Significance
Many classes of G protein-coupled receptors (GPCRs) produce truncated variants. Truncated forms of the mu opioid receptor gene Oprm1 containing only six transmembrane domains (6TM) can mediate a potent analgesia without producing many classical opioid side effects. We now show that 6TM Oprm1 splice variants are essential in the analgesic actions of delta and kappa opioids as well as α2 adrenergic drugs, but not neurotensin, cannabinoids, or muscarinic drugs. The role of the 6TM variants seems limited to analgesia because they are not involved with kappa aversion, delta-induced seizure activity, or α2 adrenergic hypolocomotion. These findings emphasize the importance of 6TM Oprm1 variants in opioid and nonopioid sensory processing and illustrate the potential importance of the vast array of other classes of truncated GPCR variants.
Keywords: GPCR, truncation, analgesia, mu opioid receptor, morphine
Abstract
The clinical management of severe pain depends heavily on opioids acting through mu opioid receptors encoded by the Oprm1 gene, which undergoes extensive alternative splicing. In addition to generating a series of prototypic seven transmembrane domain (7TM) G protein-coupled receptors (GPCRs), Oprm1 also produces a set of truncated splice variants containing only six transmembrane domains (6TM) through which selected opioids such as IBNtxA (3′-iodobenzoyl-6β-naltrexamide) mediate a potent analgesia without many undesirable effects. Although morphine analgesia is independent of these 6TM mu receptor isoforms, we now show that the selective loss of the 6TM variants in a knockout model eliminates the analgesic actions of delta and kappa opioids and of α2-adrenergic compounds, but not cannabinoid, neurotensin, or muscarinic drugs. These observations were confirmed by using antisense paradigms. Despite their role in analgesia, loss of the 6TM variants were not involved with delta opioid-induced seizure activity, aversion to the kappa drug U50,488H, or α2-mediated hypolocomotion. These observations support the existence of parallel opioid and nonopioid pain modulatory systems and highlight the ability to dissociate unwanted delta, kappa1, and α2 actions from analgesia.
Modulation of pain is complex, with contributions from a variety of neurotransmitter systems. The opioid systems are particularly prominent clinically because of the availability of many potent and effective drugs, particularly those acting through mu opioid receptors. The mu opioid receptor gene, Oprm1, is complex, containing two independent promoters that generate dozens of variants through both 5′ and 3′ alternative splicing patterns that are highly conserved among multiple species (SI Appendix, Fig. S1) (1). Like the first mu opioid receptor (Oprm1) clone, MOR-1, most of the variants are traditional seven transmembrane domain (7TM) G protein-coupled receptors (GPCRs) generated from the exon 1 (E1) promoter. Each contains exons 1, 2, and 3, differing only at the intracellular C terminus due to 3′ splicing. The exon 11 (E11) promoter, located ∼30 kb upstream of exon 1, generates a set of truncated proteins lacking the first transmembrane domain because of the absence of exon 1, resulting in only six transmembrane domains (6TM).
Classically, opioid mechanisms were defined pharmacologically by using selective agonists and antagonists, but genetic approaches offer a more precise approach toward assessing the pharmacological contributions of the various Oprm1 splice variants. Mice have been developed that have the selective loss of only 6TM variants (E11 KO) (2), only the exon 1-containing 7TM and 1TM variants (E1 KO) (3) or the loss of all Oprm1 variants (E1/E11 KO) (4) (SI Appendix, Fig. S1). These models provide insights into the differing pharmacology of the full-length 7TM and the truncated 6TM variants. Full-length 7TM variants are essential for morphine actions (3, 5–7). However, morphine analgesia is unaffected in a mouse lacking only 6TM variants (2). Truncated splice forms of GPCRs are relatively common, but were typically thought to have little significance (8). Thus, the lack of a 6TM contribution to morphine analgesia initially was not surprising. However, truncated 6TM variants are important in the actions of a number of other mu opioids (1, 2, 4, 9–11). For example, some compounds act through 6TM variants and independently of 7TM variants, to elicit potent analgesia in thermal, neuropathic, and inflammatory pain models without producing many of the undesirable effects associated with opioid drugs, such as respiratory depression, physical dependence, or reward behavior (1, 2, 4, 9–11). Reconstitution of a 6TM in a full Oprm1 knockout by using viral vectors confirmed that a 6TM was both necessary and sufficient for their activity (4). We now show that E11 variants play a more widespread role in the modulation of nociception and are essential in delta and kappa1 opioid and α2 adrenergic analgesic systems.
Results
The importance of 6TM variants in IBNtxA analgesia has been established (9–12). We now have extended our studies of these truncated forms of the mu opioid receptor to explore other classes of analgesics. First, we explored the kappa1 receptor, generated from Oprk1, another member of the opioid receptor family. The kappa opioid U50,488H elicited a robust analgesia in wild-type (WT) mice both systemically or supraspinally, but not in E11 KO mice, even at doses many fold greater than their ED50 values in the WT mice when given systemically, supraspinally (i.c.v.), or intrathecally (i.t.) (Table 1, Fig. 1A, and SI Appendix, Figs. S2 and S3). Salvinorin A is a highly selective kappa ligand (13, 14). Like U50,488H, its analgesic response was lost in E11 KO mice (Table 1 and SI Appendix, Fig. S2). The importance of the 6TM variants in kappa opioid analgesia was confirmed in an antisense paradigm (15, 16). Antisense approaches offer the ability to down-regulate expression levels in adults without impacting development and potential compensatory systems. Selective down-regulation of 6TM variants using an exon 11 antisense lowered U50,488H analgesia, whereas its mismatch control did not (Fig. 1B), confirming the KO studies. In a rescue paradigm (4), we administered lentivirus constructs expressing enhanced green fluorescent protein (eGFP) intracereboventricularly into knockout mice with disruptions of both exons 1 and 11 or only of exon 11 (SI Appendix, Methods). The control virus only expressed eGFP, whereas the experimental virus also expressed mMOR-1G. Control studies showed that viral expression following intracerebroventricular administration was diffuse and robust (SI Appendix, Fig. S3) and persisted for at least a year following virus treatment (SI Appendix, Fig. S4). Expression of the 6TM mMOR-1G restored U50,488H analgesia in both E1/E11 and in E11 KO mice (Fig. 1A). The rescue of the response in E1/E11 KO mice indicates that only 6TM variants of Oprm1 are needed for kappa analgesia because the animal expresses neither 6TM nor 7TM variants and we restored only a 6TM variant.
Table 1.
Analgesia in MOR-1 exon 11 KO mice
| Drug | Receptor target | Route | WT ED50 | E11 KO ED50 | Shift | P value |
| DPDPE | Delta | i.c.v. | 0.44 ± 0.06 | >100 | >227 | <0.01* |
| SNC80 | Delta | i.c.v. | 1 ± 0.2 | >65 | >67 | <0.001* |
| U50,488H | Kappa | s.c. | 2.5 ± 0.3 | >40 | >15 | <0.0001* |
| i.c.v. | 2 ± 0.1 | >20 | >10 | <0.001* | ||
| Salvinorin | Kappa | s.c. | 33% MPE | 5% MPE | <0.01* | |
| Clonidine | α2 Adrenergic | s.c. | 0.16 ± 0.05 | >1 | >6 | <0.0001* |
| i.c.v. | 0.53 ± 0.08 | >30 | >60 | <0.001* | ||
| Dexmedetomidine | α2 Adrenergic | s.c. | 0.034 ± 0.005 | >0.1 | >3 | <0.0001* |
| CP55,940 | Cannabinoid | s.c. | 0.65 ± 0.13 | 0.63 ± 0.16 | 0.97 | n.s.† |
| NT72 | Neurotensin | s.c. | 3.5 ± 0.8 | 5.6 ± 0.5 | 1.6 | n.s.† |
| Oxotremorine | Muscarinic | s.c. | 0.04 ± 0.01 | 0.05 ± 0.01 | 1.2 | n.s.† |
Dose–response curves were performed for each drug by using cumulative dosing. All experiments were performed 3–4 times on mice on the C57BL/6J background with similar results obtained with each determination. Doses for s.c. administration are milligrams/kilograms, whereas those i.c.v. are micrograms per mouse. Dose–response curves were calculated via nonlinear regression analysis (GraphPad Prism). Results are the means ± SEM of the independent determinations of the ED50 values, and significance was assessed by Student’s t test. In cases where ED50 values could not be determined due to insufficient analgesic response, significance was assessed with Student’s t test comparing the responses at the highest dose tested.
Significance was determined with a Student’s t test at the highest drug dose tested.
Significance determined by three independent determinations of the ED50 values.
Fig. 1.
Role of 6TM mu opioid receptor variants in kappa opioid and α2-adrenergic agonist actions. (A) Lentiviral rescue of U50,488H analgesia. Groups of 129/C57BL/6J wild-type (WT) and exon 1/exon 11 KO (E1/E11 KO) mice (n = 8–10) received U50,488H (20 mg/kg, s.c.) and were tested for analgesia 30 min later. An additional group of E1/E11 KO mice (n = 3) was injected with a lentivirus containing mMOR-1G (E1/E11 KO + lenti, i.c.v.) on days 1, 3, and 5 and tested for analgesia at least 6 wk later. Statistical significance was determined by using one-way ANOVA (P < 0.0001; F2,18 = 18.51) with Bonferroni post hoc analysis. Groups of C57BL/6J wild-type (WT) and exon 11 KO (E11 KO) mice (n = 7–9) received U50,488H (20 mg/kg, s.c.) and were tested for analgesia 30 min later. An additional group of exon 11 KO mice (n = 8) was injected with lentivirus containing mMOR-1G (E11 KO + lenti, i.c.v.) on days 1, 3, and 5 and tested for analgesia at least 6 wk later. Significant difference was calculated by one-way ANOVA (P = 0.0001; F2,21 = 14.33) with Bonferroni post hoc analysis **P < 0.01, ***P < 0.001. (B) Antisense knockdown of U50,488H analgesia. Groups of CD1 mice (n = 15) were injected with antisense (AN) oligodeoxynucleotides targeting exon 11 (E11 AN), or mismatched (MIS) control oligodeoxynucleotides i.c.v. on days 1, 3, and 5. On day 6, mice received U50,488H (10 mg/kg, s.c.) and were tested for analgesia 30 min later. Control mice (n = 15) received no injection before testing. Significant difference was calculated by one-way ANOVA (P = 0.0007; F2,42 = 8.77) with Bonferroni post hoc analysis **P < 0.01, ***P < 0.001. (C) The role of 6TM variants on U50,488H-induced conditioned place aversion. C57BL/6J wild-type (WT) and exon 11 KO (E11 KO) mice (n = 14 per group) were randomly assigned to receive U50,488H (5 mg/kg, s.c.) paired with one side and saline with the other such that 50% of the mice were conditioned to associate the drug with each side. On day 5, mice were allowed to freely explore both sides of the apparatus. The amount of time spent in each compartment postconditioning vs. preconditioning was compared by using a two-way repeated measures ANOVA (P < 0.03; F1,26 = 5.71) with post hoc Bonferroni multiple comparisons test. E11 KO mice displayed significant place aversion (*P < 0.05). (D) Lentiviral rescue of clonidine analgesia. Groups of 129/C57BL/6J wild-type (WT) and exon 1/exon 11 KO (E1/E11 KO) mice (n = 8–15) received clonidine (0.5 mg/kg, s.c.) and were tested for analgesia 30 min later. An additional group of E1/E11 KO mice (n = 3) was injected with a lentivirus containing mMOR-1G (E1/E11 KO + lenti, i.c.v.) on days 1, 3, and 5 and tested for analgesia at least 6 wk later. Statistical significance was determined by using one-way ANOVA (P < 0.0001; F2,23 = 27.83) with Bonferroni post hoc analysis. Groups of C57BL/6J wild-type (WT) and exon 11 KO (E11 KO) mice (n = 10) received clonidine (0.5 mg/kg, s.c.) and were tested for analgesia 30 min later. An additional group of exon 11 KO mice (n = 8) was injected with lentivirus containing mMOR-1G (E11 KO + lenti, i.c.v.) on days 1, 3, and 5 and tested for analgesia at least 6 wk later. Significant difference was calculated by one-way ANOVA (P = 0.0004; F2,25 = 6.95) with Bonferroni post hoc analysis *P < 0.05, **P < 0.01. (E) Antisense knockdown of clonidine analgesia. Groups of CD1 mice (n = 14–15) were injected with antisense (AN) oligodeoxynucleotides targeting exon 11 (E11 AN) or mismatched (MIS) control oligodeoxynucleotides i.c.v. on days 1, 3, and 5. On day 6, mice received clonidine (0.5 mg/kg, s.c.) and tested for analgesia. Control mice (n = 10) received no injection before testing. Significant difference was calculated by two-way ANOVA (P < 0.0001; F1,12 = 12.43) with Bonferroni post hoc analysis *P < 0.05, ****P < 0.0001 . (F) Clonidine-induced hypolocomotion. Clonidine (0.3 mg/kg, s.c.) significantly reduced locomotor behavior relative to saline, but there was no difference between C5Bl/6 wild-type (WT, n = 7) and exon 11 KO (E11 KO, n = 7) groups by using two-way repeated measures ANOVA (P < 0.0001, F1,12 = 73.48 for a main effect of drug; P = 0.67, F1,12 = 0.2 for main effect of genotype).
Despite the loss of analgesia, the E11 KO mice still showed U50,488H-induced aversion (Fig. 1C) and locomotion suppression (SI Appendix, Fig. S6). Indeed, U50,488H aversion in a conditioned place aversion paradigm was more prominent in the E11 KO mice than in the WT mice. U50,488H suppression of locomotor activity was equivalent between WT and E11 KO groups (SI Appendix, Fig. S6). Thus, kappa analgesia is E11-dependent and dissociable from kappa aversion and locomotor suppression.
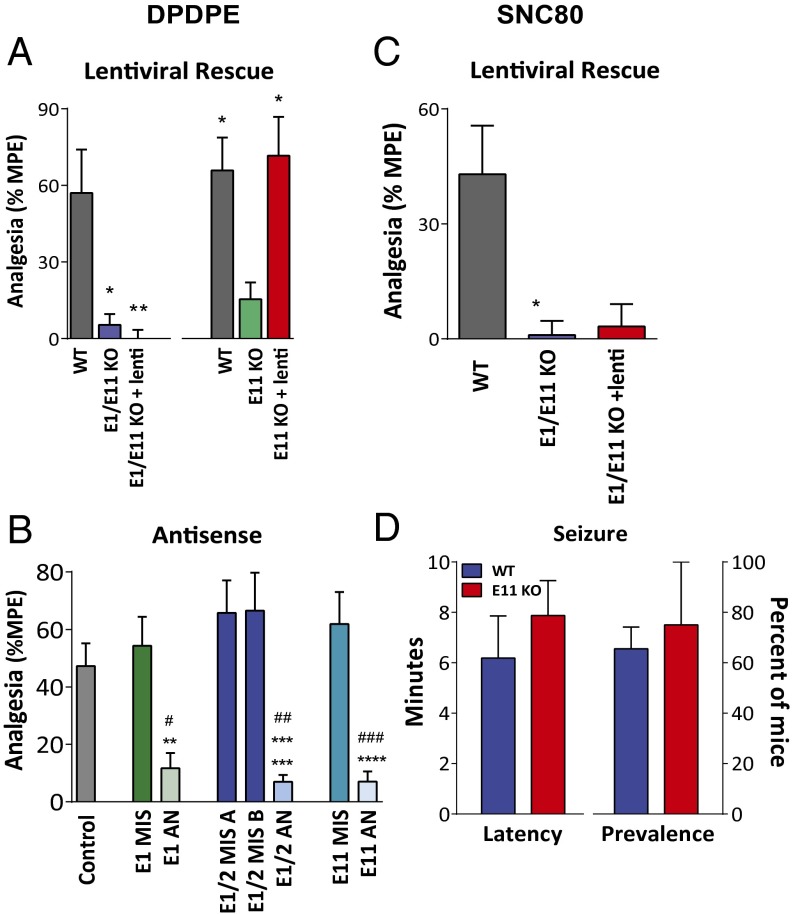
Prior work implicated mu receptors in supraspinal delta analgesia (17–19). Our results reveal that delta analgesia depended on 6TM Oprm1 variants. The delta opioid agonists DPDPE and SNC80 were inactive in E11 KO mice at doses greater than 200-fold and 60-fold their ED50 in WT mice, respectively (Table 1, Fig. 2, and SI Appendix, Figs. S2 and S3). Unlike U50,488H and IBNtxA, the lentivirus-induced expression of the 6TM mMOR-1G in the double E1/E11 KO mice failed to rescue DPDPE or SNC80 analgesia (Fig. 2). However, the mMOR-1G/lentivirus rescued DPDPE when administered to the E11 KO mice (Fig. 2). The difference between these two mu receptor KO mice is the continued expression of the 7TM variants in the E11 KO animals compared with the absence of all mu receptor variants in the E1/E11 KO mice. Together, these results suggested that delta analgesia required full-length 7TM and 6TM Oprm1 variants.
Fig. 2.
Role of 6TM and 7TM mu opioid receptor variants in delta opioid actions. (A) Lentiviral rescue of DPDPE analgesia. Groups of 129/C57BL/6J mixed background wild-type (WT) and exon 1/exon 11 KO (E1/E11 KO) mice (n = 8–10) received DPDPE (15 µg, i.c.v.) and were tested for analgesia 10 min later. All control groups were from the same background or, if mixed, from littermate controls. An additional group of E1/E11 KO mice (n = 3) was injected with a lentivirus containing mMOR-1G (E1/E11 KO + lenti, i.c.v.) on days 1, 3, and 5 and was tested for analgesia at least 6 wk later. Statistical significance was determined by using one-way ANOVA (P = 0.0057; F2,18 = 6.99) with Bonferroni post hoc analysis. Groups of C57BL/6J wild-type (WT) and exon 11 KO (E11 KO) mice (n = 8–10) received DPDPE (15 µg, i.c.v.) and tested for analgesia 10 min later. An additional group of exon 11 KO mice (n = 8) was injected with lentivirus containing mMOR-1G (E11 KO + lenti, i.c.v.) on days 1, 3, and 5 and was tested for analgesia at least 6 wk later. Significant difference was calculated by one-way ANOVA (P = 0.0082; F2,23 = 5.97) with Bonferroni post hoc analysis *P < 0.05, **P < 0.01. (B) Antisense knockdown of MOR-1 variants. Groups of CD1 mice (n = 10–15) were injected with antisense (AN) targeting exon 1 (E1 AN), the splice junction of exons 1 and 2 (E1/2 AN), exon 11 (E11 AN), or mismatched (MIS) control oligodeoxynucleotides i.c.v. on days 1, 3, and 5. On day 6, mice received DPDPE (10 µg, i.c.v.) and were tested for analgesia 10 min later. Control mice (n = 20) received no injection before testing. Significant difference was calculated by two-way ANOVA (P < 0.001; F5,78 = 9.36) with Bonferroni post hoc analysis, # indicates significantly different from control group #P < 0.05, ###P < 0.001, ####P < 0.0001, * indicates significantly different from mismatched control group *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 . (C) Lentiviral expression of mMOR-1G in E1/E11 KO mice. Expression of mMOR-1G did not rescue SNC80 analgesia. Groups of 129/C57BL/6J wild-type (WT) and exon 1/exon 11 KO (E1/E11 KO) mice (n = 5–8) received SNC80 (10 µg, i.c.v.) and were tested for analgesia 10 min later. An additional group of E1/E11 KO mice (n = 3) was injected with a lentivirus containing mMOR-1G (E1/E11 KO + lenti, i.c.v.) on days 1, 3, and 5 and was tested for analgesia at least 6 wk later. Significant difference was calculated by one-way ANOVA (P = 0.03; F2,13 = 4.66) with Bonferroni post hoc analysis *P < 0.05. (D) Role of SNC80 seizure activity in E11 KO mice. C57BL/6J wild-type (WT) and exon 11 KO (E11 KO) mice were injected with SNC80 (200 µg, i.c.v.), and the presence and latency of convulsive seizures were recorded. Data showing percent of mice with seizure are presented as mean ± SEM of three independent determinations. Latency data are presented as mean ± SEM in minutes (n = 11–12 per group). There was no significant difference between genotypes in presence of (P = 0.74; t4 = 0.36, unpaired t test) of or latency to seizure (P = 0.45; t14 = 0.78, unpaired t test).
Antisense studies support the importance of both E1-associated and E11-associated variants in supraspinal delta analgesia. The antisense targeting exon 1, but not its mismatch control, lowered DPDPE analgesia (Fig. 2B). Because 7TM and 1TM variants both contain exon 1, this antisense oligodeoxynucleotides down-regulated both. To distinguish between them, we used a previously validated second antisense oligodeoxynucleotide targeting the splice junction between exons 1 and 2 (E1/2 AN), a sequence not present in the 1TM variants because they do not contain exon 2 (20). The junctional antisense probe also lowered supraspinal DPDPE analgesia while its mismatch control was ineffective, implicating 7TM, but not 1TM variants, in delta analgesia (Fig. 2B). The decreased DPDPE response following an exon 11 antisense, but not its mismatch control, implicated a role for 6TM variants, consistent with the rescue results.
Seizures have been associated with delta opioids, potentially limiting their clinical utility (21, 22). Unlike analgesia, the incidence of SNC80-induced convulsions was equal in E11 KO and WT mice, with no difference in latency to seizure (Fig. 3D). Thus, delta opioid analgesia and seizure activity were dissociable.
Fig. 3.
Potential models of 6TM-dependent analgesia. (A) In the first model, a GPCR forms a functional heterodimer with a 6TM MOR-1 variant, which mediates analgesia. (B) In the second model, the GPCR signals converge to act through a common downstream 6TM mechanism that mediates analgesia. (C) In the last model, a GPCR signals to a specific downstream 6TM, which mediates analgesia. Models two and three might explain why nonanalgesic kappa, delta, and α2 adrenergic actions persisted in the E11 KO if they activated pathways independent of 6TM variants.
E11 variants were also implicated in nonopioid analgesic mechanisms. The α2 agonists clonidine and dexmedetomidine were potent analgesics in wild-type but not in E11 KO mice (Table 1, Fig. 1D, and SI Appendix, Fig. S2). Antisense down-regulation of 6TM variants attenuated clonidine analgesia, whereas the mismatch controls were ineffective (Fig. 1E). Clonidine analgesia was rescued with the lentivirus-expressing mMOR-1G in both exon 11 KO and in E1/E11 KO mice (Fig. 1D), indicating that 6TM, but not 7TM, variants were needed for activity. Clonidine-induced hypolocomotion (Fig. 2F) was retained in E11 KO mice with a similar time course in WT and E11 KO mice (SI Appendix, Fig. S6D). Thus, the necessity of E11 variants for α2 analgesia did not extend to all α2 actions.
Not all analgesics required 6TM variants for activity. The cannabinoid CP55,940, the neurotensin analog NT72, and the muscarinic agonist oxotremorine all produced equivalent analgesic responses in WT and E11 KO mice (Table 1 and SI Appendix, Fig. S2).
Discussion
Despite the large number of truncated GPCRs, their functions have been unclear (8). Many have been ignored or considered irrelevant. Others reportedly act as dominant negatives to modulate the activity of full-length variants, and some have been implicated in disease processes. The 6TM Oprm1 gene variants do not fit into these categories. Rather, they represent a functionally active and previously unrecognized target for opioid analgesics with a distinct and improved pharmacology (4, 9–11). Classically, opioid receptor mechanisms have been defined pharmacologically, using selective agonists and antagonists. However, genetic approaches toward classifying receptor mechanisms provide less ambiguity, although they, too, have limitations and caveats. The current findings suggest a number of independent mu opioid receptor analgesic systems. Classical mu drugs, like morphine and methadone, act only through full-length 7TM mu variants (1–3) whereas 6TM variants are both necessary and sufficient for other drugs, as illustrated by IBNtxA (4). Although IBNtxA has affinity for kappa receptors (23), its continued analgesia in a triple KO mouse that lacks kappa, as well as delta and 7TM mu, receptors, strongly argues against a role for a kappa analgesic mechanism (10). A third category of drugs, including levorphanol, butorphanol, and nalbuphine, require both 6TM and 7TM variants for full activity (10). This genetic dissection of mu opioids into three general categories may help explain some of the variability in clinical response to various opioid analgesics and incomplete tolerance.
The importance of the truncated 6TM Oprm1 variants is not limited to mu opioids. The kappa1 and α2 adrenergic drugs require their respective receptors for activity. Clonidine analgesia was markedly impaired in α2A receptor knockout mice (24) and U50,488H analgesia was lost in a kappa opioid receptor knockout mouse (25, 26). Furthermore, it is unlikely they directly label the 6TM target based on the poor affinity of both clonidine and U50,488H for the E11 binding site in brain labeled by 125I-IBNtxA (10). However, their activity still depended on 6TM variant expression.
Delta drugs were unique. In addition to delta receptors, they depended on both 6TM and 7TM Oprm1 variants for analgesia. Loss of all Oprm1 gene products in the E1/E11 KO mouse eliminated delta analgesia, which could not be rescued by the 6TM/lentivirus-expressing mMOR-1G. Although delta analgesia also was lost in exon 11 knockout mice that still expressed 7TM variants, the 6TM lentivirus rescued the response in this knockout model, presumably because of the combined presence of both 7TM and 6TM variants following virus treatment.
The role of 6TM Oprm1 variants in nonopioid analgesia was unexpected. The possibility of common mechanisms for these diverse receptor classes provides interesting avenues for future investigation in the study of pain modulation, whereas the lack of 6TM involvement in neurotensin, muscarinic, and cannabinoid analgesia illustrates the existence of multiple independent analgesic systems that may be targeted to relieve pain.
Six transmembrane variants lack the first transmembrane domain (TM1) contained in the full-length, traditional variants. The crystal structure of MOR-1 indicates that TM1 is not directly involved with the docking of opioids (27–29), raising the possibility that the 6TM protein may be capable of docking ligands directly, although this possibility has not yet been demonstrated. However, the absence of TM1 would be expected to lead to major structural alterations. Alternatively, the 6TM variants may associate with another GPCR (Fig. 3) (10), with the ligand docking into either a stabilized 6TM protein or its partner. Heterodimerization is supported by the ability to coimmunopurify a 6TM variant with a range of coexpressed GPCRs, including DOR-1, KOR-1, and α2 adrenergic receptors as well as the 7TM Oprm1 receptor MOR-1. Although these observations indicate that the 6TM variants can physically associate with other GPCRs, the assays depend on overexpression, making its relevance to native expression unclear. Heterodimerization is an unlikely mechanism for supraspinal delta analgesia because it would require a trimer comprised of delta receptors along with both 6TM and 7TM Oprm1 variants. Alternatively, 6TM variants may act in a circuit “downstream” from the other GPCRs (Fig. 3 B and C). This possibility also might help explain the persistence of other kappa, delta, and α2-adrenergic actions in E11 KO mice if the GPCRs also activated circuits independent of the 6TM variants. The 6TM circuits could converge (Fig. 3B), providing a single 6TM target for all or they could be independent of each other and act in parallel, independent circuits, each with its own 6TM components (Fig. 3C).
Overall, our findings highlight the importance of truncated 6TM Oprm1 variants in the relief of pain for several diverse classes of pharmacologically distinct compounds. Equally important, they illustrate the more general question of the overall relevance of truncated GPCRs and whether truncated forms in other systems may also provide important targets for drug development.
Methods
Drugs.
Drugs were obtained from the Research Technology branch of the National Institute on Drug Abuse, Tocris Bioscience, Polypeptide Group, or Cayman Chemical. Miscellaneous chemicals and buffers were obtained from Sigma Aldrich. Due to solubility constraints CP55,940 and Salvinorin A were dissolved in vehicle consisting of DMSO (10% vol/vol) and Tween 80 (10% vol/vol) in water. This vehicle produced no response in the tail flick analgesia assay.
Mice.
Male CD-1 mice were purchased from Charles River Laboratories (24–32 g). C57Bl/6J mice (24–37 g) were purchased from Jackson Laboratories. Oprm1 exon 11 KO animals were derived as described (2) and backcrossed at least 10 generations on a C57BL/6J background. Exon 1/exon 11 double KO mice and their mixed background littermate controls (129/C57BL/6 mixed background) were bred by our laboratory (4). Knockout studies used male and female mice. Wild-type and KO groups were matched for age and sex. Mice were given at least a 1-wk washout period after receiving a drug before repeated testing. All control groups were from the same background or, if mixed, from littermate controls. All mice were maintained on a 12-h light/dark cycle with Purina rodent chow and water available ad libitum and housed in groups of five until testing. All animal studies were approved by the Institutional Animal Care and Use Committee of Memorial Sloan–Kettering Cancer Center and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals in an Association for Assessment and Accreditation of Laboratory Animal Care accredited facility.
Analgesia Assays.
Analgesic dose–response curves were generated by using a radiant heat tail-flick paradigm with a Ugo Basile 37360 tail flick unit. A maximum cutoff latency of 10 s was used to minimize tissue damage, and data are presented as percent maximum possible effect (%MPE) according to the formula: %MPE = [(observed latency – baseline latency)/(10 s – baseline latency) × 100]. Similar results were obtained when data were analyzed quantally with analgesia defined as a doubling of baseline latency. ED50 values were determined by using nonlinear regression analysis where indicated (GraphPad Prism). Experiments were performed at least three times with similar results observed during each replication unless stated otherwise. ED50 values from independent replications were tested for significance by using Student’s t test. In cases where ED50 values could not be calculated because of insufficient analgesic response, groups were compared at the highest drug doses tested by using a Student’s t test. Group means for individual doses were compared by using one-way ANOVA with post hoc Bonferroni multiple comparisons test.
Subcutaneous U50,488H, clonidine, dexmedetomidine, NT72, and CP55,940 analgesia were tested 30 min after injection at peak effect. Salvinorin A and oxotremorine were tested 10 min after injection at peak effect. Several compounds were also delivered i.c.v. as described (30). Briefly, the mice were anesthetized with isoflurane and injections (3 µL per mouse) were made into the right lateral ventricle (2 mm caudal to bregma, 2 mm lateral to sagittal suture, and 2 mm in depth). Mice were tested for analgesia 10 min following i.c.v. injections. DPDPE, U50,488H, and clonidine were also administered i.t. via lumbar puncture (1 µL) (31, 32). Mice were tested for analgesia 15 min after i.t. injections.
Lentiviral Generation and Administration.
Detailed methods are presented in SI Appendix, Methods and have been described (4). Lentivirus was generated by using constructs containing eGFP with and without the mMOR-1G cassette. Lentivirus particles (4 µL of 1.5 × 109 transducing units/mL) were injected supraspinally (i.c.v.) on days 1, 3, and 5. Previous studies established that expression of the lentivirus proteins at the spinal level increases over 3–4 wk, corresponding to the appearance of the rescue of IBNtxA analgesia (4). eGFP expression 22 d after initiating virus injection i.c.v. revealed widespread, robust expression in the brain (SI Appendix, Fig. S4). Viral expression persists for at least 1 y at levels similar to the 6TM variants natively expressed in wild-type mice, as determined by RT-PCR (SI Appendix, Fig. S5).
Antisense Oligodeoxynucleotide Injection.
Groups of mice received the stated antisense (5–10 µg) or mismatch (5–10 µg) oligodeoxynucleotide i.c.v. under light isoflurane anesthesia on days 1, 3 and 5, as described (16). Analgesia was tested on day 6. The antisense oligodeoxynucleotides were based on the sequence of mouse MOR-1 and targeted exon 1 (E1 AN), the splice junction of exons 1 and 2 (E1/2 AN), or exon 11 (E11) (SI Appendix, Table S1). Prior studies confirmed that the E1/E2 AN selectively down-reguated the 7TM variants, but not the 1TM variants (20). Mismatch controls for E1 and E11 had the same base composition but the sequence of 2 or 4 pairs of bases was changed. Two mismatch controls were used for the E1/E2 AN, each of which was scrambled on only one side of the splice junction.
Seizure Assessment.
Mice were injected (i.c.v.) with SNC80 (200 µg) as described above. Mice were observed for 30 min following administration. Both presence of convulsive seizure and latency until the first seizure were recorded (33). Data are presented as percent of mice demonstrating seizures (mean ± SEM of replicate experiments). Average latency until first seizure (minutes) (mean ± SEM) is also reported. Unpaired t tests were used to test for significant differences between genotypes.
Conditioned Place Aversion.
Conditioned place aversion was assessed by using an unbiased two-chamber conditioned place preference insert in the activity chamber (MedAssociates ENV-510) and the animals’ position monitored using Activity Monitor software. Mice were habituated to the testing room for at least 1 h before testing each day. On day 1, mice were allowed to freely explore both sides of the apparatus. On days 2–4, mice were injected s.c. with saline and immediately placed into one side off the apparatus for 20 min. In the afternoon, they were injected s.c. with 5 mg/kg U50,488H, which was paired with the opposite side. Mice were randomly assigned to receive drug paired with one side and saline in the other such that 50% of the mice were conditioned to associate the drug with each side. On day 5, mice were again allowed to freely explore both sides of the apparatus. The amount of time spent in each compartment postconditioning vs. preconditioning was compared by using a two-way repeated measures ANOVA followed by a post hoc Bonferroni’s multiple comparisons test (GraphPad Prism). The total distance traveled by each mouse after receiving drug on each of the three conditioning days was also recorded and normalized to the distance traveled after receiving saline that morning.
Open Field Locomotor Activity.
Open field locomotor activity was obtained in a MedAssociates ENV-510 activity chamber by using MedAssociates Activity Monitor software. Mice were habituated to the testing room for at least 1 h before testing each day. Mice were injected s.c. with U50,488H (10 mg/kg), clonidine (0.3 mg/kg), or saline and immediately placed in the open field box for 60 min. Total distance traveled as well as distance traveled in 2- or 5-min bins were compared by using a repeated measures ANOVA followed by Bonferroni multiple comparisons test.
Supplementary Material
Acknowledgments
This work was supported in part by grants from Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, The Experimental Therapeutics Center of Memorial Sloan–Kettering Cancer Center (MSKCC), The Harrington Discovery Institute, the Tri-Institutional Therapeutics Discovery Institute, the Peter F. McMannus Charitable Trust and National Institute on Drug Abuse of the National Institutes of Health Grants DA06241 and DA07242 (to G.W.P.) and DA13997 (to Y.-X.P.), National Cancer Institute of the National Institutes of Health Core Grant CA08748 (to MSKCC), a fellowship from the PhRMA Foundation (to S.G.G.), and National Science Foundation Graduate Research Fellowship Grant DGE-1257284 (to G.F.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523894113/-/DCSupplemental.
References
- 1.Pasternak GW, Pan Y-X. Mu opioids and their receptors: Evolution of a concept. Pharmacol Rev. 2013;65(4):1257–1317. doi: 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan YX, et al. Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci USA. 2009;106(12):4917–4922. doi: 10.1073/pnas.0811586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuller AG, et al. Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci. 1999;2(2):151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- 4.Lu Z, et al. Mediation of opioid analgesia by a truncated 6-transmembrane GPCR. J Clin Invest. 2015;125(7):2626–2630. doi: 10.1172/JCI81070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthes HW, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature. 1996;383(6603):819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 6.Sora I, et al. Opiate receptor knockout mice define μ receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci USA. 1997;94(4):1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loh HH, et al. μ Opioid receptor knockout in mice: Effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res. 1998;54(2):321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- 8.Wise H. The roles played by highly truncated splice variants of G protein-coupled receptors. J Mol Signal. 2012;7(1):13. doi: 10.1186/1750-2187-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majumdar S, et al. Synthesis and evaluation of aryl-naloxamide opiate analgesics targeting truncated exon 11-associated μ opioid receptor (MOR-1) splice variants. J Med Chem. 2012;55(14):6352–6362. doi: 10.1021/jm300305c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majumdar S, et al. Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci USA. 2011;108(49):19778–19783. doi: 10.1073/pnas.1115231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wieskopf JS, et al. Broad-spectrum analgesic efficacy of IBNtxA is mediated by exon 11-associated splice variants of the mu-opioid receptor gene. Pain. 2014;155(10):2063–2070. doi: 10.1016/j.pain.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grinnell SG, et al. Pharmacologic characterization in the rat of a potent analgesic lacking respiratory depression, IBNtxA. J Pharmacol Exp Ther. 2014;350(3):710–718. doi: 10.1124/jpet.114.213199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prisinzano TE. Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sci. 2005;78(5):527–531. doi: 10.1016/j.lfs.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 14.McCurdy CR, Sufka KJ, Smith GH, Warnick JE, Nieto MJ. Antinociceptive profile of salvinorin A, a structurally unique kappa opioid receptor agonist. Pharmacol Biochem Behav. 2006;83(1):109–113. doi: 10.1016/j.pbb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Standifer KM, Chien C-C, Wahlestedt C, Brown GP, Pasternak GW. Selective loss of δ opioid analgesia and binding by antisense oligodeoxynucleotides to a δ opioid receptor. Neuron. 1994;12(4):805–810. doi: 10.1016/0896-6273(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 16.Rossi GC, Pan Y-X, Brown GP, Pasternak GW. Antisense mapping the MOR-1 opioid receptor: Evidence for alternative splicing and a novel morphine-6 β-glucuronide receptor. FEBS Lett. 1995;369(2-3):192–196. doi: 10.1016/0014-5793(95)00757-z. [DOI] [PubMed] [Google Scholar]
- 17.Matthes HWD, et al. Activity of the δ-opioid receptor is partially reduced, whereas activity of the kappa-receptor is maintained in mice lacking the μ-receptor. J Neurosci. 1998;18(18):7285–7295. doi: 10.1523/JNEUROSCI.18-18-07285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sora I, Funada M, Uhl GR. The μ-opioid receptor is necessary for [D-Pen2,D-Pen5]enkephalin-induced analgesia. Eur J Pharmacol. 1997;324(2-3):R1–R2. doi: 10.1016/s0014-2999(97)10016-4. [DOI] [PubMed] [Google Scholar]
- 19.Scherrer G, et al. The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: A parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur J Neurosci. 2004;19(8):2239–2248. doi: 10.1111/j.0953-816X.2004.03339.x. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, et al. Stabilization of the μ-opioid receptor by truncated single transmembrane splice variants through a chaperone-like action. J Biol Chem. 2013;288(29):21211–21227. doi: 10.1074/jbc.M113.458687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tortella FC, Robles LE, Holaday JW, Cowan A. ICI 154,129, a δ-opioid receptor antagonist raises seizure threshold in rats. Eur J Pharmacol. 1984;97(1-2):141–144. doi: 10.1016/0014-2999(84)90523-5. [DOI] [PubMed] [Google Scholar]
- 22.Chung PC, et al. Delta opioid receptors expressed in forebrain GABAergic neurons are responsible for SNC80-induced seizures. Behav Brain Res. 2015;278:429–434. doi: 10.1016/j.bbr.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majumdar S, et al. Generation of novel radiolabeled opiates through site-selective iodination. Bioorg Med Chem Lett. 2011;21(13):4001–4004. doi: 10.1016/j.bmcl.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairbanks CA, Kitto KF, Nguyen HO, Stone LS, Wilcox GL. Clonidine and dexmedetomidine produce antinociceptive synergy in mouse spinal cord. Anesthesiology. 2009;110(3):638–647. doi: 10.1097/ALN.0b013e318195b51d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simonin F, et al. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17(4):886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ansonoff MA, et al. Antinociceptive and hypothermic effects of Salvinorin A are abolished in a novel strain of kappa-opioid receptor-1 knockout mice. J Pharmacol Exp Ther. 2006;318(2):641–648. doi: 10.1124/jpet.106.101998. [DOI] [PubMed] [Google Scholar]
- 27.Huang W, et al. Structural insights into µ-opioid receptor activation. Nature. 2015;524(7565):315–321. doi: 10.1038/nature14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sounier R, et al. Propagation of conformational changes during μ-opioid receptor activation. Nature. 2015;524(7565):375–378. doi: 10.1038/nature14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manglik A, et al. Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature. 2012;485(7398):321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haley TJ, McCormick WG. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br Pharmacol Chemother. 1957;12(1):12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hylden JL, Wilcox GL. Intrathecal morphine in mice: A new technique. Eur J Pharmacol. 1980;67(2-3):313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 32.Paul D, Standifer KM, Inturrisi CE, Pasternak GW. Pharmacological characterization of morphine-6 β-glucuronide, a very potent morphine metabolite. J Pharmacol Exp Ther. 1989;251(2):477–483. [PubMed] [Google Scholar]
- 33.Comer SD, et al. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J Pharmacol Exp Ther. 1993;267(2):888–895. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.