Significance
Changes in Ca2+ concentration in the cell play important roles in cell life and death decisions. Antiapoptotic Bcl-2 family proteins help protect cells from dying by interacting with proteins at mitochondria and endoplasmic reticulum. At the endoplasmic reticulum, antiapoptotic Bcl-2 proteins interact with InsP3R Ca2+ channels that release Ca2+ into the cytoplasm. However, it is controversial how they interact with the InsP3R, as well as the functional consequences of the interactions. We found that antiapoptotic Bcl-xL interacts with InsP3Rs by unique mechanisms that change the activity of the channel depending on its concentration. We also found that disrupting these interactions diminishes cell viability. Our results provide a unifying model of the effects of antiapoptotic Bcl-2 proteins on the InsP3R.
Keywords: Bcl-2, ion channel, calcium, apoptosis, mitochondria
Abstract
Antiapoptotic Bcl-2 family members interact with inositol trisphosphate receptor (InsP3R) Ca2+ release channels in the endoplasmic reticulum to modulate Ca2+ signals that affect cell viability. However, the molecular details and consequences of their interactions are unclear. Here, we found that Bcl-xL activates single InsP3R channels with a biphasic concentration dependence. The Bcl-xL Bcl-2 homology 3 (BH3) domain-binding pocket mediates both high-affinity channel activation and low-affinity inhibition. Bcl-xL activates channel gating by binding to two BH3 domain-like helices in the channel carboxyl terminus, whereas inhibition requires binding to one of them and to a previously identified Bcl-2 interaction site in the channel-coupling domain. Disruption of these interactions diminishes cell viability and sensitizes cells to apoptotic stimuli. Our results identify BH3-like domains in an ion channel and they provide a unifying model of the effects of antiapoptotic Bcl-2 proteins on the InsP3R that play critical roles in Ca2+ signaling and cell viability.
The inositol trisphosphate receptors (InsP3R) are a family of intracellular cation channels that release Ca2+ from the endoplasmic reticulum (ER) in response to a variety of extracellular stimuli (1). Three InsP3R isoforms are ubiquitously expressed and regulate diverse cell processes, including cell viability (1). Activation of the channels by InsP3 elicits changes in cytoplasmic Ca2+ concentration ([Ca2+]i) that provide versatile signals to regulate molecular processes with high spatial and temporal fidelity (1). Regions of close proximity to mitochondria enable localized Ca2+ release events to be transduced to mitochondria (2, 3). Ca2+ released from the ER during cell stimulation modulates activities of effector molecules and is taken up by mitochondria to stimulate oxidative phosphorylation and enhance ATP production (4–6) to match energetic supply with enhanced demand. In addition, cells in vivo are constantly exposed to low levels of circulating hormones, transmitters, and growth factors that bind to plasma membrane receptors to provide a background level of cytoplasmic InsP3 (7) that generates low-level stochastic InsP3R-mediated localized or propagating [Ca2+]i signals (8–10). Such signals also play an important role in maintenance of cellular bioenergetics (8). Nevertheless, under conditions of cell stress the close proximity of mitochondria to Ca2+ release sites may result in mitochondrial Ca2+ overload and initiate Ca2+-dependent forms of cell death, including necrosis and apoptosis (11–13). It has been suggested that high levels of ER Ca2+ (14–16) and enhanced activity of the InsP3R (17–19) promote cell death by providing a higher quantity of released Ca2+ to mitochondria (3, 20, 21).
Protein interactions modulate the magnitude and quality of InsP3R-mediated [Ca2+]i signals that regulate apoptosis and cell viability. Notable in this regard is the Bcl-2 protein family. Proapoptotic Bcl-2–related proteins Bax and Bak initiate cytochrome C release from mitochondria in response to diverse apoptotic stimuli, whereas antiapoptotic Bcl-2–related proteins, including Bcl-2 and Bcl-xL, antagonize Bax/Bak by forming heterodimers that prevent their oligomerization and apoptosis initiation (22, 23). Heterodimerization is mediated by interactions of proapoptotic Bcl-2 homology 3 (BH3) domains with a hydrophobic groove on the surface of antiapoptotic Bcl-2 proteins (23) that is a therapeutic target in diseases, including cancer (22). Whereas a central feature of molecular models of apoptosis is the control of outer mitochondrial membrane permeability by Bcl-2–related proteins, a substantial body of evidence has demonstrated that these proteins localize to the ER (24, 25), bind to InsP3Rs (26–32) and, by modulating InsP3R-mediated Ca2+ release, regulate ER-mediated cell death and survival (15, 27, 32–34). Nevertheless, a unified understanding of the detailed molecular mechanisms by which Bcl-2 family proteins interact with and regulate InsP3R channel activity is lacking. The Bcl-2 family member homolog NrZ interacts with the amino-terminal InsP3-binding region via its helix 1 BH4 domain and inhibits Ca2+ release (28). Bcl-2 also interacts with the InsP3R (26) via its BH4 domain (35), but in contrast it associates with a region in the central coupling domain (35). Whereas this interaction also inhibits Ca2+ release (26), Bok interacts with the channel 500 residues C-terminal to the Bcl-2 binding sequence via its BH4 domain but does not affect Ca2+ release (29). Conversely, the Bcl-xL BH4 domain may lack this interaction (36). Inhibition of the Bcl-2 BH4 domain interaction with the channel enhanced InsP3R-mediated Ca2+ signals and apoptosis sensitivity in white blood cells (18, 35, 37). However, it is unclear if Bcl-2 inhibits Ca2+ signaling directly by binding to the channel or if it acts indirectly, as a hub in a protein complex that influences channel phosphorylation (38). Conversely, we demonstrated that Bcl-xL, Bcl-2, and Mcl-1 bind to the carboxyl (C)-terminus of all three InsP3R isoforms, and showed that these interactions activated single InsP3R channels and promoted InsP3R-mediated Ca2+ release and apoptosis resistance (27, 31, 32). Furthermore, Bcl-xL mediates an interaction of oncogenic K-RAS with the InsP3R C terminus that regulates its biochemical and functional interaction and cell survival (39). However, the molecular details of the interactions of antiapoptotic protein with the InsP3R C terminus are unknown. Furthermore, the relationship between Bcl-2 family protein binding in the coupling domain and C terminus is unclear. Thus, the mechanisms whereby Bcl-2 and Bcl-xL affect InsP3R activity and the effects of this modulation on cell viability remain to be determined.
Here, we used single-channel electrophysiology of native ER membranes to explore the detailed mechanisms of the effects of Bcl-xL on the InsP3R, and the role of this interaction on cell viability. Surprisingly, our results reveal that whereas Bcl-xL activates the channel at low concentrations, it inhibits it at higher concentrations, resulting in a biphasic response of channel activation on [Bcl-xL]. Remarkably, the Bcl-xL BH3 domain-binding pocket is required for both effects. Low [Bcl-xL] activates the channel by simultaneous binding to two BH3 domain-like helices in the channel C terminus, whereas channel inhibition at high [Bcl-xL] requires binding to only one of them and to a site previously identified as the Bcl-2 binding site in the channel-coupling domain. Disruption of these interactions diminishes cell viability. Our results provide a unifying model of the effects of antiapoptotic Bcl-2 proteins on the InsP3R that play critical roles in Ca2+ signaling and cell viability.
Results
Bcl-xL Binds to Dual BH3-Like Domains in the InsP3R Carboxyl Terminus.
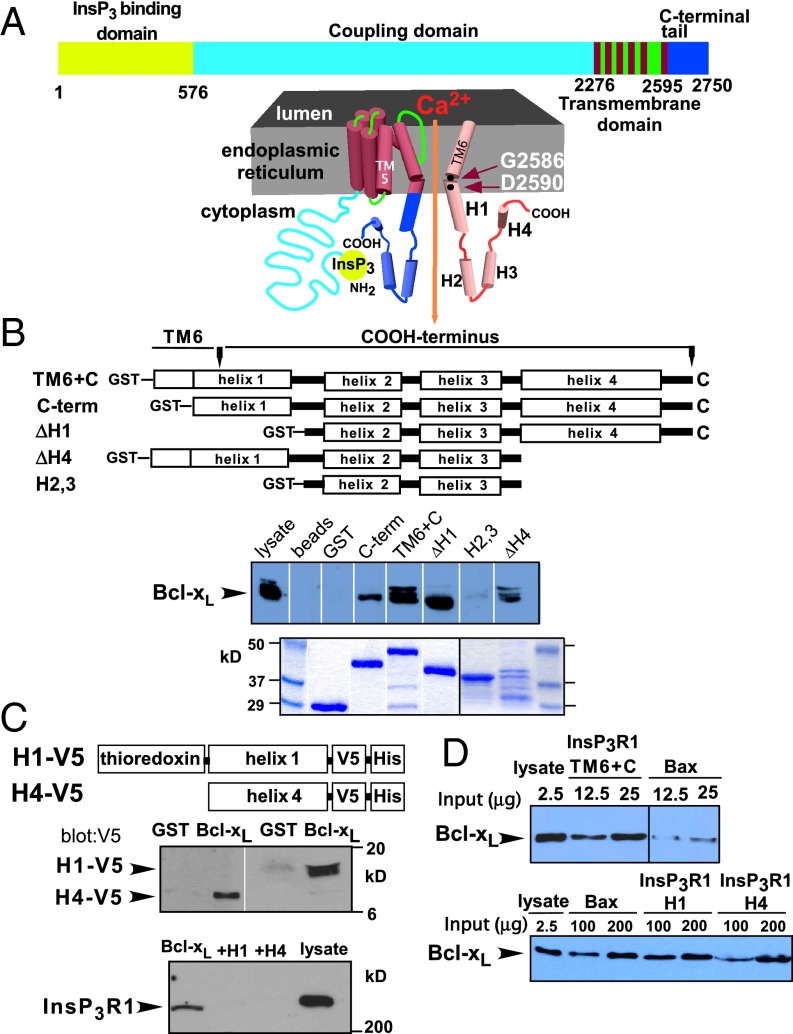
The InsP3R consists of a 600-residue N-terminal InsP3 binding domain, a cytoplasmic region (coupling domain) that links it to the transmembrane pore region that contains six transmembrane (TM) helices, and a cytoplasmic C-terminal tail of ∼175 amino acids (Fig. 1A) (1). The strength of the interaction of Bcl-xL with a region spanning from the TM5–TM6 linker to the C terminus (residues 2,512–2,750) is quantitatively equivalent to that of the full-length channel (27, 31). To define Bcl-xL binding determinants within this region, GST-fusion proteins (Fig. 1B) were immobilized on glutathione beads, the quantities of beads were titrated, and the amounts of GST-InsP3R fragments used for pull-down experiments as well as the concentrations of Bcl-xL were adjusted to equivalent levels. Bcl-xL bound to a construct containing TM6 and the C terminus (TM6+C; residues 2,571–2,750) as efficiently as to the longer construct used in refs. 27 and 31. However, a shorter construct that encompassed the terminal 7 residues of TM6 and the remaining C terminus (C-term; residues 2,589–2,750) bound Bcl-xL less efficiently (Fig. 1B). The TM6 helix is predicted to form the channel gate near residue G2586 (Fig. 1A) and to extend beyond the membrane into the cytoplasm. Distal to this helix, the remaining C terminus is predicted to contain three additional helices (H2–H4). We defined H1 as the helix containing TM6 and the 14 subsequent residues. Constructs with either H1 (ΔH1) or H4 (ΔH4) deleted bound Bcl-xL (Fig. 1B), whereas one encompassing only helices 2 and 3 failed to bind Bcl-xL (Fig. 1B), suggesting that both H1 and H4 bind Bcl-xL. In agreement, purified H1 or H4 peptides each interacted with GST-Bcl-xL (Fig. 1C). The interaction of full-length InsP3R with Bcl-xL was inhibited by purified H1 or H4 proteins in the pull-down lysate (Fig. 1C), indicating that, whereas either H1 or H4 can bind to Bcl-xL, both are required for strong binding to full-length InsP3R. This result also suggests that the C terminus is the dominant Bcl-xL binding region in the InsP3R.
Fig. 1.
Bcl-xL binds differentially to two sites in the InsP3R C terminus. (A) Schematic of full-length InsP3R (Upper) and C terminus extending from approximately halfway down final transmembrane helix 6 (TM6) into cytoplasmic region containing four putative helices, H1–H4. From PSIPRED: TM6+C: residues 2,571–2,750; C-term: 2,591–2,750; H1: 2,571–2,606; H2: 2,627–2,644; H3: 2,658–2,678; H4: 2,690–2,732. (B) Schema of GST-fusion proteins used in Bcl-xL pull-down assays. (Upper) Bcl-xL pulled down; (Lower) Coomassie stain of GST fusion proteins. Multiple Bcl-xL bands most likely represent amidated and deamidated forms (see ref. 51). (C) GST-Bcl-xL pull down of V5-tagged purified H1 and H4 peptides (Upper). Binding of full-length InsP3R blocked by H1 and H4 peptides (Lower). (D) Bcl-xL binds more strongly to InsP3R C terminus than to full-length human Bax (h-Bax) (Upper). Binding of Bcl-xL to GST-Bax and GST-H1 is comparable, whereas binding to GST-H4 is weaker (Lower).
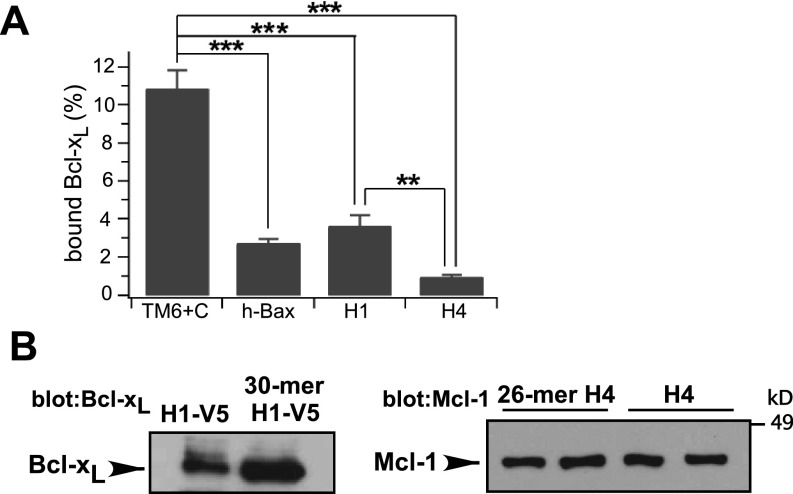
The affinity of the C terminus for Bcl-xL was estimated by quantitative comparisons with human Bax (hBax) binding to Bcl-xL. InsP3R TM6+C exhibited ∼10-fold greater binding than equivalent amounts of hBax (Fig. 1D), suggesting an apparent affinity <50 nM (40, 41). H1 bound to Bcl-xL with approximately the same affinity as hBax, whereas H4 bound with lower affinity (Fig. 1D and Fig. S1A). Binding of TM6+C was greater than expected for simple additivity of H1 and H4 binding, suggesting that H1 and H4 contribute synergistically, in agreement with the conclusions from binding competition experiments.
Fig. S1.
Bcl-2 family protein binding to InsP3R peptides. (A) Quantification of Bcl-xL binding to various InsP3R C-terminal peptides, normalized to Bcl-xL in lysate. Mean ± SEM, n = 3, **P < 0.01; ***P < 0.001. Related to Fig. 1. (B) BH3-like domain-containing InsP3R peptides bind to Bcl-xL (Left) or Mcl-1 (Right) with efficacies equal to those of the full-length helices. Related to Fig. 2.
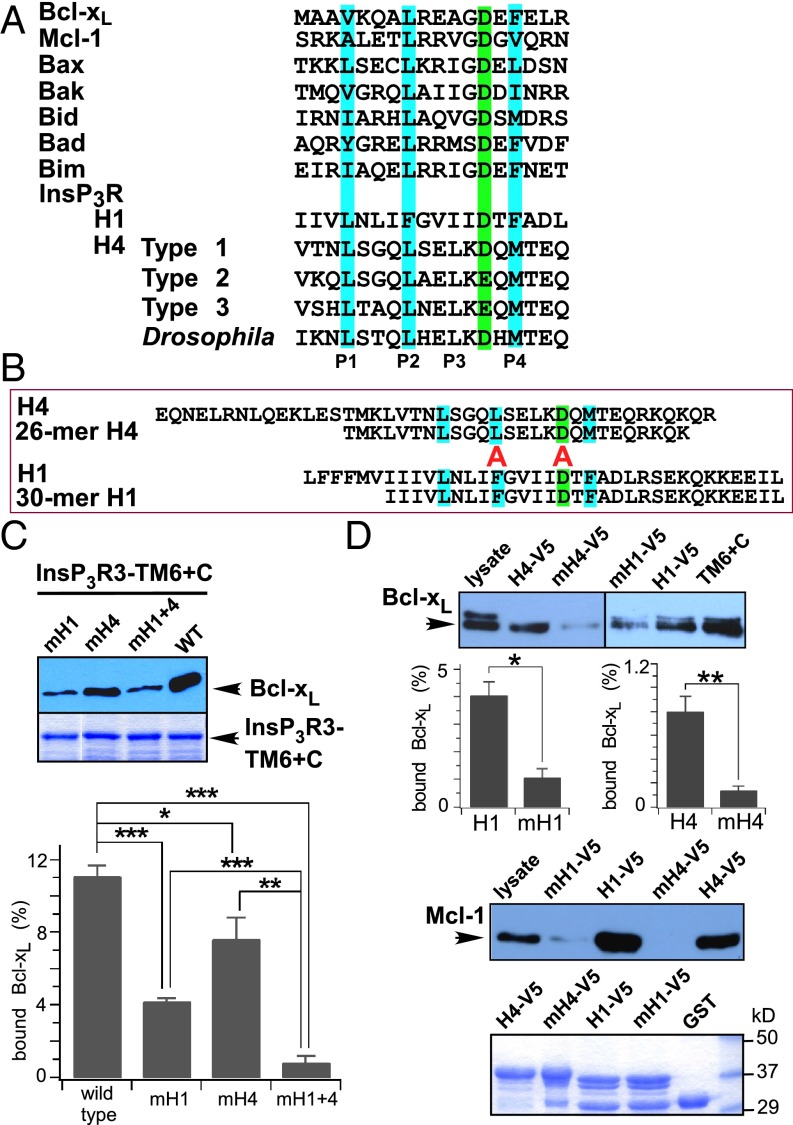
We previously demonstrated that Bcl-xL binding to the InsP3R was competitively inhibited by either t-Bid or Bax (27). Bax and t-Bid binding to Bcl-xL is mediated by their BH3 domains, amphipathic helical regions with a conserved arrangement of a key aspartic acid (Asp) and hydrophobic amino acids (Fig. 2A) (23, 42). We speculated that InsP3R interactions with Bcl-xL were similarly mediated by BH3-like sequences. Inspection of H1 and H4 revealed conserved BH3-like sequences in both (Fig. 2A). Synthetic H1 and H4 peptides that contained the putative BH3-like domains (Fig. 2B) bound to both Bcl-xL and the antiapoptotic Bcl-2 protein, Mcl-1, as efficiently as full-length H1 and H4 helices (Fig. S1B). Mutations of the key acidic residue and hydrophobic residue at P2 inhibit BH3 domain interactions (42). Mutations to alanine (Ala) of Glu and P2 phenylalanine (Phe) in H1 (mH1) strongly inhibited Bcl-xL binding to TM6+C (Fig. 2C). Similar mutations in H4 (mH4) inhibited binding to a lesser extent (Fig. 2C), whereas simultaneous mutations in both H1 and H4 (mH1+4) inhibited binding to an extent observed for mutation in H1 only (Fig. 2C), again indicating a higher binding affinity for H1. Similar results were obtained using purified WT and mutant H1 and H4 peptides (Fig. 2D). These biochemical results strongly suggest that Bcl-xL interacts with both H1 and H4 through BH3-like domains contained within each helix in the InsP3R channel C terminus.
Fig. 2.
Bcl-xL binds to the InsP3R C terminus by interaction with BH3-like domains. (A) Sequence alignment of BH3 domains from Bcl-2 related proteins, with residues critical for binding interactions with other Bcl-2 proteins highlighted. Sequences of short regions within InsP3R H1 and H4 aligned with Bcl-2 protein BH3 domains. (B) Sequence alignments of H4 and H1 with peptides encompassing their respective BH3-like domains. In mutant helices, residues aligned with red “A” were mutated to alanine. (C) Pull down of Bcl-xL by various InsP3R3 TM6+C GST-fusion proteins. Mutations to Ala of residues critical for BH3 domain interactions in either H1 (mH1) or H4 (mH4) inhibit binding of InsP3R C terminus to Bcl-xL, with mutations in H1 having most profound effects. (Lower) Quantification expressed as percent Bcl-xL in lysate. Mean ± SEM, n = 3; *P < 0.05; **P < 0.01; ***P < 0.001. (D) Pull down of Bcl-xL or Mcl-1 by various InsP3R3 constructs. Mutations in H4 and HI inhibit Bcl-xL (mean ± SEM, n = 3; *P < 0.05; **P < 0.005) and Mcl-1 (n = 3) binding.
Bcl-xL Binding to Dual InsP3R BH3-Like Domains Has Overlapping and Distinct Effects on Channel Gating.
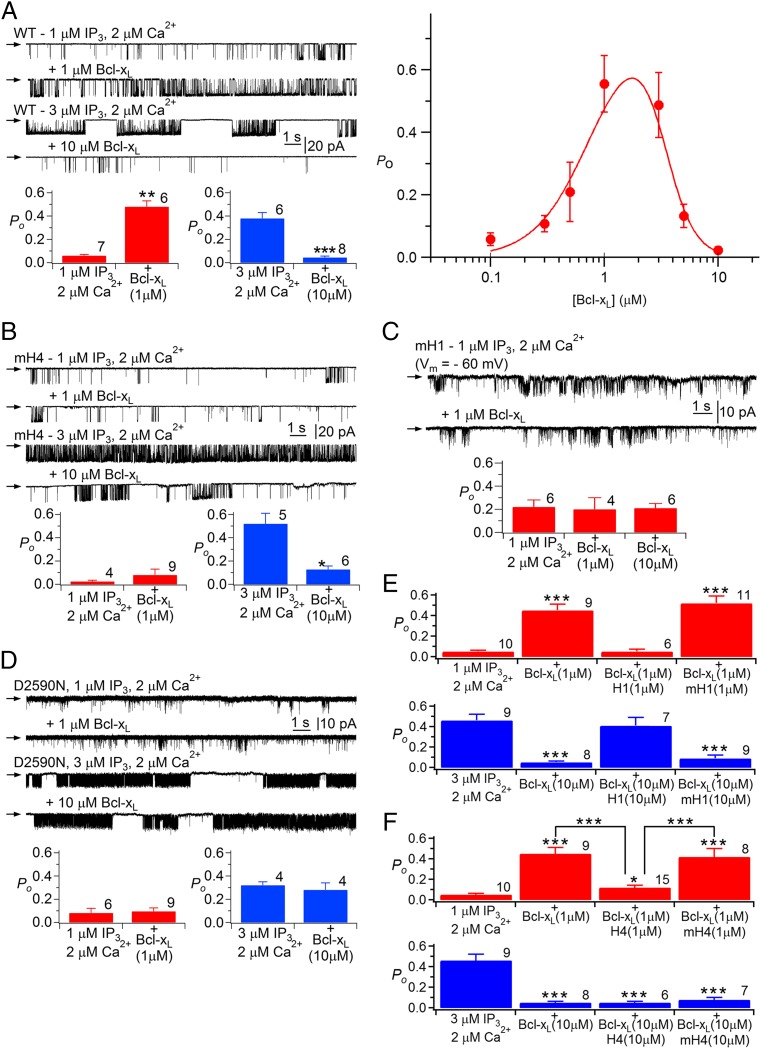
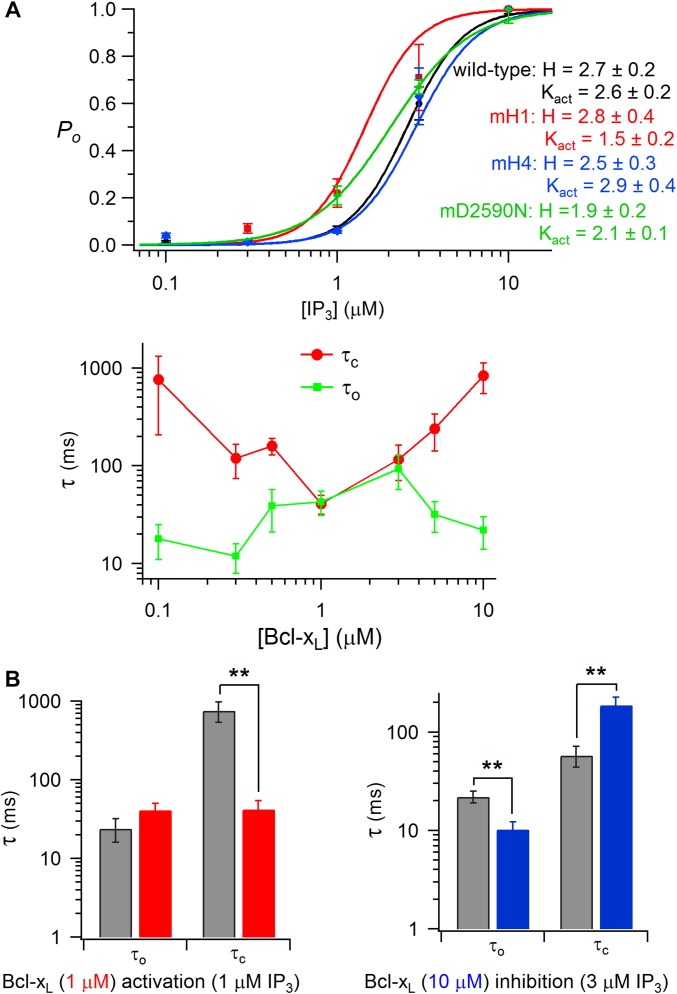
To explore the functional consequences of the interaction of Bcl-xL with the InsP3R, we recorded single InsP3R channels in native ER membranes by nuclear patch-clamp electrophysiology (1, 27, 43) using chicken DT40 cells with all InsP3R isoforms genetically deleted (DT40-KO) and engineered to stably express WT or mutant rat type 3 InsP3R (InsP3R3), the channel isoform that gates most robustly in these cells (32). InsP3R3 activated by suboptimal [InsP3] (1 μM) displayed a low open probability Po that was elevated by over an order of magnitude by inclusion of 1-μM purified recombinant full-length Bcl-xL in the pipette solution that bathes the cytoplasmic face of the channel (Fig. 3A), in agreement with previous studies (27, 32). The stimulation was dose-dependent, with an apparent KD of ∼700 nM and Hill coefficient of ∼2 (Fig. 3A), suggesting cooperative activation, consistent with Bcl-xL binding to H1 and H4. Surprisingly, [Bcl-xL] > ∼2 μM was less effective (Fig. 3A). Consequently, the dose-dependence of Bcl-xL channel activation was biphasic, with half-maximal apparent inhibition observed at ∼3.5 μM with a Hill coefficient >3 (Fig. 3A), indicating that apparent inhibition was also a cooperative process possibly reflecting multiple binding sites. To ensure that reduced efficacy of high [Bcl-xL] to activate channel gating was caused by inhibition mediated by a low-affinity Bcl-xL binding sites, InsP3R activity was recorded with higher [InsP3] (3 μM) that is more optimal for channel activity. Addition of 10 μM Bcl-xL inhibited channel gating under these conditions (Fig. 3A), demonstrating that Bcl-xL both activates and inhibits InsP3R channel gating, with high-affinity Bcl-xL binding activating the channel, and Bcl-xL binding to a low-affinity sites causing channel inhibition. At 1 μM Bcl-xL, channel activation was caused by an ∼25-fold decrease in the mean closed time. At 10 μM Bcl-xL, inhibition was caused by a threefold enhancement of the closed time and a twofold decrease in the channel open time (Fig. S2B).
Fig. 3.
Bcl-xL binding to InsP3R H1 and H4 BH3-like domains regulates channel gating. (A, Left) InsP3R3 single channel currents with pipette solution containing 1 μM InsP3 and 2 μM free Ca2+, agonist concentrations suboptimal for channel gating. In each trace, arrow indicates zero-current level; openings are downward deflections with pipette voltage = −40 mV. Addition of 1 μM full-length Bcl-xL activates channel gating (second trace). In the presence of 3 μM InsP3 and 2 μM Ca2+, conditions optimal for channel gating (third trace), 10 μM Bcl-xL inhibits gating (fourth trace). Histograms summarize activation and inhibition by Bcl-xL. In all Po histograms, number of experiments shown above each bar, and shown are mean ± SEM; *P < 0.05; **P < 0.005; ***P < 0.001. (Right) Dose-dependence of Bcl-xL modulation of channel open probability Po in the presence of 1 μM InsP3 and 2 μM Ca2+. Solid line is from data fitted with a biphasic Hill equation. (B) Effects of Bcl-xL on mH4-InsP3R3 (mH4) stably expressed in DT40-KO cells. (C) Effects of Bcl-xL on mH1-InsP3R3 (mH1) stably expressed in DT40-KO cells. (D) Effects of Bcl-xL on D2590N-InsP3R3 stably expressed in DT40-KO cells. (E and F) Effects of WT and mutant H1 (E) and H4 (F) peptides on Bcl-xL activation and inhibition of InsP3R3 channel activity.
Fig. S2.
InsP3R channel properties. (A) Dependence of normalized channel open probability Po on [InsP3] for WT, mH1-InsP3R3, mH4-InsP3R3, and D2590N-InsP3R3 channels, with optimum (2 μM) Ca2+ in pipette. Mean ± SEM, n = 4–8. (B) Mean channel open (τo) and closed (τc) times in response to Bcl-xL. (Upper) Channels activated with 1 μM InsP3 in presence of 2 μM Ca2+. Mean ± SEM, n = 4–14. (Lower Left) Kinetic effects of channel activation by 1 μM Bcl-xL in the presence of 1 μM InsP3 and 2 μM Ca2+. (Lower Right) Kinetic effects of channel inhibition by 10 μM Bcl-xL in presence of 3 μM InsP3 and 2 μM Ca2+. Mean ± SEM, n = 8–10. **P < 0.01. Related to Fig. 3.
Stable cell lines were generated that expressed InsP3R3 with mutations in either H4 (mH4-InsP3R3) or H1 (mH1-InsP3R3) that reduced Bcl-xL binding to the C terminus (Fig. 2C). mH4-InsP3R3 had normal permeation and gating properties and responses to InsP3 (Fig. S2A). However, gating activation by Bcl-xL was completely abolished (Fig. 3B). In contrast, high [Bcl-xL] inhibition was unaffected (Fig. 3B). Thus, Bcl-xL binding to H4 is required for channel activation, whereas inhibition is mediated by Bcl-xL binding to a different site. Channel gating activation by 1 μM Bcl-xL was also abolished in mH1-InsP3R3 (Fig. 3C). Raising Bcl-xL to 10 μM still failed to activate the mutant channel (Fig. 3C). mH1-InsP3R3 had normal sensitivity to InsP3 (Fig. S2A) but had altered conductance and gating properties compared with the WT channel. Single-channel conductance was reduced from 545 pS to 54 ± 6 pS (n = 4) for the mutant. Reduced single-channel conductance was caused in part by neutralization of Asp at position 2,590, because a channel with only the D2590 mutation (D2590N-InsP3R3) exhibited reduced conductance (209 ± 3 pS; n = 11) (Fig. 3D). Altered gating was primarily a consequence of mutating Phe2585, because this residue is predicted to be in close proximity to the channel gate (Fig. 1A) (44) and D2590N-InsP3R3 had normal Po, albeit with reduced open and closed times (τo ∼ τc = 2 ms) (Fig. 3D). To minimize uncertainties associated with a channel with abnormal Po, we repeated experiments using D2590N-InsP3R3. D2590N-InsP3R3 had normal sensitivity to InsP3 (Fig. S2A) but Bcl-xL failed to activate this mutant channel (Fig. 3D). Furthermore, channel-gating inhibition by high [Bcl-xL] was also abolished (Fig. 3D). Taken together, these results indicate that Bcl-xL binding to both H4 and H1 are required for activation of channel gating, whereas lower affinity Bcl-xL inhibition requires binding to H1, but not to H4. In agreement, a H4 peptide—but not a mutant peptide mH4—blocked Bcl-xL channel activation but not inhibition (Fig. 3F), whereas a H1 peptide—but not a mutant mH1—blocked both channel activation and inhibition (Fig. 3E).
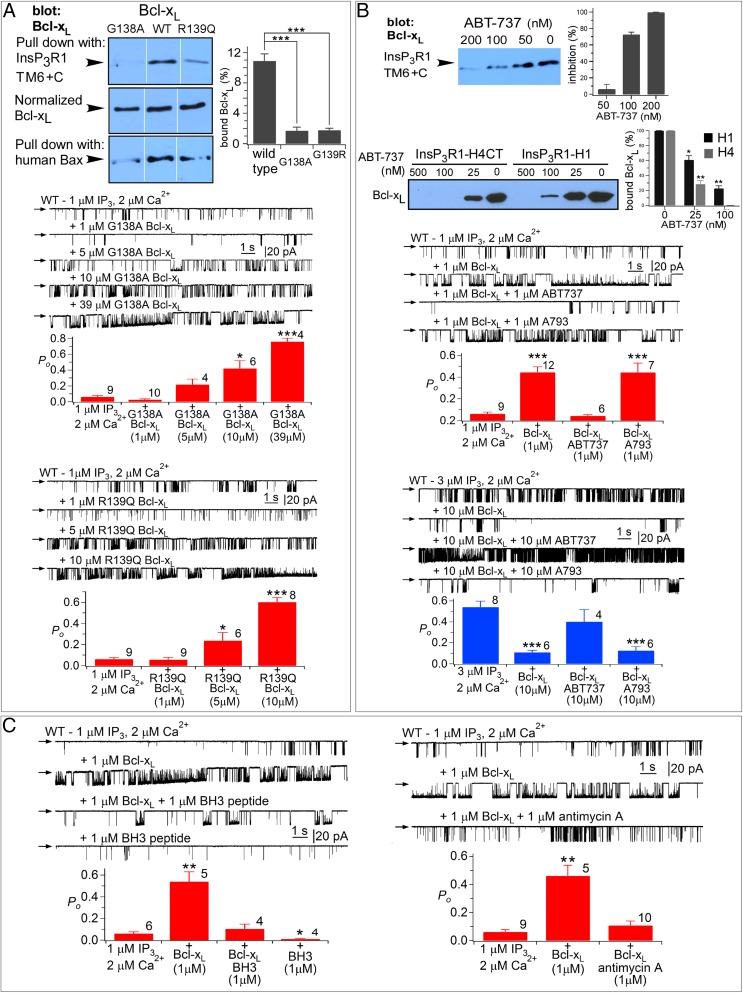
The BH3 Domain-Binding Pocket in Bcl-xL Mediates Its Biochemical and Functional Interactions with InsP3R.
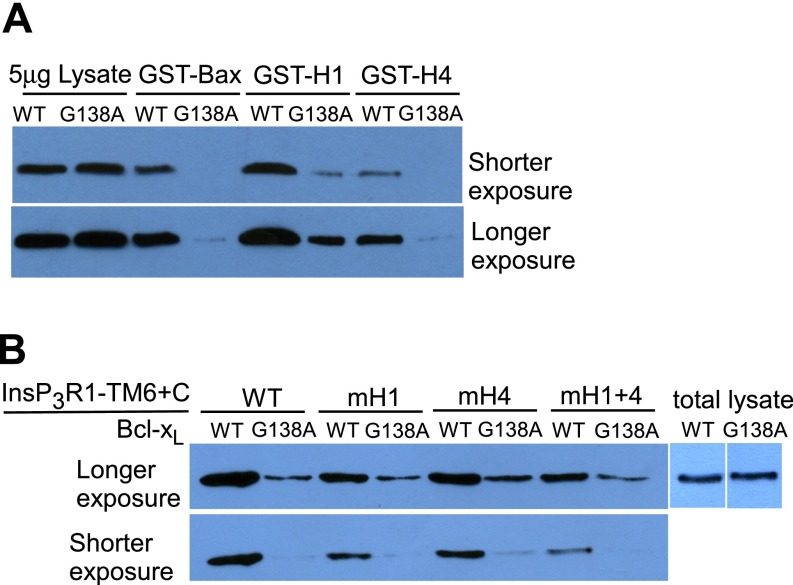
Bcl-2 protein heterodimerization is mediated by BH3 domain binding to a hydrophobic groove on the surface of its binding partner (23, 42). Key residues in the Bcl-xL binding pocket that mediate interactions with BH3 domains of Bax and t-Bid include Gly138 and Arg139 (23). The BH3 domain-like sequences in H1 and H4, and the biochemical and functional consequences of mutations in those domains, suggested that the InsP3R may interact with Bcl-xL in a manner analogous to proapoptotic Bcl-2 proteins. To explore this theory, we examined biochemical and functional interactions of the InsP3R with purified full-length Bcl-xL proteins with either Gly138 or Arg139 mutated. As a control, we verified that G138A–Bcl-xL binding to hBax was significantly reduced compared with WT Bcl-xL (Fig. 4A). Binding of G138A–Bcl-xL to TM6+C was profoundly inhibited compared with WT Bcl-xL (Fig. 4A) (P < 0.001), because of reduced binding at both H1 and H4 (Fig. S3). The weakened biochemical interactions were manifested as significantly reduced potencies at the single-channel level. Whereas 1 μM WT Bcl-xL activated the channel gating robustly, 1 μM G138A–Bcl-xL was without effect (Fig. 4A). However, higher concentrations activated gating (Fig. 4A) (P < 0.001), indicating that the G138A mutation significantly reduced the binding affinity, consistent with the biochemical data. Similar data were obtained with R139Q–Bcl-xL (Fig. 4A). These results suggest that the pocket that binds to BH3 domains of Bcl-2 family proteins also mediates biochemical and functional interactions of Bcl-xL with BH3-like domains in InsP3R. To test this further, we examined the effects of ABT-737, a small molecule that binds specifically in the Bcl-xL surface groove and prevents Bax and t-Bid binding (45). ABT-737 inhibited the interaction of TM6+C with Bcl-xL with half-maximal concentration <100 nM (Fig. 4B). Bcl-xL binding to H1 was less sensitive to ABT-737 than was H4 (Fig. 4B), consistent again with higher-affinity Bcl-xL binding to H1. ABT-737 (1 μM), but not inactive enantiomer, completely blocked channel activation by Bcl-xL (Fig. 4B). Furthermore, ABT-737 also completely blocked high [Bcl-xL] inhibition of channel gating (Fig. 4B). Other pharmacological agents that disrupt Bcl-xL–BH3 domain interactions also blocked effects of Bcl-xL on InsP3R channel gating, including a synthetic Bax BH3 domain peptide and antimycin A (46) (Fig. 4C). Together, these results implicate the BH3 binding groove in Bcl-xL in its biochemical and functional interactions with the InsP3R.
Fig. 4.
The BH3 domain-binding pocket in Bcl-xL mediates its biochemical and functional interaction with the InsP3R C terminus. (A, Top Left) Pull down of WT and mutant Bcl-xL (G138A, R139Q) by Bax and TM6+C GST fusion protein. (Top Right) Quantification expressed as percent of Bcl-xL in lysate. Mean ± SEM, n = 3, ***P < 0.001. (Middle and Lower) Effects of G138A– and R139Q–Bcl-xL on InsP3R3 channel activity. In all Po histograms, number of experiments shown above each bar, and shown are mean ± SEM; *P < 0.05; **P < 0.005; ***P < 0.001. (B, Upper gel, Left) Effects of ABT-737 on interaction of Bcl-xL with WT and mutant TM6+C InsP3R GST-fusion proteins. (Upper Right) Dose-dependent inhibition of Bcl-xL binding to TM6+C GST fusion protein. Mean ± SEM, n = 3; **P < 0.005; ***P < 0.001. (Lower gel) Effects of ABT-737 on interaction of Bcl-xL with InsP3R GST-H1 peptide and GST-H4 peptide that contained H4 extending to the C terminus. (Right) Quantification, mean ± SEM, n = 3; *P < 0.05; **P < 0.005. (Lower) Effects of ABT-737 and its inactive enantiomer A-793844 (A793) on InsP3R3 channel gating. (C) Effects of Bax BH3 peptide (Left) and antimycin-A (Right) on InsP3R3 channel gating.
Fig. S3.
Binding of GST-fusion proteins to WT Bcl-xL (WT) and G138A–Bcl-xL, related to Fig. 4. (A) Binding to Bax, H1- and H4-fusion proteins. (B) Binding to WT and mutant TM6+C constructs.
Additional Determinants in InsP3R Mediate Biochemical and Functional Interactions with Bcl-xL.
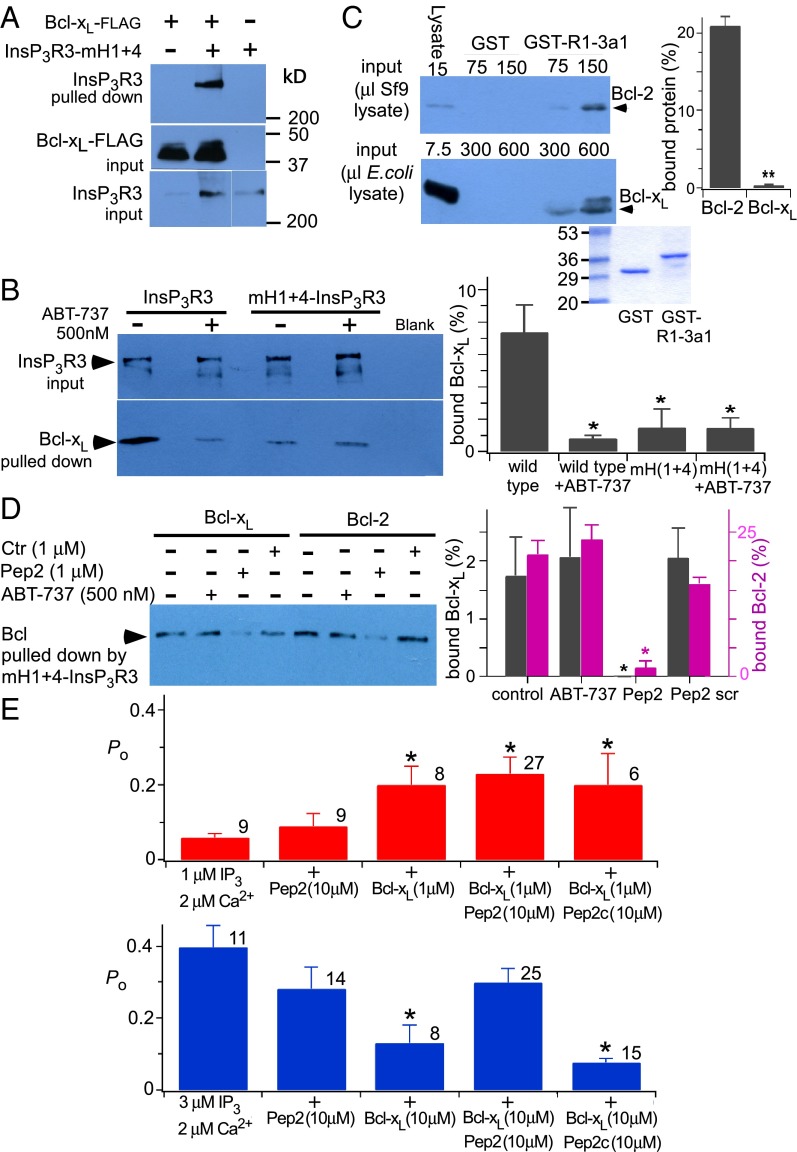
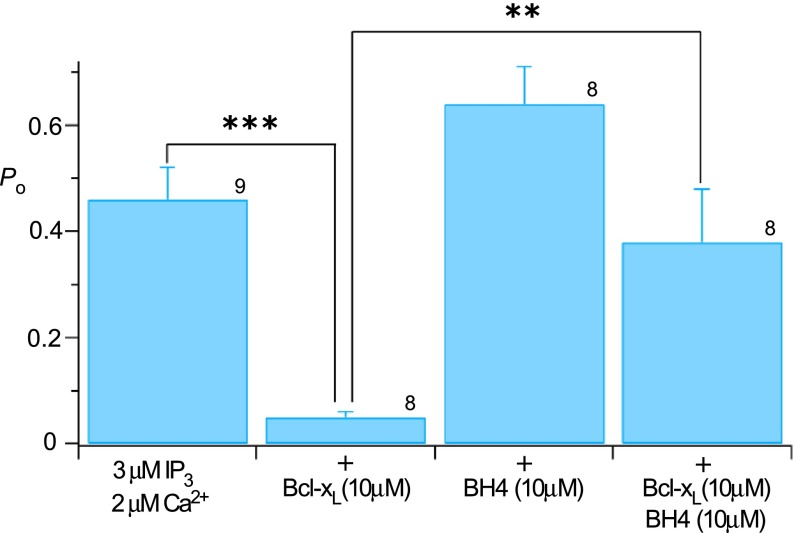
InsP3R channel activation by Bcl-xL requires binding to both H1 and H4 BH3-like domains, whereas channel inhibition by high Bcl-xL concentrations requires binding to H1 but H4 is not required. Low-affinity channel inhibition mediated by H1 is in apparent conflict with high-affinity biochemical binding of H1 to Bcl-xL (Fig. 1). This finding suggested that low-affinity inhibition of channel gating may involve determinants in addition to the H1 BH3-like domain. Indeed, Bcl-xL bound weakly to full-length InsP3R with both H1 and H4 mutated (Fig. 5 A and B) that was insensitive to ABT-737 (500 nM) (Fig. 5B). Bcl-2 interacts with a region in the InsP3R coupling domain encompassing residues 1,347–1,426, referred to as 3a1 (37). Bcl-xL bound to GST-3a1, but by comparison with Bcl-2, its binding was very much weaker (Fig. 5C). Binding of Bcl-2 as well as residual binding of Bcl-xL to mH1+4-InsP3R3, although insensitive to ABT-737, were similarly inhibited by a peptide (Pep2; P2 in ref. 37) encompassing these residues (Fig. 5D). Bcl-xL binding to the C terminus both activates and inhibits InsP3R channel activity (Fig. 3), whereas only inhibition of Ca2+ release has been reported for the interaction of Bcl-2 with the channel (17). We hypothesized that if this region contributed additional binding determinants required for H1-mediated inhibition of channel gating, inclusion of Pep2 in the patch pipette solution would inhibit high [Bcl-xL] inhibition of channel gating. Neither the peptide itself (10 μM) nor a scrambled control peptide had effects on channel gating in the absence of Bcl-xL, nor did either peptide affect 1 μM Bcl-xL activation (Fig. 5E). In contrast, the peptide completely blocked the inhibitory effects of 10 μM Bcl-xL (Fig. 5E). These results demonstrate that channel-gating inhibition by high [Bcl-xL] requires binding to both the H1 BH3-like domain and a region (3a1) localized in the channel-coupling domain. The BH4 domain of Bcl-2 has been implicated in its binding to the InsP3R (35, 36). It is possible that the BH4 domain of Bcl-xL also interacts with this channel region, because a synthetic Bcl-xL BH4 peptide reduced high [Bcl-xL]-mediated channel inhibition (Fig. S4).
Fig. 5.
Additional determinants in InsP3R mediate biochemical and functional interactions with Bcl-xL. (A) Interaction of full-length mH1+H4-InsP3R3 with FLAG-tagged Bcl-xL (Top). Flag-tagged Bcl-xL on antibody beads (Middle) and mH1+H4-InsP3R3 in lysate (Bottom) shown. (B, Left) Effects of ABT-737 on pull down of Bcl-xL (Lower) by InsP3R3 and mH1+H4-InsP3R3 (Upper) stably expressed in DT40-KO cells and bound to a GST-CaBP1 column. (Right) Quantification of Bcl-xL pulled down as percent in lysate. Mean ± SEM, n = 3; *P < 0.05. (C) Purified rat Bcl-2 (Top) and Bcl-xL (Middle) binding to InsP3R1 GST-3a1 peptide. Coomassie blue staining (Bottom) shows GST fusion protein equivalence. (Right) Quantification of protein pulled down as percent in lysate using 75 μL Bcl-2 and 300 μL Bcl-xL. Mean ± SEM, n = 3; **P < 0.005. (D, Left) Pull down of mH1+H4-InsP3R3 stably expressed in DT40-KO cells by FLAG-tagged Bcl-xL and Bcl-2 in the presence or absence of ABT-737, Pep2, or a scrambled peptide (Ctr). (Right) Quantification of Bcl-xL and Bcl-2 pulled down as percent in lysate. Mean ± SEM, n = 3; *P < 0.05. (E) Summary of InsP3R3 channel Po recorded with pipette solution containing InsP3, free Ca2+, Pep2 peptide, Pep2c scrambled peptide, or Bcl-xL, as indicated. Number of experiments shown above each bar; mean ± SEM; *P < 0.05 relative to control values (first bar of charts).
Fig. S4.
Effect of Bcl-xL BH4 peptide on Bcl-xL inhibition of InsP3-activated InsP3R3 open probability Po, related to Fig. 5. Mean ± SEM; **P < 0.01; ***P < 0.001. Number of experiments indicated above bars.
InsP3R BH3-Like Domains Regulate Cell Viability.
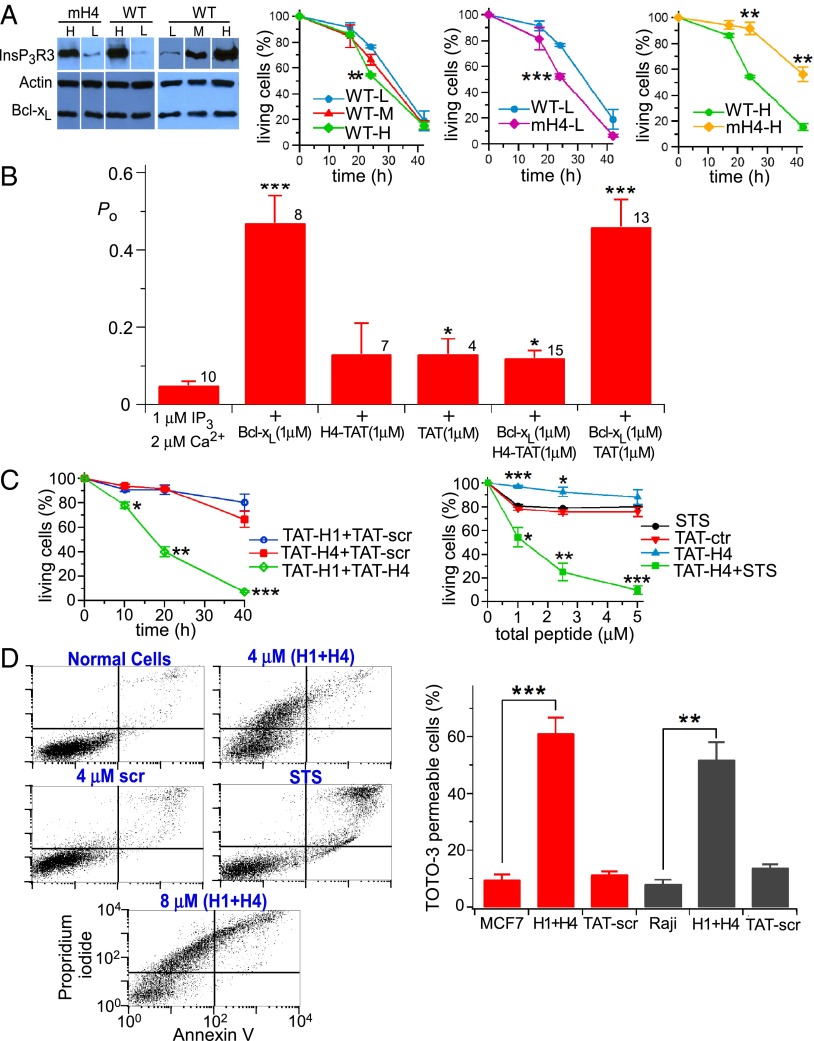
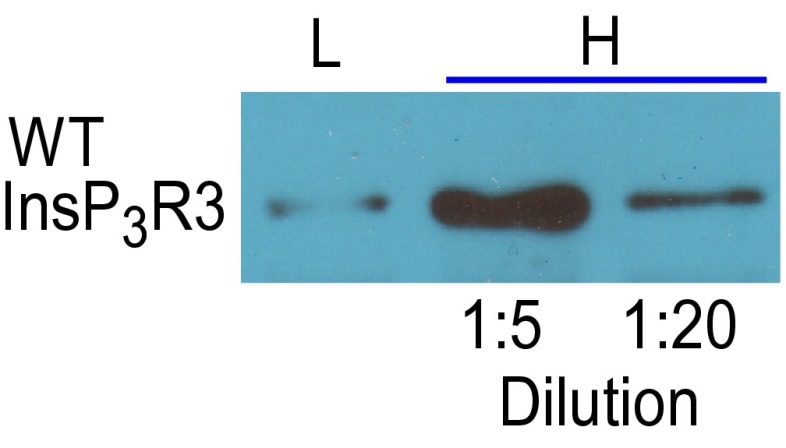
It was shown previously that Bcl-xL interaction with the InsP3R conferred apoptosis protection (27, 32), likely by stimulating low-level Ca2+ signaling that adapts cells to be resistant to stress (31). Based on the results above, we hypothesized that Bcl-xL binding to InsP3R C-terminal BH3-like domains mediates this protection. To test this theory, stable DT40-KO cells expressing human Bcl-xL (27) were engineered to express WT InsP3R3 or mH4-InsP3R3 at equivalent levels and used in cell viability assays. Because of its altered conductance and gating properties, mH1-InsP3R3 was considered inappropriate in these assays. Cell death was triggered by 500 nM staurosporine (STS) in clonal lines that expressed comparable levels of Bcl-xL and WT vs. mutant InsP3R. With mH4-InsP3R3 and InsP3R3 expressed at levels comparable to WT cells (low expressers), STS induced cell death in both lines, but the mH4-InsP3R3 cells were more sensitive (Fig. 6A). We also examined cells in which the WT and mutant InsP3R were overexpressed by >50-fold compared with normal (Fig. 6A and Fig. S5). With much higher levels of InsP3R expression, Bcl-xL stimulation of channel gating may contribute to excessive Ca2+ release to promote cell death. In this case, preventing Bcl-xL activation of InsP3R would be predicted to confer protection. In agreement, cell death was enhanced with increasing levels of strong overexpression of InsP3R3 (Fig. 6A). Importantly, mutation of the channel H4 BH3-like domain conferred significant protection in cells expressing very high levels of InsP3R (Fig. 6A).
Fig. 6.
InsP3R BH3-like domains regulate cell viability. (A) Expression levels of WT and mH4 (mH4)-InsP3R3 and Bcl-xL in DT40 cells used in viability assays in response to 500 nM STS. (Left time course) Viability (TOTO-3 uptake) of cells with different WT InsP3R3 expression levels. (Center time course) Responses of cells expressing WT and mH4-InsP3R3 at low levels. (Right time course) Responses of cells expressing WT and mH4-InsP3R3 at high levels. n = 3 experiments. Mean ± SEM; **P < 0.005; ***P < 0.001. (B) Summary of InsP3R3 Po activated by 1 μM InsP3 in presence of 2 μM Ca2+ in response to Bcl-xL and H4-TAT and control scrambled TAT peptides, as indicated. Number of patches indicated; Mean ± SEM; *P < 0.05, ***P < 0.001. (C) Cell viability (TOTO-3 uptake) of WT DT40 cells treated with STS (500 nM) or TAT fusion peptides for 24 h. (Left) 2 μM of each peptide was used. n = 3 experiments. Mean ± SEM; *P < 0.05; **P < 0.005; ***P < 0.001 compared with control scrambled TAT peptide. (D, Left) Effects of H1- and H4-TAT peptides, STS (500 nM) and scrambled TAT fusion peptides on human Raji cell viability by flow cytometry. (Right) Cell necrosis (TOTO-3 permeability) of MCF7 and Raji cells treated or not with 2 μM TAT-H1 plus 2 μM TAT-H4 peptides (H1+H4) or 4 μM scrambled TAT peptides (TAT-scr).
Fig. S5.
Expression in DT40-KO cells of rat InsP3R3, related to Fig. 6. Cells expressing low levels (L) and high levels (H) with lysate amount run on gel, diluted as indicated.
To further explore the roles of Bcl-xL interaction with the channel H1 and H4 domains, we generated TAT fusion proteins of H1 and H4 peptides to facilitate their delivery into cells. Here, we reasoned that blocking the Bcl-xL–InsP3R interaction by peptide competition would reveal a role of these domains in normal cell viability. Neither H4-TAT (1 μM) nor the control peptides (scrambled H4 sequence) affected single InsP3R channel activity, whereas H4-TAT completely blocked activation by 1 μM Bcl-xL (Fig. 6B), validating use of the peptides for cellular studies. Exposure of DT40 cells for 24 h to up to 4 μM TAT-H1 or TAT-H4 was without effect on cell viability (Fig. 6C). In contrast, combined exposure to both TAT-H1 and TAT-H4 caused a dose-dependent loss of cell viability, with nearly complete cell killing at 4 μM (Fig. 6C). In addition, although exposure to 1–5 μM TAT-H4 had no effect on viability, it strongly potentiated killing induced by 500 nM STS (Fig. 6C). Similarly, simultaneous exposure to TAT-H1 and TAT-H4 peptides caused profound killing of human Raji lymphoblasts and MCF7 breast cells, whereas control peptides (scrambled H1 and H4 sequences) were without effect (Fig. 6D). By flow cytometry analyses, cell death appeared to be distinct from apoptosis induced by STS, suggesting necrosis as the primary mechanism. TOTO-3 staining also suggested that necrosis was the major mechanism of cell death (Fig. 6D). Taken together, these results suggest that the interaction between Bcl-xL and the H1/H4 domains of the InsP3R plays an important role in regulating cell viability.
Discussion
Antiapoptotic Bcl-2 family proteins Bcl-xL, Bcl-2, and Mcl-1 bind to the InsP3R Ca2+ release channel C terminus, enhancing its channel activity and Ca2+ release that affords ER-based apoptosis protection (27, 31, 32). Bcl-xL interacts with the C terminus as strongly as with the full-length channel, suggesting that the C terminus represents the major binding determinant for Bcl-xL (27) and possibly the other antiapoptotic Bcl-2 proteins. Here, using biochemical approaches and single-channel electrophysiology, we have defined two distinct sites in the C terminus that mediate channel interactions with Bcl-xL. Furthermore, we defined determinants in Bcl-xL that mediate its interactions with both sites. Remarkably, the interactions are highly analogous to the interactions of proapoptotic Bcl-2 family proteins with Bcl-xL. Thus, we have identified two BH3-like domains in the InsP3R C terminus that interact with different affinities with the hydrophobic surface groove of Bcl-xL that binds BH3 domains in apoptotic proteins. Importantly, the biochemical results are strongly supported by electrophysiological analyses of the effects of WT and mutant Bcl-xL on the activities of single WT and mutant InsP3R channels. Interaction of Bcl-xL with the two sites has distinct functional effects, activating and inhibitory, which result in a biphasic concentration dependence of the effects of Bcl-xL on channel gating. Cell viability was compromised by interfering with the interactions, highlighting their importance. Of note, we found that a third binding site in the coupling domain, previously shown to interact with Bcl-2, participated in the inhibitory effects on channel gating of Bcl-xL binding to the C terminus, thereby providing a unifying framework for understanding the biochemical and functional interactions of antiapoptotic Bcl-2 family proteins with the InsP3R.
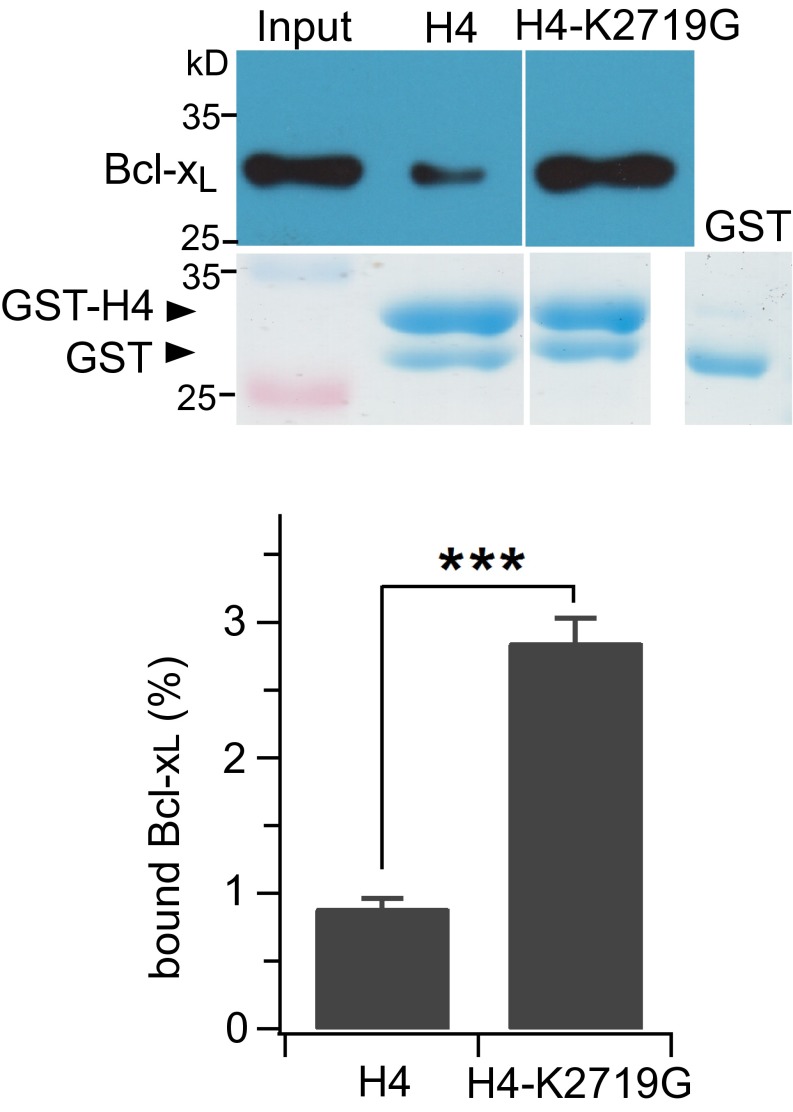
Various lines of evidence suggest that two regions in the InsP3R C terminus interact with Bcl-xL and that both are BH3 domain-like. First, the sequences of the interacting regions are reminiscent of BH3 domains. H1 and H4 have arrangements of critical negatively changed and surrounding hydrophobic residues that are present in all BH3 domains (42). In the high-affinity H1 helix, the negatively charged residue is Asp, as in bone fide BH3 domains. In H4, it is Asp in the type 1 and Drosophila InsP3Rs, and Glu in types 2 and 3 InsP3R, which preserve the critical negative charge. The hydrophobic Leu at the P2 position is conserved, except in H1, where it is hydrophobic Phe. A notable difference in the sequence of the BH3-like domains in H1 and H4 is the absence of Gly immediately preceding the Asp. In H1 it is Ile and in H4 a Lys. Both residues are bulkier than Gly, the Lys introduces a charge, and both might be expected to diminish the binding affinity to Bcl-xL. Nevertheless, H1 bound to Bcl-xL with a higher apparent affinity than the Bax BH3 domain, suggesting that Ile in lieu of Gly is well accommodated by Bcl-xL. Lys in H4 is bulkier than Ile, which together with its charge, may account for the lower apparent affinity of H4. In agreement, mutation of Lys to Gly strongly enhanced the binding of H4 to Bcl-xL (Fig. S6). Nevertheless, mutagenesis of the key Asp and hydrophobic P2 residue strongly inhibited binding of both helices to Bcl-xL. In addition, mutation of Arg139 in Bcl-xL, which interacts with the BH3 Asp, as well as Gly138, which also contributes to the binding interaction with BH3 domains, significantly reduced binding of each InsP3R helix with Bcl-xL. Finally, the biochemical interaction of the C terminus and each helix was strongly inhibited by ABT-737 and the Bax BH3 domain peptide, both of which bind specifically in the Bcl-xL surface groove. The biochemical data therefore strongly support the conclusion that interaction of Bcl-xL with the InsP3R is mediated by both H1 and H4 with the surface pocket in Bcl-xL in a manner that is highly reminiscent of the interactions of Bcl-xL with proapoptotic BH3 domains. Despite a similar strategy among Bcl-2 family proteins of BH3 domain binding to a surface hydrophobic groove, there is a high degree of specificity in the interactions (42, 47). Furthermore, the binding pockets of some Bcl-2 family members, including Bcl-xL, have considerable conformational flexibility to accommodate different BH3 domains (48, 49). Such structural flexibility may explain the interaction with the BH3-like domains in the InsP3R.
Fig. S6.
Relative binding affinities of WT and K2719G GST-H4 fusion proteins for Bcl-xL; related to Discussion. WT and GST-K2719G-H4 were used to pull down Bcl-xL expressed from E. coli (Upper gels). Amount of GST-H4CT fusion proteins normalized by SDS/PAGE stained with Coomassie blue R250 (Lower gels). Quantification: mean ± SEM, n = 3, ***P < 0.001.
Unique to the study of Bcl-2 protein interactions, real-time single molecule recordings here validated the conclusions reached from biochemical studies, and provided insights into the functional implications of the interaction of Bcl-xL with the InsP3R. Using WT and mutant proteins and single-channel electrophysiology, we found a remarkable concordance between the biochemical and electrophysiological results. First, Bcl-xL both activated and inhibited the channel, suggesting that it interacts with the channel in at least two functional sites. High-affinity channel gating activation requires Bcl-xL binding to both H1 and H4, in agreement with the biochemical demonstration that the entire C terminus had the highest affinity for Bcl-xL. In contrast, low-affinity channel inhibition required binding to only the H1 helix. Thus, the biochemistry and electrophysiology suggest that H1 and H4 are both involved in the interactions of Bcl-xL with the InsP3R, with functional consequences. The single-channel recordings also provide strong support for the conclusion that the interactions of each helix are mediated by canonical BH3 domain interactions with the surface groove in Bcl-xL. Mutation of either Gly138 or Arg139 in the Bcl-xL binding pocket strongly reduced the ability of Bcl-xL to activate channel gating. Normal channel activation by the mutant Bcl-xL proteins was achieved when [Bcl-xL] was raised by 10- to 40-fold. It is likely that the lower-affinity inhibitory site interaction was similarly affected by these mutations, but they were not observed because Bcl-xL could not be used in high-enough concentrations. This conclusion is supported by the effects of ABT-737, antimycin C, and the Bax BH3 domain peptide. All bind in the hydrophobic pocket in Bcl-xL and inhibited both channel activation and inhibition by Bcl-xL. In summary, single-channel electrophysiology provides strong validation of the biochemical studies. The studies demonstrate that the biochemical interactions have strong functional implications, and they support a model in which Bcl-xL interacts with two BH3-like domains in the channel C terminus.
After these experiments were completed, a high-resolution cryo-electron microscopy (cryo-EM) structure of rat InsP3R1 was solved (44). The structure demonstrates that the TM6 helix extends into the cytoplasm, defining what we have referred to as H1. The side-chain of D2590 (2591 in the structure) points toward the cytoplasmic vestibule of the permeation pathway, in agreement with our studies that showed that neutralization of this residue reduced channel conductance, suggesting that D2590 contributes to the electrostatic environment in the cytoplasmic vestibule that promotes cation conduction. In the structure, F2585 (2586 in the structure) appears to form the channel gate. It is not obvious how Bcl-xL could access this site. However, the channel structure was solved in the absence of InsP3 and Ca2+, and in a closed conformation whose relevance for the structure of an active liganded channel remains to be determined. Bcl-xL interaction with this region in an active channel suggests that the channel cytoplasmic vestibule must become more accessible during channel gating than is evident in the cryo-EM structure. Nevertheless, interaction with such a critical site in the channel would be predicted to influence channel gating, as we observe.
H4 is referred to as the C-terminal domain (CTD) in the cryo-EM structure (44). It is an extended α-helix that has contacts with the InsP3 binding domain and a ring of helical linker (LNK) domains, forming a connection between the InsP3 binding domain and TM6. The LNK domain contains the two helices we referred to here as H2 and H3. The Bcl-xL binding determinants we defined here are located immediately proximal to the major LNK-interacting CTD residues. The CTD is in an ideal position to transmit conformational changes associated with Bcl-xL binding to both the InsP3-binding domain and the channel-gating machinery, and may therefore provide a structural basis for the role of H4 binding in Bcl-xL sensitization of the channel to InsP3 (27) and in channel-gating activation. Nevertheless, our results indicate that simultaneous Bcl-xL binding to the H1 region is also required for channel activation, suggesting a model in which conformational changes in the CTD induced by Bcl-xL binding to H4 are transmitted to the channel regions that destabilize channel closed states only when Bcl-xL is simultaneously bound to TM6.
High [Bcl-xL] inhibition of channel gating was independent of the CTD. Inhibition of InsP3R-mediated Ca2+ release has been observed as a functional effect of Bcl-2 interaction with the channel (17). There, the interaction determinants described are different from those defined here. The Bcl-2 BH4 domain, a helix localized on the opposite face of the protein from the surface groove, interacts with a 3a1 region in the coupling domain (35, 37). We found residual binding of Bcl-xL to the channel after elimination of its binding to the C terminus, which was localized to the same 3a1 region. Although the affinity for Bcl-xL was much lower than that for Bcl-2, the interaction nevertheless appears to be critical for channel-gating inhibition by high [Bcl-xL] because a peptide that blocks the (residual) interaction of both Bcl-2 and Bcl-xL blocked the ability of Bcl-xL to inhibit channel gating. Thus, high-affinity Bcl-xL binding to H1 and simultaneous low-affinity interaction with 3a1 accounts for channel-gating inhibition by high [Bcl-xL]. Our results suggest a unifying framework that rationalizes previously discrepant results regarding the biochemical and functional implications of different antiapoptotic Bcl-2 family protein interactions with the InsP3R. It will be of interest to determine if inhibition of InsP3R-mediated Ca2+ release by Bcl-2 requires its simultaneous binding to H1. Channel activation requires at least two Bcl-xL molecules to interact with a tetrameric InsP3R channel, because the interactions involve two sites (H1, H4) with the same binding site on Bcl-xL. In contrast, a single Bcl-xL molecule could possibly mediate channel inhibition, by binding H1 with its surface groove and 3a1 with another part of the molecule, perhaps the BH4 domain on the opposite surface.
The biphasic concentration dependence of effects of Bcl-xL on channel gating suggests that functional effects in cells will vary with expression levels and physical proximity of Bcl-xL and the InsP3R. A priori it might be expected that the high-affinity, activating effects of Bcl-xL will usually dominate. Because low-level constitutive InsP3R-mediated Ca2+ release is essential for preserving normal bioenergetics under basal conditions (8), we expected that the interaction of Bcl-xL with H1 and H4 would provide resistance to stress and promote cell viability. With InsP3R expressed at approximately normal levels, intact H1 and H4 BH3-like domains play important roles in cell viability. In contrast, when InsP3R is grossly overexpressed, the domains sensitize cells to stress, possibly because of excessive Ca2+ release that activates death pathways. Both results suggest that H1 and H4 play important roles in regulating the level of channel activity in vivo, and they indicate that antiapoptotic Bcl-2 protein InsP3R C-terminal interactions play important roles in regulating the channels under normal conditions in the cell types examined.
In summary, we have identified unique molecular interactions of Bcl-xL with the InsP3R that involves dual BH3-like domains in the C terminus of the channel. The interactions of the surface hydrophobic groove of Bcl-xL with these domains have both activating and inhibitory effects on channel gating. These interactions are important in regulating channel activity in vivo in a manner that strongly influences sensitivity to cell stress and cell viability. Because drugs such as ABT-737 that target BH3–Bcl-2 family protein interactions are being developed as cancer therapeutics, our results suggest an additional target, the interaction of Bcl-2 proteins with the InsP3R, that may influence their efficacy. Furthermore, ABT-737 inhibition of enhanced InsP3R-mediated Ca2+ release in diabetic vascular smooth muscle cells (50) may suggest that this interaction could be targeted for other diseases as well.
Materials and Methods
Details regarding the sources of materials used, cell culture and generation of cell lines, plasmid construction and virus generation, protein and peptide expression, synthesis and purification, the sequences of peptides used, details regarding GST-fusion protein pull downs, fluorescence binding and Western blotting assays, cell viability assays, single-channel electrophysiology, and analyses and statistics are discussed in SI Materials and Methods.
SI Materials and Methods
Materials.
ABT-737 was a gift from Abbott Laboratories (Abbott Park, IL). STS and other chemicals were purchased from Sigma. TOTO-3, propidium iodide and Alexa 488 Annexin V assay kits were purchased from Invitrogen. Antibodies were purchased from BD.
Cell Culture and Stable Cell Line Generation.
MCF-7 and Raji cells were purchased from ATCC and grown following ATCC instructions in medium containing 1% (vol/vol) penicillin/streptomycin (Invitrogen). DT40 (Gallus gallus) cells were maintained in suspension culture media-RPMI 1640 (Invitrogen) containing 10% (vol/vol) FBS (Gemini), 1% chicken serum (Invitrogen), 1% (vol/vol) penicillin/streptomycin mixture (Invitrogen), 0.1% Fungizone Amphotericin B (Invitrogen) at 37 °C, 95% humidity, 5% air/CO2. Sf9 (Spodoptera frugiperda) cells were maintained in suspension culture at 27 °C in serum free Sf-900 II SFM medium (Invitrogen). DT40 Bcl-xL and DT40 InsP3R3 stable cell lines were generated as described previously (27, 32). Bcl-xL and InsP3R3 expression levels were evaluated by Western blotting using antibodies from BD.
Plasmid Construction and Virus Generation.
Rat (SI+, SII+, SIII+) InsP3R-1 fragments indicated in Fig. 1 were amplified by PCR and subcloned into pGEX-6P-1 (Amersham) at BamHI and Xho I sites. H1 and H4 mutations were constructed using the QuickChangeTM site-directed mutagenesis kit (Stratagene). pGEX-TAT-H4CT and scrambled peptide plasmids were constructed by PCR to add a HIV-TAT sequence at the amino terminus. Full-length rat (SI−, SII+, SIII+) InsP3R3 mutant constructs were constructed by two steps in pMXΔ-IRES-GFP retrovirus vector. Briefly, a BstZ171 site (The GTC code of V2424 was changed to GTA) was generated in pCDNA3.1.rInsP3R3 plasmid without changing the amino acid sequence. Overlap PCR was used to construct full-length InsP3R3 mutants in pCDNA3.1. Mutant full-length cDNAs were subcloned into pMXΔ-IRES-GFP at EcoRI and NotI sites. Using pET-16b-Bcl-xL plasmid as template, mutants were constructed using the Quick Change TM site-directed mutagenesis kit (Stratagene). pGEX-BAK-BH3 (human BAK residues 60–95) was constructed by PCR and cloned into pGEX-6P-1 vector at BamH1 and XhoI sites. The Bcl-xL plasmid, purified flag-tagged recombinant Bcl-xL and Bcl-2 proteins and baculoviruses were provided by Chi Li (University of Louisville, Louisville, KY).
Protein and Peptide Expression, Synthesis and Purification.
WT and mutant Bcl-xL proteins were expressed in Sf9 cells, purified and reconstituted as previously described (27, 32). InsP3R-GST fusion proteins were expressed in Escherichia coli (BL21 DE3) Lys from Invitrogen) at 25 °C for 3 h and purified using GSH beads (GE). Purified GST fusion proteins on beads were normalized using SDS/PAGE staining by Coomassie blue R250. Amino terminal-tagged TAT-H4CT and TAT-H4-scrambled peptides were expressed in E. coli and purified using GSH beads. Purified GST-TAT-H4CT and scrambled peptide coupled onto beads were released by Precision Protease (GE). The TAT-H4CT peptide (>95% purity) was characterized by HPLC, LC-MSMS and Maldi-TOF (Protein Core Facility of University of Pennsylvania). Peptide concentrations were measured using an amino acid analysis method (Dana Faber Cancer Institute, Cambridge, MA). H1-30, TAT-H1 and TAT-H1 scrambled peptides were ordered from Peptide 2.0 Ltd. Bax-BH3 peptides were purchased from Anaspec. Other peptides were purchased from GL Biochem.
Peptide Sequences.
TAT-H1: RKKRRQRRRIIVLNLIFGVIIDTFADTRSEKQKK
TAT-H1scramble: RKKRRQRRRDGDLGLMKIKAYVTKVQLENSQFRF
H1: VIIIVLNLIFGVIIDTFADTRSEKQKK
H4: EQNELRNLQEKLESTMKLVTNLSGQLSELKDQMTEQRKQKQR
TAT-H4CT: GSRKKRRQRRREQNELRNLQEKLESTMKLVTNLSGQLSELKDQMTEQRKQKQRIGLLGHPPHMNVNPQQPA
H4-TAT: EQNELRNLQEKLESTMKLVTNLSGQLSELKDQMTEQRKKRRQRRR
H4-TATscramble: TEMLDQTENRQLGSNMKSNTQLLELKEVQEQESLKLRKKRRQRRR
BH4: SQSNRELVVDFLSYKLSQKGYSWSQ
GST-Fusion Protein Pull Downs, Fluorescence Binding, and Western Blotting Assays.
Cells were harvested and lysed using 1× PBS, 10 mM DTT, 1% Triton-X 100 with protease inhibitor mixture (Sigma). After slight sonication, cells were centrifuged at 4 °C for 30 min. The supernatant was used for pull downs and Western blotting assay, as previously described (27, 32). GST beads were mixed with GST-InsP3R fragment beads in Coomassie staining gel to normalize full-length GST-InsP3R fragments and degraded GST to the same amount of beads. Protein was eluted using 1× sample buffer at 100 °C for 5 min. Total elution solution was loaded onto SDS/PAGE for Western blotting.
Cell Death Assays Using Flow Cytometry.
Cell viability was determined as described previously (31). Cells grown in suspension were passaged and grown to 0.5–1 × 106 cells/mL after 24 h, and then treated with peptides for 24 h. Cells grown on plastic were passaged and grown to 70–80% confluence after 24 h before exposure to peptides for 24 h. Cells were detached using 0.25% trypsin (Invitrogen). TOTO-3 (1 μM) taken up was measured using Fascalibur (BD). Propidium iodide and Alexa 488 Annexin V staining was performed following Invitrogen protocols.
Electrophysiology.
DT40 cells were washed twice with PBS and suspended in a nuclear isolation solution containing: 150 mM KCl, 250 mM sucrose, 1.5 mM β-mercapoethanol, 10 mM Tris⋅HCl, 0.05 mM phenylmethylsulphonyl fluoride, protease inhibitor mixture (Complete, Roche Molecular Biochemicals), pH 7.5. Nuclei were isolated using a Dounce glass homogenizer and plated onto a 1-mL glass-bottomed dish containing standard bath solution: 140 mM KCl, 10 mM Hepes and 0.5 mM BAPTA (free [Ca2+] ∼50 nM), pH 7.3. The pipette solution contained 140 mM KCl, 0.5 mM ATP, 10 mM Hepes, pH 7.3. The free [Ca2+] in all solutions was adjusted to the desired level by the addition of an appropriate Ca2+ chelator, as described previously (51). Experiments were performed at room temperature. Data were acquired using an Axopatch-1D amplifier (Axon Instruments) and single-channel analysis performed using QuB software (University of Buffalo).
Analysis and Statistics.
Data were summarized as the mean ± SEM and the statistical significance of differences between means assessed using unpaired t tests or ANOVA for repeated measures, using Fisher’s protected least-significant difference test. Differences between means were accepted as statistically significant at the 95% level (P < 0.05).
Acknowledgments
We thank Dr. Chi Li for reagents and advice, and Dr. King-Ho Cheung and Ms. Lijuan Mei for technical assistance. This study was supported by National Institutes of Health Grant GM56328 (to J.K.F.) and Shanghai Institute of Planned Parenthood Research Grant PD2012-4 (to J.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517935113/-/DCSupplemental.
References
- 1.Foskett JK, White C, Cheung KH, Mak D-OD. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87(2):593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Brito OM, Scorrano L. An intimate liaison: Spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 2010;29(16):2715–2723. doi: 10.1038/emboj.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naon D, Scorrano L. At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochim Biophys Acta. 2014;1843(10):2184–2194. doi: 10.1016/j.bbamcr.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82(3):415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 5.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. 1999;96(24):13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duchen MR. Mitochondria and calcium: From cell signalling to cell death. J Physiol. 2000;529(Pt 1):57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luzzi V, Sims CE, Soughayer JS, Allbritton NL. The physiologic concentration of inositol 1,4,5-trisphosphate in the oocytes of Xenopus laevis. J Biol Chem. 1998;273(44):28657–28662. doi: 10.1074/jbc.273.44.28657. [DOI] [PubMed] [Google Scholar]
- 8.Cárdenas C, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142(2):270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith IF, Shuai J, Parker I. Active generation and propagation of Ca2+ signals within tunneling membrane nanotubes. Biophys J. 2011;100(8):L37–L39. doi: 10.1016/j.bpj.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ju YK, Woodcock EA, Allen DG, Cannell MB. Inositol 1,4,5-trisphosphate receptors and pacemaker rhythms. J Mol Cell Cardiol. 2012;53(3):375–381. doi: 10.1016/j.yjmcc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Szalai G, Krishnamurthy R, Hajnóczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999;18(22):6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernardi P. Mitochondrial transport of cations: Channels, exchangers, and permeability transition. Physiol Rev. 1999;79(4):1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 13.Orrenius S, Gogvadze V, Zhivotovsky B. Calcium and mitochondria in the regulation of cell death. Biochem Biophys Res Commun. 2015;460(1):72–81. doi: 10.1016/j.bbrc.2015.01.137. [DOI] [PubMed] [Google Scholar]
- 14.Pinton P, et al. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: Significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20(11):2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scorrano L, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science. 2003;300(5616):135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 16.Oakes SA, Opferman JT, Pozzan T, Korsmeyer SJ, Scorrano L. Regulation of endoplasmic reticulum Ca2+ dynamics by proapoptotic BCL-2 family members. Biochem Pharmacol. 2003;66(8):1335–1340. doi: 10.1016/s0006-2952(03)00482-9. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg EF, Lavik AR, Distelhorst CW. Bcl-2 regulation of the inositol 1,4,5-trisphosphate receptor and calcium signaling in normal and malignant lymphocytes: Potential new target for cancer treatment. Biochim Biophys Acta. 2014;1843(10):2205–2210. doi: 10.1016/j.bbamcr.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong F, et al. Induction of Ca²+-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2-IP3 receptor interaction. Blood. 2011;117(10):2924–2934. doi: 10.1182/blood-2010-09-307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boehning D, et al. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5(12):1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari D, et al. Endoplasmic reticulum, Bcl-2 and Ca2+ handling in apoptosis. Cell Calcium. 2002;32(5-6):413–420. doi: 10.1016/s0143416002002014. [DOI] [PubMed] [Google Scholar]
- 21.Rizzuto R, et al. Calcium and apoptosis: Facts and hypotheses. Oncogene. 2003;22(53):8619–8627. doi: 10.1038/sj.onc.1207105. [DOI] [PubMed] [Google Scholar]
- 22.Delbridge AR, Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015;22(7):1071–1080. doi: 10.1038/cdd.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta. 2004;1644(2-3):83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Germain M, Shore GC. Cellular distribution of Bcl-2 family proteins. Sci STKE. 2003;2003(173):pe10. doi: 10.1126/stke.2003.173.pe10. [DOI] [PubMed] [Google Scholar]
- 25.Szegezdi E, Macdonald DC, Ní Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296(5):C941–C953. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]
- 26.Chen R, et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol. 2004;166(2):193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White C, et al. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol. 2005;7(10):1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonneau B, et al. The Bcl-2 homolog Nrz inhibits binding of IP3 to its receptor to control calcium signaling during zebrafish epiboly. Sci Signal. 2014;7(312):ra14. doi: 10.1126/scisignal.2004480. [DOI] [PubMed] [Google Scholar]
- 29.Schulman JJ, Wright FA, Kaufmann T, Wojcikiewicz RJ. The Bcl-2 protein family member Bok binds to the coupling domain of inositol 1,4,5-trisphosphate receptors and protects them from proteolytic cleavage. J Biol Chem. 2013;288(35):25340–25349. doi: 10.1074/jbc.M113.496570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanson CJ, Bootman MD, Distelhorst CW, Wojcikiewicz RJ, Roderick HL. Bcl-2 suppresses Ca2+ release through inositol 1,4,5-trisphosphate receptors and inhibits Ca2+ uptake by mitochondria without affecting ER calcium store content. Cell Calcium. 2008;44(3):324–338. doi: 10.1016/j.ceca.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Eckenrode EF, Yang J, Velmurugan GV, Foskett JK, White C. Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J Biol Chem. 2010;285(18):13678–13684. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, et al. Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci USA. 2007;104(30):12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis A, Hayashi T, Su TP, Betenbaugh MJ. Bcl-2 family in inter-organelle modulation of calcium signaling; roles in bioenergetics and cell survival. J Bioenerg Biomembr. 2014;46(1):1–15. doi: 10.1007/s10863-013-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13(8):1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- 35.Rong YP, et al. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc Natl Acad Sci USA. 2009;106(34):14397–14402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monaco G, et al. Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ. 2012;19(2):295–309. doi: 10.1038/cdd.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rong YP, et al. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2's inhibition of apoptotic calcium signals. Mol Cell. 2008;31(2):255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang MJ, et al. Feedback regulation mediated by Bcl-2 and DARPP-32 regulates inositol 1,4,5-trisphosphate receptor phosphorylation and promotes cell survival. Proc Natl Acad Sci USA. 2014;111(3):1186–1191. doi: 10.1073/pnas.1323098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sung PJ, et al. Phosphorylated K-Ras limits cell survival by blocking Bcl-xL sensitization of inositol trisphosphate receptors. Proc Natl Acad Sci USA. 2013;110(51):20593–20598. doi: 10.1073/pnas.1306431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ku B, Liang C, Jung JU, Oh BH. Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res. 2011;21(4):627–641. doi: 10.1038/cr.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fletcher JI, et al. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc Natl Acad Sci USA. 2008;105(47):18081–18087. doi: 10.1073/pnas.0808691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kvansakul M, Hinds MG. The Bcl-2 family: Structures, interactions and targets for drug discovery. Apoptosis. 2015;20(2):136–150. doi: 10.1007/s10495-014-1051-7. [DOI] [PubMed] [Google Scholar]
- 43.Mak DO, Foskett JK. Inositol 1,4,5-trisphosphate receptors in the endoplasmic reticulum: A single-channel point of view. Cell Calcium. 2015;58(1):67–78. doi: 10.1016/j.ceca.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan G, et al. Gating machinery of InsP3R channels revealed by electron cryomicroscopy. Nature. 2015;527(7578):336–341. doi: 10.1038/nature15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 46.Tzung SP, et al. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat Cell Biol. 2001;3(2):183–191. doi: 10.1038/35055095. [DOI] [PubMed] [Google Scholar]
- 47.Chen L, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17(3):393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 48.Rajan S, Choi M, Baek K, Yoon HS. Bh3 induced conformational changes in Bcl-Xl revealed by crystal structure and comparative analysis. Proteins. 2015;83(7):1262–1272. doi: 10.1002/prot.24816. [DOI] [PubMed] [Google Scholar]
- 49.Feng W, Huang S, Wu H, Zhang M. Molecular basis of Bcl-xL’s target recognition versatility revealed by the structure of Bcl-xL in complex with the BH3 domain of Beclin-1. J Mol Biol. 2007;372(1):223–235. doi: 10.1016/j.jmb.2007.06.069. [DOI] [PubMed] [Google Scholar]
- 50.Velmurugan GV, White C. Calcium homeostasis in vascular smooth muscle cells is altered in type 2 diabetes by Bcl-2 protein modulation of InsP3R calcium release channels. Am J Physiol Heart Circ Physiol. 2012;302(1):H124–H134. doi: 10.1152/ajpheart.00218.2011. [DOI] [PubMed] [Google Scholar]
- 51.Deverman BE, et al. Bcl-xL deamidation is a critical switch in the regulation of the response to DNA damage. Cell. 2002;111(1):51–62. doi: 10.1016/s0092-8674(02)00972-8. [DOI] [PubMed] [Google Scholar]