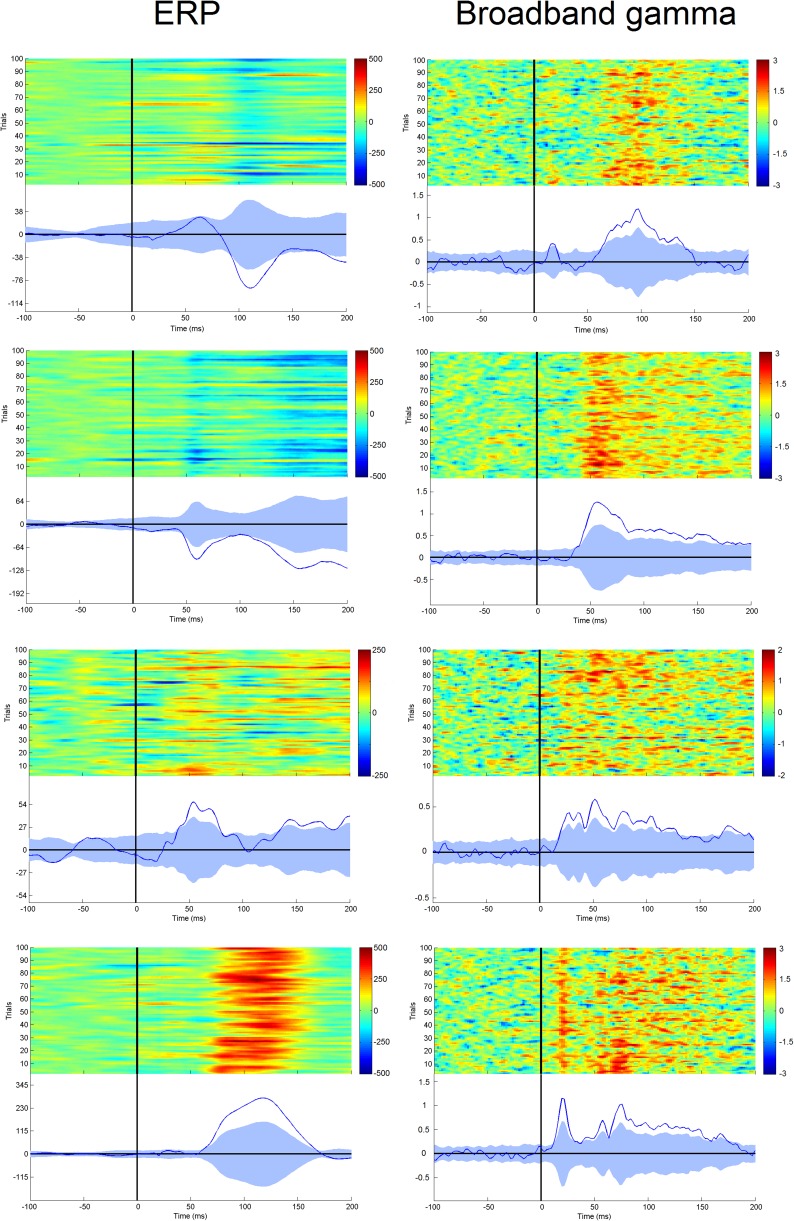
Fig. S9.
Comparison of intracranial ERP (left column) and broadband gamma (right column). Responses in four leads (four different patients) recording from area OP1. The color scale indicates voltage and z scores, respectively. Note that two of the four leads (first and last rows) exhibit a mixed pattern, composed of a phasic activity followed by a tonic one. Although this pattern is poorly visible in the ERP response, it appears evidently in the gamma band time course.
Responsiveness Maps Document the Wide Extent of the Cortical Somatosensory Network.
The overall responsiveness (Fig. 2) and weighted amplitude maps (Fig. 5) reveal the cortical network involved in processing somatosensory stimuli and document its time course. This network encompasses several areas the involvement of which remained controversial so far. Indeed, beside primary somatosensory cortices and SII/insula region (39), we revealed the activation of motor and premotor cortex (13), SMA (10, 40), superior and inferior parietal lobules (12, 41, 42). Thus, despite the very stringent response criteria we used, computed maps indicate a widespread network (over 10% of the surface in both hemispheres) devoted to processing median nerve input. This result is even more notable given that the present report is limited to stimulation of only one of the three nerves innervating the hand.
In addition, two additional regions were identified as responding to median nerve stimulation. The first is located in both hemispheres in the same sector of parieto-ventral region that has been called human PR (19). This area appears to be the counterpart of monkey PR, which is connected to IPL, area 4, and premotor cortex (19, 43). Disbrow et al. (18) reported that human PR responded to passive tactile stimulation, although in only 60% of tested participants. Investigating the temporal relationship between areas S2 and PR using MEG, Hinkley et al. (44) concluded that both S2 and PR contribute to the somatomotor integration needed for manual exploration and object discrimination, with S2 activity preceding that of PR. The tonic time course observed in human PR in the present study supports the involvement of this region in these discriminative functions.
The second site was revealed by leads exploring the ventral medial–superior–temporal area (pMSTv), part of the MT cluster (22), and neighboring posterior MTG in the right hemisphere (Fig. 2B). This region is classically considered involved in high-order visual processing, whereas its contribution to somatosensory processing is controversial. van Kemenade et al. (45) reported that different neuronal populations in human middle temporal (hMT)+/V5 process tactile and visual motion information. In contrast, Jiang et al. (46) reported only a weak (2% of voxels) response to tactile stimulation. Given the high sensitivity of intracranial recordings, we were able to show that indeed somatosensory information reaches pMSTv, suggesting a possible integration between tactile and visual information (47). If pMSTv functions in the same capacity as its monkey counterpart, this integration may serve to control tracking of moving objects not only with eyes, as described by Newsome et al. (48), but also with arms (49).
Four-Dimensional Maps Provide Previously Unidentified Information About Interareal Dynamics.
There is an ongoing debate of whether SI and SII operate serially or in parallel (14, 15). Both views are compatible with anatomical findings demonstrating that SI is connected to SII via cortico–cortical connections (50, 51) and that various thalamic nuclei project in parallel to SI and SII (52). Neuroimaging studies addressed this issue using causal modeling approach, but with mixed results: Kalberlah et al. (53) and Khoshnejad et al. (54) supported serial processing, whereas Chung et al. (55) and Liang et al. (56) the parallel model. Even intracranial studies yielded conflicting results. Barba et al. (57) reported a negative ERP peaking at 30 ms in parietal operculum following the first negative component occurring at 20 ms waveform, thus favoring the serial interpretation. On the contrary, Karhu and Tesche (58) observed synchronous neuronal population activity in contralateral SII area 20–30 ms after stimulation, coinciding in time with the first responses generated in SI, thus supporting the parallel hypothesis.
In the present study, the phasic component in OP1 coincided in time with the phasic activity in SI, thus favoring the view of a parallel propagation of sensory information to the two areas. However, because of the 10-ms time bin of our analysis, this conclusion should be taken with caution. Interestingly, our findings distinguished different patterns of activation in peri-sylvian areas, with OP1 presenting the strongest phasic component followed by a tonic pattern, and OP2, OP3, and OP4 exhibiting mainly tonic activity (Fig. 4C). The relative timing of phasic and tonic activities in operculoinsular areas is consistent with previous MEG data (59, 60). Hence, OP1 appears to play the role of a hub dispatching information to neighboring opercular cortices (61). To settle this point, further studies are needed using for example functional connectivity techniques like cortico–cortical evoked potentials (62).
Finally, our data showed that insular cortex was consistently activated by median nerve stimulation and presented a markedly tonic time course (Fig. 3). This activation was limited to the posterior part of insula, anatomically corresponding to the insular long gyri. This finding is in agreement with the metaanalysis by Kurth et al. (63), which demonstrated that this part of the insula is a specific sector related to somatosensory processing. Our finding of a tonic activity of this sector may be related to the involvement of posterior insula in the integration of somatosensory inputs with other sensory modalities (64).
Materials and Methods
Participants.
Stereo-EEG data were collected from 99 patients (52 male, 47 female) suffering from drug-resistant focal epilepsy. Only patients presenting with no anatomical alterations (n = 78) or with small abnormalities outside of the sensorimotor areas (n = 21), as evident on MR, were included. Fifteen of the patients with positive MR showed alterations of the temporal lobe [4 hippocampal sclerosis, 4 minimal periventricular nodular heterotopia, 2 cavernomas of temporal pole, 2 focal cortical dysplasia (FCDII), and 3 post ischemic injuries]. Four patients presented alteration of the posterior parietal lobe (no overlap with active ROIs reported in the study), of whom, two patients had FCDII, one had cavernoma, and one had post-ischemic injury. Finally, one patient presented a FCDII of the occipital lobe, and one patient had an anterior periventricular nodular heterotopia.
These patients were stereotactically implanted with intracerebral electrodes as part of their presurgical evaluation, at the Centro per la chirurgia dell’Epilessia “Claudio Munari.” Implantation sites were selected solely on clinical grounds, using seizure semiology, scalp-EEG, and neuroimaging as guide. Patients were fully informed regarding the electrode implantation and sEEG recordings. The present study received the approval of the Ethics Committee of Ospedale Ca’Granda-Niguarda (ID 939-2.12.2013) and informed consent was obtained. Intracerebral recordings were performed according to sEEG methodology to define the cerebral structures involved in the onset and propagation of seizure activity (65, 66). No seizure occurred, no alterations in the sleep/wake cycle were observed, and no additional pharmacological treatments were applied during the 24 h before the experimental recording. Neurological examination was unremarkable in all cases; in particular, no motor or sensory deficit was found in any patient.
Electrode Implantation.
Most implantations were unilateral, because clinical evidence generally indicates the hemisphere generating the seizures. Only 8 of the 99 patients were implanted bilaterally, resulting in a total of 107 implanted hemispheres. A number of depth electrodes (range: 9–19; average: 13) were implanted in different regions of the hemisphere using stereotactic coordinates. Each cylindrical electrode had a diameter of 0.8 mm and consisted of eight to eighteen 2-mm-long contacts (leads), spaced 1.5 mm apart (DIXI Medical, Besancon, France).
Immediately after the implantation, cone-beam computed tomography (CBCT) was obtained with the O-arm scanner (Medtronic) and registered to preimplantation 3D T1-weighted MR images. Subsequently, multimodal scenes were built with the 3D Slicer software package (67), and the exact position of each lead was determined, at the single patient level, looking at multiplanar reconstructions (68). Following clinical conventions, all leads are identified by a letter corresponding to the electrode shaft, followed by a number starting from the tip of the electrode.
Median Nerve Stimulation.
The day after the implantation, patients were admitted to the neurology ward, to undergo clinical and neuropsychological tests to functionally characterize the recording leads. To map leads involved in processing hand somatosensory information, a median nerve stimulation test was administered to the patients lying in bed with the eyes closed. The median nerve opposite to the recorded hemisphere was stimulated at the wrist, using 100 constant-current pulses (0.2-ms duration) at 1 Hz. The intensity and exact site of stimulation were varied until an observable thumb twitch was obtained. The motor threshold in our sample ranged from 3.2 to 5.8 mA. The stimulation intensity was set at 10% above the motor threshold. As a control, most of the patients (n = 70) were also tested with a stimulation intensity 20% below the motor threshold.
Anatomical Reconstruction of Electrodes.
The aim of this computational stage was to precisely locate the recording leads in individual cortical surfaces using the multimodal explorations performed in each patient, and import these locations into a common template. Each patient underwent a structural MRI (Achieva; Philips Medical Systems) before electrodes implantation. The T1 images were segmented using FreeSurfer software (69) to identify the pial and the white matter surfaces for the native mesh of each patient. The quality of segmentation was verified by visual inspection of the resulting surfaces. The midthickness surface, i.e., the average surface lying between the pial and white matter surfaces, was extracted. Moreover, starting from the pial surface, the sulci pattern was extracted through a procedure that evaluates the normalized geometric depth of each point [folding index 70)]. The more negative this parameter, the deeper the point is buried in a sulcus. After the implantation, each patient underwent a volumetric brain CT, to locate precisely the recording leads using the artifacts generated on CT images (Fig. 1B). The MRI and CT datasets were coregistered [FMRIB's linear image registration tool (FLIRT), 6 df, mutual information (71)] to get the anatomical brain data and the implanted electrodes into the same coordinate space. Thresholding of the CT signal intensity was used to segregate the recording leads (Fig. 1B) and to reconstruct their position in the CT volume. During this process, the knowledge of the number of implanted electrodes, the number of leads on each electrode, and the electrodes' entry and target points were used as constraints to ensure a reliable reconstruction.
Subtracting white-matter from pial surfaces yielded a ribbon surface corresponding to the gray matter thickness. Because the spatial resolution of CT images exceeded that of the MRI imaging and the CT images were reduced to binary information (1: presence of a recording lead; 0: absence of it), the ribbon was oversampled at a resolution of 0.4 mm in all directions. The intersection of CT images with the ribbon surface detected the contact points located in the cortical gray matter (Fig. 1B, red dots). Thus, at this stage, a lead located in the gray matter is represented by the number of 0.064-mm3 voxels it shares with the gray matter. For those leads in the gray matter, the centroid of the artifact was computed and projected onto the nearest nodes of the midthickness native surface, thus obtaining a single surface node representing the projection of a lead located in gray matter.
Finally, the individual midthickness surface was resampled to match the number of nodes (163.842) of the template (Fs-LR-average) and coregistered with this template using Freesurfer_to_fs_LR pipeline (brainvis.wustl.edu/wiki/index.php/Caret:Download). In this step, single-lead representations were enhanced by arbitrarily adding six nodes (on the hexagonal mesh) surrounding the original single nodes on the native surfaces, to ensure that all leads in the individual surfaces were maintained on the template surface. To quantify the goodness of fit between the native meshes and the template, we computed two local indices [i.e., deformation and distortion, evaluated for each node of the mesh (72)], indexing the shrinkage and translation of each node during the transformation. These indices were used to calculate the surface area of nodes in a group taking into account individual cortical surface area.
In conclusion, the recording leads located in the gray matter are represented in three different formats in the reconstruction procedure: as the number of 0.064-mm3 native voxels and as a fixed number (7) of nodes in the native and in the template meshes. Whereas the first format provides the precise location in the cortical depth, the latter two provide precise information about location in the cortical surface (at midthickness).
SEEG Data Recording and Processing.
For each implanted patient, the initial recording procedure included the selection of an intracranial reference, which was chosen by clinicians using both anatomical and functional criteria. The reference was computed as the average of two adjacent leads both exploring white matter. These leads were selected time-by-time because they did not present any response to standard clinical stimulations, including somatosensory (median, tibial, and trigeminal nerves), visual (flash), and acoustical (click) stimulations. Nor did the leads’ electrical stimulation evoke any sensory and/or motor behavior. The sEEG trace was recorded with a Neurofax EEG-1100 (Nihon Kohden System) at 1-kHz sampling rate.
Clinicians visually inspected recordings to verify for ictal epileptic discharges (IEDs) during the stimulation protocol. In 87 patients, the ROIs were devoid of any IED. In the remaining 12 patients, sparse IEDs were recorded during the stimulation protocol. In these cases, however, false-positive responses are very unlikely because none of the patients included showed reflex IEDs, and thus IEDs were not synchronized to the stimulus. This absence was confirmed by visual inspection of the quality of the data averaged.
The recordings from all leads in the gray matter were filtered (band-pass: 0.015–500 Hz; notch: 50 Hz) to avoid aliasing effects and decomposed into time–frequency plots using complex Morlet’s wavelet decomposition. Power in the gamma (50- to 150-Hz) frequency band was extracted in a window extending from 100 ms before to 500 ms after the electrical stimulation, and subdivided into 60 nonoverlapping 10-ms bins. Following previous intracranial studies (35, 73, 74), gamma power was estimated for 10 adjacent nonoverlapping 10-Hz frequency bands.
To identify the leads responsive to median nerve stimulation, the gamma band power in each poststimulus bin was compared with baseline using a t test. Significance was Bonferroni corrected for 50 comparisons (P = 0.001), and to decrease the false-positive ratio, only leads with significant gamma increases in at least three time bins were designated as responsive. To normalize data across patients and leads, power in poststimulus bins was transformed into z scores relative to the prestimulus interval.
A k mean clustering was applied to the gamma band time course of the responsive leads to group them according to the time course of the response, independently of their location. Nineteen clustering procedures were performed imposing increasing numbers of clusters (3–20) and computing silhouette values (75) to evaluate clustering validity. The optimal clustering was defined by the maximal average silhouette value, and leads with negative silhouette values were not considered in the evaluation of the optimal clustering using a repeated-measurement ANOVA with time and cluster as factors. t tests (P < 0.001 to account for 50 time bins) were used to evaluate post hoc comparisons.
Finally, to compare cortical areas, leads were grouped by their location in a ROI-based analysis to evaluate differences among primary sensory areas (3a, 3b, 1, and 2), motor areas (4, medial premotor area, PMd, PMv, and phAIP), and the secondary somatosensory areas (OP1, OP2, OP3, OP4, and LgI). These ROIs were defined either by cytoarchitectonical or functional criteria (see Continuous Maps). For each of these groups, we performed a two-way repeated-measure ANOVA with time and area as factors. Significant interactions were explored in post hoc analysis with planned-comparisons t tests (P < 0.001 to account for the 50 time bins).
Continuous Maps.
To provide a continuous view of the topographic pattern of active leads, we built a circular mask based on the geodesic distance between two cortical points (76) (i.e., the minimum pathway within the gray matter connecting the source and the target nodes). For each cortical node, we defined the nodes within a 1-cm geodesic distance from the original node and weighted the contribution of each node by a sigmoid function. Node weight was defined as a logistic function with unitary amplitude, a steepness of 2 and a midpoint at 7.5 mm. As a result, each node of the cortical mesh was associated with a collection of surrounding nodes (averaging 806 and 813 nodes for the right and left hemispheres), with all nodes within 5 mm of the origin maximally weighted, whereas those between 5 and 10 mm were gradually reduced in weight, to avoid edge effects.
By this approach, we computed five different functional variables:
-
i)
Cortical sampling density [i.e., number of explored leads per cm2, using the fixed number (7) of nodes per lead and the average surface of a disk].
-
ii)
Overall responsiveness [i.e., the number of responsive nodes (Nr) as a percent of the number of explored nodes (Ne) within a disk]. Given the fixed number of nodes representing a lead (7), this variable is equivalent to the number of responsive leads as a percent of the number of explored leads within a disk. This time-independent variable provides an overall picture of the cortical responsiveness, ranging from 0% to 100%, directly comparable to neuroimaging studies. Data were thresholded at 10% so as to exclude the contribution of sparsely responsive regions.
-
iii)
Relative responsiveness [i.e., the number of nodes (leads) exhibiting a specific temporal response pattern in percent of the number of responsive nodes (leads) within a disk]. This variable indexes the degree to which an area responds with a specific temporal pattern, information to which neuroimaging studies are completely blind. Results were thresholded at a 1/N value, where N represents the number of clusters, to show only areas in which proportions of single clusters exceed chance.
-
iv)
The response amplitude for each time bin (i.e., the average z score across all responsive nodes in the disk). This variable indexes the strength of a response, regardless of how reliably it occurs within a disk. No minimum threshold was set for the z score, but results were masked for overall responsiveness exceeding 10%.
-
v)The weighted amplitude for each time bin, i.e., a combination of response amplitude and overall responsiveness, depicting the variation over the cortical surface of the response amplitude weighted by the responsiveness:
where Ne is the number of explored nodes (leads) and Nr is the number of responsive nodes (leads) in a disk. Computation was restricted to regions whose overall responsiveness was higher than 10%.
These maps were plotted using CARET software (77) (www.nitrc.org/projects/caret) and directly compared with retinotopic regions in the occipital cortex (72) to confidence ellipses of rostral intraparietal sulcus (78), the most rostral of which corresponds to phAIP, and to cytoarchitectonic sensorimotor regions (79), opercular areas (17), and area 44 (80). The subdivision between dorsal and ventral premotor areas was made according to Tomassini et al. (81).
Supplementary Material
Acknowledgments
The authors thank Dr. S. Raiguel for revising the English version of the manuscript. This study was supported by European Research Council (ERC) “Cogsystem” project no. 250013 (to G.R.) and ERC “Parietalaction” project no. 323606 (to G.A.O.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601889113/-/DCSupplemental.
References
- 1.Van Essen DC. Cartography and connectomes. Neuron. 2013;80(3):775–790. doi: 10.1016/j.neuron.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopell NJ, Gritton HJ, Whittington MA, Kramer MA. Beyond the connectome: The dynome. Neuron. 2014;83(6):1319–1328. doi: 10.1016/j.neuron.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hari R, Parkkonen L. The brain timewise: How timing shapes and supports brain function. Philos Trans R Soc Lond B Biol Sci. 2015;370(1668):pii: 20140170. doi: 10.1098/rstb.2014.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burle B, et al. Spatial and temporal resolutions of EEG: Is it really black and white? A scalp current density view. Int J Psychophysiol. 2015;97(3):210–220. doi: 10.1016/j.ijpsycho.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debener S, Ullsperger M, Siegel M, Engel AK. Single-trial EEG-fMRI reveals the dynamics of cognitive function. Trends Cogn Sci. 2006;10(12):558–563. doi: 10.1016/j.tics.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Cardinale F, Lo Russo G. Stereo-electroencephalography safety and effectiveness: Some more reasons in favor of epilepsy surgery. Epilepsia. 2013;54(8):1505–1506. doi: 10.1111/epi.12222. [DOI] [PubMed] [Google Scholar]
- 7.Noy N, et al. Intracranial recordings reveal transient response dynamics during information maintenance in human cerebral cortex. Hum Brain Mapp. 2015;36(10):3988–4003. doi: 10.1002/hbm.22892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitmer D, Worrell G, Stead M, Lee IK, Makeig S. Utility of independent component analysis for interpretation of intracranial EEG. Front Hum Neurosci. 2010;4:184. doi: 10.3389/fnhum.2010.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferretti A, et al. Cortical brain responses during passive nonpainful median nerve stimulation at low frequencies (0.5-4 Hz): An fMRI study. Hum Brain Mapp. 2007;28(7):645–653. doi: 10.1002/hbm.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manganotti P, et al. Steady-state activation in somatosensory cortex after changes in stimulus rate during median nerve stimulation. Magn Reson Imaging. 2009;27(9):1175–1186. doi: 10.1016/j.mri.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Arienzo D, et al. Somatotopy of anterior cingulate cortex (ACC) and supplementary motor area (SMA) for electric stimulation of the median and tibial nerves: An fMRI study. Neuroimage. 2006;33(2):700–705. doi: 10.1016/j.neuroimage.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Ruben J, et al. Somatotopic organization of human secondary somatosensory cortex. Cereb Cortex. 2001;11(5):463–473. doi: 10.1093/cercor/11.5.463. [DOI] [PubMed] [Google Scholar]
- 13.Boakye M, Huckins SC, Szeverenyi NM, Taskey BI, Hodge CJ., Jr Functional magnetic resonance imaging of somatosensory cortex activity produced by electrical stimulation of the median nerve or tactile stimulation of the index finger. J Neurosurg. 2000;93(5):774–783. doi: 10.3171/jns.2000.93.5.0774. [DOI] [PubMed] [Google Scholar]
- 14.Rowe MJ, Turman AB, Murray GM, Zhang HQ. Parallel organization of somatosensory cortical areas I and II for tactile processing. Clin Exp Pharmacol Physiol. 1996;23(10-11):931–938. doi: 10.1111/j.1440-1681.1996.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 15.Forss N, Hietanen M, Salonen O, Hari R. Modified activation of somatosensory cortical network in patients with right-hemisphere stroke. Brain. 1999;122(Pt 10):1889–1899. doi: 10.1093/brain/122.10.1889. [DOI] [PubMed] [Google Scholar]
- 16.Kaukoranta E, Hämäläinen M, Sarvas J, Hari R. Mixed and sensory nerve stimulations activate different cytoarchitectonic areas in the human primary somatosensory cortex SI. Neuromagnetic recordings and statistical considerations. Exp Brain Res. 1986;63(1):60–66. doi: 10.1007/BF00235646. [DOI] [PubMed] [Google Scholar]
- 17.Eickhoff SB, Grefkes C, Zilles K, Fink GR. The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex. 2007;17(8):1800–1811. doi: 10.1093/cercor/bhl090. [DOI] [PubMed] [Google Scholar]
- 18.Disbrow E, Roberts T, Krubitzer L. Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: Evidence for SII and PV. J Comp Neurol. 2000;418(1):1–21. doi: 10.1002/(sici)1096-9861(20000228)418:1<1::aid-cne1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J Comp Neurol. 2003;462(4):382–399. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- 20.Amunts K, et al. Broca’s region: Novel organizational principles and multiple receptor mapping. PLoS Biol. 2010;8(9):e1000489. doi: 10.1371/journal.pbio.1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amunts K, Zilles K. Architecture and organizational principles of Broca’s region. Trends Cogn Sci. 2012;16(8):418–426. doi: 10.1016/j.tics.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Kolster H, Peeters R, Orban GA. The retinotopic organization of the human middle temporal area MT/V5 and its cortical neighbors. J Neurosci. 2010;30(29):9801–9820. doi: 10.1523/JNEUROSCI.2069-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malikovic A, et al. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: A probabilistic, stereotaxic map of area hOc5. Cereb Cortex. 2007;17(3):562–574. doi: 10.1093/cercor/bhj181. [DOI] [PubMed] [Google Scholar]
- 24.Caspers S, et al. The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33(2):430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 25.Caspers S, et al. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct. 2008;212(6):481–495. doi: 10.1007/s00429-008-0195-z. [DOI] [PubMed] [Google Scholar]
- 26.Miller KJ, et al. Real-time functional brain mapping using electrocorticography. Neuroimage. 2007;37(2):504–507. doi: 10.1016/j.neuroimage.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 27.Burke JF, et al. Synchronous and asynchronous theta and gamma activity during episodic memory formation. J Neurosci. 2013;33(1):292–304. doi: 10.1523/JNEUROSCI.2057-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conner CR, Chen G, Pieters TA, Tandon N. Category specific spatial dissociations of parallel processes underlying visual naming. Cereb Cortex. 2014;24(10):2741–2750. doi: 10.1093/cercor/bht130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidesco I, et al. Spatial and object-based attention modulates broadband high-frequency responses across the human visual cortical hierarchy. J Neurosci. 2013;33(3):1228–1240. doi: 10.1523/JNEUROSCI.3181-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dykstra AR, et al. Individualized localization and cortical surface-based registration of intracranial electrodes. Neuroimage. 2012;59(4):3563–3570. doi: 10.1016/j.neuroimage.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadipasaoglu CM, et al. Development of grouped icEEG for the study of cognitive processing. Front Psychol. 2015;6:1008. doi: 10.3389/fpsyg.2015.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juphard A, et al. Direct evidence for two different neural mechanisms for reading familiar and unfamiliar words: An intra-cerebral EEG study. Front Hum Neurosci. 2011;5:101. doi: 10.3389/fnhum.2011.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lachaux J-P, Axmacher N, Mormann F, Halgren E, Crone NE. High-frequency neural activity and human cognition: Past, present and possible future of intracranial EEG research. Prog Neurobiol. 2012;98(3):279–301. doi: 10.1016/j.pneurobio.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ossandón T, et al. Efficient “pop-out” visual search elicits sustained broadband γ activity in the dorsal attention network. J Neurosci. 2012;32(10):3414–3421. doi: 10.1523/JNEUROSCI.6048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidal JR, et al. Category-specific visual responses: An intracranial study comparing gamma, beta, alpha, and ERP response selectivity. Front Hum Neurosci. 2010;4:195. doi: 10.3389/fnhum.2010.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popivanov ID, Jastorff J, Vanduffel W, Vogels R. Heterogeneous single-unit selectivity in an fMRI-defined body-selective patch. J Neurosci. 2014;34(1):95–111. doi: 10.1523/JNEUROSCI.2748-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray S, Maunsell JHR. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 2011;9(4):e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadipasaoglu CM, et al. Surface-based mixed effects multilevel analysis of grouped human electrocorticography. Neuroimage. 2014;101:215–224. doi: 10.1016/j.neuroimage.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Kaas JH. Somatosensory System. In: Paxinos G, Mai JK, editors. The Human Nervous System. 2nd Ed. Elsevier Academic; New York: 2004. pp. 1059–1092. [Google Scholar]
- 40.Korvenoja A, et al. Activation of multiple cortical areas in response to somatosensory stimulation: Combined magnetoencephalographic and functional magnetic resonance imaging. Hum Brain Mapp. 1999;8(1):13–27. doi: 10.1002/(SICI)1097-0193(1999)8:1<13::AID-HBM2>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forss N, Salmelin R, Hari R. Comparison of somatosensory evoked fields to airpuff and electric stimuli. Electroencephalogr Clin Neurophysiol. 1994;92(6):510–517. doi: 10.1016/0168-5597(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 42.Gelnar PA, Krauss BR, Szeverenyi NM, Apkarian AV. Fingertip representation in the human somatosensory cortex: An fMRI study. Neuroimage. 1998;7(4 Pt 1):261–283. doi: 10.1006/nimg.1998.0341. [DOI] [PubMed] [Google Scholar]
- 43.Padberg J, Disbrow E, Krubitzer L. The organization and connections of anterior and posterior parietal cortex in titi monkeys: Do New World monkeys have an area 2? Cereb Cortex. 2005;15(12):1938–1963. doi: 10.1093/cercor/bhi071. [DOI] [PubMed] [Google Scholar]
- 44.Hinkley LB, Krubitzer LA, Nagarajan SS, Disbrow EA. Sensorimotor integration in S2, PV, and parietal rostroventral areas of the human sylvian fissure. J Neurophysiol. 2007;97(2):1288–1297. doi: 10.1152/jn.00733.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Kemenade BM, et al. Tactile and visual motion direction processing in hMT+/V5. Neuroimage. 2014;84:420–427. doi: 10.1016/j.neuroimage.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Jiang F, Beauchamp MS, Fine I. Re-examining overlap between tactile and visual motion responses within hMT+ and STS. Neuroimage. 2015;119:187–196. doi: 10.1016/j.neuroimage.2015.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricciardi E, Bonino D, Pellegrini S, Pietrini P. Mind the blind brain to understand the sighted one! Is there a supramodal cortical functional architecture? Neurosci Biobehav Rev. 2014;41:64–77. doi: 10.1016/j.neubiorev.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60(2):604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- 49.Ilg UJ, Schumann S. Primate area MST-l is involved in the generation of goal-directed eye and hand movements. J Neurophysiol. 2007;97(1):761–771. doi: 10.1152/jn.00278.2006. [DOI] [PubMed] [Google Scholar]
- 50.Jones EG, Powell TP. Connexions of the somatic sensory cortex of the rhesus monkey. I. Ipsilateral cortical connexions. Brain. 1969;92(3):477–502. doi: 10.1093/brain/92.3.477. [DOI] [PubMed] [Google Scholar]
- 51.Jones EG, Powell TP. Connexions of the somatic sensory cortex of the rhesus monkey. II. Contralateral cortical connexions. Brain. 1969;92(4):717–730. doi: 10.1093/brain/92.4.717. [DOI] [PubMed] [Google Scholar]
- 52.Almeida TF, Roizenblatt S, Tufik S. Afferent pain pathways: A neuroanatomical review. Brain Res. 2004;1000(1-2):40–56. doi: 10.1016/j.brainres.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 53.Kalberlah C, Villringer A, Pleger B. Dynamic causal modeling suggests serial processing of tactile vibratory stimuli in the human somatosensory cortex--an fMRI study. Neuroimage. 2013;74:164–171. doi: 10.1016/j.neuroimage.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 54.Khoshnejad M, Piché M, Saleh S, Duncan G, Rainville P. Serial processing in primary and secondary somatosensory cortex: A DCM analysis of human fMRI data in response to innocuous and noxious electrical stimulation. Neurosci Lett. 2014;577:83–88. doi: 10.1016/j.neulet.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 55.Chung YG, et al. Intra- and inter-hemispheric effective connectivity in the human somatosensory cortex during pressure stimulation. BMC Neurosci. 2014;15:43. doi: 10.1186/1471-2202-15-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang M, Mouraux A, Iannetti GD. Parallel processing of nociceptive and non-nociceptive somatosensory information in the human primary and secondary somatosensory cortices: Evidence from dynamic causal modeling of functional magnetic resonance imaging data. J Neurosci. 2011;31(24):8976–8985. doi: 10.1523/JNEUROSCI.6207-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barba C, Frot M, Mauguière F. Early secondary somatosensory area (SII) SEPs. Data from intracerebral recordings in humans. Clin Neurophysiol. 2002;113(11):1778–1786. doi: 10.1016/s1388-2457(02)00261-4. [DOI] [PubMed] [Google Scholar]
- 58.Karhu J, Tesche CD. Simultaneous early processing of sensory input in human primary (SI) and secondary (SII) somatosensory cortices. J Neurophysiol. 1999;81(5):2017–2025. doi: 10.1152/jn.1999.81.5.2017. [DOI] [PubMed] [Google Scholar]
- 59.Hari R, et al. Functional organization of the human first and second somatosensory cortices: A neuromagnetic study. Eur J Neurosci. 1993;5(6):724–734. doi: 10.1111/j.1460-9568.1993.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 60.Simões C, Jensen O, Parkkonen L, Hari R. Phase locking between human primary and secondary somatosensory cortices. Proc Natl Acad Sci USA. 2003;100(5):2691–2694. doi: 10.1073/pnas.0437944100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazzola L, Faillenot I, Barral F-G, Mauguière F, Peyron R. Spatial segregation of somato-sensory and pain activations in the human operculo-insular cortex. Neuroimage. 2012;60(1):409–418. doi: 10.1016/j.neuroimage.2011.12.072. [DOI] [PubMed] [Google Scholar]
- 62.David O, et al. Probabilistic functional tractography of the human cortex. Neuroimage. 2013;80:307–317. doi: 10.1016/j.neuroimage.2013.05.075. [DOI] [PubMed] [Google Scholar]
- 63.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214(5-6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 65.Munari C, et al. Stereo-electroencephalography methodology: Advantages and limits. Acta Neurol Scand Suppl. 1994;152(Suppl.c):56–67. doi: 10.1111/j.1600-0404.1994.tb05188.x. [DOI] [PubMed] [Google Scholar]
- 66.Cossu M, et al. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy: A retrospective analysis of 215 procedures. Neurosurgery. 2005;57(4):706–718. [PubMed] [Google Scholar]
- 67.Fedorov A, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 69.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Essen DC, Drury HA. Structural and functional analyses of human cerebral cortex using a surface-based atlas. J Neurosci. 1997;17(18):7079–7102. doi: 10.1523/JNEUROSCI.17-18-07079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 72.Abdollahi RO, et al. Correspondences between retinotopic areas and myelin maps in human visual cortex. Neuroimage. 2014;99:509–524. doi: 10.1016/j.neuroimage.2014.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caruana F, et al. Human cortical activity evoked by gaze shift observation: An intracranial EEG study. Hum Brain Mapp. 2014;35(4):1515–1528. doi: 10.1002/hbm.22270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caruana F, Sartori I, Lo Russo G, Avanzini P. Sequencing biological and physical events affects specific frequency bands within the human premotor cortex: An intracerebral EEG study. PLoS One. 2014;9(1):e86384. doi: 10.1371/journal.pone.0086384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rousseeuw PJ. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math. 1987;20:53–65. [Google Scholar]
- 76.Knutsen AK, et al. A new method to measure cortical growth in the developing brain. J Biomech Eng. 2010;132(10):101004. doi: 10.1115/1.4002430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Essen DC. Cortical cartography and Caret software. Neuroimage. 2012;62(2):757–764. doi: 10.1016/j.neuroimage.2011.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jastorff J, Begliomini C, Fabbri-Destro M, Rizzolatti G, Orban GA. Coding observed motor acts: Different organizational principles in the parietal and premotor cortex of humans. J Neurophysiol. 2010;104(1):128–140. doi: 10.1152/jn.00254.2010. [DOI] [PubMed] [Google Scholar]
- 79.Geyer S, Schormann T, Mohlberg H, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage. 2000;11(6 Pt 1):684–696. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- 80.Amunts K, et al. Broca’s region revisited: Cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412(2):319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 81.Tomassini V, et al. Diffusion-weighted imaging tractography-based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomical and functional specializations. J Neurosci. 2007;27(38):10259–10269. doi: 10.1523/JNEUROSCI.2144-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.