Significance
Humans can remember the features of three or four visual objects for short periods of time. Individual differences in this working memory capacity, which accurately predict fluid intelligence and performance in numerous cognitive tasks, have been hypothesized to reflect variations in attentional processes that govern access to the memory system. However, the specific attention mechanism that differentiates high- and low-capacity individuals is unknown. Here, we show that differences in working memory capacity are specifically related to distractor-suppression activity in visual cortex. Our electrophysiological measures reveal that although high-capacity individuals are able to actively suppress distractors, low-capacity individuals cannot suppress them in time to prevent distractors from capturing attention.
Keywords: suppression, attention, working memory, event-related potentials, distractor positivity
Abstract
According to contemporary accounts of visual working memory (vWM), the ability to efficiently filter relevant from irrelevant information contributes to an individual’s overall vWM capacity. Although there is mounting evidence for this hypothesis, very little is known about the precise filtering mechanism responsible for controlling access to vWM and for differentiating low- and high-capacity individuals. Theoretically, the inefficient filtering observed in low-capacity individuals might be specifically linked to problems enhancing relevant items, suppressing irrelevant items, or both. To find out, we recorded neurophysiological activity associated with attentional selection and active suppression during a competitive visual search task. We show that high-capacity individuals actively suppress salient distractors, whereas low-capacity individuals are unable to suppress salient distractors in time to prevent those items from capturing attention. These results demonstrate that individual differences in vWM capacity are associated with the timing of a specific attentional control operation that suppresses processing of salient but irrelevant visual objects and restricts their access to higher stages of visual processing.
Each day, human observers perform numerous tasks that require temporary storage of information about objects in the surrounding visual environment. Laboratory studies have revealed substantial variability across neurologically healthy adults in the ability to keep such visuospatial information in mind (1–4). Originally, this variability was attributed to individual differences in the capacity of visual working memory (vWM). According to this account, the maximum amount of information that can be entered into vWM at one time, or the number of “slots” available to store the information, varies across individuals (3, 5–8). Other contemporary accounts, however, relate the individual differences in vWM performance to variability in attentional control, as well as capacity (9–12). One such attention-based perspective holds that when faced with multiple visual objects, low-capacity individuals have difficulty filtering relevant from irrelevant information (11–15). More specifically, this filtering-efficiency hypothesis proposes that attention regulates the flow of sensory information to the limited-capacity vWM system and that consuming capacity with task-irrelevant information effectively reduces storage capacity for task-relevant items. This hypothesis helps to explain why low-capacity individuals sometimes store more items in vWM than do high-capacity individuals: whereas high-capacity individuals encode only task-relevant items, low-capacity individuals encode irrelevant items along with task-relevant items (15).
Although there is mounting evidence for the filtering-efficiency hypothesis, little is known about the precise mechanism responsible for controlling access to vWM or how its operation differs in low- and high-capacity individuals. Theoretically, filtering can be achieved by enhancing the representation of a to-be-remembered item or by suppressing the representation of a to-be-ignored item (16). Accordingly, the inefficient filtering observed in low-capacity individuals might be linked to problems enhancing relevant items, problems suppressing irrelevant items, or both. Precise characterization of individual differences in filtering efficiency requires not only a method for determining what items gain access to vWM but also a method for isolating processes associated with the two diametrically opposed facets of filtering. Behavioral measures (e.g., negative priming) have been used to study the link between attention and vWM capacity (17, 18), but given the difficulty in linking such measures to specific processes (e.g., perceptual inhibition, memory retrieval), existing behavioral results do not clearly indicate whether individual differences in capacity are related to selective enhancement or suppression.
Researchers have started to develop event-related potential (ERP) methods to determine how attention-filtering capabilities vary as a function of vWM capacity. In one pair of studies (19, 20), participants were cued in advance to attend to the location of an impending visual target that was accompanied by at least one distractor item on the same side of fixation (with an equal number of items on the opposite side of fixation). After a brief interval, bilateral “probe” stimuli were presented to assess the spatial gradient of attention. ERPs elicited by the probes were used to compute an attention-gradient index, which was positive when attention was tightly focused at the target location and was near zero when attention was broadly distributed across the items in the cued hemifield. Low-capacity individuals were found to have a broader distribution of attention than high-capacity individuals. This finding could indicate that low-capacity individuals are unable to prevent the inadvertent capture of attention by nearby distractors (19), to boost the target’s representation over and above those of nearby distractors, or to maintain a tight focus of attention at the cued location before the appearance of the target display. At present, it is impossible to distinguish between these alternatives in part because the attention-gradient index that was used did not isolate target-selection and distractor-suppression processes separately.
In the present study, we recorded ERPs during a unidimensional variant of the additional singleton search paradigm and isolated specific components known to reflect stimulus selection (N2pc) and active suppression (distractor positivity, PD). The N2pc, an enhanced negative potential observed contralateral to attended targets, is a well-known electrophysiological index of attentional selection that emerges over the posterior scalp 180–200 ms after the appearance of a search array (21, 22). In contrast, the PD is an enhanced positive potential observed contralateral to task-irrelevant distractors in the same time interval (23, 24). Two key pieces of evidence indicate that the PD is associated with an active suppression process. First, the PD is present when observers must carefully inspect another task-relevant item (target) but is absent when observers merely have to detect the target (23). Second, the amplitude of the PD is predictive of the speed with which participants respond to a target on a trial-by-trial basis, with faster response times (i.e., less distraction) associated with larger PD amplitudes (24, 25). These findings indicate that the visual system resolves attentional competition in demanding identification tasks by suppressing potentially distracting items, but that the ability to suppress, and thus to prevent distraction, varies across trials.
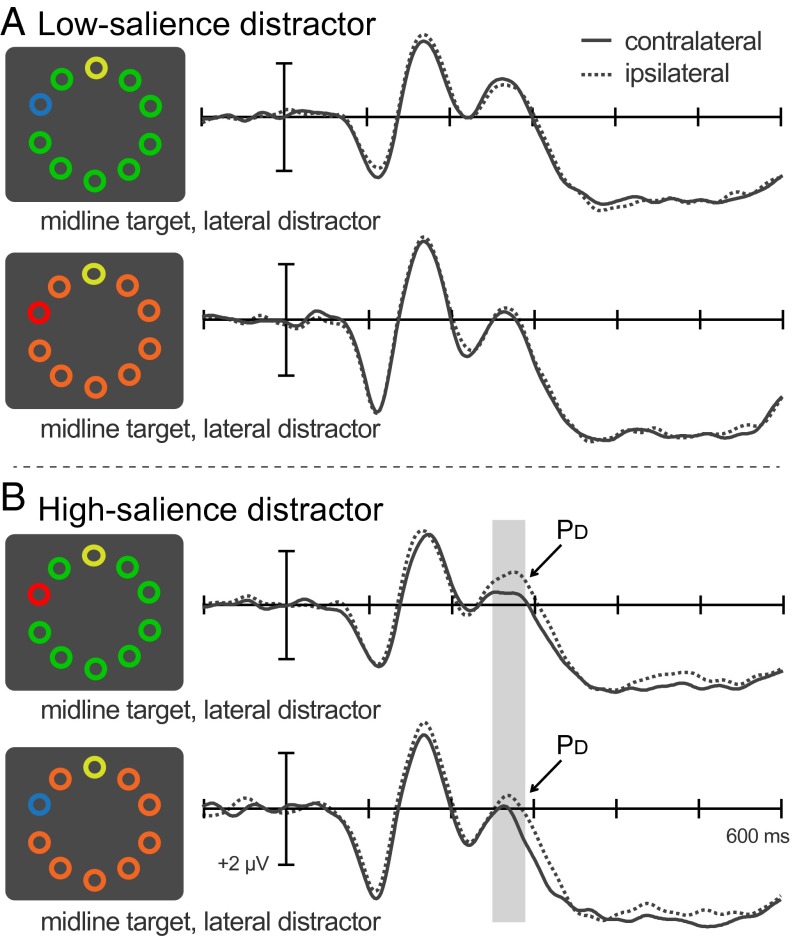
Armed with these two electrophysiological indices of attention, we asked whether individuals with higher vWM capacities are better able to select items of interest or to suppress potentially distracting items. Participants searched multiitem displays for a prespecified color singleton while attempting to ignore other, task-irrelevant color singletons that could appear in the same displays. Each display contained eight or nine same-color nontargets, one yellow target, and on distractor-present trials, one red or blue distractor (Fig. 1). The color of the nontargets was varied (all green or all orange) to disentangle distractor salience from distractor color. Specifically, the red distractor was the most salient singleton against green nontargets, whereas the blue distractor was the most salient singleton against orange nontargets (this was confirmed in a behavioral pilot experiment; SI Results). Target- and distractor-related ERPs were measured separately for individuals with low, medium, and high vWM capacities to determine whether the attentional deficits associated with low capacity are attributable to difficulties selecting an object of interest, actively suppressing irrelevant objects, or both.
Fig. 1.
ERPs elicited by displays containing a midline target and a lateral distractor for each nontarget condition. Time 0 reflects the onset of the search display, and negative voltage deflections are plotted above the x-axis, by convention. Waveforms were recorded over the lateral occipital scalp (electrodes PO7 and PO8). (A) ERPs recorded contralateral and ipsilateral to a low-salience distractor. (B) ERPs recorded contralateral and ipsilateral to a high-salience distractor.
SI Results
An initial singleton-detection task was performed to confirm that in the main experiment, only one distractor (per condition) was considerably more salient than the target. In the pilot, one of the singletons used in the main experiment appeared among either all green or all orange nontargets, and participants pressed a button when they detected the singleton. The mean RTs for the pilot were analyzed in a repeated-measures ANOVA with factors for nontarget color (green, orange) and singleton color (yellow, blue, red). With green nontargets, RTs were shorter for the red singleton (363 ms) than for the blue or yellow singleton (387 ms, 405 ms). With orange nontargets, RTs were shorter for the blue singleton (364 ms) than for the red or yellow singleton (411 ms, 402 ms). This pattern of results led to a significant interaction between the nontarget color and the singleton color [F(2,28) = 12.4; P < 0.001]. Planned Bonferroni-corrected t tests confirmed the following: on green-nontarget trials, RTs to the yellow singleton were longer than RTs to the red singleton [t(7) = 4.9; P = 0.003] but not the blue singleton [t(7) = 2.1; P = 0.268] (2); on orange-nontarget trials, RTs to the yellow singleton were significantly longer than RTs to the blue singleton [t(7) = 4.4; P = 0.008] but not the red singleton [t(7) = 1.0; P = 0.366].
Results
Behavior in Change-Detection Task.
In addition to the search task, participants performed in a change detection task used to measure vWM capacity (4). The average vWM capacity estimate was 2.5, with scores ranging from 1.8 to 4.0. The capacity estimates did not differ significantly across the two nontarget-color conditions [2.47 vs. 2.52; t(46) = 0.3; P = 0.778].
Behavior in Search Task.
Median reaction times (RTs) were computed for each participant, separately for the display configurations of interest. Interparticipant mean RTs were then derived by averaging participant median RTs across the different trial types. We first analyzed RTs from distractor-present trials as a function of distractor color (red, blue) and nontarget color (green, orange). Neither main effect was significant, Fs < 1, but the distractor color × nontarget color interaction was highly significant [F(1, 46) = 55.2; P < 0.001]. For green nontarget displays, RTs were longer when the distractor was red (636 ms) than when it was blue (620 ms) [t(23) = 4.9; P < 0.001]. Conversely, for orange nontarget displays, RTs were longer when the distractor was blue (620 ms) than when it was red (607 ms) [t(23) = 6.6; P < 0.001].
The result of the preliminary RT analysis confirmed that distractor salience, not distractor color per se, modulated performance in the additional singleton task used here. Accordingly, RT data from the two distractor colors were combined with RT data from the two nontarget colors to yield high- and low-salience distractor types. A distractor-absent level was added to assess the overall effects of high- and low-salience distractors on search performance. Interparticipant mean RTs were shortest on distractor-absent trials (605 ms), intermediate for low-salience distractor trials (613 ms), and longest in the high-salience distractor trials (628 ms), leading to a significant main effect of distractor type [F(2, 92) = 75.5; P < 0.001]. Interparticipant mean RTs across the three levels were all found to be statistically different from one another by pair-wise comparison (Ps < 0.001). These findings demonstrate that although both distractors delayed search, the high-salience distractor caused a longer delay (22 ms) than did the low-salience distractor (8 ms).
In addition to RT interference, we examined median RTs and the variability in each participant’s RTs to assess attention control capabilities. Individuals with improved attention-control capabilities have fewer lapses of attention, and thus perform more consistently (26–28). In terms of speeded response performance, both average/median RT and variability in RT have been shown to correlate negatively with general intelligence (29). Here, following previous behavioral and theoretical papers linking attention control to vWM capacity (2, 10, 30), we predicted that median RT and RT variability would correlate negatively with vWM capacity. This is exactly what was found (rs > −0.39; P < 0.007). That is, higher capacity was associated with faster and less variable responses.
Lateralized ERPs to Distractors.
To assess the processing of distractors, we analyzed ERPs elicited by displays containing a lateral distractor and a midline target. Differences between ERP waveforms recorded contralateral and ipsilateral to the distractor can be ascribed to distractor processing because midline color singletons do not trigger lateralized ERP activity (23, 31). On the basis of our recent findings (24), we anticipated a PD 250–290 ms after the onset of a high-salience distractor when averaged across all participants in the study. It was less clear whether the low-salience distractor would also elicit a PD in this time range.
ERP waveforms recorded contralateral and ipsilateral to a low-salience distractor largely overlapped throughout the plotting window (Fig. 1). For these trials, no PD was evident in either nontarget-color condition [green: t(23) = −1.0 (P = 0.345); orange: t(23) = 0.7 (P = 0.493); no difference between conditions: t(46) = 1.1 (P = 0.266)]. As expected, however, displays with lateral high-salience distractors were found to elicit the PD in both nontarget-color conditions [green, t(23) = 2.5 (P = 0.02); orange, t(23) = 2.1 (P = 0.046)]. This pattern indicates that an active suppression mechanism is called on to deal with irrelevant distractors, but only when the distractor is more salient than the target.
For high-salience distractors, neither the timing nor the amplitude of the PD was found to differ significantly between the two conditions [onset latencies: 274 ms vs. 293 ms; tc(33.4) = −1.3 (P = 0.19); mean amplitudes: 0.53 vs. 0.44 μV; t(46) = 0.3 (P = 0.756)]. As a consequence, the contralateral-ipsilateral difference waves were collapsed across the two conditions for all subsequent analyses. Unsurprisingly, the PD was highly significant in the full sample [t(47) = 3.3; P = 0.002]. PD amplitudes computed from two independent halves of trials were moderately correlated (r = 0.57; P < 0.001). This internal reliability is on par with the previously reported internal reliability of N2pc waves triggered by task-irrelevant images (32).
Distractor-Suppression Activity Predicts Individual Differences in Working Memory Capacity.
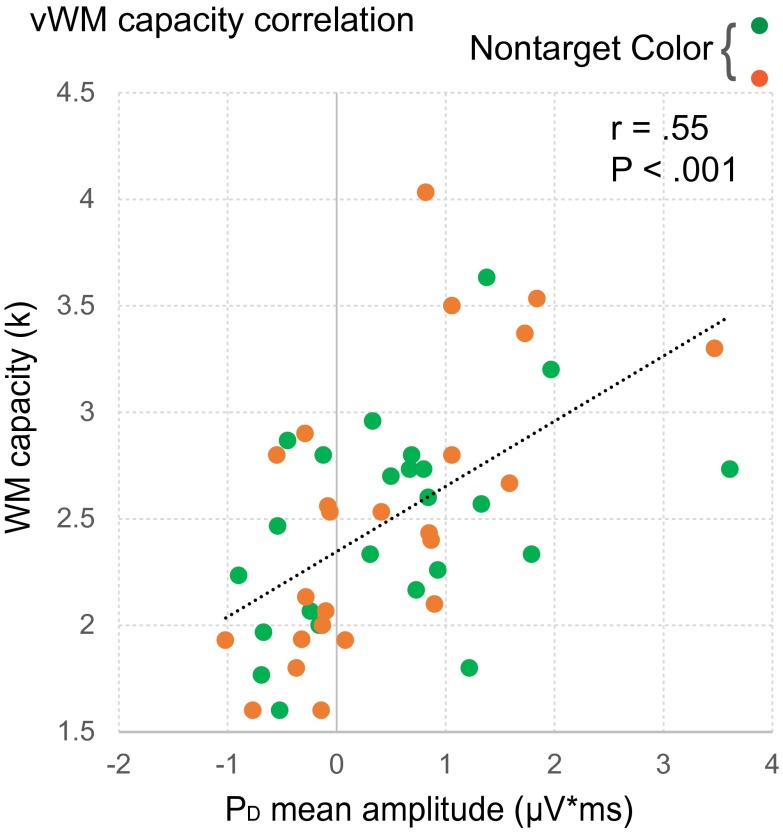
Participants with higher vWM capacity scores tended to have large positive amplitudes in the PD time interval, whereas participants with lower vWM capacity scores tended to have negative amplitudes in the PD time interval (Fig. S1). This led to a highly significant positive correlation between PD mean amplitude and vWM capacity across the 48 individuals in the study (r = 0.55; P < 0.001). Whereas the positive ERP amplitude can be ascribed to the PD, the negative ERP amplitude likely reflects a distractor-elicited N2pc (as the PD and N2pc time intervals overlapped considerably). Accordingly, the correlation indicates that the high-salience distractor elicited either a PD or an N2pc, depending in large part on an individual’s vWM capacity.
Fig. S1.
Neural activity associated with salient distractor suppression predicts visual working memory capacity. Correlation between memory capacity (k) and PD mean amplitude.
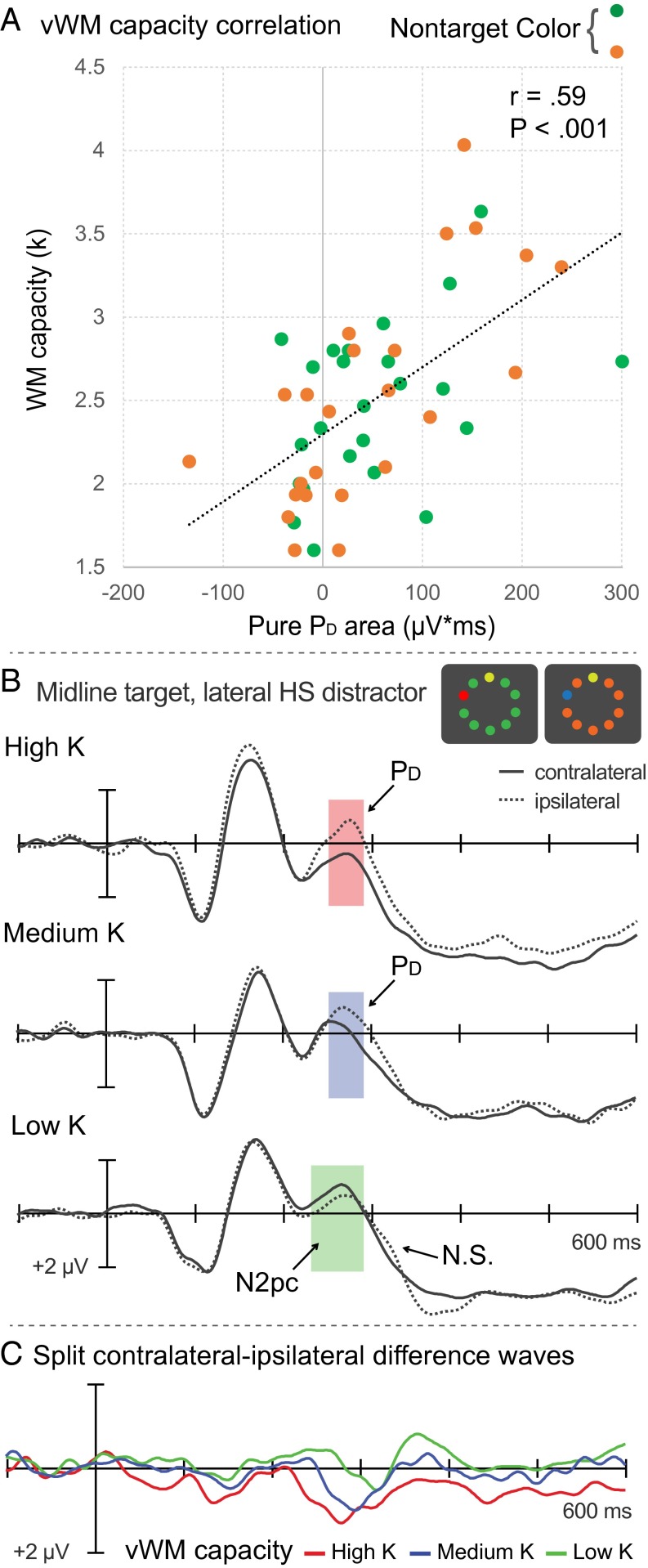
To further test the potential relationship between PD magnitude and vWM capacity, we isolated the PD from overlapping N2pc waves by computing the signed positive area within a 150-ms time window that allowed for considerable variability in the timing of PD across individuals. The signed positive area in an equally wide prestimulus baseline (noise) interval was subtracted from the signed positive area within the 200–350-ms window to obtain a measure of PD area that is not inflated by noise (herein, we use the term “pure” PD area to refer to this corrected measure). The mean of the resultant pure-PD area measurements was found to be internally reliable (r = 0.61; P < 0.001) and significant over the 150-ms PD window [t(47) = 4.1; P < 0.001]. Critically, a significant correlation between vWM capacity and pure-PD area was in evidence (r = 0.59; P < 0.001), thereby demonstrating that PD magnitude increased as a function of vWM capacity.
Next, we sought to determine whether vWM capacity was related to the timing of the PD. This analysis was premised on the hypothesis that high- and low-capacity individuals differ in terms of processing speed (33). This general hypothesis translated into a specific prediction that high-capacity individuals implement a distractor-suppression mechanism faster than do low-capacity individuals. To test this speed-of-suppression prediction, we computed jackknifed estimates of PD onset latency and correlated them with individual participant vWM capacity estimates (34, 35). The correlation was found to be statistically significant (r = −0.39; P < 0.006), thereby confirming the speed-of-suppression prediction.
To help visualize the relationship between vWM capacity and distractor-elicited ERPs, we sorted participants into three groups on the basis of their vWM capacity scores (Low-K, Medium-K, High-K) and constructed separate ERP waveforms for each group (Fig. 2 B and C). The PD was visibly reduced and delayed for the low-capacity group compared with the medium- and high-capacity groups. One-way ANOVAs confirmed that PD magnitude varied significantly across the three groups [mean amplitude: F(2,45) = 8.4 (P < 0.001); positive area: F(2,45) = 8.6 (P < 0.001)]. Follow-up t tests confirmed the presence of the PD for the medium- and high-capacity groups (Ps < 0.006), but the diminutive PD in the low-capacity group was nonsignificant (Ps < 0.18). A single, planned, pair-wise comparison revealed that the PD was significantly larger for the high-K group than for the low-K group [mean amplitude: t(30) = 3.8 (P < 0.001); positive area: t(30) = 4.0 (P < 0.001)].
Fig. 2.
Neural activity associated with salient distractor suppression predicts vWM capacity. (A) Correlation between memory capacity (k) and pure PD area (Materials and Methods). (B) ERP waveforms recorded contralateral and ipsilateral to the salient distractor plotted separately for high-, medium-, and low-capacity groups. (C) Contralateral-minus-ipsilateral difference waveforms for high-, medium-, and low-capacity groups.
As can be seen in Fig. 2 B and C, the low-capacity group’s diminutive PD was preceded by a contralateral negativity starting ∼230 ms after display onset. This early contralateral negativity occurred squarely in the typical N2pc time range, and thus almost certainly reflects a distractor-elicited N2pc similar to that observed previously (36). A t test revealed that the distractor N2pc was significant [t(15) = 2.3; P = 0.03). Together with the absence of a statistically significant PD in the low-capacity individuals, the presence of a distractor N2pc suggests that low-capacity individuals inadvertently oriented attention to salient distractors, whereas higher-capacity individuals managed to prevent salience-driven capture via suppression.
Target-Selection Activity Does Not Predict Individual Differences in Working Memory Capacity.
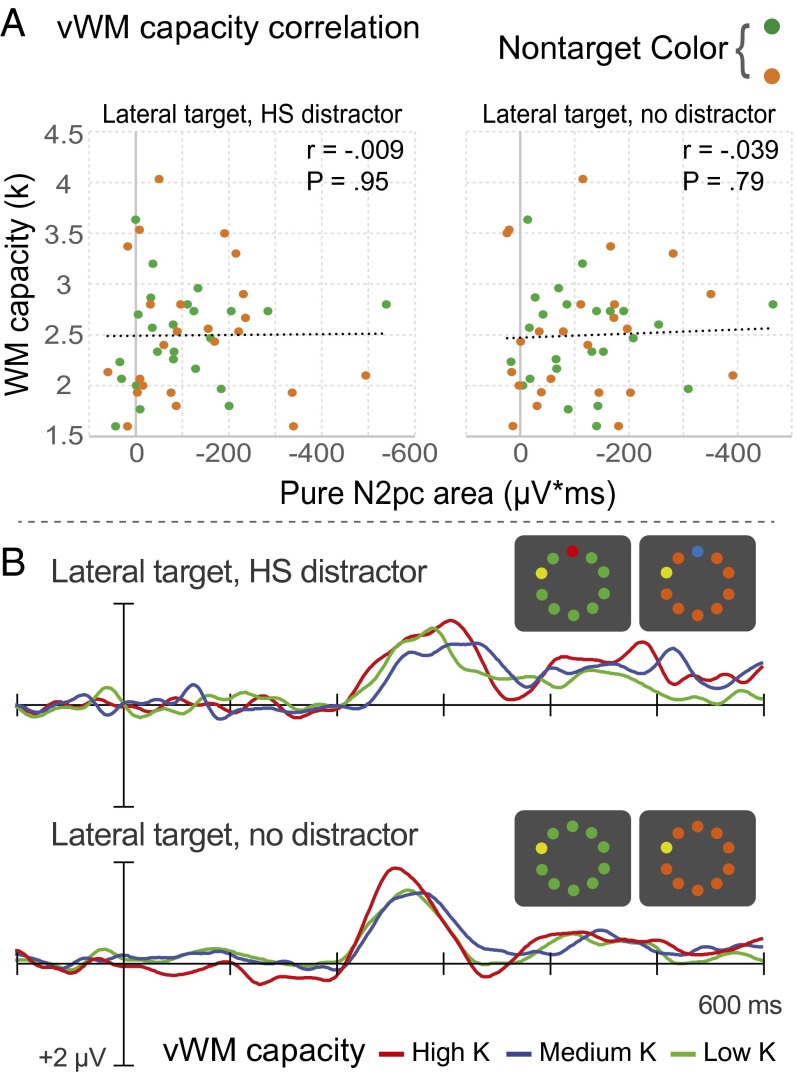
To assess the relationship between selective target processing and vWM capacity, we isolated target N2pc waves for two display configurations: lateral target, midline high-salience distractor, and lateral target, no distractor. As expected, the target elicited an N2pc in each configuration. Statistical analysis of both mean amplitude and signed negative area confirmed the presence of significant target N2pc waves (ts > −5.2; Ps < 0.001) and indicated that the N2pc measures were internally reliable (rs > 0.67; Ps < 0.001). Despite the clear presence of N2pc, however, neither the mean amplitude nor the signed negative area of N2pc was found to correlate with vWM capacity in either display configuration (rs < 0.08; Ps > 0.59; Fig. 3A). Furthermore, no significant relationship was found between Jackknife N2pc onset latencies and vWM capacity (rs < 0.13; Ps > 0.38).
Fig. 3.
Neural activity associated with target processing not predictive of visual working memory capacity. (A) Correlation between memory capacity (k) and pure N2pc area for lateral-target displays of interest. (B) Contralateral-minus-ipsilateral difference waveforms for high-, medium-, and low-capacity groups.
Fig. 3B shows the target N2pc waves elicited by the two display configurations of interest, separately for Low-K, Medium-K, and High-K groups (collapsed over nontarget-color conditions). Consistent with the correlational analyses, one-way ANOVAs revealed no significant variation across groups in N2pc magnitude (mean amplitude: Fs < 0.7, Ps > 0.512; negative area: Fs < 0.5, Ps > 0.620) or N2pc onset latency (Fs < 0.6; Ps > 0.552).
Revisiting Response Times.
As noted earlier, RT medians and SDs were found to correlate negatively with vWM capacity. Here we asked whether any target- or distractor-elicited ERP component correlated with the two behavioral performance measures. PD latency was correlated with median RT (r = 0.23; P = 0.119) and with RT SD (r = 0.25; P = 0.093), although the correlations were only marginally significant by two-tailed tests. In addition, target N2pc latency was found to correlate with RT SD (r = 0.29; P = 0.05). These results indicate that as the latencies of the PD and target N2pc increased, speeded RT performance became more variable. No other correlation was statistically significant.
Discussion
Researchers have hypothesized that individual differences in attention-control capabilities contribute to variations in vWM capacity (2, 30). Supporting evidence for this hypothesis has come from studies looking at behavioral measures of attention control, such as negative priming (17, 18) and enumeration (37). Across a wide variety of tasks, low-capacity individuals appear to have greater difficulty in restricting controlled attention processes to task-relevant visual stimuli and in preventing irrelevant information from accessing vWM and other capacity-limited systems (13, 15). On the basis of behavior measures alone, however, it is difficult to determine whether difficulties in suppressing irrelevant stimuli or difficulties in selecting relevant stimuli underlie the relationship between attention control and vWM capacity. Researchers have considered whether high- and low-capacity individuals differ in the ability to prevent distractors from capturing attention (19), but the extant ERP findings have not supported this hypothesis (20).
We resolved this enduring issue by isolating neural measures of attentional processing during a competitive visual search task. Whereas prior studies used relatively general measures of attentional processing, the N2pc and PD enabled us to pinpoint specific processes and to determine whether the magnitude or timing of these processes correlated with vWM capacity. The N2pc was originally ascribed to suppression of unattended items (22), but more recent work indicates the contralateral negativity reflects selective processing of the attended item (23, 38, 39), whereas the PD reflects activity associated with the suppression of unattended distractors (23, 24; for a review, see ref. 34). Here, the target N2pc was found to be unrelated to vWM capacity, suggesting low- and high-capacity individuals do not differ in their capabilities to select target singletons that pop out from the rest of the search display. In contrast, variability in the timing and magnitude of the PD was found to be highly predictive of individual differences in vWM capacity. Namely, low-capacity individuals were less able to suppress a salient distractor in time to prevent that item from diverting attention.
In the present study, processing of low-salience distractors was not predictive of individual differences in vWM capacity. This finding appears to be at odds with prior change-detection studies, in which observers were asked to remember some visual items but not other, equally salient items (15). Specifically, in a variety of change-detection tasks, high-capacity individuals successfully prevented low-salient distractors from accessing vWM, whereas low-capacity individuals were unable to do so. Why, then, was such a difference not apparent in the present study? One possibility is that the rejection of low-salience distractors requires more effort during change-detection tasks than during fixed-feature search and that the observed links between distractor processing and vWM capacity are determined by the degree to which each requires effort. This possibility is in line with attention-control theories of vWM capacity, according to which capacity and performance differences emerge only when the task requires controlled processing (37). From this perspective, it is entirely conceivable that vWM capacity would correlate with suppression of low-salient distractors in more challenging visual search tasks.
The present study has important implications for the long-standing debate over the degree to which visual attention is stimulus-driven or under top-down control. According to the stimulus-driven capture theory, attention is first deployed to the most salient item in the display, irrespective of an observer’s intentions (40, 41). According to other theories, voluntary control of attention can prevent salient distractors from capturing attention, thereby enabling rapid deployment of attention to task-relevant objects (42–44). Recent ERP evidence has indicated that salient distractors do not capture attention (24, 44–48), but those studies were designed to determine whether the average individual attends to salient distractors. Functional neuroimaging studies have reported frontal lobe activity that correlates negatively with behavioral measures of attention capture) (49, 50). Similar to these latter studies, the present ERP study highlights the importance of looking at individual differences and reveals substantial variation in the ability to prevent stimulus-driven attention capture. We conclude that although many individuals often manage to prevent salience-driven capture of attention, low-capacity individuals frequently fail to do so.
Materials and Methods
The Research Ethics Board at Simon Fraser University approved the research protocol used in this study.
Participants.
Fifty-five students from Simon Fraser University participated after giving informed consent. The students received course credit for their participation as part of a departmental research participation program. Seven subjects were excluded because more than 25% of the trials were rejected as a result of excessive ocular artifacts (rejection criterion set in advance). Of the remaining 48 participants, 24 participated in Condition 1 (20 women, age 20.8 ± 2.2 y), and 24 participated in Condition 2 (13 women, age 20.0 ± 2.85 y; 1 left-handed). All subjects reported normal or corrected-to-normal visual acuity and were tested for typical color vision, using Ishihara color test plates.
Stimuli and Apparatus.
Stimuli were presented on a 23-inch, 120-Hz LCD monitor viewed from a distance of 57 cm. Visual search arrays comprised 10 unfilled circles presented equidistant (9.2°) from a central fixation point. Each circle was 3.4° in diameter with a 0.3° thick outline. Eight or nine of the circles were uniformly colored nontargets, one was a target color singleton, and one was a distractor color singleton (on distractor-present trials). The target was dark yellow (x = 0.416, y = 0.519, 7.88 cd/m2), and the distractor was either red (x = 0.640, y = 0.324, 6.95 cd/m2) or blue (x = 0.179, y = 0.199, 7.87 cd/m2). A randomly oriented vertical or horizontal gray line (x = 0.295, y = 0.361, 7.89 cd/m2) was contained within each of the circles. In Condition 1, the nontarget circles were green (x = 0.288, y = 0.636, 7.85 cd/m2), and in Condition 2, they were orange (x = 0.563, y = 0.402, 7.87 cd/m2). All stimuli were presented on a uniform black background (0.5 cd/m2).
The three singleton colors were selected so that the salience of the target would be approximately equal to that of one distractor and considerably less than that of the other distractor. Salience was considered in two ways: as the local contrast between the (uniform) nontargets and each color singleton, and as the rapidity with which each singleton could be detected. Local contrast was measured as the distance in CIE [Commission Internationale de l’Éclairage (International Commission on Illumination)] chromaticity space between the nontarget color and a singleton color (herein called color distance). In each condition, the color distance was considerably larger for one distractor (e.g., red distractor vs. green nontargets) than for the target (e.g., yellow target vs. green nontargets). After candidate colors for all five items were selected, a behavioral experiment was conducted, wherein participants (n = 8) were required to detect any singleton (yellow, red, or blue) appearing with either the green or orange nontargets. The six combinations of colors were presented in separate blocks, and on each trial, there was an equal probability the target would be present or absent (SI Results).
Procedure.
On each trial, a search display was presented after an 800–1,200-ms fixation period, during which only the central fixation point was visible. Participants were instructed to maintain fixation on the central point and to identify the orientation of the gray line inside the target singleton by pressing one of two response buttons as quickly as possible. The search array remained visible for 100 ms after a response was registered, at which point the next trial began.
Displays contained a target singleton and one distractor singleton on 66% of trials (distractor-present trials). On half of these trials, the distractor singleton was more salient than the target (high-salience distractor trials), and on the other half of these trials, it was no more salient than the target (low-salience distractor trials). On the remaining 33% of trials, the target was the only singleton in the array (distractor-absent trials).
Target and distractor locations were varied to produce the following display configurations: lateral target, no distractor (22.0%); midline target, no distractor (11.3%); lateral target, midline distractor (14.7%); lateral target, ipsilateral distractor (14.7%); lateral target, contralateral distractor (14.7%); midline target, lateral distractor (14.7%); midline target, midline distractor (8.0%). There were equal numbers of both high- and low-salience distractors that appeared within each block of trials. These display configurations were randomly intermixed across trials. Each experimental block comprised 108 trials, with a 5-s break after every 36 trials. At the end of the block, participants were given a minimum 5-s rest period and could begin the subsequent block when ready. The experiment contained 12 blocks, for a total of 1,296 trials per participant. At least 36 practice trials were given to each participant before commencing the experiment.
Working Memory Capacity.
Before the main experiment, participants completed a change detection task to assess working memory capacity. Trials (n = 120) consisted of a 150-ms memory array display containing two, four, six, or eight colored squares, followed by a probe item in one of the previously occupied locations. The participant's task was to indicate whether the color of the probe matched that of the probed memory array item or not. vWM capacity, K, was computed separately for the four set sizes, using a standard equation (1, 51). K scores were then averaged across all sets. All other methods were identical to those in ref. 4.
Electrophysiological Recording and Analysis.
EEG and electrooculogram (EOG) were recorded from active sintered Ag-AgCl electrodes (Biosemi Active Two system) from 125 standard sites and three nonstandard subinion sites. EEG recording, filtering, and artifact rejection were performed using our standard methods (24) (SI Materials and Methods). Lateralized ERP waveforms were created for each condition for the following display configurations: midline target, lateral high-salience distractor, and midline target, lateral low-salience distractor. ERPs to these search displays were created by collapsing across left and right visual hemifields and left and right electrodes (P07 and P08) to produce waveforms recorded ipsilateral and contralateral to distractor stimuli (Fig. 1). Negative voltages were plotted upward, so that the N2pc and PD would appear in these difference waveforms as upward and downward deflections, respectively. Lateralized ERP difference waveforms were then derived by subtracting the ipsilateral waveform from the corresponding contralateral waveform. All ERP statistics were computed using contralateral minus ipsilateral difference values.
PD and N2pc magnitudes were measured using a conventional mean-amplitude approach and a new signed-area approach. By convention, we selected electrodes and time windows a priori based on existing studies that measured mean amplitudes (thereby avoiding problems of multiple implicit comparisons) (34). Both components were measured at lateral occipital electrodes PO7/PO8, as in most previous papers. The N2pc window was 230–290 ms (36), whereas the PD window was 250–290 ms (24). The slight offset of these two measurement windows is consistent with studies showing a slightly later onset for the PD than for the N2pc (23).
Our signed area approach was developed to complement the conventional mean-amplitude approach, which both permits very little interparticipant variability in the timing of an ERP component and can have difficulty isolating one component of interest (e.g., PD) when other, opposite-polarity components can occur in the same time window (e.g., distractor N2pc). Isolated PD and N2pc magnitudes were measured as the signed positive area and the signed negative area, respectively, within a 200–350-ms window that spanned the conventional measurement windows. These signed area measures were computed from each individual participant’s contralateral-ipsilateral difference waveform so that we could assess the relationship between the area of each ERP component and vWM capacity across participants. However, noise can contribute to the signed area measures described here, thereby inflating the type 1 error rate (25, 34). To prevent this inflation, we measured the signed (positive or negative) area within a 150-ms interval immediately preceding stimulus onset and subtracted it from the corresponding “raw” area measure on the grounds that the prestimulus fluctuations in the difference waveforms are attributable to noise. The resultant “pure” signed area measures were then analyzed using parametric statistical measures (t, F, Pearson’s r) that are robust against moderately large deviations from normality (52).
Onset latencies of PD and N2pc were measured as the 50% fractional peak amplitude within the 200–350-ms window. Statistical tests on latencies were performed using conventional jackknife procedures (53, 54).
Split-half reliability analyses were conducted by computing correlations of the half-data averages of randomized trials. All split-half correlations were corrected for using the Spearman–Brown equation (55).
To visualize how differences in working memory capacity related to distractor-related processing, ERPs were constructed for three subgroups of participants based on rank-ordered K scores (n = 16 per group; Fig. 2). K scores ranged from 2.73 to 4.03 for the high-capacity group, from 2.16 to 2.73 for the medium-capacity group, and from 1.60 to 2.13 for the low-capacity group.
SI Materials and Methods
Horizontal EOGs were recorded using two electrodes positioned 1 cm lateral to the external canthi, and vertical EOGs were recorded using two electrodes positioned above and below the right eye. All EEG and EOG signals were digitized at 512 Hz, referenced in real time to an active common-mode electrode, and low-pass filtered using a fifth-order sinc filter with a −3 dB cutoff at 104 Hz. Electrode offsets were monitored to ensure the quality of the data. After the data acquisition, EEG data for each channel were high-pass filtered (−3 dB point at 0.05 Hz) and then converted from 24-bit to 12-bit integers.
EEG processing and ERP averaging were performed using event-related potential software system (ERPSS) (University of California, San Diego). A semiautomated procedure was used to discard epochs of EEG contaminated by blinks, eye movements, or excessive noise (56). Any trial with an artifact within a 1-s interval (−200–800 ms poststimulus) was rejected. Artifact-free epochs associated with the various display configurations of interest were then averaged separately to create ERP waveforms. The resulting ERPs were digitally low-pass filtered (−3 dB point at 32 Hz) and digitally rereferenced to the average of the left and right mastoids. All ERP amplitudes and baselines were computed using a 200-ms prestimulus window. The averaged event-related horizontal EOGs did not exceed 2 μV for any individual participant, indicating their gaze remained within 0.3° of the fixation point for a majority of the trials (57).
Acknowledgments
We thank Ashley Livingstone and Alannah Wallace for assistance with data collection. This study is supported by grants from the Natural Sciences and Research Council of Canada, Canadian Foundation for Innovation, and Canada Research Chairs program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 3422.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523471113/-/DCSupplemental.
References
- 1.Cowan N. Metatheory of storage capacity limits. Behav Brain Sci. 2001;24(01):154–176. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- 2.Engle RW, Kane MJ, Tuholski SW. Individual differences in working memory capacity and what they tell us about controlled attention, general fluid intelligence, and functions of the prefrontal cortex. In: Miyake A, Shah P, editors. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge Univ Press; Cambridge: 1999. pp. 102–134. [Google Scholar]
- 3.Vogel EK, Woodman GF, Luck SJ. Storage of features, conjunctions and objects in visual working memory. J Exp Psychol Hum Percept Perform. 2001;27(1):92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]
- 4.Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428(6984):748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez GA, Cavanagh P. The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychol Sci. 2004;15(2):106–111. doi: 10.1111/j.0963-7214.2004.01502006.x. [DOI] [PubMed] [Google Scholar]
- 6.Awh E, Barton B, Vogel EK. Visual working memory represents a fixed number of items regardless of complexity. Psychol Sci. 2007;18(7):622–628. doi: 10.1111/j.1467-9280.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 7.Barton B, Ester EF, Awh E. Discrete resource allocation in visual working memory. J Exp Psychol Hum Percept Perform. 2009;35(5):1359–1367. doi: 10.1037/a0015792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Luck SJ. Discrete fixed-resolution representations in visual working memory. Nature. 2008;453(7192):233–235. doi: 10.1038/nature06860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conway AR, Engle RW. Working memory and retrieval: A resource-dependent inhibition model. J Exp Psychol Gen. 1994;123(4):354–373. doi: 10.1037//0096-3445.123.4.354. [DOI] [PubMed] [Google Scholar]
- 10.Kane MJ, Bleckley MK, Conway AR, Engle RW. A controlled-attention view of working-memory capacity. J Exp Psychol Gen. 2001;130(2):169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- 11.Shipstead Z, Zach S, Lindsey DRB, Marshall RL, Engle RW. The mechanisms of working memory capacity: Primary memory, secondary memory, and attention control. J Mem Lang. 2014;72:116–141. [Google Scholar]
- 12.Unsworth N, Fukuda K, Awh E, Vogel EK. Working memory and fluid intelligence: Capacity, attention control, and secondary memory retrieval. Cognit Psychol. 2014;71:1–26. doi: 10.1016/j.cogpsych.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Awh E, Vogel EK. The bouncer in the brain. Nat Neurosci. 2008;11(1):5–6. doi: 10.1038/nn0108-5. [DOI] [PubMed] [Google Scholar]
- 14.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11(1):103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- 15.Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438(7067):500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- 16.LaBerge D. Attentional Processing: The Brain’s Art of Mindfulness. Harvard University Press; Cambridge, MA: 1995. [Google Scholar]
- 17.Conway AR, Tuholski SW, Shisler RJ, Engle RW. The effect of memory load on negative priming: An individual differences investigation. Mem Cognit. 1999;27(6):1042–1050. doi: 10.3758/bf03201233. [DOI] [PubMed] [Google Scholar]
- 18.Long DL, Prat CS. Working memory and stroop interference: An individual differences investigation. Mem Cognit. 2002;30(2):294–301. doi: 10.3758/bf03195290. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda K, Vogel EK. Human variation in overriding attentional capture. J Neurosci. 2009;29(27):8726–8733. doi: 10.1523/JNEUROSCI.2145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda K, Vogel EK. Individual differences in recovery time from attentional capture. Psychol Sci. 2011;22(3):361–368. doi: 10.1177/0956797611398493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31(3):291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 22.Luck SJ, Hillyard SA. Spatial filtering during visual search: Evidence from human electrophysiology. J Exp Psychol Hum Percept Perform. 1994;20(5):1000–1014. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- 23.Hickey C, Di Lollo V, McDonald JJ. Electrophysiological indices of target and distractor processing in visual search. J Cogn Neurosci. 2009;21(4):760–775. doi: 10.1162/jocn.2009.21039. [DOI] [PubMed] [Google Scholar]
- 24.Gaspar JM, McDonald JJ. Suppression of salient objects prevents distraction in visual search. J Neurosci. 2014;34(16):5658–5666. doi: 10.1523/JNEUROSCI.4161-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawaki R, Geng JJ, Luck SJ. A common neural mechanism for preventing and terminating the allocation of attention. J Neurosci. 2012;32(31):10725–10736. doi: 10.1523/JNEUROSCI.1864-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen AR. The importance of intraindividual variation in reaction time. Pers Individ Dif. 1992;13(8):869–881. [Google Scholar]
- 27.Kane MJ, Engle RW. Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. J Exp Psychol Gen. 2003;132(1):47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- 28.Schmiedek F, Oberauer K, Wilhelm O, Süss H-M, Wittmann WW. Individual differences in components of reaction time distributions and their relations to working memory and intelligence. J Exp Psychol Gen. 2007;136(3):414–429. doi: 10.1037/0096-3445.136.3.414. [DOI] [PubMed] [Google Scholar]
- 29.Jensen AR. Why Is Reaction Time Correlated With Psychometric g? Curr Dir Psychol Sci. 1993;2(2):53–56. [Google Scholar]
- 30.Engle RW. Working Memory Capacity as Executive Attention. Curr Dir Psychol Sci. 2002;11(1):19–23. [Google Scholar]
- 31.Woodman GF, Luck SJ. Serial deployment of attention during visual search. J Exp Psychol Hum Percept Perform. 2003;29(1):121–138. doi: 10.1037//0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]
- 32.Kappenman ES, Farrens JL, Luck SJ, Proudfit GH. Behavioral and ERP measures of attentional bias to threat in the dot-probe task: Poor reliability and lack of correlation with anxiety. Front Psychol. 2014;5:1368. doi: 10.3389/fpsyg.2014.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jannati A, McDonald JJ, Di Lollo V. Individual differences in rate of encoding predict estimates of visual short-term memory capacity (K) Can J Exp Psychol. 2015;69(2):213–220. doi: 10.1037/cep0000048. [DOI] [PubMed] [Google Scholar]
- 34.Luck SJ. An Introduction to the Event-Related Potential Technique. MIT Press; Cambridge, MA: 2014. [Google Scholar]
- 35.Stahl J, Gibbons H. The application of jackknife-based onset detection of lateralized readiness potential in correlative approaches. Psychophysiology. 2004;41(6):845–860. doi: 10.1111/j.1469-8986.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- 36.Hickey C, McDonald JJ, Theeuwes J. Electrophysiological evidence of the capture of visual attention. J Cogn Neurosci. 2006;18(4):604–613. doi: 10.1162/jocn.2006.18.4.604. [DOI] [PubMed] [Google Scholar]
- 37.Tuholski SW, Engle RW, Baylis GC. Individual differences in working memory capacity and enumeration. Mem Cognit. 2001;29(3):484–492. doi: 10.3758/bf03196399. [DOI] [PubMed] [Google Scholar]
- 38.Mazza V, Turatto M, Caramazza A. Attention selection, distractor suppression and N2pc. Cortex. 2009;45(7):879–890. doi: 10.1016/j.cortex.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Eimer M. ERP modulations indicate the selective processing of visual stimuli as a result of transient and sustained spatial attention. Psychophysiology. 1996;33(1):13–21. doi: 10.1111/j.1469-8986.1996.tb02104.x. [DOI] [PubMed] [Google Scholar]
- 40.Theeuwes J. Perceptual selectivity for color and form. Percept Psychophys. 1992;51(6):599–606. doi: 10.3758/bf03211656. [DOI] [PubMed] [Google Scholar]
- 41.Theeuwes J. Top-down and bottom-up control of visual selection. Acta Psychol (Amst) 2010;135(2):77–99. doi: 10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. J Exp Psychol Hum Percept Perform. 1992;18(4):1030–1044. [PubMed] [Google Scholar]
- 43.Wolfe JM. Guided Search 2.0 A revised model of visual search. Psychon Bull Rev. 1994;1(2):202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- 44.Sawaki R, Luck SJ. Capture versus suppression of attention by salient singletons: Electrophysiological evidence for an automatic attend-to-me signal. Atten Percept Psychophys. 2010;72(6):1455–1470. doi: 10.3758/APP.72.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jannati A, Gaspar JM, McDonald JJ. Tracking target and distractor processing in fixed-feature visual search: Evidence from human electrophysiology. J Exp Psychol Hum Percept Perform. 2013;39(6):1713–1730. doi: 10.1037/a0032251. [DOI] [PubMed] [Google Scholar]
- 46.Leblanc E, Prime DJ, Jolicoeur P. Tracking the location of visuospatial attention in a contingent capture paradigm. J Cogn Neurosci. 2008;20(4):657–671. doi: 10.1162/jocn.2008.20051. [DOI] [PubMed] [Google Scholar]
- 47.Wykowska A, Schubö A. Irrelevant singletons in visual search do not capture attention but can produce nonspatial filtering costs. J Cogn Neurosci. 2011;23(3):645–660. doi: 10.1162/jocn.2009.21390. [DOI] [PubMed] [Google Scholar]
- 48.McDonald JJ, Green JJ, Jannati A, Di Lollo V. On the electrophysiological evidence for the capture of visual attention. J Exp Psychol Hum Percept Perform. 2013;39(3):849–860. doi: 10.1037/a0030510. [DOI] [PubMed] [Google Scholar]
- 49.de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291(5509):1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- 50.Leber AB. Neural predictors of within-subject fluctuations in attentional control. J Neurosci. 2010;30(34):11458–11465. doi: 10.1523/JNEUROSCI.0809-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pashler H. Familiarity and visual change detection. Percept Psychophys. 1988;44(4):369–378. doi: 10.3758/bf03210419. [DOI] [PubMed] [Google Scholar]
- 52.Keppel G, Wickens TD. Design and Analysis: A Researcher’s Handbook. Pearson College Division; Upper Saddle River, NJ: 2004. [Google Scholar]
- 53.Ulrich R, Miller J. Using the jackknife-based scoring method for measuring LRP onset effects in factorial designs. Psychophysiology. 2001;38(5):816–827. [PubMed] [Google Scholar]
- 54.Kiesel A, Miller J, Jolicoeur P, Brisson B. Measurement of ERP latency differences: A comparison of single-participant and jackknife-based scoring methods. Psychophysiology. 2008;45(2):250–274. doi: 10.1111/j.1469-8986.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- 55.Anastasi A, Urbina S. Psychological Testing. Pearson College Division; Upper Saddle River, NJ: 2009. [Google Scholar]
- 56.Green JJ, Conder JA, McDonald JJ. Lateralized frontal activity elicited by attention-directing visual and auditory cues. Psychophysiology. 2008;45(4):579–587. doi: 10.1111/j.1469-8986.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- 57.McDonald JJ, Ward LM. Spatial relevance determines facilitatory and inhibitory effects of auditory covert spatial orienting. J Exp Psychol Hum Percept Perform. 1999;25(5):1234–1252. [Google Scholar]