Significance
Inbreeding depression is the decrease in fitness with increased genome-wide homozygosity that occurs in the offspring of related parents. Estimation of its effect in wild populations has been challenging, and while evidence of inbreeding depression in juvenile traits is widespread, examples during later life stages remain rare. Here, in a species with extended maternal care, genomic inbreeding coefficients, but not pedigree-based ones, revealed inbreeding depression in annual breeding success in both sexes, and in offspring rearing success in females. This contributed to inbreeding depression in estimates of lifetime fitness in both sexes. Our work illustrates that inbreeding depression in adult traits can be as large as in juvenile traits but requires more powerful methods to be detected.
Keywords: fitness, adult traits, parental inbreeding, red deer, Single-Nucleotide Polymorphism
Abstract
Inbreeding depression is of major concern for the conservation of threatened species, and inbreeding avoidance is thought to be a key driver in the evolution of mating systems. However, the estimation of individual inbreeding coefficients in natural populations has been challenging, and, consequently, the full effect of inbreeding on fitness remains unclear. Genomic inbreeding coefficients may resolve the long-standing paucity of data on inbreeding depression in adult traits and total fitness. Here we investigate inbreeding depression in a range of life history traits and fitness in a wild population of red deer (Cervus elaphus) in Scotland using individual inbreeding coefficients derived from dense Single-Nucleotide Polymorphism (SNP) data (). We find associations between and annual breeding success in both sexes, and between maternal inbreeding coefficient and offspring survival. We also confirm previous findings of inbreeding depression in birth weight and juvenile survival. In contrast, inbreeding coefficients calculated from a deep and comparatively complete pedigree detected inbreeding depression in juvenile survival, but not in any adult fitness component. The total effect of inbreeding on lifetime breeding success (LBS) was substantial in both sexes: for , a value resulting from a half-sib mating, LBS declined by 72% for females and 95% for males. Our results demonstrate that SNP-based estimates of inbreeding provide a powerful tool for evaluating inbreeding depression in natural populations, and suggest that, to date, the prevalence of inbreeding depression in adult traits may have been underestimated.
Decreasing fitness with increasing inbreeding is a widespread phenomenon (1), which occurs because mating between relatives increases homozygosity at loci carrying rare recessive deleterious alleles or exhibiting overdominance (2). The magnitude of inbreeding depression is relevant to many disciplines within biology, including the conservation of small, isolated populations (3), animal and plant breeding (4), trait variation in humans (5), and the evolution of mating systems (2, 6). In wild animal populations, evidence for inbreeding depression typically comes in the form of decreased juvenile survival (1, 6). The detrimental effects of inbreeding on adult traits such as fecundity, longevity, offspring birth weight, and milk production are well known in agricultural and zoo populations (e.g., refs. 4 and 7), but their prevalence and magnitude in wild populations remain hitherto unclear.
There is no a priori genetic or ecological reason to expect inbreeding depression to be reduced late in the life cycle, other than the higher opportunity for selection in juveniles (8). The lack of widespread evidence for adult inbreeding depression could partly be explained by the general acceptance that inbred individuals may be rare, due to inbreeding avoidance (6), and inbred adults even rarer when there is selection against inbred juveniles (1). More importantly, estimating inbreeding in natural populations is not trivial.
Pedigree-based inbreeding coefficients (), the traditional estimate of genome-wide homozygosity through identity by descent (IBD), are not available for most natural populations, as accurate and sufficiently deep pedigrees are difficult to construct. Short or incomplete pedigrees generate downward biased estimates of inbreeding coefficients, which decreases the power to detect inbreeding depression (9, 10). Bias may be reduced through exclusion of individuals with little or no ancestry information, such as founders, immigrants, and their offspring, but this can drastically reduce sample size (9). Consequently, relatively few studies of inbreeding depression in wild populations are based on pedigrees, although there are some notable exceptions (see ref. 1).
As an alternative to pedigrees, average homozygosity at genetic markers has been widely used to estimate an individual’s inbreeding status. However, for many natural populations, genetic resources have hitherto been limited to small panels of markers, typically microsatellites, yielding estimates of marker homozygosity that are often poorly correlated with genome-wide homozygosity (9, 11). Consequently, correlations between microsatellite homozygosity and phenotypic trait values are typically small, although a metaanalysis showed an overall weak effect of decreasing fitness with increasing microsatellite homozygosity (12).
In recent years, genome-wide high-density marker data, such as that provided by panels of Single-Nucleotide Polymorphisms (SNPs), are becoming available for an increasing number of species. Theory and simulations predict that average homozygosity at a large number of SNPs provides a more precise estimate of genome-wide homozygosity than inbreeding coefficients from even a perfect pedigree, because it can capture the variation in IBD around the pedigree expectation, brought about by Mendelian segregation and recombination (13, 14). By chance, and because of physical linkage, some individuals with the same pedigree inbreeding coefficient (e.g., after a mating between full siblings) inherit a larger proportion of their alleles from the same ancestral copy than expected, including at causal loci. The performance of genomic relative to pedigree-based estimators of inbreeding and pairwise relatedness has been shown in simulations (14) and, among others, humans (5, 15) and cattle (16). The potential of large SNP panels in the wild was recently demonstrated in a small nonpedigreed sample of harbor seals (17), in which SNP heterozygosity showed a strong and highly significant association with parasite burden, whereas microsatellite heterozygosity did not.
However, in general it is not fully clear how novel SNP-based metrics of inbreeding will compare with the traditional pedigree-based measures in studies of wild populations. Their performance may not be as immaculate in real datasets as in simulations, as genomic estimators, like microsatellite markers, rely on a correlation in homozygosity between loci within individuals (9, 11). This so-called identity disequilibrium comes about through a fraction of systematic consanguineous matings, genetic drift, or admixture (9), but its magnitude can be difficult to predict.
Here, we combine dense SNP data with detailed life history data for a large number of individuals, to examine whether genomic estimates reveal inbreeding depression in fitness and various fitness components, in particular during adult life stages. We use a population of red deer (Cervus elaphus) in the North Block of the Isle of Rum, Scotland, which have been individually studied for over four decades. Our data are relatively rare in that they yield estimates of individual fitness (lifetime production of offspring), as well as various fitness components, and factors affecting each are well known (18, 19). Previous studies found inbreeding depression in birth weight and juvenile survival (20–22), as well as an association between microsatellite heterozygosity and lifetime breeding success (LBS) (23). In this species with extensive maternal care (18), an effect of the maternal inbreeding coefficient on offspring fitness has been hypothesized but not confirmed (22).
First, we compared genomic inbreeding coefficients with traditional pedigree-based inbreeding coefficients. Next, we tested for inbreeding depression in several fitness components and correlated traits, including juvenile survival, and annual survival and annual breeding success (ABS) in adults of both sexes, using both inbreeding measures. For traits expressed in juveniles, we considered the effects of both offspring and maternal inbreeding coefficients. Lastly, we investigated the cumulative effect of inbreeding on all traits, by considering the association between inbreeding coefficient and LBS in each sex, as well as with female lifetime reproductive success (LRS), the number of offspring which survived to independence.
Results
Level of Inbreeding.
Based on the pedigree (details of which are given in SI Materials and Methods and Table S1), close inbreeding resulting in is rare ( instances), and occurred only via father−daughter matings (in line with refs. 22 and 24). To minimize bias, -based analysis was restricted to individuals for whom it was, at minimum, clear whether or not they were the product of close inbreeding, i.e., for which at least both parents and the maternal grandfather were known. In this restricted dataset, 45% of individuals had an greater than zero (899/2,012), of which 125 individuals (6%) had .
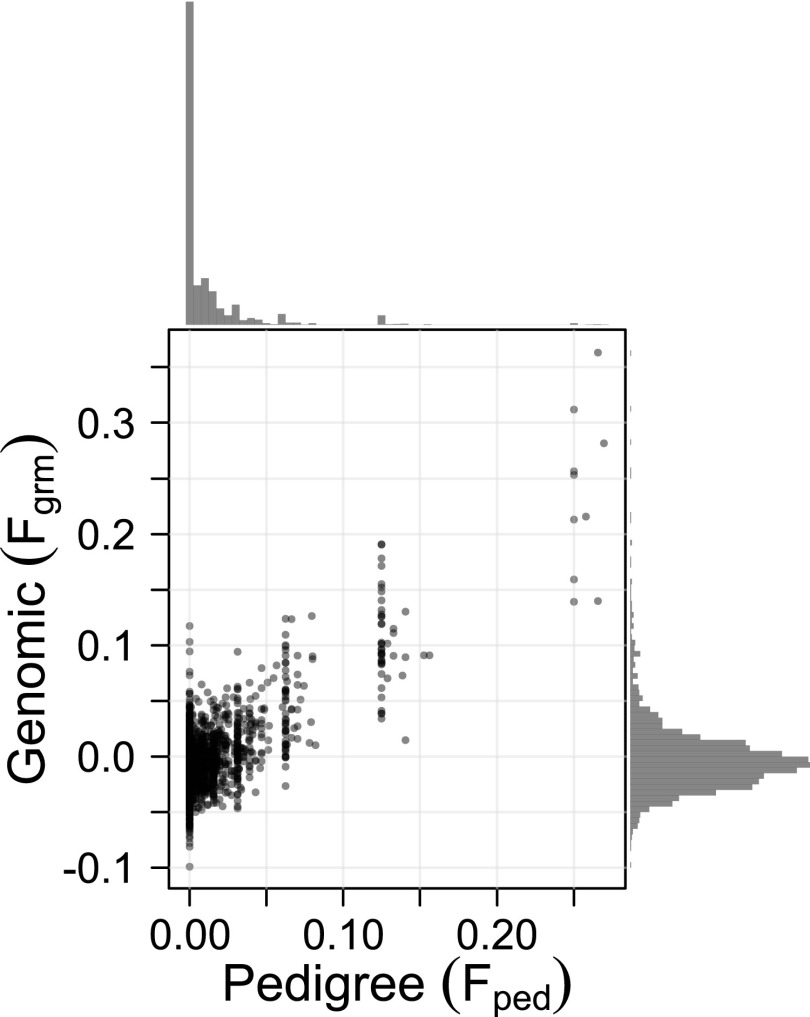
Our genomic inbreeding estimator (, for Genomic Relatedness Matrix; details in SI Materials and Methods and Fig. S1) was strongly correlated with (, Fig. 1) and with average homozygosity (r = 0.94, Fig. S2). This estimator by Yang et al. (25) estimates how similar the gametes were that made up an individual’s genome, relative to a random draw from a reference population (here: a random-mating population with the same allele frequencies as among our sampled individuals), and can take negative values (see Fig. 1). The distribution of is more convenient statistically than , as the high, narrow peak at becomes an approximately normal distribution centered at (histograms in Fig. 1). The variation in within classes was expected (see the Introduction), and is due to both pedigree incompleteness and variation in realized genome-wide IBD around the pedigree expectation. Identity disequilibrium estimated from marker loci differed significantly from zero in the complete dataset [; bootstrap confidence interval (CI) 0.0010–0.0014; details in SI Materials and Methods and SI Results] as well as in all trait-specific data subsets (Fig. S3C), indicating the data meet the requirements to detect inbreeding depression, if any is present (9).
Fig. S1.
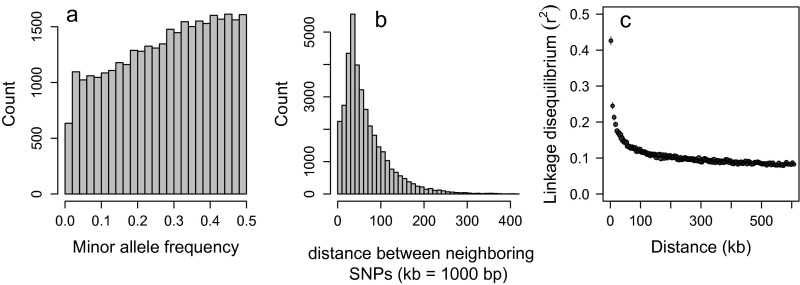
(A) Minor allele frequencies of the 37,292 autosomal SNPs that passed quality control; (B) the distribution of putative physical distance between neighboring SNPs; and (C) the decay of LD () with putative distance. Pairwise LD is calculated with the 10 nearest neighbors of each SNP, and averages per bin spanning 5 kb are shown, with error bars indicating 1 SE.
Fig. 1.
Pedigree and genomic inbreeding coefficients for 1,968 individuals for whom both measures were available, including individuals with at least both parents and the maternal grandfather known for (see Results, Level of Inbreeding); , . Histograms show the distributions of (top) and (right). At , the scatter of is in line with the theoretical distribution of realized inbreeding coefficients for a mating between half-siblings [2.5–97.5 percentile: 0.05–0.20 (13); based on a human genome, which is of similar total length as the red deer genome].
Fig. S2.
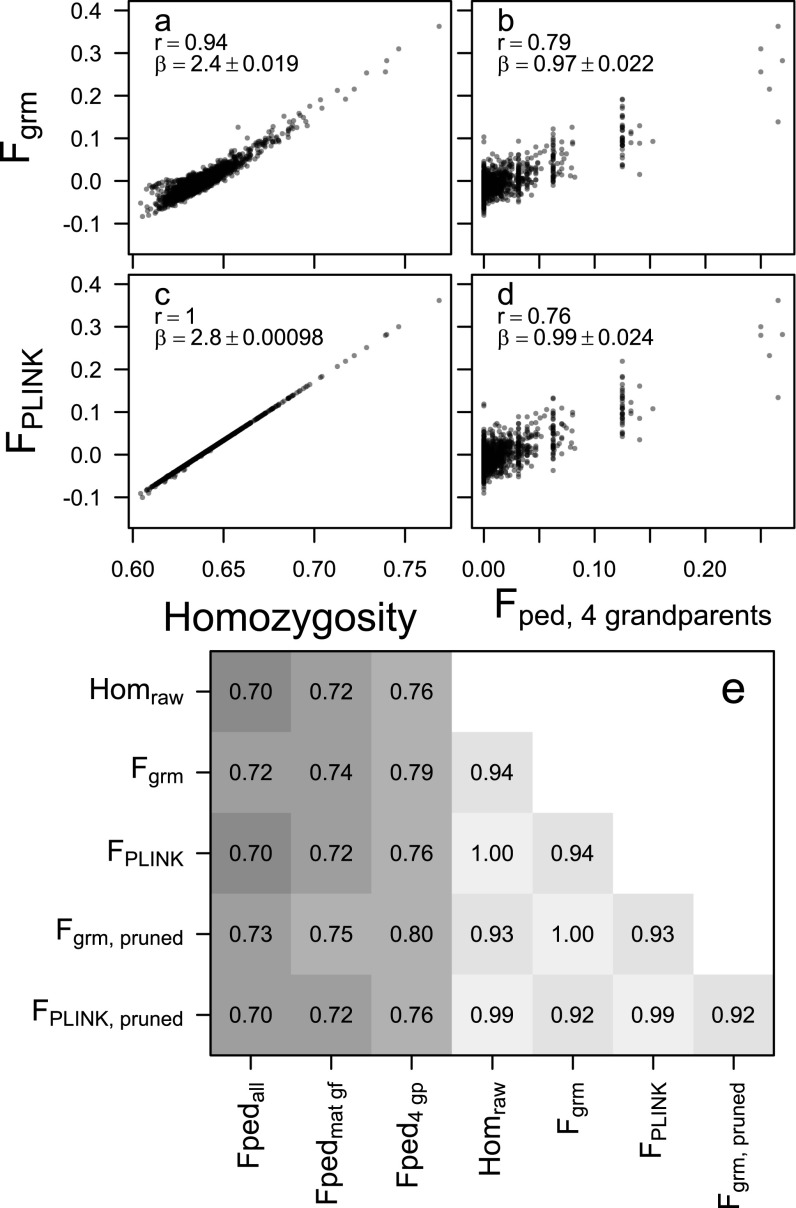
Relationships between inbreeding estimated as (A and B) or (C and D) and average SNP homozygosity (A and C, n = 2,248) or pedigree inbreeding for individuals with all four grandparents known (B and D, n = 1,198). Correlations (r) between a wider range of estimators are given in E, with a pairwise sample size of n = 2,248 between all genomic estimators, n = 1944 between genomic estimators and Fpedmat gf (both parents plus maternal grandfather known), and n = 1,198 between genomic estimators and Fped4 gp. “Pruned” refers to using a subset of about 90% of SNPs after pruning on LD (details in SI Materials and Methods).
Fig. S3.
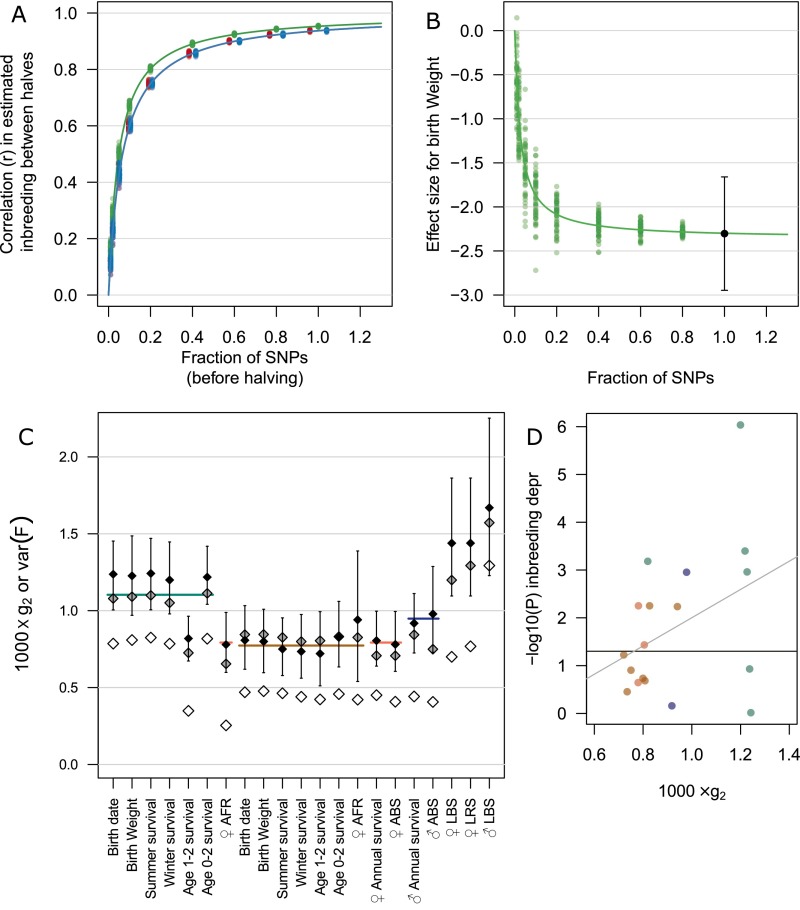
(A) Correlations between average marker homozygosity (red), (green), and (blue) calculated within the two halves of SNP subsets of increasing size, using 50 replicates for each subset (details in SI Materials and Methods). (B) Estimated effect size for birth weight, using individual calculated in each SNP subset as the explanatory variable. Vertical error bar shows 1 SE around the estimate when all SNPs are used. In A and B, curves show nonlinear least square regressions of type , with, in A, for homozygosity, 14.96 for , and 20.27 for and, in B, and . (C) Identity disequilibrium calculated in various data subsets using the approach in ref. 17 (black dots; error bars show 95% CI from 200 boostrap replications), and variance in (gray) or (white). Horizontal colored lines denote averages for juveniles (green), mothers (brown), adult females (red), and adult males (blue). (D) Relationship between log10 P values of estimated inbreeding depression (depr.) in the traits considered, using , and values (black dots in C), using the same color coding as the horizontal lines in C; LBS is omitted. The gray regression line is given by (, adj. ), and the horizontal line indicates .
Juvenile Traits.
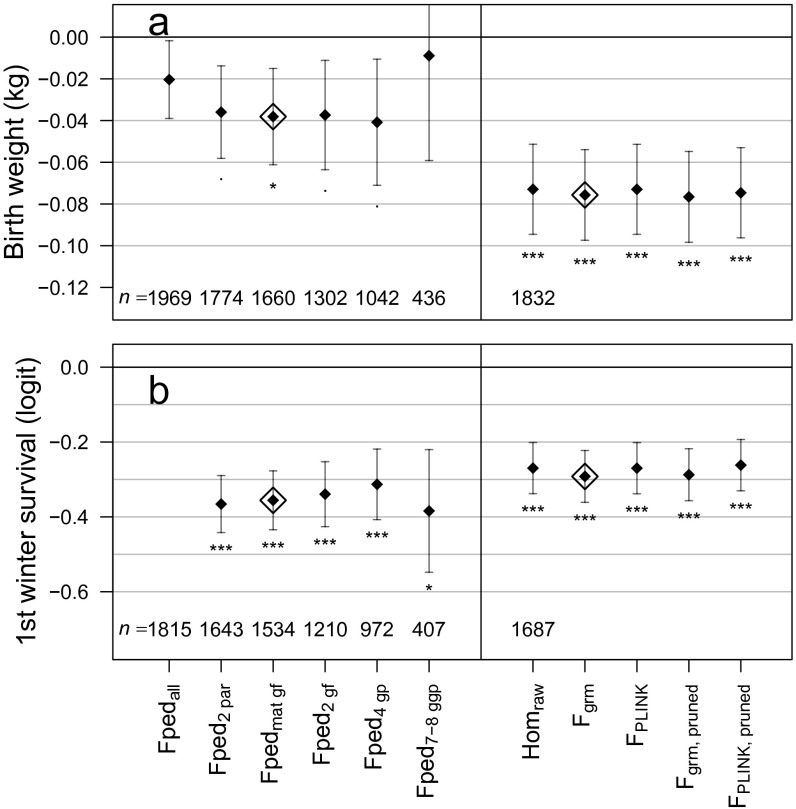
We found that increased , but not , was associated with significantly lower birth weight (model M2 in Table 1; sample sizes for all traits are given in Table S2, and estimates for all fitted fixed and random effects are given in Table S3). Estimated effect sizes were highly similar when different ancestry information thresholds were used for Fped, or when using alternative estimators of genomic inbreeding instead of Fgrm (Fig. S4). Inbred calves with (i.e., a value that might result from a half-sib mating, and which is the 99th percentile among newborns in this dataset) were kg lighter () than outbred calves (taken as ). This is of similar magnitude to the difference in average birth weight between the sexes ( kg, model M2 in Table S3), and constitutes a 4.4% decrease relative to the average birth weight of 6.39 kg. Interactions between sex and or on birth weight were nonsignificant ().
Table 1.
Estimated effect sizes for pedigree and genomic inbreeding coefficients ( and ) in a range of fitness correlated traits
| Model | Focal’s | Mother’s | Focal’s | Mother’s | |||||||||
| No. | Trait | β | SE | P | β | SE | P | β | SE | P | β | SE | P |
| Juvenile traits | |||||||||||||
| M1 | Birth date* | 2.49 | (13.4) | 0.85 | −15.0 | (19.9) | 0.45 | −16.3 | (10.4) | 0.12 | −17.9 | (14.2) | 0.21 |
| M2 | Birth weight | −1.29 | (0.86) | 0.14 | −0.58 | (2.12) | 0.78 | −2.25 | (0.69) | 0.001 | −1.95 | (1.46) | 0.18 |
| M3 | Summer survival† | 1.12 | (3.11) | 0.72 | −3.47 | (4.18) | 0.41 | 0.11 | (2.63) | 0.97 | −4.93 | (3.21) | 0.12 |
| M4 | Winter survival† | −17.3 | (3.55) | <0.001 | −4.65 | (4.68) | 0.32 | −12.4 | (2.53) | <0.001 | −3.06 | (3.29) | 0.35 |
| M5 | Age 1–2 y survival† | −9.82 | (4.94) | 0.05 | −5.56 | (5.39) | 0.30 | −12.3 | (3.60) | <0.001 | −7.99 | (4.24) | 0.06 |
| Combined juvenile traits | |||||||||||||
| M6 | Age 0–2 y survival | −13.4 | (3.31) | <0.001 | −5.02 | (4.48) | 0.26 | −13.5 | (2.46) | <0.001 | −8.71 | (3.30) | 0.006 |
| Adult traits | |||||||||||||
| M7 | Female AFR* | −1.96 | (3.51) | 0.56 | 0.56 | (2.70) | 0.86 | −2.14 | (1.97) | 0.28 | 3.46 | (2.04) | 0.09 |
| M8 | Female annual survival | −2.07 | (4.97) | 0.65 | NF | −6.84 | (3.46) | 0.05 | NF | ||||
| M9 | Male annual survival | −8.41 | (4.66) | 0.08 | NF | −1.19 | (3.80) | 0.74 | NF | ||||
| M10 | Female ABS | −6.24 | (4.32) | 0.15 | NF | −8.35 | (3.22) | 0.008 | NF | ||||
| M11 | Male ABS | −11.9 | (7.37) | 0.08 | NF | −13.0 | (3.43) | <0.001 | NF | ||||
Estimates for all fitted fixed and random effects in each model are given in Table S3. For the juvenile traits (M1–M6), the sexes were analyzed together, and either or of both focal individual and mother were fitted in the same model. Annual survival (age 2+ y) and ABS (age 5+ y) were analyzed for each sex separately, and AFR was analyzed for females only. Significant effects (P ≤ 0.05) are indicated in bold. Sample sizes ranged from 232 to 1783 (see Table S2). NF, not fitted.
Multiplied by −1, as larger values are associated with decreased fitness.
Birth date and birth weight fitted as covariates.
Fig. S4.
Comparison between estimated effect sizes of inbreeding depression using different ancestry thresholds (Left) or different genomic inbreeding estimators (Right) in models for two traits known to show inbreeding depression, birth weight (A) and first winter survival (B; birth weight accounted for). Each estimator was scaled to have an SD of 1 before fitting the model, for this comparison only, and no maternal inbreeding coefficients were fitted; models are otherwise identical to those used in the main text. The pedigree and genomic estimators used in the main text are indicated by open diamonds. Sample sizes are given for each model, and all models with genomic estimators have the same sample size. Asterisks denote standard levels of significance. The subset of individuals with both parents and at least one grandparent known is omitted, as it was identical to the subset with both parents known. The model for first winter survival using including all individuals (Fpedall) failed to converge. “Pruned” refers to using a subset of about 90% of SNPs after pruning on LD (details in SI Materials and Methods).
As previously reported (e.g., refs. 22 and 26), lower birth weight was associated with reduced survival of neonates, of calves during the first winter of life, and of yearlings (models M3–M5 in Table S3). Over and above the effect of birth weight, inbreeding coefficients (both and ) were negatively associated with calf survival over the first winter and with yearling survival. There is a similar negative effect of later birth date on survival during each of these periods (models M3–M5 in Table S3), but no association was found between a calf’s date of birth and its inbreeding coefficient (M1 in Table 1). Overall, these results show that inbreeding depression in juvenile survival is channeled both via decreased birth weight and, as the calf develops, via other mechanisms that affect survival.
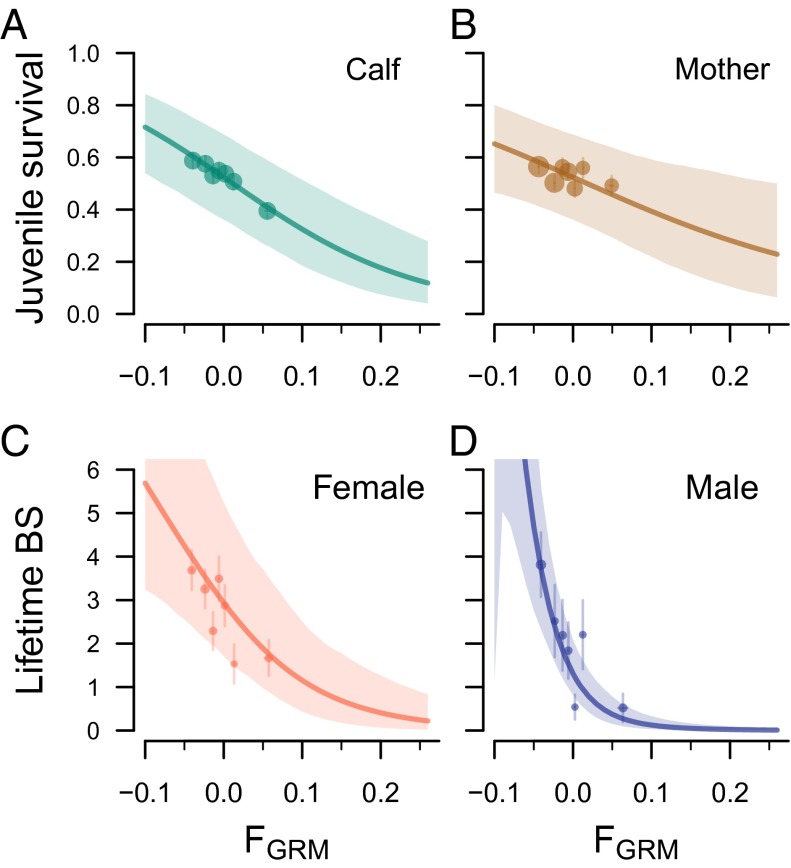
The combined effects of a juvenile’s inbreeding coefficient on its birth weight (M2) and survival during the three juvenile periods (M3–M5) resulted in a large decrease in the probability of surviving to independence at age 2 y (Table 1, model M6). As an example, an inbred female calf ( ) showed a 44% decrease (95% CI: 26–60%) in predicted survival probability compared with a female calf of , and a similarly inbred male calf was 49% (30–66%) less likely to survive (Fig. 2A, depicting average over both sexes).
Fig. 2.
The relationship between and juvenile survival and LBS. Survival from birth to age 2 y decreases with the genomic inbreeding coefficient of the offspring (A), as well as with of the mother (B), and LBS decreases with in both females (C) and males (D). Points show observations, grouped into seven bins using the septiles among newborns (−0.029, −0.018, −0.010, −0.002, 0.007, and 0.020), with point sizes proportional to number of observations in each bin, and error bars indicating 1 SE around the mean. Lines show the fitted models, and shaded areas show the 95% CI; estimated slopes and SEs are given in Table 1. ranged from −0.10 to 0.36 among neonates, and from −0.10 to 0.19 among adults.
Effect of the Mother’s Inbreeding Coefficient on Offspring Traits.
Increased maternal Fgrm was strongly associated with a decrease in offspring survival from birth to age 2 y (Fig. 2B, model M6 in Table 1), whereas for maternal Fped the effect was much smaller and nonsignificant. The effect of maternal inbreeding coefficient on offspring fitness has an effect size amounting to two-thirds that of offspring (M6). This effect appears diffuse but cumulative in nature: There was no significant effect of maternal inbreeding on parturition date, offspring birth weight, or offspring survival through any of the subperiods to age 2 y, although all coefficients were negative (models M1–M5).
Adult Traits.
There was no association between a female’s own inbreeding coefficient and her age at first reproduction (M7, typically age 3–5 y). Daughters of more inbred mothers tended to have earlier age at first reproduction (AFR), although this was not statistically significant () (see also SI Discussion).
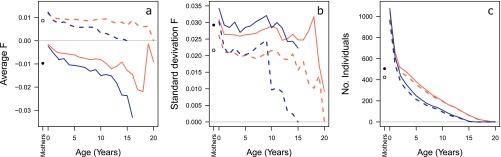
There was no significant association between annual survival after age 2 y and or , in either females (M8) or males (M9), although we did observe a decrease in both average and average with age for both sexes (Fig. S5A), and survival tended to be lower in females with higher , and in males with higher (M8 + M9, respectively).
Fig. S5.
Means (A) and SDs (B) of inbreeding coefficients and number of individuals (C) at each age in females (light red lines) and males (dark blue, immigrants are excluded), for (dashed lines) and (solid lines). The erratic patterns of mean and variance at older ages are due to limited sample size for those age classes. Points indicate the respective values among mothers, for Fped (white) and Fgrm (black).
Among both females and males aged 5 y and over, we found that increased was associated with decreased ABS (models M10 and M11 in Table 1 and Table S3). There was no significant association between and ABS in either sex.
Lifetime Fitness.
The negative association between inbreeding coefficients and each of the aforementioned traits should contribute to inbreeding depression in LBS (the number of offspring produced) and, for females, LRS (the number of offspring surviving to 2 y). To estimate total inbreeding depression, we fitted a hurdle Poisson model, which accounts for the excess of zeros in the distributions of LBS and LRS (details in SI Materials and Methods). The probability of having at least one offspring (and so passing the hurdle) decreased strongly with increasing inbreeding coefficient in both sexes, for both and (“Prob. LBS > 0” in Table 2, “hurdle” in Table S3).
Table 2.
Estimated effects of genomic inbreeding coefficients and on LBS (number of calves produced) in each sex, and on LRS (number of calves surviving to independence at age 2 y) in females
| Model | |||||||||||||
| Prob. LBS* > 0 | LBS* when >0 | Prob. LBS* > 0 | LBS* when >0 | ||||||||||
| No. | Trait | β | SE | P | β | SE | P | β | SE | P | β | SE | P |
| M12 | Female LBS | −13.7 | (6.20) | 0.02 | −0.08 | (1.86) | 0.98 | −16.8 | (4.69) | <0.001 | −2.12 | (1.44) | 0.13 |
| M13 | Female LRS | −14.2 | (6.77) | 0.03 | −9.59 | (3.78) | 0.003 | −18.7 | (5.62) | <0.001 | −4.13 | (2.07) | 0.05 |
| M14 | Male LBS | −31.7 | (11.8) | <0.001 | −23.3 | (13.0) | 0.06 | −18.6 | (5.25) | 0.001 | −14.9 | (3.92) | <0.001 |
The models simultaneously estimate the effect of inbreeding coefficients on the probability of breeding (“Prob. LBS > 0,” binomial part) and on the number of offspring, conditional on having at least one offspring (“LBS when > 0,” truncated Poisson part). Significant effects (P ≤ 0.05) are indicated in bold. The estimates for the random effects in each model are given in Table S3. Sample sizes ranged from 384 to 458 (see Table S2).
LRS, rather than LBS, is used for M13.
In females, there was no further effect of inbreeding on LBS, the number of offspring born (M12, “when > 0” in Table 2, “Poisson” in Table S3). However, there was an effect on LRS, the number of offspring that survived to independence at age 2 y (M13), in line with the effect of maternal inbreeding coefficient on offspring survival (M6). Similarly, among males that sired at least one offspring (LBS 0), those with a higher sired significantly fewer offspring (M14). There was no significant association with (Table S3).
Combining the effects of inbreeding on the hurdle and on the truncated Poisson distribution, inbred females with had a predicted 72% reduction in LBS, compared with an average female with (95% credibility region: 43–88% reduction) (Fig. 2C), and a 79% reduction in LRS (52–92%). Males with had a predicted 95% (86–98%) reduction in LBS (Fig. 2D). Note that, for both sexes, individuals with had a higher LBS than those with (Fig. 2 C and D), making a conservative benchmark to use as “outbred” individuals.
SI Materials and Methods
DNA Extraction.
DNA was extracted for n = 2,530 individuals. Samples were predominantly neonatal ear punches and postmortem ear samples (n = 2,348), extracted using the Qiagen DNeasy blood and tissue kit in 96-well-plate or spin column format, following the manufacturer’s protocol, except that, in the final step, DNA was eluted twice in 50 μL elution buffer. Phenol chloroform extraction was used on 27 postmortem muscle samples, using a standard protocol. For 117 males, we obtained high DNA yields from cast antlers, using a protocol adapted from ref. 42: Antlers were drilled along the edges of the base, and drillings were pulverized with pestle and mortar while frozen with liquid nitrogen; 20–50 mg of antler powder was digested overnight in 380 μL buffer EDTA + 0.5% N-lauryl-sarcosine and 20 μL proteinase K, and, subsequently, a slightly modified version of the Qiagen extraction protocol was followed. DNA concentration was quantified using pico green (dsDNA BR Assay Kit; Invitrogen) on a Fluorostar Omega microplate reader (BMG Labtech) or Qubit Fluorometer (Thermo Fisher Scientific). Samples in the range 25–45 ng/μL were vacuum concentrated to achieve the necessary concentration (>50 ng/μL), whereas samples below 25 ng/μL were reextracted where possible, and otherwise not used further.
SNP Genotyping.
Genotyping was performed using the cervine Illumina Infinium iSelect HD Custom BeadChip on an iScan instrument at the Wellcome Trust Clinical Research Facility Genetics Core. SNP discovery is described in Brauning et al. available at biorxiv.org/content/early/2015/09/23/027318. To be able to check for consistency between batches, and to calculate the repeatability of each SNP, the same positive control was included twice on each 96-well plate.
No linkage map for deer is available yet, and SNPs were mapped to the bovine reference genome (as described in Brauning et al.). This resulted in a typical pattern of linkage disequilibrium (LD) decay with putative physical distance (Fig. S1C).
Pedigree Reconstruction.
Because we could not find an available method that would make the most of the SNP data in pedigree reconstruction, the pedigree was reconstructed using a novel likelihood approach applicable when large numbers of SNP markers are available. First, a subset of SNPs were selected with high minor allele frequency (MAF > 0.4) and in low LD with each other () using PLINK (36). This resulted in 440 SNPs, which were subsequently treated as independent. Parents were assigned by first excluding highly unlikely parent−offspring pairs (opposite homozygotes at more than nine SNPs), then phasing the possible pairs based on birth years. Subsequently, for each candidate parent in turn, we calculated the likelihood of the candidate parent being (i) a parent, (ii) a full sibling, (iii) a half-sibling, (iv) a grandparent, (v) a full aunt/uncle, (vi) a half-aunt/uncle or great-grandparent, or (vii) unrelated, and assigned the candidate as parent if hypothesis i was more likely than any alternative, using a threshold log10 likelihood ratio (LLR) of 2.0. Subsequent likelihoods were calculated conditional on the already assigned parent.
Subsequently, among those with no father assigned this way, for all pairs of individuals, we calculated the likelihoods under hypotheses i−vii, conditional on the maternal genotype where known, and including age-based prior information. Pairs were ordered by decreasing LLR paternal siblings/unrelated, and sequentially either added to an existing paternal sibship cluster and assigned a dummy father or used to found a new sibship, if this was more likely (again comparing hypotheses i−vii with respect to the sibship). The procedure was repeated to create maternal sibship clusters, conditional on membership of paternal sibships. This procedure was then repeated twice more for both paternal and maternal siblings, including merging of clusters if this resulted in a higher likelihood.
Lastly, grandparents were sought for each sibship, among those individuals at least 6 y older than the oldest sibling (double the earliest age of reproduction), following a procedure analogous to the parentage assignment. This included potential assignment of the dummy parent of one sibships as dummy grandparent of another sibship. The described approach is implemented in the R package sequoia, available on GitHub (https://github.com/JiscaH/sequoia).
The thus reconstructed pedigree was compared with the existing pedigree, based on observational and microsatellite data (37). No mismatches in maternity were observed, and all observation-based maternities were added to the pedigree. Assignment of nongenotyped males to paternal sibship clusters was done following ref. 37, based on the mother’s harem membership during an 11-d window around their oestruses. Of the 2,189 paternities assigned in the previous pedigree, 67 proved to be erroneous (3%); in at least 13 of these cases, paternity was assigned to a half-brother of the true father. For cases where SNP genotyping failed, the SNP and observation-based pedigree was supplemented with microsatellite-based paternities, provided this did not create any mismatches e.g., between the previously assigned father and known offspring of the focal individual.
The resulting pedigree had a higher rate of paternity assignment, a smaller number of founders, and a higher number of individuals with nonzero than the previous microsatellite-based pedigree (37) (previous: 483 out of 3,090 with , currently 936 out of 3,233). The correlation between inbreeding estimates from the two pedigrees was high ().
Genomic Inbreeding Estimators.
Several estimators have been developed with the intention of improving estimates of genome-wide homozygosity, relative to average marker homozygosity, by taking into account the allele frequencies at the markers used. Comparisons between estimators have been performed on simulated datasets, humans, and cattle (e.g., refs. 14 and 43), and, generally, the low sampling variance inbreeding estimator () in GCTA (25) is (among) the best performing. It is defined as
| [S1] |
where is the allele frequency at locus i, and is the number of copies of the reference allele (0, 1, or 2). This estimator will here be referred to as , for Genomic Relatedness Matrix, and gives more weight to homozygosity for rare alleles, using weights for minor homozygotes, for major homozygotes, and for heterozygotes.
As it was not fully clear how the results from simulations and other studies translate to the relatedness structure of wild populations in general, and this population in particular, we compared to (i) unstandardized average marker homozygosity, the proportion of SNPs at which an individual is homozygous, out of the SNPs for which it was successfully genotyped (on average 99.9% after QC), and (ii) the inbreeding estimator in PLINK (36), given by
| [S2] |
where the expected number of homozygotes is calculated assuming Hardy−Weinberg equilibrium, as . We omitted runs of homozygosity and other estimators depending on the order and proximity of SNPs from our comparison, as the precise map order of our SNPs is, as yet, uncertain.
Both and implicitly assume independence between markers, and we therefore also calculated these metrics on a pruned subset of 32,945 SNPs, after removal of the SNPs in highest LD with neighboring SNPs. Pruning was done using a sliding window approach in PLINK, with a window size of 100 SNPs (assuming the bovine map order), sliding with an overlap of 25, and a threshold for the multiple correlation coefficient for a SNP being regressed on all other SNPs simultaneously of .
Evaluation of Identity Disequilibrium and Marker Number.
To assess whether there was a significant correlation in homozygosity between loci within individuals, we estimated , a measure of identity disequilibrium in the population, defined (9) as
| [S3] |
where are the true realized inbreeding coefficients. This parameter can be estimated directly from SNP homozygosity, as described in the supporting information of ref. 17. Code was kindly made available by those authors as a beta version of the R package InbreedR, which provides a bootstrap confidence interval. We calculated for each of the trait-specific data subsets and compared these values with the variance in , as the correlation between estimated inbreeding coefficients (average marker homozygosity in ref. 9, here or ) and can be estimated as
| [S4] |
To assess whether marker density and marker coverage of the genome was sufficient, or if an increase in the number of markers would be likely to change estimates of inbreeding depression, we calculated Homozygosity−Homozygosity Correlations (HHC) for each of the three estimators, and investigated the relationship between HHC and marker number. First, we randomly sampled 1%, 2%, 5%, 10%, 20%, 40%, 60%, 80%, or 100% of SNPs (without prior LD pruning). Then, we divided the SNPs at random into two equal-sized subsets and calculated the inbreeding estimates within each subset and the correlation (HHC) between these two estimates. This was repeated 50 times for each fraction. These calculations were performed using custom Fortran scripts, available upon request.
In addition, individual inbreeding estimates calculated within each fraction (over both halves, for each of the 50 repeats) were used as explanatory variables in the model of birth weight, to assess the effect of number of SNPs used on the estimated inbreeding depression.
Statistical Analysis: (Generalized) Linear Mixed Models.
Each trait was fitted in a univariate (generalized) linear mixed model using R (40). The fixed and random effects fitted for each trait are given in Table S3, and their descriptions are given below. Birth date (M1) and birth weight (M2) were fitted as Gaussian traits using ASREML-R, and juvenile survival traits (M3–M5) were fitted in generalized linear mixed models (GLMM) with library LME4, using a logit link function (results were highly similar when using a probit link function). Bootstrap confidence intervals for the random effects were obtained with the R function “confint,” using 100 bootstrap replicates. Survival from birth to age 2 y (M6), adult annual survival (M8 and M9), and female ABS (M10) were analyzed using a binomial error structure in MCMCglmm, as convergence for M6 in LME4 was slow when birth weight was not fitted as covariate, and to better take into account, and be more conservative about, the repeated measures structure of M8 and M9. The prior for the residual variance was fixed to 1, and we used the standard prior V = 1/4, nu = 0.002 for each of the random effects. For female age at first reproduction (M7) and male ABS (M11), we fitted quasi-Poisson models using MCMCglmm.
Following suggestions in the course notes for MCMCglmm (41), available online, thinning intervals for each MCMCglmm model were chosen to ensure an autocorrelation < 0.1 between subsequent stored iterations, aiming for an effective sample of 2,000 but at least 1,000 iterations. Traces were visually inspected to confirm convergence. See footnotes of Table S3 for the number of iterations and the burn-in and thinning intervals used per model. For each model, we used the recommended prior in these course notes. To allow direct comparison with the GLMM, SEs of the estimates were computed as the SD of the posterior distributions.
Statistical Analysis: Hurdle Poisson Models.
For LBS and LRS (M12–M14), we fitted hurdle Poisson models, which jointly estimate the probability of having zero offspring () and the mean of the truncated Poisson distribution (), of the likelihood,
| [S5] |
as described in ref. 41. The prior for the residual variance was a diagonal matrix with nu = 0.002, with the residual variance for fixed at 1, and the prior for the random effects was a diagonal matrix with nu = 0.002.
Predictions from the hurdle Poisson model (see Results, and lines in Fig. 2B) were made by, first, for each stored iteration x, calculating for a range of values of the pedigree or genomic inbreeding coefficient f the parameters
where is the intercept and is the slope for , and VCV1 and VCV2 are the random effects on the truncated Poisson and hurdle, respectively (41). We then used and to calculate the probability P of having n offspring (n from 0 to 50), and subsequently calculated the weighted sum of the number of offspring for each iteration x and each value of the inbreeding coefficient f. Finally, we took the mean, SD, and 95% interval over iterations as the point estimate, SE, and credibility region, respectively.
Statistical Analysis: Explanation of Fixed and Random Covariates.
For the LBS and LRS models, only male immigrant status (see below) was fitted as fixed effect in M13, and for juvenile survival (M6) we only included several maternal characteristics. For the remaining models, we initially fitted all known or plausible effects in a full model. To ensure the binomial models of juvenile survival converged (M3–M5), continuous explanatory variables were scaled to zero mean and unit variance, and nonsignificant terms were dropped. To ease comparison, these covariates were scaled in all of the models of juvenile traits (M1–M6). For scaling, we used the following means and variances: for birth date (days since 1 April), mean and SD ; capture age (hours) , ; birth weight (kilograms) , ; maternal age (variance scaled only), maternal age squared ; population size , ; winter rainfall (mm) , ; spring temperature (°C) , .
The fixed effect maternal reproductive status is a four-level factor indicative of the condition of a female, based on whether she is a first time breeder (“Naïve”), whether she cared for her previous calf during winter and throughout pregnancy (“Milk”), or if the previous calf died during winter (“Winter”) or before the first of October or she did not breed at all of the previous year (“Yeld”, the reference level); see ref. 18 for further details. For female annual survival, we fitted a variable denoting whether or not the female had given birth in the spring of that year (“Calf”). For female ABS, the probability of conceiving depends on a female’s body condition during the rut in autumn, which is correlated to whether she is nursing a calf at that point in time (“Calf October 1”). Earlier AFR in females was previously shown to be associated with increased ABS later in life (44), and this was fitted as a fixed covariate with three levels: “early” (age 3 y), “mid” (age 4 y, the majority) or “late” (age 5–7 y). AFR was not fitted for female annual survival (age 2+ y), as this would lead to exclusion of all individuals who died before their first breeding attempt. Population size has been shown to affect, among other traits, birth weight and female fecundity (26), and was fitted throughout for consistency. Population size was measured as the number of females older than 1 y seen in at least 10% of censuses between January and May (20, 22). For birth date, birth weight, calf summer survival, and ABS, we fitted the population size during the preceding winter, whereas for calf winter survival, yearling survival, and adult annual survival, we fitted the population density during the winter over which survival was measured (note that we used “deer years” running from 1 May in year t to 30 April in year ; most calves are born in May and June). Following previous studies (22, 26), we additionally fitted spring temperature (average between 5 February and 23 April) in the model for birth weight, and winter rainfall (November–January) for calf winter survival and juvenile survival.
The random effect “Spatial region” consisted of six categories (each region in figure 1 in ref. 45 was split into two), and is based on the average location of the female during censuses from January to May (of the calf’s mother in the calendar year of birth for juvenile traits). Prior explorations showed that these regions explained similar variation in birth weight as the matrilines derived from females alive in 1971 used in previous publications (e.g., ref. 27), as female relatives tend to live in the same region for many generations (18, 27). Adult males tend to live outside the study area for most of the year; therefore this term was not fitted for male traits. Instead, a distinction was made between local-born and “Immigrant” males for only; was unknown for all immigrant males. A test for genetic differences between local and immigrant males is given in SI Discussion. Year of birth was fitted as a random covariate for all traits to account for cohort effects, and year of observation was fitted for annual traits. For birth date, year was additionally fitted as a fixed effect, to account for the temporal trend due to climate change (26). There was no temporal trend in any of the other traits considered, consistent with previous findings (26). To account for repeated measurements on the same mother, maternal ID was fitted as a random effect for all traits. For immigrant males, several pairs of maternal siblings were identified during pedigree reconstruction and were assigned to a dummy mother; the remainder of immigrants were given unique maternal dummy IDs. To account for repeated measures on the same individual for ABS, “own ID” was here fitted as random effect.
SI Results
Quality Control of SNP Data.
In total, 2,880 samples were genotyped, including two positive controls on each of the 30 96-well plates. The array contained 51,248 attempted SNPs, of which 50,541 were successfully scored. A subset of samples with a call frequency above 0.95 was used for automatic clustering in Genome Studio, followed by manual reclustering for 1,452 SNPs with a separation score below 0.4 and/or replication errors [following the manufacturers guidelines (Illumina)], and exclusion of 278 SNPs for which reclustering was not possible. During subsequent quality control in PLINK (36) (after readding samples with call rate below 0.95), we first removed 2,743 monomorphic SNPs (MAF < 0.0001; the chip contains SNPs intended to be diagnostic between red deer, wapiti, and sika deer), positive control samples, duplicated samples, and 50 samples with a call rate below 0.9. Then, we excluded 2,364 SNPs with a call frequency below 0.99, 5,261 SNPs with MAF , 1,956 SNPs on the X chromosome, and 388 SNPs which deviated from Hardy−Weinberg equilibrium ().
The resulting dataset consisted of 37,410 autosomal informative SNPs for 2,622 individuals, of which 2,254 were born since 1981, with an average individual call rate of 0.9992 and no replication errors for any of the SNPs. The MAF distribution had an overrepresentation of common alleles (Fig. S1A). Mean and median spacing between neighboring SNPs were 66.5 kb and 49.1 kb, respectively, when mapped to the bovine genome (Fig. S1B), and average LD at median spacing was (Fig. S1C).
Correlations Between Inbreeding Estimators.
had a higher correlation with pedigree inbreeding than raw homozygosity or , both when calculated over all SNPs and when calculated over a subset of pruned SNPs (Fig. S2). As expected, the correlations between genomic and pedigree-based inbreeding estimates increased with increasing ancestry information, as individuals with incomplete ancestry information had downward-biased (Fig. S2E). Both and showed slightly higher correlations with the pedigree inbreeding coefficients when calculated over the subset of SNPs pruned on LD (excluding about 10% of SNPs) rather than over all SNPs (Fig. S2E), but the difference was marginal. is very strongly correlated with average marker homozygosity in this dataset (r = 0.99986), which is a direct consequence of the low rate of missing genotypes per individual (%), such that the expected homozygosity and number of genotyped SNPs (Eq. S2) are virtually constant across individuals. is also strongly correlated with homozygosity (, Fig. S2), implying a relatively small contribution of population allele frequencies to the estimates (Eq. S1). The dependency of on the allele frequencies is responsible for a slight bulge above and to the left of the main cloud in Fig. S2A; these are all offspring of a single nongenotyped immigrant male (“TURKS”), who most likely carried a range of alleles rare to the study population.
Variance in Inbreeding.
The variance in inbreeding was larger for (0.00114) than for (0.00084), and both values are considerably lower than a previously reported value for this population (0.0025) (24). The previous value was based on a study considering close () and moderate () inbreeding only, and overestimated the proportion of matings between paternal siblings [11 out of 103 individuals with both maternal and paternal grandfather known (24), versus 39 out of 1,543 here].
Between our various trait-specific subsets, variance in inbreeding was largest in the subsets used for estimating LBS (Fig. S3C). These subsets are much smaller than the ones used for juvenile survival (Table S2), while containing both some of the most inbred and the most outbred individuals. For none of the subsets did the variance in lie outside the 95% confidence interval of (Fig. S3C), suggesting that the correlation between and true realized inbreeding coefficients (Eq. S4) did not differ significantly from 1.
Versus Parental Genomic Relatedness.
An additional line of evidence that is closely correlated with true realized inbreeding coefficients comes from the regression of on parental genomic relatedness, calculated in GCTA (25), which nearly perfectly matches the theoretical expectation [expected: , (46), observed: , , for n = 1,128 trios]. For comparison, the regression coefficient of on parental pedigree relatedness is half the coefficient of on (Fig. 1), as is by definition half the parental relatedness, and is , with .
Temporal Trend in F.
During the 33 y that the dataset spans, after excluding immigrant males, both and increased very slightly. In a linear regressions of F on birth year, increased by 1.3E-4 E-5 SE per year (, adj. ), and increased by 3.2E-4 E-5 per year (, adj ). The trend in was not explained by the lower sampling rate in the earliest years, particularly of individuals who died young, which may have underestimated the proportion of inbred individuals in that period. The smaller increase in than in suggests that the contribution of accumulating pedigree depth is minimal, at least among the subset used for which at least both parents and maternal grandfather were known (note that the earliest records in the pedigree go back to the 1960s).
SI Discussion
Genetic Differences Between Local and Immigrant Males.
Calculations of expected homozygosity assume complete random mating in the (reference) population, which may not always be realistic or relevant. Here, immigrant males had slightly higher than locally born adult males (, ), whereas the two groups did not differ in homozygosity (, ). Because female deer are highly philopatric (27), SNP allele frequencies are likely to differ across the island, and allele frequencies among residents in the study area may deviate from those among immigrants. Reassuringly, the effect of homozygosity on male ABS and LBS was very similar to the effect of ( for ABS, and for both the hurdle and Poisson parts of LBS).
Although did not differ between individuals sired by immigrant males () and those sired by local-born males () (, ), homozygosity was slightly lower among [0.636 () versus 0.638 (), , ]. Note that this difference is much smaller than the difference in between the groups [average 0.0058 () versus 0.0153 (), , ]. The latter thus mainly reflects differences in ancestry information between the two groups, rather than differences in homozygosity.
AFR.
Earlier AFR typically indicates higher individual quality (44) (M8 and M10 in Table S3), and there was a trend for daughters of more-inbred mothers to have an earlier AFR (,). This may indicate selective disappearance, a compensatory effect in inbred mothers, or be a false positive [maternal inbreeding on AFR had the smallest sample size (S2), although one of the highest (Fig. S3C)]. An alternative, biological, hypothesis is that daughters of inbred mothers have a smaller skeletal size, and consequently reach breeding condition (ratio of body weight to skeletal size) earlier. However, we found no significant association between maternal and postmortem jaw length, a proxy for skeletal size, in daughters that died as adults (age 2+ y; , , ; with daughter’s age at death fitted as covariate).
Potential for Population-Level Consequences.
Despite the considerable effects of inbreeding on LBS in both sexes, under current conditions, we believe that inbreeding is unlikely to have population-level consequences in our study population. Male reproductive success will rarely limit population growth rates of a polygynous species, and juvenile survival and female fecundity have been shown to be density-dependent in this population (18, 26). Deer numbers in the study area are limited by food availability during winter (18), and total population size over the last three decades has been relatively stable (45), so that the selection acting on inbreeding is probably soft selection (1): If an inbred individual had not died, another one probably would have. Nonetheless, in populations with a higher proportion of inbred individuals, or which are less constrained by density dependence, this degree of inbreeding depression might very well be ecologically relevant, as has been shown in various species (3).
Discussion
The substantial inbreeding depression in lifetime fitness found here is partly due to inbreeding depression in juvenile survival, which has been reported in previous studies of this population (20, 22) and in many other species (1). In addition, we found that increased genomic inbreeding coefficients were associated with decreased ABS in adults of both sexes. Moreover, increased maternal inbreeding coefficients decreased offspring survival to independence. In contrast, when using pedigree-derived inbreeding coefficients, we could detect significant inbreeding depression in juvenile survival but not in any of the adult traits.
Variance in Inbreeding and Identity Disequilibrium.
The Rum red deer study population was expected to have high variance in inbreeding coefficients, due to a limited population size, recent admixture with red deer from the mainland (21), ongoing admixture with red deer from other parts of the island, a strongly polygynous mating system, and higher levels of consanguineous matings than expected under random mating (27). Despite this, the variance in inbreeding appears relatively low compared with other pedigreed vertebrate populations, at and among neonates (Fig. S5B), compared with (median 0.0031) among 18 mammal and bird species listed by Grueber et al. (28). However, this list includes various populations with a very small number of founders; data on the typical variance of inbreeding in animal populations is currently lacking.
Variance in inbreeding coefficients typically decreases with age, due to the selective disappearance of the most inbred individuals (see Fig. S5B), resulting in a lower identity disequilibrium () among adults. Here, compared with neonates, was 33% lower among adult females and 21% lower among adult males (including immigrant males; see also Fig. S3C and SI Discussion). Consequently, estimating inbreeding depression in adult traits is even more challenging than in juveniles, and more markers are required to estimate genome-wide homozygosity accurately. The large number of markers used here ensured that was significantly different from zero for all data subsets (Fig. S3C), although, across traits, there was a (nonsignificant) positive trend between and estimated inbreeding depression (Fig. S3D).
Genomic Versus Pedigree-Based Estimates.
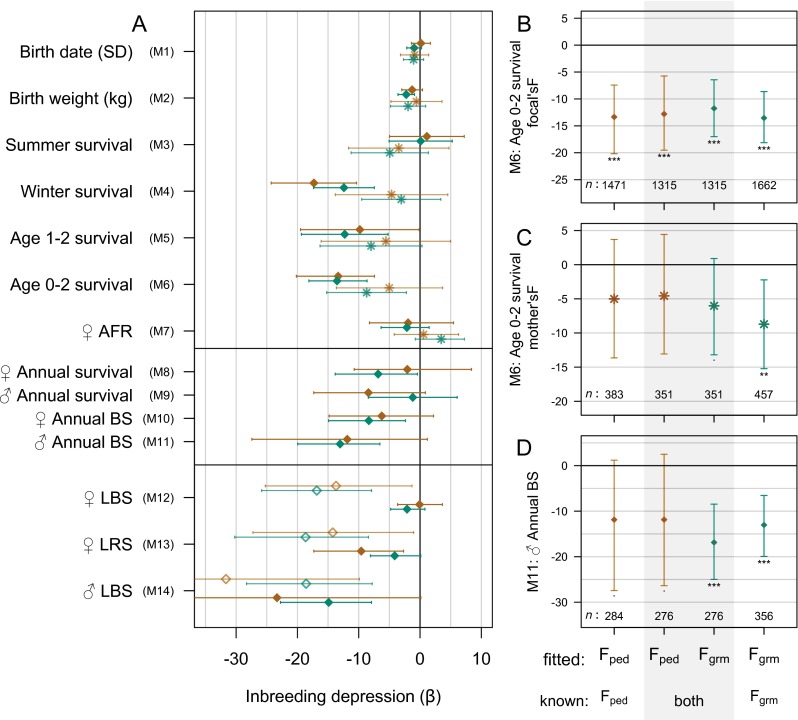
More - than -based estimates of inbreeding depression differed significantly from zero; the main reason for this was that the SEs when using were consistently smaller than when using (Table 1), in line with expectations based on other studies (14, 16). For most (but not all) traits, we found that additionally resulted in slightly larger point estimates, but none of the differences between the estimates were significant (Table 1 and Fig. S6A). Exceptions to the general pattern of larger estimates for may thus be due to chance alone.
Fig. S6.
(A) Estimated slopes and 95% CIs of trait values on (brown) and (green) of the focal individual (diamonds) or its mother (asterisks), on datascale (M1, M2), log scale (M7, M11), or logit scale (M3–M6, M8–M10). For the hurdle Poisson models (M12–M14), the effect of F on the hurdle is denoted by open diamonds, and, on the truncated Poisson, is denoted by filled diamonds. B−D show, for M6 (B for focal and C for mother) and M11 (D), the estimated slopes when or is fitted in a smaller dataset, in which both metrics are known for all individuals (middle bars, gray shaded area), compared with the larger datasets in which either (outer left) or (outer right) is known (these are identical to the bars in A). Sample sizes are given under each bar, and asterisks indicate standard levels of significance.
The difference in performance between and can be attributed to a number of factors. First, there was a difference in the available sample size (Table S2). However, even, when using a smaller data set including only individuals for whom both metrics were known, the narrower confidence intervals for compared with remained (Fig. S6 B–D). Second, although was based on a standardized minimum amount of pedigree information, this metric will contain error due to missed inbreeding in the highly variable depth of pedigree available for different individuals. Third, as described in the Introduction, captures variation in IBD around the pedigree expectation. Note that the pattern is unlikely to have been caused by the somewhat different scales of the two estimators because, all else being equal, the wider range of observed values for (–0.36) than for (0–0.27) would result in shallower slopes of trait values against than against .
The difference between pedigree and genomic estimates of inbreeding depression reported here is much more pronounced than in a similar comparison focusing on the estimation of heritability and genetic covariances in a study population of Soay sheep on St Kilda, NW Scotland (29). One reason for this is that individuals who lack some ancestry information may have a highly imprecise Fped, while having accurate pedigree relationships with their many (half-)siblings, descendants, and known ancestors. All these pairwise relationships contribute to the estimate of heritability, whereas only the relatively small number of direct ancestors contribute to Fped.
Inbreeding Depression in Fitness Components.
Generally, inbreeding depression is expected to be stronger in traits more closely associated with fitness (30). Our findings were in line with this expectation, with, for example, individuals with showing only a small reduction in birth weight (%) compared with the reduction in juvenile survival (%), and an even larger reduction in LBS (% to %).
This trend was not followed by the two adult fitness components considered. Previous studies in this red deer population showed that female LBS is more strongly determined by longevity than by ABS, whereas male LBS is about equally determined by both components (19, 31). This is a consequence of the breeding biology of red deer, with a maximum of one calf per year for females, and hence a greater advantage of longevity over ABS compared with males. Based on this, one would expect females to show stronger inbreeding depression in survival than in ABS, and that estimates for both traits would be similar in males. In contrast, in inbred females (again using ), we found a stronger reduction in ABS (%) than in adult survival (%), and the difference was even larger for males (ABS %, survival %). The reason for this unforeseen pattern is not clear, and seems unrelated to sample size (Table S2) or identity disequilibrium (Fig. S3C). The relative magnitudes of inbreeding depression in these traits are likely to be species-specific, but, interestingly, in song sparrows, a similar pattern was found, with a larger effect of inbreeding coefficients on male than female annual reproductive success (ARS), and no detectable effect on adult survival (10). Similarly, in Darwin’s finches, inbreeding depression in the annual probability of breeding was more consistent than in annual survival (32).
Inbred Mothers.
An effect of the mother’s inbreeding coefficient on offspring survival is rarely reported, but possibly contributed to the documented reduced ARS in song sparrows (10) and bottlenose dolphins (33). In the deer, offspring of inbred females ( ) had a 31% lower probability of survival to independence, which, together with the reduced ABS, resulted in a 47% decrease in ARS for inbred females (assuming independent effects on ABS and offspring survival). The relative magnitudes of inbreeding depression in ABS and offspring survival, and the most affected offspring age class, are likely to vary between species. For example, an earlier study in the song sparrows documented a negative effect of female on decreased hatching rates, but not on egg number or subsequent fledging rates (8), whereas, in wandering albatross, microsatellite homozygosity was associated with offspring fledging rates, but not number of hatched offspring (34).
On Rum, red deer females do not conceive in years when they are in poor condition at the rut, and this may provide an opportunity for compensation in inbred females. By foregoing the cost of reproduction on survival (M8) and subsequent fecundity (M10), the net effect of a female’s inbreeding coefficient on LBS is diminished, and so was undetectable among those who bred at least once (M12). Similar discrepancies between effects on annual or seasonal and lifetime measures of fitness due to compensation can be found, for example, when inbreeding affects brood success in birds, but inbred females increase the number of breeding attempts (35). These results emphasize the importance of considering the entire life cycle, including effects of parental inbreeding coefficients on juvenile survival, when estimating the total effect of inbreeding depression.
Inbreeding Depression in Fitness.
We found inbreeding depression both in whether or not a male sired any offspring, and, if he did, in how many offspring he sired during his lifetime (model M14). In contrast, in females, although there was inbreeding depression in whether or not a female bred, if a female did breed, there was no association between inbreeding coefficient and her LBS (M12), but there was a negative association with her lifetime number of recruits (LRS, M13). These results for LBS are consistent with a previous heterozygosity fitness correlation of LBS using microsatellites, in which the slope was steeper for males than for females (25). The difference between the sexes is probably partly a consequence of the polygynous breeding system of red deer with strong male−male competition, which leads to higher variance in LBS in males than in females. In song sparrows, which are somewhat polygynous (but much less so than red deer), the magnitude of inbreeding depression in LRS was twice as large in males as in females (10). However, in general, this hypothesis is difficult to verify, as few other vertebrate studies have documented the effect of inbreeding coefficients on LBS or LRS in both sexes.
Materials and Methods
Study Population.
The study area is located in the North Block of the Isle of Rum off the west coast of Scotland, and contains about one-quarter of the adult red deer on the island. Red deer are highly seasonal, polygynous breeders, and, during the autumn rut, adult males defend groups of females against competitors for the chance to mate. Deer are free to move in and out of the study area, and are unmanaged within it, but are subject to annual culls on the remainder of the island. For further details, see refs. 18 and 22. The research was conducted following approval of the University of Edinburgh's Animal Welfare and Ethical Review Body and under appropriate UK Home Office licenses.
We considered all individuals born between 1981 (when the population reached carrying capacity following cessation of culling in 1973) and 2013 for which sufficient life history information was available (see SI Materials and Methods for details). Sample sizes for each trait are given in Table S2. Data are available on FigShare (10.6084/m9.figshare.2075584.v1).
Phenotypic Traits.
Birth weight.
Most calves are caught within a few days of birth, to take measurements and apply artificial markings. In the models of birth weight, capture weight was used as a response variable, and age at capture (in hours) was fitted as a covariate. When birth weight was used as a covariate, it was estimated from a linear regression of body mass on age at capture (slope: 0.01696 kg/h).
Survival.
Regular censuses throughout the year and mortality searches during winter provide accurate information on death date for most individuals. Individuals who were shot, emigrated, or with unknown fate were excluded from survival analysis in that particular year only. Annual survival was evaluated between 1 May and 1 May, rather than in calendar years, as most mortality occurs in winter.
Breeding success.
Females give birth to, at most, a single offspring per year. The regular censuses, plus intensive observations in the calving season, provide pregnancy statuses and parturition dates for all resident females, as well as AFR, typically between 3 y and 5 y. For ABS, all individuals age 5 y and over seen in censuses during the rut (males) or calving season (females) in a given year were included in the data. This lower age limit avoided confounding ABS with AFR in the females, and enabled comparison among potentially reproducing mature males only (0.7% of individuals were sired by males aged 2–4 y).
LBS.
LBS (birth–death) was calculated for all individuals in the cohorts 1981–2000 who had either died a natural death or were still alive in 2013 (n = 4 males) or 2015 (n = 9 females). This approach, following ref. 23, minimizes bias toward individuals who died young, whereas the few individuals still alive had obtained almost all of their LBS: 1.9% of pregnancies were after age 15 y, and of calves were sired by males over age 13 y (we know the mothers but not fathers of calves born in 2014 and 2015). For LRS (total number of offspring who survived to age 2 y, birth–death of focal individual), we considered the cohorts 1981–1998, otherwise using the same criteria as for LBS.
DNA Extraction and SNP Data.
DNA was extracted from neonatal ear punches, postmortem tissue, and cast antlers (details in SI Materials and Methods). Genotyping was performed using the cervine Illumina BeadChip, and quality control was done in Genome Studio (Illumina) and PLINK (36) (SNP call rate , individual call rate , minor allele frequency ; further details in SI Results and Fig. S1). In total, 2,254 individuals were genotyped at 37,410 polymorphic SNPs. The number of markers did not limit the precision of our estimates (SI Materials and Methods, SI Results, and Fig. S3B).
Inbreeding Coefficients.
The existing pedigree (37) was extended and improved, using the better resolution of the SNP data compared with the previously used microsatellite markers, and the additional individuals genotyped for the SNPs. A likelihood-based pedigree reconstruction method was developed that identified parents as well as second-degree relatives, using 440 SNPs (details in SI Materials and Methods). was calculated in the R-package Pedantics (38) using Wright’s path approach (39).
The genomic inbreeding estimator used [ in Yang et al. (25)] estimates the correlation between uniting gametes, Wright’s original definition of the inbreeding coefficient (39). It is highly correlated to average marker homozygosity, and to an estimator that corrects for expected homozygosity (36) (details in SI Materials and Methods and Figs. S4 and S2) but has a lower sampling variance (25) (Fig. S3).
Statistical Analysis.
Detailed statistical analyses for many traits in the study population have been published before (18, 19), and effects of multiple explanatory variables other than inbreeding on the focal traits are well known. To maximize sample sizes, we included all individuals with either or known, rather than using a smaller, identical set of individuals with both metrics known. For each trait, all known fixed and random covariates were fitted in addition to the inbreeding coefficient(s), and we attempted to use the same covariates for each of the juvenile traits and each of the adult traits, where appropriate (see Table S3). All analysis was done in R (40), using ASREML-R for the normally distributed traits, LME4 for the juvenile survival traits, and MCMCglmm (41) for the remaining traits. To ensure convergence of models in LME4, fixed effects were standardized. Details of the statistical analysis including covariates are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
Thanks go to F. Guinness, A. Morris, S. Morris, M. Baker, and many others for collecting field data; S. Albon for his contributions to the long-term project; and M. Stoffel for providing code to calculate g2. We are grateful to C. Berenos, S. Johnston, and H. Froy for discussion; and W. G. Hill, C. Walling, J. Slate, and three anonymous reviewers for comments on the manuscript. We also thank Scottish Natural Heritage for permission to work on the Isle of Rum National Nature Reserve, and Wellcome Trust Clinical Research Facility Genetics Core in Edinburgh for performing the genotyping. The long-term project is funded by the UK Natural Environment Research Council, while the SNP genotyping and current work were supported by a European Research Council advanced grant (to J.M.P.). L.E.B.K. is supported by an Australian Research Council Future Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited on Figshare, https://dx.doi.org/10.6084/m9.figshare.2075584.v1.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518046113/-/DCSupplemental.
References
- 1.Keller L, Waller D. Inbreeding effects in wild populations. Trends Ecol Evol. 2002;17(5):230–241. [Google Scholar]
- 2.Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annu Rev Ecol Syst. 1987;18:237–268. [Google Scholar]
- 3.Frankham R, Briscoe D, Ballou J. Introduction to Conservation Genetics. Cambridge Univ Press; New York: 2002. [Google Scholar]
- 4.Mc Parland S, Kearney JF, Rath M, Berry DP. Inbreeding effects on milk production, calving performance, fertility, and conformation in Irish Holstein-Friesians. J Dairy Sci. 2007;90(9):4411–4419. doi: 10.3168/jds.2007-0227. [DOI] [PubMed] [Google Scholar]
- 5.Joshi PK, et al. BioBank Japan Project Directional dominance on stature and cognition in diverse human populations. Nature. 2015;523(7561):459–462. doi: 10.1038/nature14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pusey A, Wolf M. Inbreeding avoidance in animals. Trends Ecol Evol. 1996;11(5):201–206. doi: 10.1016/0169-5347(96)10028-8. [DOI] [PubMed] [Google Scholar]
- 7.Boakes EH, Wang J, Amos W. An investigation of inbreeding depression and purging in captive pedigreed populations. Heredity (Edinb) 2007;98(3):172–182. doi: 10.1038/sj.hdy.6800923. [DOI] [PubMed] [Google Scholar]
- 8.Keller L. Inbreeding and its fitness effects in an insular population of song sparrows (Melospiza melodia) Evolution. 1998;52(1):240–250. doi: 10.1111/j.1558-5646.1998.tb05157.x. [DOI] [PubMed] [Google Scholar]
- 9.Szulkin M, Bierne N, David P. Heterozygosity-fitness correlations: A time for reappraisal. Evolution. 2010;64(5):1202–1217. doi: 10.1111/j.1558-5646.2010.00966.x. [DOI] [PubMed] [Google Scholar]
- 10.Reid JM, et al. Pedigree error due to extra-pair reproduction substantially biases estimates of inbreeding depression. Evolution. 2014;68(3):802–815. doi: 10.1111/evo.12305. [DOI] [PubMed] [Google Scholar]
- 11.Slate J, et al. Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: Theoretical expectations and empirical data. Heredity (Edinb) 2004;93(3):255–265. doi: 10.1038/sj.hdy.6800485. [DOI] [PubMed] [Google Scholar]
- 12.Chapman JR, Nakagawa S, Coltman DW, Slate J, Sheldon BC. A quantitative review of heterozygosity-fitness correlations in animal populations. Mol Ecol. 2009;18(13):2746–2765. doi: 10.1111/j.1365-294X.2009.04247.x. [DOI] [PubMed] [Google Scholar]
- 13.Hill WG, Weir Variation in actual relationship as a consequence of Mendelian sampling and linkage. Genet Res. 2011;93(1):47–64. doi: 10.1017/S0016672310000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kardos M, Luikart G, Allendorf FW. Measuring individual inbreeding in the age of genomics: Marker-based measures are better than pedigrees. Heredity (Edinb) 2015;115(1):63–72. doi: 10.1038/hdy.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visscher PM, et al. Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. PLoS Genet. 2006;2(3):e41. doi: 10.1371/journal.pgen.0020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pryce JE, Haile-Mariam M, Goddard ME, Hayes BJ. Identification of genomic regions associated with inbreeding depression in Holstein and Jersey dairy cattle. Genet Sel Evol. 2014;46:71. doi: 10.1186/s12711-014-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman JI, et al. High-throughput sequencing reveals inbreeding depression in a natural population. Proc Natl Acad Sci USA. 2014;111(10):3775–3780. doi: 10.1073/pnas.1318945111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clutton-Brock T, Guinness F, Albon S. Red Deer: Behaviour and Ecology of Two Sexes. Univ Chicago Press; Chicago: 1982. [Google Scholar]
- 19.Kruuk LE, et al. Heritability of fitness in a wild mammal population. Proc Natl Acad Sci USA. 2000;97(2):698–703. doi: 10.1073/pnas.97.2.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coulson T, Albon S, Slate J, Pemberton J. Microsatellite loci reveal sex-dependent responses to inbreeding and outbreeding in red deer calves. Evolution. 1999;53(6):1951–1960. doi: 10.1111/j.1558-5646.1999.tb04575.x. [DOI] [PubMed] [Google Scholar]
- 21.Slate J, Pemberton J. Comparing molecular measures for detecting inbreeding depression. J Evol Biol. 2002;15(1):20–31. [Google Scholar]
- 22.Walling CA, et al. Inbreeding depression in red deer calves. BMC Evol Biol. 2011;11(1):318. doi: 10.1186/1471-2148-11-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slate J, Kruuk LE, Marshall TC, Pemberton JM, Clutton-Brock TH. Inbreeding depression influences lifetime breeding success in a wild population of red deer (Cervus elaphus) Proc Biol Sci. 2000;267(1453):1657–1662. doi: 10.1098/rspb.2000.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall TC, et al. Estimating the prevalence of inbreeding from incomplete pedigrees. Proc Biol Sci. 2002;269(1500):1533–1539. doi: 10.1098/rspb.2002.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: A tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stopher K, Bento A, Clutton-Brock T, Pemberton J, Kruuk L. Multiple pathways mediate the effects of climate change on maternal reproductive traits in a red deer population. Ecology. 2014;95(11):3124–3138. [Google Scholar]
- 27.Stopher KV, et al. Re-mating across years and intralineage polygyny are associated with greater than expected levels of inbreeding in wild red deer. J Evol Biol. 2012;25(12):2457–2469. doi: 10.1111/j.1420-9101.2012.02626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grueber CE, Waters JM, Jamieson IG. The imprecision of heterozygosity-fitness correlations hinders the detection of inbreeding and inbreeding depression in a threatened species. Mol Ecol. 2011;20(1):67–79. doi: 10.1111/j.1365-294X.2010.04930.x. [DOI] [PubMed] [Google Scholar]
- 29.Bérénos C, Ellis PA, Pilkington JG, Pemberton JM. Estimating quantitative genetic parameters in wild populations: A comparison of pedigree and genomic approaches. Mol Ecol. 2014;23(14):3434–3451. doi: 10.1111/mec.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeRose M, Roff D. A comparison of inbreeding depression in life-history and morphological traits in animals. Evolution. 1999;53(4):1288–1292. doi: 10.1111/j.1558-5646.1999.tb04541.x. [DOI] [PubMed] [Google Scholar]
- 31.Walling CA, et al. A multivariate analysis of genetic constraints to life history evolution in a wild population of red deer. Genetics. 2014;198(4):1735–1749. doi: 10.1534/genetics.114.164319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller LF, Grant PR, Grant BR, Petren K. Environmental conditions affect the magnitude of inbreeding depression in survival of Darwin’s finches. Evolution. 2002;56(6):1229–1239. doi: 10.1111/j.0014-3820.2002.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 33.Frère C, et al. Inbreeding tolerance and fitness costs in wild bottlenose dolphins. Proc R Soc Lond B Biol Sci. 2010;277(1694):2667–2673. doi: 10.1098/rspb.2010.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amos W, et al. The influence of parental relatedness on reproductive success. Proc Biol Sci. 2001;268(1480):2021–2027. doi: 10.1098/rspb.2001.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grueber CE, Laws RJ, Nakagawa S, Jamieson IG. Inbreeding depression accumulation across life-history stages of the endangered Takahe. Conserv Biol. 2010;24(6):1617–1625. doi: 10.1111/j.1523-1739.2010.01549.x. [DOI] [PubMed] [Google Scholar]
- 36.Purcell S, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walling CA, Pemberton JM, Hadfield JD, Kruuk LE. Comparing parentage inference software: Reanalysis of a red deer pedigree. Mol Ecol. 2010;19(9):1914–1928. doi: 10.1111/j.1365-294X.2010.04604.x. [DOI] [PubMed] [Google Scholar]
- 38.Morrissey MB, Wilson AJ. PEDANTICS: An R package for pedigree-based genetic simulation and pedigree manipulation, characterization and viewing. Mol Ecol Resour. 2010;10(4):711–719. doi: 10.1111/j.1755-0998.2009.02817.x. [DOI] [PubMed] [Google Scholar]
- 39.Wright S. Coefficients of inbreeding and relationship. Am Nat. 1922;56(645):330–338. [Google Scholar]
- 40.R Core Team . R: A Language and Environment for Statistical Computing. R Found Stat Comput; Vienna: 2014. [Google Scholar]
- 41.Hadfield J. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. J Stat Softw. 2010;33(2):1–22. [Google Scholar]
- 42.Hoffmann GS, Griebeler EM. An improved high yield method to obtain microsatellite genotypes from red deer antlers up to 200 years old. Mol Ecol Resour. 2013;13(3):440–446. doi: 10.1111/1755-0998.12068. [DOI] [PubMed] [Google Scholar]
- 43.Keller MC, Visscher PM, Goddard ME. Quantification of inbreeding due to distant ancestors and its detection using dense single nucleotide polymorphism data. Genetics. 2011;189(1):237–249. doi: 10.1534/genetics.111.130922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nussey DH, Kruuk LE, Donald A, Fowlie M, Clutton-Brock TH. The rate of senescence in maternal performance increases with early-life fecundity in red deer. Ecol Lett. 2006;9(12):1342–1350. doi: 10.1111/j.1461-0248.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 45.Nussey DH, et al. Rapidly declining fine-scale spatial genetic structure in female red deer. Mol Ecol. 2005;14(11):3395–3405. doi: 10.1111/j.1365-294X.2005.02692.x. [DOI] [PubMed] [Google Scholar]
- 46.Falconer D, Mackay T. Introduction to Quantitative Genetics. 4th Ed Longman; New York: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.