Significance
Depression is the most common and debilitating psychiatric disorder in the world. However, the precise mechanisms underlying depression remain largely unknown. Recent evidence suggests that soluble epoxide hydrolase (sEH) plays a key role in inflammation, which is involved in depression. The sEH inhibitor, TPPU, showed antidepressant effects in animal models of depression. Expression of sEH protein was increased in the brain of chronically stressed (susceptible) mice and depressed patients. Prophylactic sEH inhibition or sEH-KO resulted in resilience to repeated social defeat stress, associated with increased BDNF-TrkB signaling in prefrontal cortex and hippocampus of KO mice. This study shows that sEH plays a key role in the pathophysiology of depression, and that its inhibitors could be potential therapeutic drugs for depression.
Keywords: brain-derived neurotrophic factor, depression, epoxyeicosatrienoic acid, soluble epoxide hydrolase, resilience
Abstract
Depression is a severe and chronic psychiatric disease, affecting 350 million subjects worldwide. Although multiple antidepressants have been used in the treatment of depressive symptoms, their beneficial effects are limited. The soluble epoxide hydrolase (sEH) plays a key role in the inflammation that is involved in depression. Thus, we examined here the role of sEH in depression. In both inflammation and social defeat stress models of depression, a potent sEH inhibitor, TPPU, displayed rapid antidepressant effects. Expression of sEH protein in the brain from chronically stressed (susceptible) mice was higher than of control mice. Furthermore, expression of sEH protein in postmortem brain samples of patients with psychiatric diseases, including depression, bipolar disorder, and schizophrenia, was higher than controls. This finding suggests that increased sEH levels might be involved in the pathogenesis of certain psychiatric diseases. In support of this hypothesis, pretreatment with TPPU prevented the onset of depression-like behaviors after inflammation or repeated social defeat stress. Moreover, sEH KO mice did not show depression-like behavior after repeated social defeat stress, suggesting stress resilience. The sEH KO mice showed increased brain-derived neurotrophic factor (BDNF) and phosphorylation of its receptor TrkB in the prefrontal cortex, hippocampus, but not nucleus accumbens, suggesting that increased BDNF-TrkB signaling in the prefrontal cortex and hippocampus confer stress resilience. All of these findings suggest that sEH plays a key role in the pathophysiology of depression, and that epoxy fatty acids, their mimics, as well as sEH inhibitors could be potential therapeutic or prophylactic drugs for depression.
Depression is the most severe and debilitating of the psychiatric illnesses. The World Health Organization estimates that more than 350 million individuals of all ages suffer from depression (1). Almost one million lives are lost annually because of suicide, which translates to 3,000 deaths daily (1). Although antidepressants are generally effective in the treatment of depression, it can still take weeks before patients feel the full antidepressant effects. However, approximately two-thirds of depressed patients fail to respond fully to pharmacotherapy. Furthermore, there is a high rate of relapse, and depressed patients have a high risk of committing suicide (2–4).
Accumulating evidence suggests that inflammation plays a central role in the pathophysiology of depression (5–9). Meta-analyses showed higher blood levels of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6), in drug-free depressed patients compared with healthy controls (10–13). Studies using postmortem brain samples showed elevated gene expression of proinflammatory cytokines in the frontal cortex of people with a history of depression (14, 15). Taking these data together, we find that it is likely that both peripheral and central inflammations are associated with depression and that antiinflammatory drugs, such as cyclooxygenase inhibitors, could ameliorate depressive symptoms in depressed patients (16, 17).
Epoxyeicosatrienoic acids (EETs), which are produced from arachidonic acid by the action of cytochrome P450s, have potent antiinflammatory actions. These mediators are broken down into the corresponding diols by soluble epoxide hydrolase (sEH), and inhibition of sEH enhances the beneficial effects of EETs (18–21). It is also reported that sEH inhibitors have potent antiinflammatory effects in a number of animal models (18–20, 22, 23). Although sEH has been associated with the onset of anorexia nervosa (24), the role of sEH in the pathophysiology of depression has not been studied to date.
The purpose of this study was to examine the role of sEH in the pathophysiology of depression using a potent sEH inhibitor and sEH knockout (KO) mice. Furthermore, we examined the role of brain-derived neurotrophic factor (BDNF) and its receptor TrkB signaling in selected brain regions, because BDNF-TrkB signaling plays a key role in the pathophysiology of depression (25–30).
Results
TPPU and 14,15-EET Enhance Nerve Growth Factor-Induced Neurite Outgrowth.
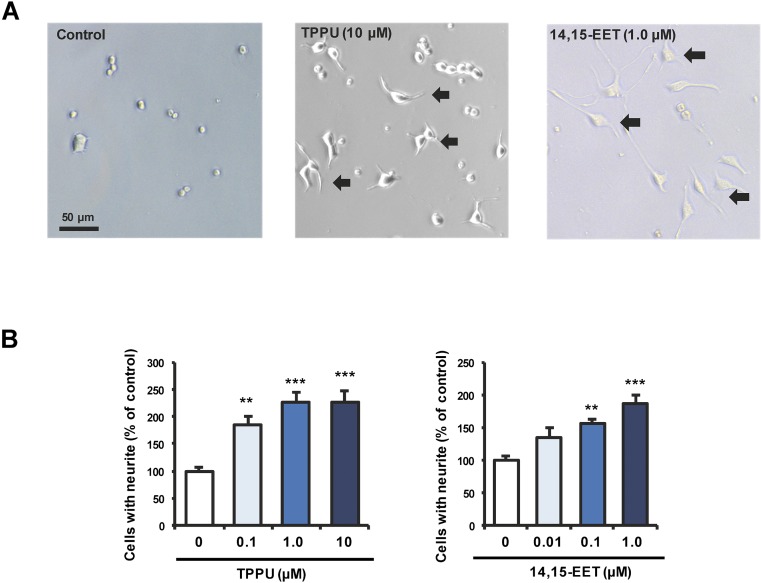
Because antidepressants are known to affect the neuronal plasticity, we examined the effects of 1-trifluoromethoxyphenyl-3-(1-propionylpiperidine-4-yl)urea (TPPU: a potent sEH inhibitor) (31–33) and the endogenous eicosanoid 14,15-EET on nerve growth factor (NGF)-induced neurite outgrowth in PC12 cells. Both TPPU and 14,15-EET potentiated NGF-induced neurite outgrowth in PC12 cells, in a concentration-dependent manner (Fig. S1). The 14,15-EET was shown to enhance axonal growth neuronal cell cultures (34). These findings suggest that TPPU and 14,15-EET can enhance neuronal plasticity, which is implicated in the action of antidepressants.
Fig. S1.
TPPU and 14,15-EET potentiated NGF-induced neurite outgrowth in PC12 cells. (A) Representative photomicrographs in PC12 cells. Control: NGF (2.5 ng/mL) alone; TPPU (10 μM): NGF (2.5 ng/mL) + TPPU (10 μM); 14,15-EET (1.0 μM): NGF (2.5 ng/mL) + 14,15-EET (1.0 μM). The arrow is the cells with neurite outgrowth. (Scale bar, 50 μm.) (B) Effects of TPPU and 14,15-EET on NGF-induced neurite outgrowth in PC12 cells. TPPU [0.1, 1.0, or 10 μM: one-way ANOVA, F(3,44) = 15.59, P < 0.001] and 14,15-EET [0.01, 0.1, or 1.0 μM; one-way ANOVA, F(3,38) = 12.69, P < 0.001] potentiated NGF-induced neurite outgrowth in PC12 cells, in a concentration-dependent manner. Data show the mean ± SEM (n = 6–12). **P < 0.01, ***P < 0.001 compared with control group (post hoc Tukey test).
TPPU Has Antidepressant Effects in an Inflammation-Induced Model of Depression.
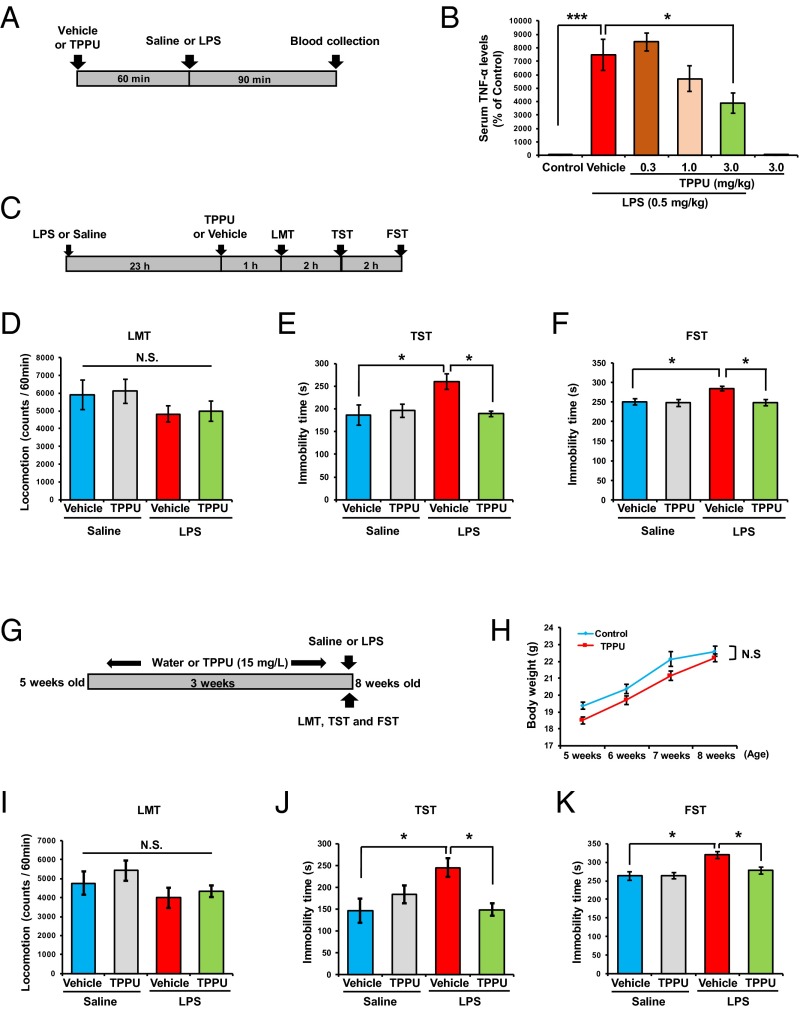
Oral administration to mice of TPPU (0.3, 1.0, or 3.0 mg/kg, 60 min before) attenuated LPS (0.5 mg/kg)-induced increase of TNF-α serum levels in a dose-dependent manner (Fig. 1 A and B), confirming its ability to reduce inflammation. TPPU (3.0 mg/kg, orally) gave no effect on serum levels of TNF-α in the control mice. Next, we examined whether TPPU showed antidepressant effects in mice pretreated with LPS (0.5 mg/kg) (Fig. 1C). There were no differences in locomotion among the four groups (Fig. 1D). In the tail suspension test (TST) and forced swim test (FST), TPPU (3 mg/kg, orally) significantly reduced the increased immobility time in LPS-treated mice (Fig. 1 E and F).
Fig. 1.
Effects of TPPU in an inflammation model of depression. (A) Schedule of treatment and blood collection. (B) Pretreatment with TPPU (0.3, 1.0, or 3.0 mg/kg, orally) attenuated increased serum levels of TNF-α after a single administration of LPS (0.5 mg/kg, intraperitoneally), in a dose-dependent manner. Data are shown as mean ± SEM (n = 5 or 6). *P < 0.05, ***P < 0.001 compared with vehicle + LPS group [one-way ANOVA, F(5,27) = 26.67, P < 0.001, post hoc Tukey test]. (C) Schedule of treatment and behavioral tests. Vehicle or TPPU (3 mg/kg, orally) was administered 23 h after a single administration of LPS (0.5 mg/kg, intraperitoneally) or saline. Behavioral tests, including the LMT, TST, and FST were performed. (D–F) Two-way ANOVA revealed the results: LMT [LPS: F(1,26) = 3.040, P = 0.093; TPPU: F(1,26) = 0.078, P = 0.783; interaction: F(1,26) = 0.001, P = 0.970], TST [LPS: F(1,28) = 5.357, P = 0.028; TPPU: F(1,28) = 4.428, P = 0.044; interaction: F(1,28) = 5.937, P = 0.021], and FST [LPS: F(1,27) = 5.974, P = 0.021; TPPU: F(1,27) = 6.747, P = 0.015; interaction: F(1,27) = 5.738, P = 0.024]. Data are shown as mean ± SEM (n = 7–9). *P < 0.05 (post hoc Tukey test); N.S., not significant. (G) Schedule of treatment and behavioral tests. Water alone or water including TPPU (15 mg/L) was given for 3 wk before a single administration of LPS (0.5 mg/kg, intraperitoneally). The LMT, TST, and FST were performed 24, 26, and 28 h after LPS administration. (H) There were no changes for body weight increase of two groups [repeated one-way ANOVA, F(3,29) = 1.894, P = 0.153]. N.S., not significant. (I–K) Two-way ANOVA revealed the results: LMT [TPPU: F(1,20) = 0.725, P = 0.405; LPS: F(1,20) = 2.415, P = 0.136; interaction: F(1,20) = 0.083, P = 0.776], TST [TPPU: F(1,20) = 4.814, P = 0.040, LPS: F(1,20) = 5.529, P = 0.029; interaction: F(1,20) = 13.93, P = 0.001], and FST [TPPU: F(1,20) = 6.708, P = 0.017, LPS: F(1,20) = 9.939, P = 0.005; interaction: F(1,20) = 4.542, P = 0.046]. Data are shown as mean ± SEM (n = 6). *P < 0.05 (post hoc Tukey test); N.S., not significant.
Furthermore, chronic intake of TPPU (15 mg/L for 3 wk) in the drinking water significantly prevented LPS (0.5 mg/kg)-induced depression-like behavior in mice, although body weight was not different in the two groups (Fig. 1 G–K). These data suggest that oral administration of TPPU has therapeutic and prophylactic effects in the inflammation model of depression.
Pharmacokinetic Study of TPPU in Mice.
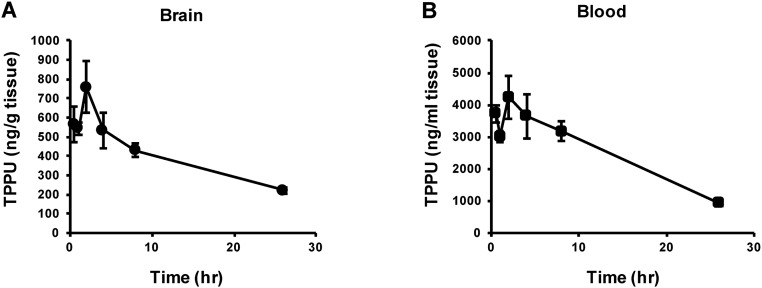
Following single oral administration of TPPU (3 mg/kg), concentration of TPPU in the blood and brain increased rapidly. The average concentration of TPPU in the blood and brain 2 h after oral administration was 4,240 ng/mL and 760 ng/g tissue, respectively. The half-life of TPPU in the plasma and cerebral cortex was 17.8 and 10.7 h, respectively (Fig. S2 A and B). The pharmacokinetic data suggest that TPPU can enter into the brain, consistent with a recent report (35).
Fig. S2.
Pharmacokinetic profile of TPPU in mice. The concentration of TPPU in the brain (A) and blood (B) increased rapidly after a single administration of TPPU (3 mg/kg, orally). The half-life of TPPU in the blood and cerebral cortex was 17.8 and 10.7 h, respectively. Data at each time point are shown as mean ± SEM (n = 3).
TPPU Has Antidepressant Effect in a Social Defeat Stress Model.
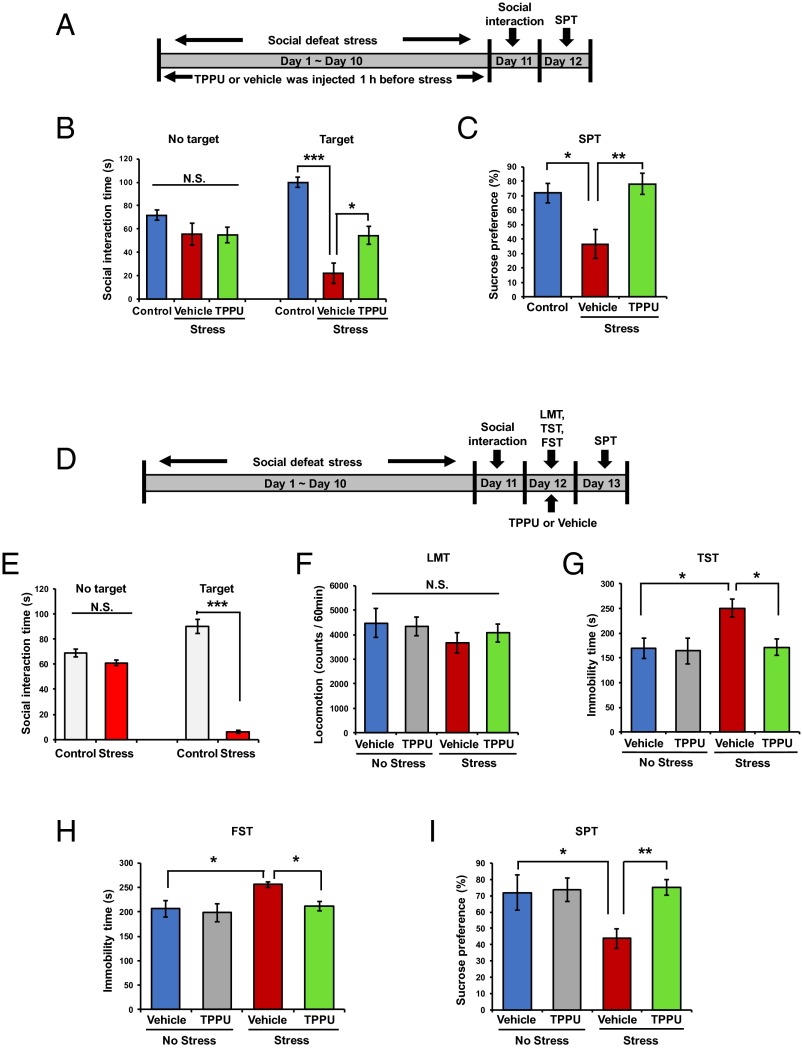
First, we examined the effects of TPPU pretreatment (3 mg/kg/d for 10 d, orally, 60 min before each stress) on the depression-like behavior after repeated social defeat stress (Fig. 2A). In the social interaction test, TPPU-pretreated mice showed the increased social interaction time in the chronically stressed mice after social defeat stress compared with vehicle-treated mice (Fig. 2B). In the 1% sucrose preference test (SPT), TPPU-pretreated mice showed increased sucrose preference compared with vehicle-treated mice (Fig. 2C). These findings suggest that pretreatment with TPPU confers resilience to repeated social defeat stress.
Fig. 2.
Effects of TPPU in repeated social defeat stress model of depression. (A) Schedule of treatment, social defeat stress, and behavioral tests. Vehicle or TPPU (3 mg/kg/d for 10 d, day 1 to day 10) was administered orally 60 min before each social defeat stress. One percent SPT was performed 24 h after the social interaction test. (B and C) One-way ANOVA revealed the results: social interaction time (s); [no target: F(2,24) = 1.859, P = 0.178; target: F(2,24) = 29.97, P < 0.001] and SPT [F(2,23) =7.362, P = 0.003]. Data are shown as mean ± SEM (n = 7–10). *P < 0.05, **P < 0.01, ***P < 0.001 (post hoc Tukey test); N.S., not significant. (D) Schedule of social defeat stress, drug treatment, and behavioral tests. Repeated social defeat stress model was performed (day 1 to day 10). Vehicle or TPPU (3 mg/kg, orally) was administered into depressed mice 24 h after social interaction test. Behavioral tests, including the LMT, TST, and FST were performed 2, 4, and 6 h after a single administration of vehicle or TPPU, respectively. One percent SPT was performed 48 h after a single administration of vehicle or TPPU (3 mg/kg, orally). (E) Mice with depression-like behaviors were selected by social interaction test [social interaction time (s); no target: t = 1.990, P = 0.052; target: t = 21.46, P < 0.001]. ***P < 0.001 (Student t test). N.S., not significant. (F–I): Two-way ANOVA showed the results: LMT [stress: F(1,39) = 1.412, P = 0.242; TPPU: F(1,39) = 0.088, P = 0.769; interaction: F(1,39) = 0.363, P = 0.551], TST [stress: F(1,34) = 4.495, P = 0.025; TPPU: F(1,34) = 5.666, P = 0.023; interaction: F(1,34) = 4.600, P = 0.039], FST [stress: F(1,35) = 7.752, P = 0.009; TPPU: F(1,35) = 4.490, P = 0.041; interaction: F(1,35) = 4.262, P = 0.046], and SPT [stress: F(1,39) = 4.920, P = 0.032; TPPU: F(1,39) = 7.122, P = 0.011; interaction: F(1,39) = 5.875, P = 0.020]. Data are shown as mean ± SEM (n = 7–16). *P < 0.05; **P < 0.01 (post hoc Tukey test); N.S., not significant.
Next, we examined the effects of TPPU treatment (3 mg/kg, orally) on the depression-like behavior in mice after repeated social defeat stress (Fig. 2D). In the social interaction test, susceptible mice were used in the subsequent behavioral test (Fig. 2E). There were no differences in locomotion among the four groups (Fig. 2F). In the TST and FST, TPPU significantly reduced the increased immobility time in the mice after social defeat stress (Fig. 2 G and H). In the SPT, TPPU significantly increased the reduced preference in the mice after social defeat stress (Fig. 2I). In contrast, TPPU did not affect the sucrose preference in the control mice (Fig. 2I). These findings suggest that TPPU showed a rapid antidepressant effect in the social defeat stress model.
sEH KO Mice Show Resilience to Repeated Social Defeat Stress.
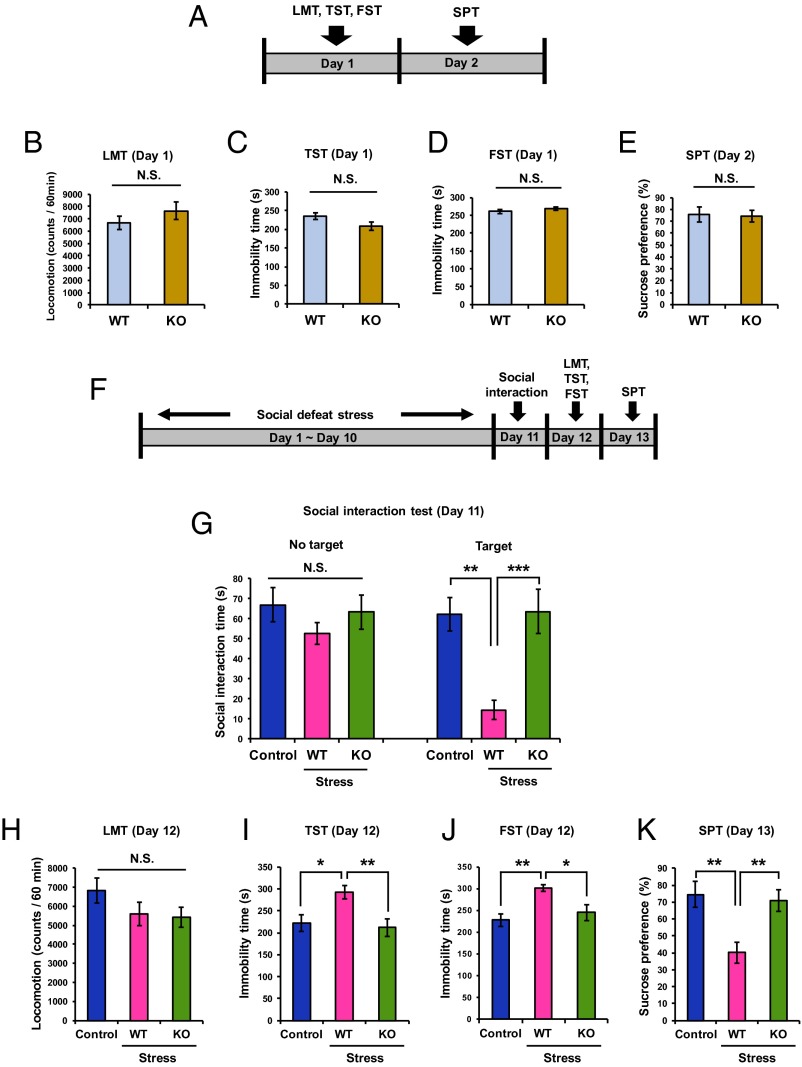
Behavioral tests [locomotion (LMT), TST, FST, SPT] were first performed on the WT and the sEH KO mice (Fig. 3A). There were no differences in the all of the behavioral tests among the two groups (Fig. 3 B–E). Next, the behavioral tests were performed after repeated social defeat stress (Fig. 3F). In the social interaction test, after social defeat stress, the social interaction time of KO mice was significantly higher than that of WT mice, and was similar to control no-stress mice (Fig. 3G). There were no differences in the LMT among the three groups (Fig. 3H). In the TST and FST, the immobility time of KO mice was significantly lower than that of WT mice after social defeat stress (Fig. 3 I and J). In the SPT, the sucrose preference of KO mice was significantly higher and comparable to control animals than that of WT mice after social defeat stress (Fig. 3K). Overall, these data suggest that sEH KO mice show resilience to repeated social defeat stress.
Fig. 3.
Effect of social defeat stress in sEH KO mice. (A) Schedule of behavioral tests. Behavioral tests, including the LMT, TST, FST, and 1% SPT were performed at day 1 and day 2. (B–E) Analysis showed the results: LMT (t = 1.130, P = 0.395), TST (t = 1.952, P = 0.386), FST (t = 0.879, P = 0.387), and SPT (t =1.069, P = 0.367). Data are shown as mean ± SEM (n = 12–16). N.S., not significant. (F) Schedule of social defeat stress and behavioral tests. Repeated social defeat stress was performed from day 1 to day 10. Social interaction test was performed on day 11. Behavioral tests, including LMT, TST, FST, and 1% SPT were performed at day 12 and day 13. (G) One-way ANOVA revealed the results [social interaction time (s); no target: F(2,30) = 0.951, P = 0.398; target: F(2,32) = 11.91, P < 0.001]. **P < 0.01; ***P < 0.001 (post hoc Tukey test). N.S., not significant. (H–K) One-way ANOVA showed the results: LMT [F(2,26) = 1.505, P = 0.241], TST [F(2,26) = 5.849, P = 0.008], FST [F(2,23) = 6.956, P = 0.004], and SPT [F(2,29) = 8.197, P = 0.002]. Data are shown as mean ± SEM (n = 8–16). *P < 0.05; **P < 0.01 (post hoc Tukey test); N.S., not significant.
Protein Levels of sEH in the Brain from Mice with Depression-Like Phenotype After LPS Administration or Social Defeat Stress.
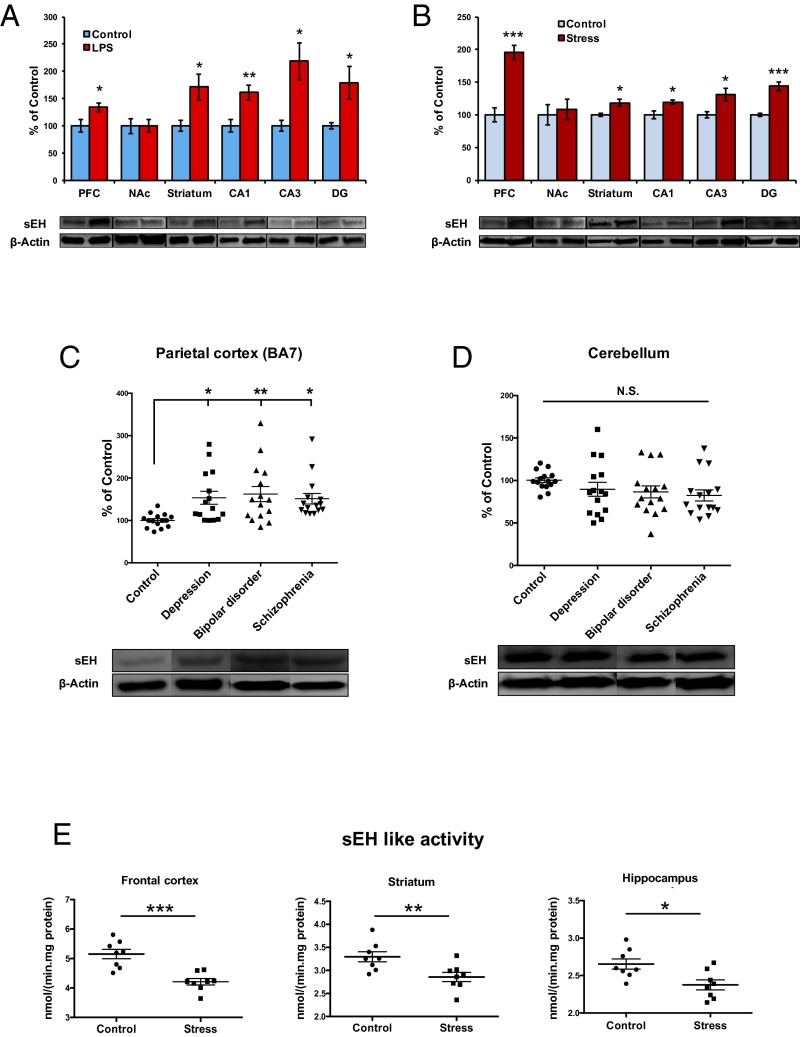
Previous reports demonstrated that the prefrontal cortex (PFC), CA3, and dentate gyrus (DG) of the hippocampus, striatum, and nucleus accumbens (NAc) play a role in the depression-like behaviors in rodents after inflammation, social defeat stress, and learned helplessness (36–40). We examined whether sEH protein is altered in the brain tissues from mice after LPS (0.5 mg/kg) administration (Fig. 4A) or repeated social defeat stress (Fig. 4B). We found significant increases of sEH protein in the PFC, striatum, CA1, CA3, and DG, but not the NAc, of both models of depression.
Fig. 4.
Protein levels of sEH and enzyme activity in the brain from mice with depression-like phenotype and depressed patients. (A) Brain regions were collected 24 h after a single administration of saline or LPS (0.5 mg/kg, intraperitoneally). Western blot analysis of sEH protein was performed. PFC (t = 2.511, P = 0.031), NAc (t = 0.035, P = 0.973), striatum (t = 2.523, P = 0.030), CA1 (t = 3.458, P = 0.006), CA3 (t = 2.439, P = 0.041), DG (t = 2.608, P = 0.026). The values are the mean ± SEM (n = 5–7). *P < 0.05, **P < 0.01 compared with control group (Student t test). (B) Social defeat stress was performed 10 d. Twenty-four hours after the final stress the social interaction test was performed. Brain regions [PFC, NAc, striatum, hippocampus (CA1, CA3, DG)] from chronically stressed (susceptible) mice were collected. Western blot analysis of sEH protein was performed: PFC (t = 6.356, P < 0.001), NAc (t = 0.345, P = 0.738), striatum (t = 3.059, P = 0.010), CA1 (t = 3.016, P = 0.017), CA3 (t = 2.755, P = 0.022), DG (t = 6.483, P < 0.001). The values represent the mean ± SEM (n = 5–7). *P < 0.05, ***P < 0.001 compared with control group (Student t test). (C) Western blot analysis of sEH in the parietal cortex (BA7) from control (n = 15), depression (n = 15), bipolar disorder (n = 15), and schizophrenia (n = 15). Protein levels of sEH in the parietal cortex from depression, bipolar disorder, and schizophrenia were significantly higher than those on controls. One-way ANOVA showed the results [F(3,56) = 4.364, P = 0.008]. Data are shown as mean ± SEM (n = 15). *P < 0.05, **P < 0.01 compared with control group (post hoc Tukey test). (D) Western blot analysis of sEH in the cerebellum from control (n = 15), depression (n = 15), bipolar disorder (n = 15), and schizophrenia (n = 15). Protein levels of sEH in the cerebellum from depression, bipolar disorder, and schizophrenia were not different among the four groups [F(3,56) =1.389, P = 0.256]. Data are shown as mean ± SEM (n = 15). N.S., not significant. (E) Repeated social defeat stress was performed 10 d. Twenty-four hours after the final stress, the social interaction test was performed. Brain regions (frontal cortex, striatum, hippocampus) from chronically stressed (susceptible) mice were used for analysis of sEH-like enzyme activity. Frontal cortex (t = 4.817, P < 0.001), striatum (t = 2.975, P = 0.010), and hippocampus (t = 2.920, P = 0.012). The values represent the mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 compared with control group (Student t test).
Increased Levels of sEH Protein in the Brain of Depressed Patients.
Using postmortem brain samples from the Neuropathology Consortium of the Stanley Medical Research Institute (41) (Table S1), we examined whether sEH protein was also altered in the brain of patients suffering from depression, bipolar disorder, and schizophrenia. Protein levels of sEH in the parietal cortex (Brodmann area 7: BA7) from depression (n = 15), bipolar disorder (n = 15), and schizophrenia (n = 15) patients were significantly higher than those of controls (n = 15) (Fig. 4C). In contrast, protein levels of sEH in the cerebellum were not different among the four groups (Fig. 4D). These findings suggest that increased levels of sEH in the parietal cortex may be implicated in the pathogenesis of these psychiatric disorders.
Table S1.
Characteristics of the postmortem brain tissues from Neuropathology Consortium of the Stanley Medical Research Institute
| Characteristics | Normal control (n = 15) | Bipolar disorder (n = 15) | Major depression (n = 15) | Schizophrenia (n = 15) | P value |
| Age at death (y) | 48.1 ± 10.7 (29–68) | 42.3 ± 11.7 (25–61) | 46.5 ± 9.3 (30–65) | 44.5 ± 13.1 (25–62) | 0.540* |
| Gender (male/female) | 9/6 | 9/6 | 9/6 | 9/6 | |
| PMI (h) | 23.7 ± 9.9 | 32.5 ± 16.1 | 27.5 ± 10.7 | 33.7 ± 14.6 | 0.147* |
| Brain pH | 6.27 ± 0.24 | 6.18 ± 0.23 | 6.18 ± 0.22 | 6.16 ± 0.26 | 0.616* |
| Brain hemispheres (right/left) | 7/8 | 8/7 | 6/9 | 6/9 | 0.864† |
| Brain weight (g) | 1501.0 ± 164.1 | 1441.2 ± 171.5 | 1462.0 ± 142.1 | 1471.7 ± 108.2 | 0.740* |
| Storage days | 338.2 ± 234.3 | 620.5 ± 172.3 | 434.1 ± 290.0 | 621.1 ± 233.1 | 0.003* |
| Age of onset (y) | 21.5 ± 8.3 | 33.9 ± 13.3 | 23.2 ± 8.0 | 0.003* | |
| Duration of disease (y) | 20.1 ± 9.7 | 12.7 ± 11.1 | 21.3 ± 11.4 | 0.068* | |
| History of Psychosis | 11 with (4 without) | 15 | 0.100‡ | ||
| Fluphenazine equivalent (mg) | 20,827 ± 24,016 (3 never) | 52,267 ± 62,062 (1 never) | 0.078§ | ||
| Cause of death | 0.192† | ||||
| Suicide | 0 | 9 | 7 | 4 | |
| Cardiopulmonary | 13 | 4 | 7 | 8 | |
| Accident | 2 | 1 | 1 | 2 | |
| Other | 0 | 1 | 1 | 1 |
One-way ANOVA.
χ2 Test for independence.
Fisher’s exact probability test.
Unpaired t test.
Enzyme Activity of sEH and Oxylipin Profile of Brain from Mice with Depression-Like Phenotype.
Because the levels of sEH protein were increased in the brain samples from mice with depression-like behaviors, we examined whether enzyme activity of sEH and eicosanoids in the brain regions are altered in the brain from chronically stressed (susceptible) mice. Unexpectedly, enzyme activity of sEH in the frontal cortex, hippocampus, and striatum from chronically stressed (susceptible) mice was significantly lower than that of control mice (Fig. 4E).
Next, we measured tissue levels of eicosanoids metabolites (Fig. S3) in the PFC, hippocampus, and striatum from control and repeated social defeat stress (susceptible) mice. There were no changes for metabolites including EETs, and their metabolite dihydroxyeicosatrienoic acids (DHETs) in the three regions (Tables S2–S4).
Fig. S3.
Eicosanoids measured in the brain regions from control and chronically stress (susceptible) mice.
Table S2.
Levels of eicosanoids metabolites in the PFC from control and depressed mice
| Metabolite | Control | Depression | P value |
| 6-Keto-PGF1a | 53.8 ± 8.9 | 55.7 ± 4.1 | 0.849 |
| TXB2 | 239.5 ± 17.9 | 312.2 ± 33.2 | 0.075 |
| 9,12,13-TriHOME | 14.7 ± 2.4 | 15.1 ± 3.0 | 0.932 |
| 9,10,13-TriHOME | 10.0 ± 2.2 | 10.6 ± 2.0 | 0.854 |
| PGF2a | 247.4 ± 24.6 | 326.2 ± 26.7 | 0.048* |
| PGE2 | 56.7 ± 6.7 | 83.3 ± 10.9 | 0.057 |
| PGD2 | 309.4 ± 31.8 | 344.3 ± 48.9 | 0.560 |
| PGJ2 | 23.7 ± 2.7 | 13.8 ± 1.5 | 0.006** |
| 12,13-DiHOME | 2.9 ± 0.7 | 1.4 ± 0.2 | 0.141 |
| 9,10-DiHOME | 2.5 ± 0.7 | 1.6 ± 0.2 | 0.237 |
| 11,12-DHET | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.919 |
| 15-Deoxy-PGJ2 | 2.9 ± 0.4 | 1.5 ± 0.2 | 0.009** |
| 15-HEPE | 1.9 ± 0.8 | 4.0 ± 1.6 | 0.429 |
| 12-HEPE | 74.2 ± 38.3 | 287.4 ± 240.3 | 0.466 |
| 13-HODE | 30.3 ± 4.4 | 24.3 ± 2.6 | 0.263 |
| 9-HODE | 21.9 ± 2.9 | 21.0 ± 2.1 | 0.791 |
| 15-HETE | 400.8 ± 165.0 | 384.5 ± 149.4 | 0.943 |
| 11-HETE | 322.5 ± 101.8 | 319.7 ± 112.5 | 0.986 |
| 8-HETE | 15.4 ± 6.8 | 12.3 ± 2.2 | 0.800 |
| 12-HETE | 26.9 ± 4.9 | 89.0 ± 26.9 | 0.040* |
| 15(S)-HETrE | 17.1 ± 8.5 | 16.9 ± 6.6 | 0.984 |
| 5-HETE | 21.0 ± 9.0 | 15.9 ± 5.5 | 0.636 |
| 19 (20)-EpDPE | 78.4 ± 25.5 | 602.0 ± 434.1 | 0.249 |
| 12 (13)-EpOME | 15.8 ± 5.3 | 101.5 ± 56.2 | 0.151 |
| 14 (15)-EET | 349.3 ± 88.8 | 1711.7 ± 981.4 | 0.188 |
| 9 (10)-EpOME | 11.4 ± 4.6 | 82.4 ± 46.8 | 0.153 |
| 10 (11)-EpDPE | 67.9 ± 21.1 | 247.5 ± 137.1 | 0.216 |
| 11 (12)-EET | 478.3 ± 132.0 | 2046.0 ± 1198.6 | 0.215 |
| 7 (8)-EpDPE | 5730.9 ± 1755.5 | 22,852.5 ± 12,652.7 | 0.201 |
| 8 (9)-EET | 218.8 ± 59.4 | 944.2 ± 547.7 | 0.209 |
| 5 (6)-EET | 4031.3 ± 2321.3 | 47576.8 ± 29365.3 | 0.161 |
Student t test: *P < 0.05, **P < 0.01.
Table S4.
Levels of eicosanoids metabolites in the hippocampus from control and depressed mice
| Metabolite | Control | Depression | P value |
| 6-Keto-PGF1a | 51.2 ± 3.3 | 77.9 ± 9.3 | 0.009** |
| TXB2 | 305.5 ± 31.5 | 357.7 ± 47.6 | 0.031* |
| 9,12,13-TriHOME | 28.0 ± 6.4 | 20.3 ± 1.6 | 0.706 |
| 9,10,13-TriHOME | 18.5 ± 4.7 | 15.3 ± 3.4 | 0.772 |
| PGF2a | 324.0 ± 33.7 | 405.7 ± 31.4 | 0.059 |
| PGE2 | 48.7 ± 3.6 | 69.5 ± 5.2 | 0.011* |
| PGD2 | 236.9 ± 28.4 | 291.5 ± 54.9 | 0.219 |
| PGJ2 | 17.0 ± 1.4 | 11.2 ± 1.1 | 0.544 |
| 12,13-DiHOME | 2.7 ± 0.6 | 1.5 ± 0.4 | 0.204 |
| 9,10-DiHOME | 2.9 ± 0.5 | 2.5 ± 0.5 | 0.875 |
| 11,12-DHET | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.301 |
| 15-Deoxy-PGJ2 | 2.1 ± 0.3 | 1.2 ± 0.2 | 0.450 |
| 15-HEPE | 4.6 ± 1.4 | 7.9 ± 1.9 | 0.202 |
| 12-HEPE | 61.6 ± 7.5 | 113.4 ± 35.6 | 0.125 |
| 13-HODE | 38.8 ± 8.2 | 30.7 ± 4.9 | 0.590 |
| 9-HODE | 29.4 ± 6.2 | 25.1 ± 3.1 | 0.662 |
| 15-HETE | 289.0 ± 54.4 | 435.7 ± 94.2 | 0.104 |
| 11-HETE | 245.5 ± 39.5 | 456.5 ± 125.4 | 0.076 |
| 8-HETE | 7.3 ± 1.9 | 12.3 ± 2.8 | 0.228 |
| 12-HETE | 123.6 ± 95.6 | 104.3 ± 33.1 | 0.630 |
| 15(S)-HETrE | 6.4 ± 4.1 | 20.9 ± 5.5 | 0.011* |
| 5-HETE | 17.7 ± 5.2 | 24.9 ± 9.0 | 0.308 |
| 19 (20)-EpDPE | 232.2 ± 82.3 | 177.9 ± 68.3 | 0.658 |
| 12 (13)-EpOME | 33.0 ± 13.2 | 36.7 ± 12.5 | 0.928 |
| 14 (15)-EET | 957.0 ± 411.1 | 863.1 ± 339.4 | 0.679 |
| 9 (10)-EpOME | 35.1 ± 15.3 | 23.0 ± 10.6 | 0.434 |
| 10 (11)-EpDPE | 142.5 ± 63.9 | 86.1 ± 42.9 | 0.359 |
| 11 (12)-EET | 1477.3 ± 784.1 | 1161.9 ± 492.7 | 0.524 |
| 7 (8)-EpDPE | 19,478.4 ± 8391.2 | 10,095.0 ± 4691.2 | 0.267 |
| 8 (9)-EET | 623.1 ± 286.6 | 425.6 ± 203.7 | 0.433 |
| 5 (6)-EET | 17,414.8 ± 9708.9 | 14,116.5 ± 8585.6 | 0.651 |
Student t test: *P < 0.05, **P < 0.01.
Table S3.
Levels of eicosanoids metabolites in the striatum from control and depressed mice
| Metabolite | Control | Depression | P value |
| 6-Keto-PGF1a | 38.3 ± 5.1 | 72.7 ± 9.2 | 0.006** |
| TXB2 | 229.1 ± 32.5 | 325.9 ± 47.3 | 0.114 |
| 9,12,13-TriHOME | 23.5 ± 5.3 | 26.7 ± 4.8 | 0.656 |
| 9,10,13-TriHOME | 13.3 ± 3.3 | 23.1 ± 5.4 | 0.144 |
| PGF2a | 233.0 ± 36.0 | 320.2 ± 27.5 | 0.075 |
| PGE2 | 43.4 ± 10.5 | 46.4 ± 1.8 | 0.782 |
| PGD2 | 221.6 ± 33.5 | 236.7 ± 31.7 | 0.749 |
| PGJ2 | 13.6 ± 2.2 | 13.4 ± 1.3 | 0.951 |
| 12,13-DiHOME | 2.2 ± 1.1 | 3.5 ± 1.3 | 0.545 |
| 9,10-DiHOME | 2.5 ± 0.4 | 4.2 ± 1.1 | 0.156 |
| 11,12-DHET | 1.2 ± 0.3 | 1.3 ± 0.1 | 0.714 |
| 15-Deoxy-PGJ2 | 1.4 ± 0.3 | 1.3 ± 0.2 | 0.839 |
| 15-HEPE | 1.0 ± 0.4 | 11.7 ± 3.9 | 0.093 |
| 12-HEPE | 93.8 ± 43.7 | 123.2 ± 28.8 | 0.646 |
| 13-HODE | 24.0 ± 1.8 | 32.2 ± 5.7 | 0.190 |
| 9-HODE | 21.0 ± 1.5 | 29.6 ± 4.8 | 0.110 |
| 15-HETE | 310.8 ± 81.2 | 418.5 ± 97.8 | 0.411 |
| 11-HETE | 272.6 ± 64.4 | 327.0 ± 85.9 | 0.620 |
| 8-HETE | 17.8 ± 8.1 | 7.5 ± 1.9 | 0.306 |
| 12-HETE | 56.0 ± 20.3 | 150.1 ± 108.2 | 0.407 |
| 15(S)-HETrE | 10.4 ± 3.9 | 14.8 ± 5.8 | 0.537 |
| 5-HETE | 14.0 ± 3.4 | 36.1 ± 13.8 | 0.144 |
| 19 (20)-EpDPE | 141.5 ± 29.3 | 96.8 ± 35.5 | 0.370 |
| 12 (13)-EpOME | 16.8 ± 3.9 | 32.7 ± 19.4 | 0.466 |
| 14 (15)-EET | 551.4 ± 95.9 | 518.8 ± 300.1 | 0.924 |
| 9 (10)-EpOME | 21.0 ± 5.4 | 13.5 ± 5.3 | 0.357 |
| 10 (11)-EpDPE | 101.4 ± 20.3 | 38.4 ± 12.7 | 0.023* |
| 11 (12)-EET | 647.4 ± 111.9 | 435.0 ± 187.2 | 0.372 |
| 7 (8)-EpDPE | 10,779.9 ± 2125.3 | 5171.9 ± 1978.1 | 0.084 |
| 8 (9)-EET | 286.5 ± 57.1 | 174.1 ± 62.5 | 0.224 |
| 5 (6)-EET | 7466.5 ± 1913.8 | 3279.7 ± 1522.0 | 0.119 |
Student t test: *P < 0.05, **P < 0.01.
Role of BDNF-TrkB Signaling and Synaptogenesis in the Stress Resilience of sEH KO Mice.
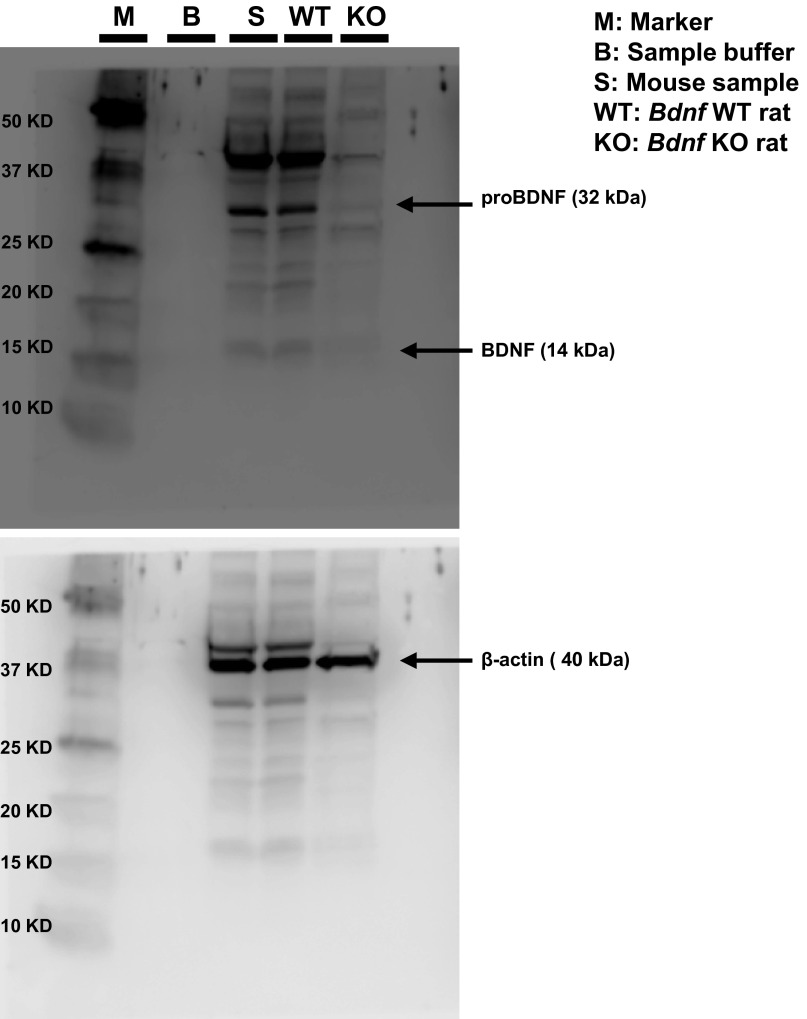
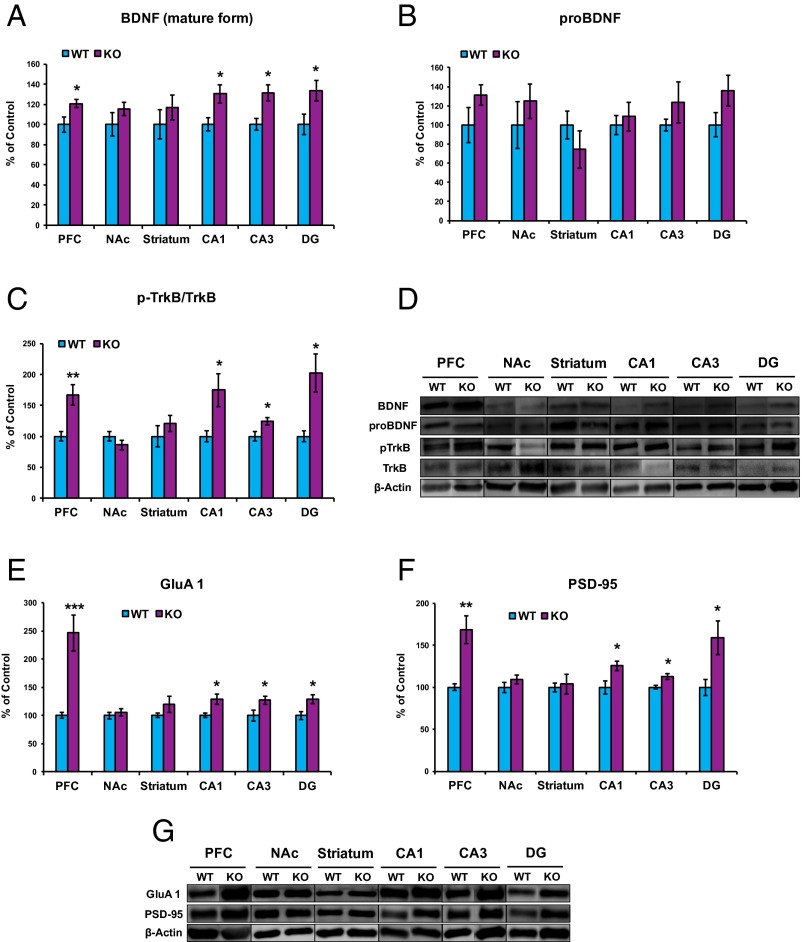
Because the BDNF-TrkB signaling pathway plays a key role in depression-like phenotype in rodents (25–30), we examined this signaling pathway in selected brain regions of sEH KO mice. First, we performed Western blot analysis of BDNF antibody in the Bdnf KO rat brain sample. The bands for BDNF (mature form) and its precursor proBDNF were not detected in the brain sample from KO rats, indicating that these bands can recognize both BDNF (mature form) and proBDNF (Fig. S4). Subsequently, Western blot analyses of BDNF, its precursor proBDNF, TrkB, and phosphorylated TrkB (p-TrkB) in the selected brain regions (PFC, NAc, striatum, DG, CA1, and CA3 of the hippocampus) in WT mice and sEH KO mice were performed. Levels of BDNF in the PFC, CA1, CA3, DG, but not the NAc and striatum, of KO mice were significantly higher than those of WT mice (Fig. 5 A and D). In contrast, tissue levels of proBDNF in the all tested regions did not differ between the two groups (Fig. 5 B and D).
Fig. S4.
Western blot analysis of proBDNF and BDNF (mature form) in the brain samples from Bdnf KO rat, WT rat, and mouse brain sample. The bands of proBDNF (approximately 32 KDa) and mature BDNF (approximately 13 KDa) were not detected in the brain samples from Bdnf KO rat (SAGE Laboratories). In this assay, the bands of β-actin were same.
Fig. 5.
Increased levels of BDNF, TrkB phosphorylation, GluA1, and PSD-95 in the brain regions from sEH KO mice. (A and B) Western blot analysis of BDNF (A: mature form) and its precursor proBDNF (B) in the PFC, NAc, striatum, CA1, CA3, and DG from sEH KO mice and WT mice was performed. The values are expressed as a percentage of that of control mice. (A) BDNF (mature form): PFC (t = 2.438, P = 0.041), NAc (t = 1.146, P = 0.285), striatum (t = 0.876, P = 0.407), CA1 (t = 2.752, P = 0.025), CA3 (t = 3.130, P = 0.014), DG (t = 2.383, P = 0.044). *P < 0.05 (Student t test). (B) proBDNF: PFC (t = 1.478, P = 0.178), NAc (t = 0.820, P = 0.436), striatum (t = 1.050, P = 0.324), CA1 (t = 0.485, P = 0.641), CA3 (t = 1.048, P = 0.325), DG (t = 1.772, P = 0.114). (C) The ratio of p-TrkB to total TrkB in the brain regions is shown. Total levels of TrkB protein in the all regions are not different between the two groups. p-TrkB/TrkB: PFC (t = 3.591, P = 0.007), NAc (t = 1.255, P = 0.245), striatum (t = 0.984, P = 0.354), CA1 (t = 2.673, P = 0.028), CA3 (t = 2.501, P = 0.037), DG (t = 3.168, P = 0.013). The values represent the mean ± SEM (n = 5). *P < 0.05, **P < 0.01 (Student t test). (D) Representative data of Western blot analyses of BDNF (mature form), proBDNF, p-TrkB, TrkB, and β-actin in the mouse brain regions. (E) GluA1: PFC (t = 4.472, P = 0.001), NAc (t = 0.590, P = 0.566), striatum (t = 1.185, P = 0.266), CA1 (t = 3.083, P = 0.013), CA3 (t = 2.827, P = 0.018), DG (t = 2.699, P = 0.024). *P < 0.05; ***P < 0.001 (Student t test). (F) PSD-95: PFC (t = 4.072, P = 0.002), NAc (t = 1.197, P = 0.254), striatum (t = 0.326, P = 0.751), CA1 (t = 2.652, P = 0.026), CA3 (t = 2.819, P = 0.023), DG (t = 2.723, P = 0.021). The values represent the mean ± SEM (n = 5–7). *P < 0.05, **P < 0.01 (Student t test). (G) Representative data of Western blot analyses of GluA1, PSD-95, and β-actin in the mouse brain regions.
To clarify the role of TrkB phosphorylation in the stress resilience of sEH KO mice, we performed Western blot analyses of TrkB and p-TrkB, an activated form of TrkB, in samples from the PFC, NAc, striatum, and hippocampus (CA1, CA3, DG). Tissue levels of TrkB in the all tested regions did not differ among the four groups (Fig. 5D). KO mice showed an increased ratio of p-TrkB/TrkB protein in the PFC, CA1, CA3, and DG, but not the NAc and striatum (Fig. 5C). These findings suggest that increased BDNF-TrkB signaling in the PFC and hippocampus (CA1, CA3, DG) of KO mice might be involved in the resilience to repeated social defeat stress.
Next, we performed Western blot analysis on the synaptogenesis markers, GluA1 (a subtype of AMPA receptor) and postsynaptic density protein 95 (PSD-95), in selected brain regions (Fig. 5 E–G). Levels of GluA1 and PSD-95 in the PFC, CA1, CA3, DG, but not NAc and striatum, of KO mice were significantly higher than those of WT mice (Fig. 5 E–G).
Discussion
Overall, our results demonstrate a key role of sEH in the pathogenesis of depression. The major findings of the present study are: First, a potent sEH inhibitor TPPU and 14,15-EET potentiated NGF-induced neurite outgrowth in PC12 cells, suggesting that sEH inhibitors can enhance neuronal plasticity associated with depression. Second, TPPU showed prophylactic and therapeutic effects in the inflammation and social defeat stress models of depression. Third, protein levels of sEH in the brain from mice with depression-like behaviors or postmortem brain from depressed patients were higher than those of controls. Fourth, sEH KO mice show resilience to social defeat stress, and increased BDNF-TrkB signaling in the PFC and hippocampus of KO mice might be implicated in the stress resilience. These all findings suggest that sEH inhibitors would be potential therapeutic drugs for depression.
In this study, we found that a single dose of TPPU has a rapid antidepressant effect in both the inflammation and the repeated social defeat stress models of depression. Interestingly, current antidepressants (paroxetine and venlafaxine) do not have any effect in the LPS-induced inflammation model of depression (36). In addition, most current antidepressants can take weeks before patients or animal models feel the full antidepressant effects (42, 43). Recently, we reported that a single dose of N-methyl-D-aspartate (NMDA) receptor antagonist ketamine (or R-ketamine) showed a rapid antidepressant effect in the social defeat stress model (37, 39), consistent with rapid antidepressant effects of ketamine in treatment-resistant patients with depression (44–46). However, ketamine leads to psychotomimetic side effects and abuse liability that appears to be absent in the case of TPPU. These findings suggest that sEH inhibitors have the ability to be more effective, faster acting, and have fewer side effects than current antidepressant drugs.
Tissue levels of sEH protein in the PFC, striatum, and hippocampus of mice with depression-like behaviors were higher than those of control mice. Interestingly, we also found that levels of sEH in the parietal cortex from patients with major psychiatric disorders (depression, bipolar disorder, and schizophrenia) were higher than controls. Inflammation is also implicated in these psychiatric disorders (6–10, 47–50). Recent studies showed that peripheral IL-6 is critical in regulating stress-related depression-like phenotypes in rodents (51–53). Because sEH plays an active role in the inflammatory response (18–20), it is possible that increased levels of sEH protein in the parietal cortex may play a role in the pathogenesis of these psychiatric disorders. In contrast, the enzyme activity of sEH in these regions from mice with depression-like phenotype was lower than that of control mice. In addition, we found no changes in the eicosanoid metabolites, such as EETs and their metabolites DHETs. Although the reasons underlying this discrepancy are currently unclear, it seems that compensatory response by increased levels of sEH protein in mice with depression-like phenotype may be involved.
Accumulating evidence suggests that BDNF-TrkB signaling plays a key role in the depression-like phenotype in rodents (25–30). In this study, we found that BDNF protein in the PFC and hippocampus, but not the NAc, of sEH KO mice was higher than that of WT mice, and that the p-TrkB/TrkB ratio in the PFC and hippocampus of sEH KO mice was also higher than that of WT mice, indicating increased BDNF-TrkB signaling in the PFC and hippocampus in the sEH KO mice. Previously, we reported that inflammation, social defeat stress, and learned helplessness caused decreased BDNF-TrkB signaling in the PFC and hippocampus, while increasing signals in the NAc, inducing depression-like behavior in rodents (36–40). Interestingly, we reported that regional differences in BDNF levels in the PFC and hippocampus of rat brain may contribute to resilience to inescapable stress (38). A recent study demonstrated that 14,15-EET could promote the production of BDNF from astrocyte (54). Because sEH KO mice show a higher level of 14,15-EET, it is likely that increased level of 14,15-EET by sEH deletion might contribute to increased BDNF expression in the frontal cortex and hippocampus, although the precise mechanisms are unknown. Given the key role of BDNF-TrkB signaling in the depression-like phenotype, it is likely that increased BDNF-TrkB signaling in the PFC and hippocampus may contribute to the stress resilience of sEH KO mice. Furthermore, we did not find any change of BDNF in the NAc of sEH KO mice. Because the NAc plays a key role in the depression, it is of interest to study the role of sEH in the NAc.
Many depressed patients become chronically ill, with several relapses (early return of symptoms within the expected duration of a current episode, of perhaps 3–12 mo) or later recurrences (new episodes) following initial short-term improvement or remission (55, 56). Recurrence rates are over 85% within a decade of an index depressive episode, and average ∼50% or more within 6 mo of apparent clinical emission (56). Therefore, the prevention of relapse and recurrence is very important in the management of depression. In this study, we found the prophylactic effects of TPPU in the inflammation and repeated social defeat stress models of depression, suggesting that TPPU could prevent the onset of depression-like phenotype by inflammation or repeated social defeat stress. Therefore, it is likely that sEH inhibitors could be prophylactic drugs to prevent or minimize the relapse by inflammation or stress in the remission state of depressed patients.
In conclusion, our study shows that a single dose of the sEH inhibitor TPPU can produce a rapid antidepressant effect in the inflammation and social defeat stress models of depression. Furthermore, it is likely that increased BDNF-TrkB signaling in the PFC and the hippocampus in sEH KO mice may confer stress resilience. Finally, unlike ketamine, sEH inhibitors appear to be rapid antidepressants without psychotomimetic side effects and abuse liability.
Materials and Methods
Male adult C57BL/6 mice, aged 8 wk (body weight 20–25 g, Japan SLC, Inc.), and male adult CD1 (ICR) mice, aged 13–15 wk (body weight >40 g; Japan SLC, Inc.) were used for the social defeat stress model. A colony of sEH KO mice with targeted deletion of the sEH gene (Ephx2), which is backcrossed to C57BL/6 background, was used (57). Animals were housed under controlled temperatures and 12-h light/dark cycles (lights on between 0700 and 1900 hours), with ad libitum food (CE-2; CLEA Japan, Inc.) and water. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (58). The protocol was approved by the Chiba University Institutional Animal Care and Use Committee.
Details of the experimental protocols, including materials, cell culture, inflammation model, social defeat stress model, behavioral tests of antidepressant effects, pharmacokinetic study, enzyme activity, analysis of oxylipins, Western blot analysis, and statistical analysis are given in SI Materials and Methods.
SI Materials and Methods
Materials.
The sEH inhibitor TPPU was synthesized in-house as previously described (31). TPPU (0.3–3.0 mg/kg) was dissolved in 20% (vol/vol) polyethylene glycol 400 (PEG400: Wako Pure Chemical). Other reagents were purchased commercially.
Effects of TPPU and 14,15-EET on Neurite Outgrowth.
PC12 cells (RIKEN Cell Bank) were cultured at 37 °C, 5% CO2 in DMEM, supplemented with 5% heat-inactivated FBS, 10% heat-inactivated horse serum, and 1% penicillin-streptomycin. Medium was changed two to three times a week. PC12 cells were plated onto 24-well tissue culture plates coated with poly-d-lysine/laminin. Cells were plated at relatively low density (0.25 × 104 cells/cm2) in DMEM containing 0.5% FBS, 1% penicillin-streptomycin. Medium containing a minimal level of serum (0.5% FBS) was used as previously reported (59–61). In this study, 2.5 ng/mL of NGF (Alomone Labs) was used to study the potentiating effects of TPPU and 14,15-EET on neurite outgrowth. Twenty-four hours after plating, the medium was replaced with DMEM containing 0.5% FBS and 1% penicillin-streptomycin with NGF (2.5 ng/mL), with or without TPPU (0.1, 1.0, or 10 μM) or 14,15-EET (0.01, 0.1, or 1.0 μM). Four days after incubation with NGF (2.5 ng/mL) with or without specified compounds, morphometric analysis was performed on digitized images of live cells taken under phase-contrast illumination with an inverted microscope linked to a camera. Images of three fields per well were taken with an average of 100 cells per field. Differentiated cells were counted by visual examination of the field; only cells that had at least one neurite with a length equal to the cell body diameter were counted, and were then expressed as a percentage of the total cells in the field. Counting was performed in a blinded manner.
Inflammation Model of Depression.
The procedure of inflammation model of depression was performed as previously reported (36). Behavioral tests were performed 24 h after a single administration of LPS (0.5 mg/kg). For the experiment of cytokine level, TPPU (0.3, 1.0, or 3.0 mg/kg) was administered orally 60 min before LPS (0.5 mg/kg) administration. Serum sample was collected 90 min after LPS administration, and serum levels of TNF-α were measured using a Ready-SET-Go ELISA kit (eBioscience), according to the manufacturer’s instructions. To examine the effect of a single dose of TPPU, vehicle or TPPU (3.0 mg/kg) was administered orally 23 h after saline (10 mL/kg, intraperitoneally) or LPS (0.5 mg/kg, intraperitoneally) administration. Behavioral tests including the LMT, TST, and FST were performed 1, 3, and 5 h after administration. To examine the prophylactic effect of TPPU in the drinking water, mice were randomized to receive TPPU (15 mg/L) (33) in the drinking water or water alone for 3 wk before saline (10 mL/kg, intraperitoneally) or LPS (0.5 mg/kg, intraperitoneally) administration. Behavioral tests including the LMT, TST, and FST were performed 24, 26, and 28 h after administration of LPS.
Social Defeat Stress Model of Depression.
The procedure of social defeat stress was performed as previously reported (37, 39, 62, 63). Every day the C57BL/6 mice were exposed to a different CD1 aggressor mouse for 10 min, for a total for 10 d. When the social defeat session ended, the resident CD1 mouse and the intruder mouse were housed in one half of the cage separated by a perforated Plexiglas divider to allow visual, olfactory, and auditory contact for the remainder of the 24-h period. At 24 h after the last session, all mice were housed individually. On day 11, a social avoidance test was performed to identify subgroups of mice that were susceptible and unsusceptible to social defeat stress. This was accomplished by placing mice in an interaction test box (42 × 42 cm) with an empty wire-mesh cage (10 × 4.5 cm) located at one end. The movement of the mice was tracked for 2.5 min, followed by 2.5 min in the presence of an unfamiliar aggressor confined in the wire-mesh cage. The duration of the subject’s presence in the “interaction zone” (defined as the 8-cm-wide area surrounding the wire-mesh cage) was recorded by a stopwatch. The interaction ratio was calculated as time spent in an interaction zone with an aggressor/time spent in an interaction zone without an aggressor. An interaction ratio of 1 was set as the cut-off: mice with scores <1 were defined as “susceptible” to social defeat stress and those with scores ≥1 were defined as “unsusceptible” (63). Only susceptible mice were used in the subsequent experiments.
Behavioral Tests of Antidepressant Effects.
Behavioral tests were performed as reported previously (36, 37, 39).
Locomotion test: the locomotor activity was measured by an animal movement analysis system SCANETMV-40 (Melquest), the mice were placed in experimental cages (length × width × height: 560 × 560 × 330 mm). The cumulative exercise was recorded for 60 min. Cages were cleaned between testing sessions.
Tail suspension test: A small piece of adhesive tape placed ∼2 cm from the tip of the tail for mouse. A single hole was punched in the tape and mice were hung individually, on a hook. The immobility time was recorded for 10 min. Mice were considered immobile only when they hung passively and completely motionless.
Forced swimming test: The FST was tested by an automated forced-swim apparatus SCANETMV-40 (Melquest). The mice were placed individually in a cylinder (diameter: 23 cm; height: 31 cm) containing 15 cm of water, maintained at 23 ± 1 °C. Immobility time from activity time as (total) – (active) time was calculated by the apparatus analysis software. The immobility time for each mouse was recorded for 6 min. The TST and FST were also performed 2 and 4 h after the LMT, respectively.
Sucrose preference test: Mice were exposed to water and 1% sucrose solution for 24 h, followed by 4 h of water and food deprivation and a 1-h exposure to two identical bottles: one of water and another of 1% sucrose solution. The bottles containing water and sucrose were weighed before and at the end of this period and the sucrose preference was determined.
Pharmacokinetic Study of TPPU in Mice.
TPPU (3 mg/kg) was administered orally into adult mice. Blood and cerebral cortex were collected at 0.5, 1.0, 2.0, 4.0, 8.0, and 26 h after a single oral administration of TPPU. Concentration of TPPU in the blood and cerebral cortex was determined using the previous method (35, 64).
Enzyme Activity in the Brain Samples.
Enzyme activity of sEH in the brain samples was measured using a previously described method (65, 66). Brain regions, including the frontal cortex, hippocampus, and striatum were resuspended in 1 mL of chilled buffer, sodium phosphate buffer (20 mM pH 7.4) containing 5 mM EDTA, 1 mM PMSF, and 1 mM DTT, and then homogenized with Ultraturax (position 5.5 for 15–20 s). The extract was centrifuged at 10,000 × g for 20 min at 4 °C. The supernatant was used for further analysis. The sEH activity: The supernatant was diluted in sodium phosphate buffer (0.1 M pH 7.4) containing 0.1 mg/mL BSA. The reaction was started by adding 1 µL of [3H]-trans-diphenylpropene oxide (t-DPPO) to 100 µL of diluted extract [(S)final = 50 µM]. The reaction was carried at 37 °C. The reaction was stopped by adding 60 µL of methanol, and extracted by either 200 µL of isooctane or 200 µL of hexanol. The formed diol was measured using liquid scintillation counter in water phase. Protein concentration was measured using BCA method with BSA as standard.
Oxylipin Profiling.
Measurement of eicosanoids was performed on brain samples from the control mice and chronically stressed (susceptible) mice by repeated social defeat stress. Concentration of eicosanoids in the frontal cortex, hippocampus, and striatum was determined using a previously described method (63, 67).
Western Blot Analysis.
To examine the selectivity of anti-BDNF (H-117; Santa Cruz Biotechnology) in the rat brain samples, we performed Western blot analysis of brain samples from WT and Bdnf KO rats (SAGE Labs). The bands for proBDNF and mature BDNF in the brain sample from Bdnf KO rat were not detected (Fig. S4). Therefore, the anti-BDNF in this study could recognize both proBDNF and mature BDNF in mouse brain samples.
Western blot analysis was performed as reported previously (36, 37, 39, 68). Mice were killed by cervical dislocation and brains were rapidly removed from the skull. Approximately 1-mm-thick coronal sections were cut and bilateral tissue punches of PFC, NAc, striatum, CA1, CA3, and DG of the hippocampus were dissected on ice using a SZ-LED Kenis light microscope, and stored at −80 °C. Basically, tissue samples were homogenized in Laemmli lysis buffer. Aliquots (20 μg) of protein were measured using the DC protein assay kit (Bio-Rad), and incubated for 5 min at 95 °C, with an equal volume of 125 mM Tris-HCl, pH 6.8, 20% glycerol, 0.1% bromophenol blue, 10% β-mercaptoethanol, 4% SDS, and subjected to SDS polyacrylamide gel electrophoresis using AnyKD minigels (Mini-PROTEAN TGX Precast Gel; Bio-Rad). Proteins were transferred onto PVDF membranes using a Trans Blot Mini Cell (Bio-Rad). For immunodetection, the blots were blocked with 2% BSA in TBST (TBS + 0.1% Tween-20) for 1 h at room temperature, and kept with primary antibodies overnight at 4 °C. The following primary antibodies were used: rabbit serum against mouse sEH (1:5,000; prepared at University of California, Davis), BDNF (1:200; H-117, Cat#: sc-20981, Santa Cruz Biotechnology), phosphor-TrkB (Tyr-706) (1:200; Cat#: sc-135645, Santa Cruz Biotechnology), TrkB (80E3) (1:1,000; Cat#: 4603, Cell Signaling Technology), AMPA glutamate receptor 1 (GluA1) (1 µg/mL; Abcam), and postsynaptic density protein 95 (PSD-95) (1 µg/mL; Invitrogen). The next day, blots were washed three times in TBST, and incubated with horseradish peroxidase-conjugated anti-rabbit antibody (1:10,000) 1 h, at room temperature. After a final three washes with TBST, bands were detected using enhanced chemiluminescence (ECL) plus the Western Blotting Detection system (GE Healthcare Bioscience). The blots then were washed three times in TBST and incubated with the primary antibody directed against β-actin (1:10,000; Sigma-Aldrich). Images were captured with a Fuji LAS3000-mini imaging system (Fujifilm), and immunoreactive bands were quantified.
Statistical Analysis.
The data show as the mean ± SEM. Analysis was performed using PASW Statistics 20 (formerly SPSS Statistics; SPSS). Comparisons between groups were performed using the one-way ANOVA or two-way ANOVA, followed by post hoc Tukey tests. Comparisons between two groups were performed using Student t test. The P values of less than 0.05 were considered statistically significant.
Acknowledgments
We thank The Stanley Medical Research Institution for providing the postmortem brain samples. This study was supported by a Grant-in-Aid 24116006 for Scientific Research on Innovative Areas of the Ministry of Education, Culture, Sports, Science and Technology, Japan (to K.H.); a Research Fellowship for Young Scientists of the Japan Society for the Promotion of Science (to Q.R.); National Institute of Environmental Health Sciences Grant R01 ES002710; National Institute of Environmental Health Sciences Superfund Research Program Grant P42 ES004699; and the National Institutes of Health U24 DK097154 West Coast Comprehensive Metabolomics Center.
Footnotes
Conflict of interest statement: As sponsor, B.D.H. has a possible conflict of interest having worked in the epoxide hydrolase field for many years. C.M., J.Y., K.M.W., and B.D.H. are authors on University of California patents in the soluble epoxide hydrolase area. Some of these patents have been licensed by EicOsis Human Health for the development of a pharmaceutical. EicOsis is only following a neuropathic pain indication in preclinical research and has not licensed technology on depression or other CNS diseases. There was no industrial support for this work.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601532113/-/DCSupplemental.
References
- 1.World Health Organization 2012 Depression. Fact sheet No. 369/October 2012. Available at www.who.int/mediacentre/factsheets/fs369/en. Accessed February 24, 2016.
- 2.Steinert C, Hofmann M, Kruse J, Leichsenring F. Relapse rates after psychotherapy for depression—Stable long-term effects? A meta-analysis. J Affect Disord. 2014;168:107–118. doi: 10.1016/j.jad.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 3.Biesheuvel-Leliefeld KE, et al. Effectiveness of psychological interventions in preventing recurrence of depressive disorder: Meta-analysis and meta-regression. J Affect Disord. 2015;174:400–410. doi: 10.1016/j.jad.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Guidi J, Tomba E, Fava GA. The sequential integration of pharmacotherapy and psychotherapy in the treatment of major depressive disorder: A meta-analysis of the sequential model and a critical review of the literature. Am J Psychiatry. 2016;173(2):128–137. doi: 10.1176/appi.ajp.2015.15040476. [DOI] [PubMed] [Google Scholar]
- 5.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raison CL, Lowry CA, Rook GA. Inflammation, sanitation, and consternation: Loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch Gen Psychiatry. 2010;67(12):1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto K. Inflammatory biomarkers as differential predictors of antidepressant response. Int J Mol Sci. 2015;16(4):7796–7801. doi: 10.3390/ijms16047796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry. 2015;20(1):32–47. doi: 10.1038/mp.2014.163. [DOI] [PubMed] [Google Scholar]
- 10.Dowlati Y, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 11.Young JJ, Bruno D, Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord. 2014;169:15–20. doi: 10.1016/j.jad.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 12.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strawbridge R, et al. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur Neuropsychopharmacol. 2015;25(10):1532–1543. doi: 10.1016/j.euroneuro.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Dean B, Tawadros N, Scarr E, Gibbons AS. Regionally-specific changes in levels of tumour necrosis factor in the dorsolateral prefrontal cortex obtained postmortem from subjects with major depressive disorder. J Affect Disord. 2010;120(1-3):245–248. doi: 10.1016/j.jad.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Shelton RC, et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. 2011;16(7):751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Na KS, Lee KJ, Lee JS, Cho YS, Jung HY. Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:79–85. doi: 10.1016/j.pnpbp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Köhler O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: A systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12):1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 18.Morisseau C, Hammock BD. Epoxide hydrolases: Mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 19.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8(10):794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris TR, Hammock BD. Soluble epoxide hydrolase: Gene structure, expression and deletion. Gene. 2013;526(2):61–74. doi: 10.1016/j.gene.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner K, Vito S, Inceoglu B, Hammock BD. The role of long chain fatty acids and their epoxide metabolites in nociceptive signaling. Prostaglandins Other Lipid Mediat. 2014;113-115:2–12. doi: 10.1016/j.prostaglandins.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.López-Vicario C, et al. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: Role for omega-3 epoxides. Proc Natl Acad Sci USA. 2015;112(2):536–541. doi: 10.1073/pnas.1422590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shih PB, et al. Dysregulation of soluble epoxide hydrolase and lipidomic profiles in anorexia nervosa. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nestler EJ, et al. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto K, Shimizu E, Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res Brain Res Rev. 2004;45(2):104–114. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: An historical overview and future directions. Psychiatry Clin Neurosci. 2010;64(4):341–357. doi: 10.1111/j.1440-1819.2010.02113.x. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: Emerging links between cardiovascular disease and depression. Prog Neurobiol. 2013;100:15–29. doi: 10.1016/j.pneurobio.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Björkholm C, Monteggia LM. BDNF—A key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose TE, et al. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010;53(19):7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu JY, et al. Substituted phenyl groups improve the pharmacokinetic profile and anti-inflammatory effect of urea-based soluble epoxide hydrolase inhibitors in murine models. Eur J Pharm Sci. 2013;48(4-5):619–627. doi: 10.1016/j.ejps.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sirish P, et al. Unique mechanistic insights into the beneficial effects of soluble epoxide hydrolase inhibitors in the prevention of cardiac fibrosis. Proc Natl Acad Sci USA. 2013;110(14):5618–5623. doi: 10.1073/pnas.1221972110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdu E, et al. Epoxyeicosatrienoic acids enhance axonal growth in primary sensory and cortical neuronal cell cultures. J Neurochem. 2011;117(4):632–642. doi: 10.1111/j.1471-4159.2010.07139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostermann AI, et al. Oral treatment of rodents with soluble epoxide hydrolase inhibitor 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl)rea (TPPU): Bioavailability, resulting drug levels and modulation of oxylipin pattern. Prostaglandins Other Lipid Mediat. 2015;121(Part A):131–137. doi: 10.1016/j.prostaglandins.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JC, et al. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol. 2015;18(4):pyu077. doi: 10.1093/ijnp/pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang JC, et al. Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 2015;232(23):4325–4335. doi: 10.1007/s00213-015-4062-3. [DOI] [PubMed] [Google Scholar]
- 38.Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K. Regional differences in brain-derived neurotrophic factor levels and dendritic spine density confer resilience to inescapable stress. Int J Neuropsychopharmacol. 2015;18(7):pyu121. doi: 10.1093/ijnp/pyu121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Yang C, et al. R-ketamine: A rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirayama Y, et al. Alterations in brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain regions of a learned helplessness rat model and the antidepressant effects of a TrkB agonist and antagonist. Eur Neuropsychopharmacol. 2015;25(12):2449–2458. doi: 10.1016/j.euroneuro.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Torrey EF, Webster M, Knable M, Johnston N, Yolken RH. The Stanley Foundation Brain Collection and Neuropathology Consortium. Schizophr Res. 2000;44(2):151–155. doi: 10.1016/S0920-9964(99)00192-9. [DOI] [PubMed] [Google Scholar]
- 42.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 43.Krishnan V, Nestler EJ. Linking molecules to mood: New insight into the biology of depression. Am J Psychiatry. 2010;167(11):1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol Psychiatry. 2013;73(12):1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monteggia LM, Zarate C., Jr Antidepressant actions of ketamine: From molecular mechanisms to clinical practice. Curr Opin Neurobiol. 2015;30:139–143. doi: 10.1016/j.conb.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newport DJ, et al. APA Council of Research Task Force on Novel Biomarkers and Treatments Ketamine and other NMDA antagonusts: Early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 47.Munkholm K, Braüner JV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: A systematic review and meta-analysis. J Psychiatr Res. 2013;47(9):1119–1133. doi: 10.1016/j.jpsychires.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Dargél AA, Godin O, Kapczinski F, Kupfer DJ, Leboyer M. C-reactive protein alterations in bipolar disorder: A meta-analysis. J Clin Psychiatry. 2015;76(2):142–150. doi: 10.4088/JCP.14r09007. [DOI] [PubMed] [Google Scholar]
- 49.Potvin S, et al. Inflammatory cytokine alterations in schizophrenia: A systematic quantitative review. Biol Psychiatry. 2008;63(8):801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 50.Fernandes BS, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: Meta-analysis and implications. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.87. [DOI] [PubMed] [Google Scholar]
- 51.Hodes GE, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci USA. 2014;111(45):16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K. Peripheral interleukin-6 promotes resilience versus susceptibility to inescapable electric stress. Acta Neuropsychiatr. 2015;27(5):312–316. doi: 10.1017/neu.2015.36. [DOI] [PubMed] [Google Scholar]
- 53.Yang C, Hashimoto K. Peripheral IL-6 signaling: A promising therapeutic target for depression? Expert Opin Investig Drugs. 2015;24(7):989–990. doi: 10.1517/13543784.2015.1055669. [DOI] [PubMed] [Google Scholar]
- 54.Yuan L, et al. 14,15-epoxyeicosatrienoic acid promotes production of BDNF from astrocytes and exerts neuroprotective effects during ischemic injury. Neuropathol Appl Neurobiol. 2015 doi: 10.1111/nan.12291. [DOI] [PubMed] [Google Scholar]
- 55.Forte A, et al. Long-term morbidity in bipolar-I, bipolar-II, and unipolar major depressive disorders. J Affect Disord. 2015;178:71–78. doi: 10.1016/j.jad.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Sim K, Lau WK, Sim J, Sum MY, Baldessarini RJ. Prevention of relapse and recurrence in adults with major depressive disorder: Systematic review and meta-analyses of controlled trials. Int J Neuropsychopharmacol. 2015;18:pyv076. doi: 10.1093/ijnp/pyv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sinal CJ, et al. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275(51):40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 58.National Institutes of Health . Guide for the Care and Use of Laboratory Animals. 8th Ed National Institutes of Health; Bethesda, MD: 2011. [Google Scholar]
- 59.Hashimoto K, Ishima T. 2010. A novel target of action of minocycline in NGF-induced neurite outgrowth in PC12 cells: Translation initiation [corrected] factor eIF4AI. PLoS One 5(11):e15430, erratum in PLoS One (2010) 5(12): 10.1371/annotation/afc0a9a2-01c0-4e58-8d69-e0ed4ff953fa.
- 60.Ishima T, Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by ifenprodil: The role of sigma-1 and IP3 receptors. PLoS One. 2012;7(5):e37989. doi: 10.1371/journal.pone.0037989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishima T, et al. Potentiation of neurite outgrowth by brexpiprazole, a novel serotonin-dopamine activity modulator: A role for serotonin 5-HT1A and 5-HT2A receptors. Eur Neuropsychopharmacol. 2015;25(4):505–511. doi: 10.1016/j.euroneuro.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 62.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6(8):1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao T, et al. Effects of chronic social defeat stress on behavior and choline acetyltransferase, 78-kDa glucose-regulated protein, and CCAAT/enhancer-binding protein (C/EBP) homologous protein in adult mice. Psychopharmacology (Berl) 2013;228(2):217–230. doi: 10.1007/s00213-013-3028-6. [DOI] [PubMed] [Google Scholar]
- 64.Inceoglu B, et al. Epoxy fatty acids and inhibition of the soluble epoxide hydrolase selectively modulate GABA mediated neurotransmission to delay onset of seizures. PLoS One. 2013;8(12):e80922. doi: 10.1371/journal.pone.0080922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borhan B, Mebrahtu T, Nazarian S, Kurth MJ, Hammock BD. Improved radiolabeled substrates for soluble epoxide hydrolase. Anal Biochem. 1995;231(1):188–200. doi: 10.1006/abio.1995.1520. [DOI] [PubMed] [Google Scholar]
- 66.Kitamura S, et al. Potent natural soluble epoxide hydrolase inhibitors from Pentadiplandra brazzeana baillon: Synthesis, quantification, and measurement of biological activities in vitro and in vivo. PLoS One. 2015;10(2):e0117438. doi: 10.1371/journal.pone.0117438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81(19):8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ren Q, et al. BDNF-TrkB signaling in the nucleus accumbens shell of mice has key role in methamphetamine withdrawal symptoms. Transl Psychiatry. 2015;5:e666. doi: 10.1038/tp.2015.157. [DOI] [PMC free article] [PubMed] [Google Scholar]