Abstract
Oncolytic viruses (OVs) are unique anticancer agents based on their pleotropic modes of action, which include, besides viral tumor cell lysis, activation of antitumor immunity. A panel of diverse viruses, often genetically engineered, has advanced to clinical investigation, including phase 3 studies. This diversity of virotherapeutics not only offers interesting opportunities for the implementation of different therapeutic regimens but also poses challenges for clinical translation. Thus, manufacturing processes and regulatory approval paths need to be established for each OV individually. This review provides an overview of clinical-grade manufacturing procedures for OVs using six virus families as examples, and key challenges are discussed individually. For example, different virus features with respect to particle size, presence/absence of an envelope, and host species imply specific requirements for measures to ensure sterility, for handling, and for determination of appropriate animal models for toxicity testing, respectively. On the other hand, optimization of serum-free culture conditions, increasing virus yields, development of scalable purification strategies, and formulations guaranteeing long-term stability are challenges common to several if not all OVs. In light of the recent marketing approval of the first OV in the Western world, strategies for further upscaling OV manufacturing and optimizing product characterization will receive increasing attention.
Introduction
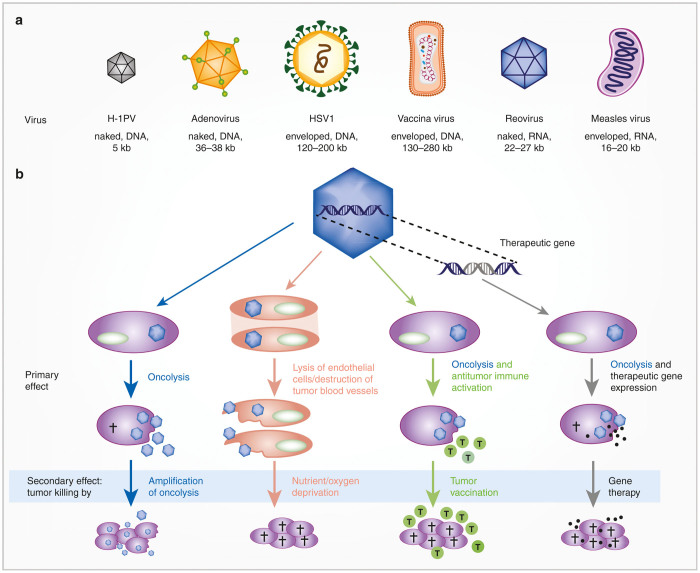
With recent marketing approval in the United States and recommendation for marketing approval in Europe of the first oncolytic virus (OV) in the Western world, T-Vec (brand name Imlygic),1,2 this new class of cancer drugs is now complementing surgery, chemotherapy, irradiation, targeted small molecules, and antibodies in routine clinical oncology. OVs implement a unique mode of action, tumor-restricted viral infection, replication, cell lysis, and spread.3,4 Notably, recent preclinical and clinical research has revealed pleiotropic therapeutic activity of OVs (Figure 1): (i) viral tumor cell lysis has been shown to trigger systemic antitumor immunity in animal models and patients,5–8 (ii) the insertion of therapeutic genes can trigger bystander killing by different means, depending on the chosen gene9, and (iii) endothelial cells specifically in tumor vessels were shown to be susceptible to OVs, resulting in vascular shut down and indirect destruction of tumor cells.10 As multitasking agents, OVs offer promising opportunities for treatment of heterogeneous tumors, avoidance of resistance development, and implementation of combination therapies. Research and clinical translation has recently focused especially on the vaccination effect and on the combination with (other) immunotherapies, foremost immune checkpoint inhibition.6–8
Figure 1.
Diversity of oncolytic viruses and their modes of action. (a) Oncolytic viruses covered in this review. (Adapted by permission from Macmillan Publishers: Nature Reviews Microbiology, (Cattaneo et al.45), copyright (2008)). (b) Modes of action implemented by oncolytic viruses. Each of the described activity has been reported in animal models and in cancer patients (tumor cells in purple, endothelial cells in orange).
The fact that besides herpesvirus T-Vec OVs derived from several classes of viruses are being developed highlights the future potential of viral oncolysis3,11 (Figure 1). They represent a panel of pharmacophores that differ considerably with respect to their structure, size, genome, replication mechanisms, and host interactions including triggered host immune responses (Table 1). This diversity is further extended by the opportunity to genetically engineer OVs by a panel of different strategies (Table 1) aiming at better biodistribution and tumor specificity or at extending therapeutic applications, for example, by transgene expression as mentioned above. Moreover, by iterative translation cycles in a bench-to-bed-and-back approach, virus engineering facilitates overcoming emerging roadblocks to therapeutic efficacy. Notably, viruses from nine virus families are already in clinical investigation, and for some of these viruses, derivatives with different genetic modifications are being studied.3,11
Table 1. Properties of selected oncolytic viruses covered in this review.
| Herpesvirus: T-Vec (Imlygic) | Vaccinia virus | Reovirus: REOLYSIN (pelareorep) | Adenovirus | Measles virus | Parvovirus: H-1 | |
|---|---|---|---|---|---|---|
| Genome | dsDNA | dsDNA | dsRNA | dsDNA | Nonsegmented negative-strand RNA | Linear ssDNA |
| Capsid | Icosahedral | Brick shaped | Double capsid | Icosahedral | Helical nucleocapsid | Icosahedral |
| Envelope | Yes | Yes | No | No | Yes | No |
| Genome/particle size | ~152 kbp/155–240 nm | 190 Kb/360 × 270 × 250 nm | 23,560 bp/85 nm | ~36 kbp/~90 nm | 15,894 nt (unmodified)/100–300 nm | 5,176 nt/~26 nm |
| Host species | Human | Unknown | Pan mammalian | Human | Human | Rat |
| Reported virus modifications for applications as oncolytics | T-Vec: Deletion of ICP34.5 gene Deletion of ICP47 gene Insertion of transgene (encoding GM-CSF) Others: Deletion of further viral genes Envelope modifications for specific oncotropism (entry “re-targeting”) Insertion of cellular promoters for tumor-targeted replication | Pexa-Vec Deletion of thymidine kinase gene Natural mutation of B18R gene Inclusion of human GM-CSF cDNA Beta galactosidase gene JX-929 Deletion of thymidine kinase gene and VGF gene | Eleven amino acid substitutions from the parental strain (primarily located in the L1 segment – RNA polymerase) | Full or partial deletion of viral genes for tumor-targeted replication Insertion of cellular promoters for tumor-targeted replication Insertion of miRNA target sequences for increased safety Insertion of aptamers for inducible replication Insertion of transgenes for therapeutic potentiation, biomonitoring, immunomodulation Capsid modification for enhanced/targeted entry into tumor cells | Insertion of transgenes for therapeutic potentiation, biomonitoring, immunomodulation Envelope modifications for specific oncotropism (entry “re-targeting”) Insertion of miRNA target sequences for increased safety Insertion of aptamers for inducible replication | H-1: none Production of second-generation viruses through engineering/selection (preclinical) |
| Specific features | Insertion of GM-CSF gene | Multiple isoforms facilitate cell to cell spread and immune evasion Stable in human serum Excellent human safety record as multiple vaccine strains Large capacity for encoding transgenes (50 Kb) Demonstrated ability to be delivered as an intravenous agent even in previously immunized patients Antitumor vascular activity | Growth advantage in human cells | Particle and genomic stability No integration into host genome Low pathogenicity High capacity for insertion of transgenes High-titer production Excellent knowledge of structure, genome, and replication cycle allows for modification >50 serotypes infecting humans | Genomic stability No integration into host genome Vaccine strain provides safe applications intrinsically Preferred oncotropism Adjustable gene expression No persistence in infected individuals Crossing of physiological membranes | Stability Crossing of physiological membranes Safety and low natural infectivity of wild-type virus in humans No preexisting immunity in humans Availability of various serotypes Natural oncoselectivity Efficient expression vectors (preclinical) No integration into cell genome Induction of alternative immunogenic tumor cell death pathways Cryptic persistence in infected individuals |
| Clinical studies (numbers and phases, MTD, therapeutic activity) | T-Vec: Phase 1 all comers (completed) Phase 1/2, pancreatic cancer (completed), i.t., NCT00402025 Phase 3, melanoma (completed), i.t., NCT00769704 Phase 2, melanoma (ongoing, to evaluate correlation between intratumoral CD8+ cell density and objective response rate), i.t., NCT02366195 Phase 1b/2, combination with ipilimumab in melanoma (ongoing), NCT01740297 phase 1b/3, combination with pembrolizumab in melanoma (underway), NCT02263508 Phase 3, combination with cisplatin and radiotherapy in HNSCC (terminated), NCT01161498 | Pexa-Vec: Multiple completed or ongoing phase 1 to phase 2b trials. Routes - intratumoral46, 47: Phase 1, various solid tumors (completed), NCT01169584 Phase 1, HCC (completed), NCT00629759 Phase 1/2, melanoma (completed), NCT00429312 Phase 2a, HCC (completed), NCT00554372 i.v.43: Phase 1, various solid tumors (completed), NCT00625456 i.v. followed by repeat i.t., i.v. dosing: Phase 2b, HCC (completed), NCT01387555 Phase 1b, CRC (ongoing), NCT01380600 Well-tolerated, flu-like symptoms. Demonstration of tumor site replication following i.v., antitumor vascular activity,10 antitumor immune response.50 Tumor response in multiple cancer types. Survival benefit following i.t. treatment in small phase 2 study (front line HCC),42 NCT00554372. combination studies51: Phase 1/2a, combination with irinotecan in CRC (ongoing), NCT01394939 Phase 2, combination with sorafenib in CRC (ongoing), NCT01171651 JX-929: Phase 1, various solid tumors, i.v., NCT00574977 Well-tolerated, flu-like symptoms. | Reolysin: Twenty-one completed or ongoing single arm or randomized studies in the phase 1 or phase 2 setting. Single agent objective responses have been demonstrated following i.t. or i.v. administration.48,49 Rate of response as monotherapy is modest, e.g. Phase 2, stage i.v. melanoma, (completed), NCT00651157 Higher response rates were observed when combined with pretreatments like ionizing radiation and chemotherapy, e.g. Phase 2, combination with paclitaxel and carboplatin in HNSCC, (completed), NCT01166542 Phase 2, combination with paclitaxel and carboplatin in NSCLC with KRAS or EGFR activation, NCT00861627 1 and 2 year OS were 57% and 30% respectively. Phase 2, combination with gemcitabine in advanced pancreatic adenocarcinoma (completed), NCT00998322 Evidence of viral replication in tumor following systemic delivery.52 | DNX-2401: Phase 1, glioma), i.t., NCT02197169 Phase 1/2, glioma, i.t., NCT01582516, NCT01956734 CG0070: Phase 2, bladder, intravesical, NCT02365818 ICOVIR-5: Phase 1, melanoma, endovenous, NCT01864759 Phase 2, solid tumors, i.v., NCT01844661 VCN-01: Phase 1, solid tumors, i.t., NCT02045589; i.v., NCT02045602 ONCOS-102: Phase 1, solid tumors (completed), i.t. and i.v., NCT01598129 Colo-Ad1: Phase 1/2, solid tumors, i.t. or i.v., NCT02053220; i.v., NCT02028442; intraperitoneal injection, NCT02028117 | MV-CEA: Phase 1, glioblastoma multiforme (clinical assessment in progress), i.t., NCT00390299 MTD not reached MV-NIS: Phase 1, pleural mesothelioma, intrapleural injection, NCT01503177 Phase 1, HNSCC (ongoing), i.t., NCT01846091 Phase 1/2, multiple myeloma (ongoing), i.v., NCT00450814 Phase 2, multiple myeloma (ongoing), i.v., NCT02192775 Phase 2, ovarian, fallopian or peritoneal cancer, i.p. injection, NCT02364713 MV-CEA / MV-NIS: phase 1, ovarian cancer (clinical assessment in progress), intraperitoneal injection, NCT00408590 MTD not reached (dose escalation up to E09 TCID50). Safety demonstrated; evidence of biological activity. | ParvOryx01: Phase 1/2a, glioblastoma (completed), i.t. or i.v., NCT01301430 MTD not reached. Evidence of virus replication, cytotoxicity and immunostimulation in treated tumors. Virus crossing of the blood–brain barrier after i.v. administration. Clinical assessment in progress |
| References | 53–55 | 10, 42, 43, 46, 47, 50, 51 | 48, 49, 52, 56–64 | 13, 65–68 | 12, 33, 68–70 | 71–73 and Leuchs B et al., manuscript submitted |
CRC, colorectal cancer; GM-CSF, granulocyte-macrophage colony-stimulating factor; HCC, hepatocellular carcinoma; MTD, maximum tolerated dose.
The diversity of OVs clearly establishes promising therapeutic opportunities. The following are three examples: oncolytic measles viruses located to tumor lesions and triggered durable complete remission after single systemic application in a patient with disseminated myeloma12; T-Vec demonstrated immune-mediated systemic antitumor activity for metastatic melanoma patients after repeated intratumoral injections5; and oncolytic vaccinia viruses showed the above-mentioned antivascular effect in patients with solid tumors.10 However, virus diversity also poses challenges for translation of viral oncolysis into clinical application: safety and manufacturing issues need to be addressed for each virus individually, because their relevant properties, such as genetic stability, biodistribution, and immunogenicity, might differ considerably.
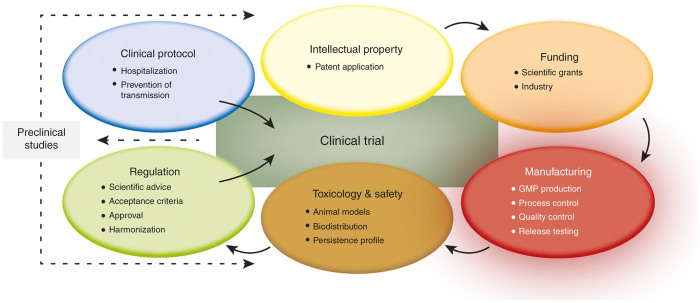
This review provides a concise overview of clinical-grade manufacturing of six classes of OVs that are currently in clinical development: adeno-, herpes-, measles, parvo-, reo-, and vaccinia viruses. Key facts and numbers for these viruses and their manufacturing are provided in Tables 1 and 2. As a condensed overview, five additional, essential aspects during the translation process of OVs into the clinics are presented in Figure 2. Major issues, challenges, and opportunities for virus manufacturing are discussed for each virus individually followed by a synopsis.
Table 2. Overview of production, purification, and characterization of clinical grade oncolytic viruses.
| Herpesvirus: T-Vec (Imlygic) | Vaccinia virus | Reovirus: REOLYSIN (pelareorep) | Adenovirus | Measles virus | Parvovirus: H-1 | |
|---|---|---|---|---|---|---|
| Producer cell(s) (name, species, tissue, genetic modifications if applicable) | Vero (african green monkey, kidney) BHK (hamster, kidney) | HeLa cells (human cervical carcinoma, HPV sequences) Vero (African green monkey, kidney) | HEK293, suspension (human, kidney, inserted adenoviral sequences) | HEK293 (human, kidney, inserted adenoviral sequences) Per.C6, (human, retinoblast cells, inserted adenoviral sequences)16 A549-derived pTG6559 (human, lung adenocarcinoma, inserted adenoviral sequences) N52.E6 (human, aminocytes, inserted adenoviral sequences)16 All cells contain contiguous adenovirus E1A and E1B sequences | Vero cells (african green monkey, kidney), adherent | Human newborn kidney cells (SV40 transformed nonproducer cells), adherent |
| Culture vessels | Flasks, roller bottles, cell factories or bioreactors | Adherent culture vessels, cell factories, RC-40 roller bottle packs | 100 l stir tank reactor | Spinner, roller bottle, bioreactor, single-use bags | Cell factory | Cell factory |
| Culture media; volume for final amplification step; cell density, or total number of cells | DMEM; up to 100 l | DMEM containing 10% fetal bovine serum, pH 7.3, 40 l for 40 extended surface roller bottles (34,000 cm2). Approximately 4E05 cells/cm2 and 1 l of DMEM per bottle | Custom media optimized for HEK293 growth and viral production; 100 l | Medium without serum CD293, SFM II, AEM (Thermo Fischer Scientific); 10–100 l; 1 to 2E06 cells/ml | VP-SFM (serum-free, Invitrogen), glutamine; 25–50 l ~1E05 cells/cm2; ~2E10 total | MEM, 5% FBS, glutamine; production with ~2E10 cells, 3.6E04 cells/cm2 |
| Virus purification method | Centrifugation, size exclusion and ion exchange chromatography, membrane chromatography and filtration | Whole cell harvest followed by hypo-osmotic shock, clarification of cell lysate by depth filtration, DNase (Benzonase) and protease treatment (Tryp LE), tangential flow filtration and diafiltration. | Clarification of feed stock followed by UF/DF; final steps are anion exchange chromatography followed by size exclusion chromatography; terminal 0.22-µm filtration | a) CsCl-density gradient ultracentrifugation b) HPLC | Physical virus release from cells; clarification (filtration 3 µm); DNase treatment (Benzonase); tangential flow filtration and diafiltration; polishing (filtration 1.2 µm) | Physical virus release from cells; DNase treatment; clarification (filtration); density gradient centrifugation |
| Storage buffer formulation | PBS plus sugars | Tris Buffer plus sugars | Modified PBS | 5% glycerol, 25 mmol/l NaCl, 20 mmol/l Tris | 5% sucrose, 50 mmol/l Tris–HCl (pH 7.4), 2 mmol/l MgCl2 | Visipaque/Ringer solution (approved for human use) |
| Recovery (vp or ip per total volume or per cell) | Confidential | ip: 5E12 (from 40 l) | vp: 4.31E16; ip: 2.45E15 TCID50 (from 100 l) | vp: 1E15; ip: 5E13 (from 100 l) | vp: 5E13; ip: 5E11 (from 30 l) | 1E05 vp/cell; 1E03 ip/cell |
| Max. virus concentration (ip/ml) | 1E09 | 1E09 | 3.62E11 TCID50 | 4E10 to 5E11 | 4E09 | 1E11 |
| Typical ratio vp/ip | 10–50 | 50–100 | 15 | 10–50 | 50–100 | 100–1,000 |
| Method to determine v.p.; i.p. titers | ip: plaque assay or TCID50 assay | ip: plaque assay vp: qPCR | vp: HPLC; ip: TCID50 assay | vp: OD 260 nm, real-time PCR; ip: plaque assay, end-point dilution assay, electron microscopy | vp: qRT-PCR (N gene copies per ml); ip: TCID50 assay | vp: qPCR (full particles), capsid-ELISA (full + empty particles); ip: plaque assay |
| Application route (i.t., i.p., i.v.) | i.t.: 1E06 to 1E0853 | i.t.: 1E08 to 1E09 (refs. 46,47) i.v.: 1E09 (ref. 43) | i.v.: standard dose is 3E10 TCID5060–62,64 | i.t.: 3E10 to 3E12 vp74 i.v.: 1 to 6E12 vp 75 | i.t.: 1E09 ip i.p.: 1E10 ip69,70 i.v.: up to 1E11 ip 12 | i.t.: 1E06 to 5E09 ip i.v.: 5E07 to 1E09 ip71 |
| Molecular characterization of virus identity | Virus species identity and presence of any inserted genes confirmed using antibody based assays, Southern blot and/or PCR | Q-PCR for presence of transgenes, Q-PCR for confirmation of gene deletions Western blotting and bioactivity assays for transgenes. Sanger sequencing if applicable | Identity by western blotting, Q-PCR and Sanger sequencing; | Endpoint PCR, restriction analysis, sequencing | Identity: RNA by RT-PCR, sequencing; protein by western blot; transgene expression (if applicable) | Identity: DNA by sequencing, PCR)/protein by western blot/virus particles by capsid ELISA, electron microscopy |
| Purity, toxicity and release criteriaa | Absence of process related impurities (residual host cell protein, host cell DNA, critical ingredients e.g. residual DNase, residual CsCl (adenovirus)), adventitious agents (e.g., cell dissociation enzyme) Absence of product related impurities (e.g., vp/ip ratio, for adenovirus: replication-competent (wild-type) Ad testing) and sub visible particles Absence of contaminants: mycoplasma, sterility, bioburden, abnormal toxicity (virus unrelated) and extraneous agents (in vitro and in vivo) Titer, identity (see above), pH, osmolality, appearance, potency, extractable volume | |||||
| Assays for analysis of efficiency of virus preparation | Virus titration and potency of any inserted genes | Virus titration and potency/bioactivity of inserted transgenes | Virus titration | a) Potency assay at low MOI in different tumor cells versus normal cells, b) In vivo xenograft model | Virus titration; potency assay on test cells | Potency assay on target tumor cells |
| Other procedures/features | None | Stability at RT, 4 °C, -20 °C and -65 °C | None | In vivo: a) Biodistribution studies by PCR b) Virus replication in lungs of Syrian hamster | Stability (>6 years at ≤ -65 °C) | Stability (>4 years at ≤ -60 °C) |
GMP, good manufacturing practice; ip, infectious particles; MOI, multiplicity of infection; vp, (physical) virus particles.
According to USP or/and Ph.Eur. in compliance with e.g. US Food and Drug Administration, European Medicines Agency, GMP International Council of Harmonization.
Figure 2.
Essential steps involved in the translation of OVs into the clinics. Starting from preclinical studies showing proof of concept in terms of therapeutic efficacy of a novel oncolytic agent, securing intellectual property (i.e., filing a patent) is necessary in order to successfully apply for funding, particularly with regard to finding an industrial partner who can perform the challenging and cost-intensive manufacturing under GMP conditions. Pharmacological and toxicological studies using appropriate animal models demonstrating a safe application of the OV is one of the prerequisites to submit an investigational new drug application to the regulatory authorities that contains all of the information regarding production and testing of the clinical grade OV. Additionally, a clinical protocol thoroughly considering the application of replication-competent viruses to patients has to be designed in order to get the approval for the clinical study. GMP, good manufacturing practice; OV, oncolytic virus.
Adenoviruses
Adenoviruses (Ads) are attractive oncolytic agents, based on their potent lytic activity, detailed knowledge of their structure and replication cycle, ample opportunities for virus engineering, and the high stability of virus particles and their genome. Different oncolytic Ads have been investigated in clinical studies, and the Ad Oncorine (H101) obtained marketing approval in China in 2005 (ref. 13). As with any new therapies, the translation of oncolytic (adeno-) viruses from the laboratory bench to their use in the clinic is subject to specific regulations and must meet several well-defined criteria for selectivity, potency, stability, identity, and product characterization (Table 2). Particular attention should be directed toward a detailed characterization of a stable good manufacturing practice (GMP) process, including the establishment of a master cell bank and a master seed virus and the development of sophisticated release assays.14–16 Thus, it is advisable that developers of oncolytic Ads contact local regulatory authorities at an early time point to discuss these issues.
For the production of oncolytic Ads, many producer cells,17,18 such as HEK293, are available and produce high Ad titer. However, since these cells possess genomic insertions of adenoviral E1 genes and oncolytic Ads contain E1 deletions or modifications, recombinations can occur reverting the modified E1 gene of oncolytic Ads to wild-type sequence. This problem can be encountered by the selection of a thoroughly characterized cell line that does not have integrated adenoviral sequences.18 An additional challenge is a consistent surveillance of master cell bank and master seed virus used in the production of oncolytic Ads that included tests for the cellular identity and the absence of any adventitious contaminants, such as mycoplasma and bovine/porcine viruses.14
Different production methods for viral particle (vp) have been described in the literature,19–23 including the growth of producer cells in suspension using serum-free medium. However, the production of oncolytic Ads under serum-free conditions causes a significant reduction in virus yield. In addition, the production of oncolytic Ads is also affected by the so-called cell density effect that limits the production of Ads at cell densities of ~1 × 106 cells/ml.
The most popular method for the purification of oncolytic Ads is the use of two rounds of cesium chloride density gradient ultracentrifugation. This method can provide sufficient material for phase 1 trials but has the disadvantage of not being scalable. Therefore, for large-scale manufacturing of clinical-grade oncolytic vectors exceeding ≥1015 vp, column chromatographic methods, including anion exchange chromatography, have been developed.24–26 During virus production and purification steps, the upstream and downstream processing is time consuming and causes considerable production costs. Thus, it is important to establish a method, which enables the monitoring of virus titer in every single production step during upstream and downstream processing.
Purified viruses should be tested for potency, identity (endpoint PCR, restriction analysis, or sequencing), sterility, purity, endotoxin, contaminating host cell DNA (≤5 pg/1011 vp), and proteins (Table 2). Total vp and infectious particles (ip) are two critical parameters for comparison of different production processes and should be determined carefully.14 The Food and Drug Administration recommends a vp/ip ratio of 30. A high deviation from this value should be avoided (although a certain ratio is not specified by the European regulatory agency).
It is of decisive importance to choose an appropriate immunocompetent animal model for testing toxicity and biodistribution studies of oncolytic Ad. In this regard, it has been shown that Syrian hamster support viral replication in several organs.27 Such studies might be performed with “lab-derived material” if the comparability of the “lab material” and the GMP material has been shown unequivocally.
Herpesviruses
Herpes simplex virus (HSV) is a large, enveloped, double-stranded DNA virus, which between the envelope and capsid contains a region known as the tegument, containing over 20 HSV proteins, which is essential for infectivity. The envelope carries 12 glycoproteins, and the capsid is composed of 7 different proteins. As such, manufacturing of HSV presents a number of challenges. These include the large size of the virus (155–240 nm), including that it is enveloped, which needs to be kept intact throughout the purification process. As HSV is complex, full characterization of the final product can also be challenging. The 152 Kb genome has both long and short unique regions each flanked by terminal repeat regions which are present in the four possible orientations in relation to each other. The repeated regions mean that some HSV genes (e.g., ICP34.5—see later) are present twice in the genome, and the different orientations means that an HSV stock contains capsids containing each of these isomers.28 Both of these properties of the HSV genome can also complicate characterization.
Oncolytic versions of HSV have one or more nonessential genes deleted, in some cases combined with the insertion of a therapeutic transgene.8 A nonessential gene is unnecessary for replication in vitro but is required for pathogenicity in vivo. Those deleted or mutated genes in candidate oncolytic viruses include ICP34.5 (the neurovirulence factor that provides the greatest attenuation of pathogenicity in vivo while still allowing replication in vivo), thymidine kinase (not only necessary for sensitivity to acyclovir and similar drugs but also required for efficient replication in vivo), ICP6 (the large subunit of ribonucleotide reductase), and ICP47 (which blocks antigen presentation by transporter associated with antigen processing). Insertions include a number of cytokines, including IL-12 and granulocyte-macrophage colony-stimulating factor (GM-CSF). Talimogene laherparepvec (T-Vec, brand name Imlygic, Amgen, Thousand Oaks, CA) is the most advanced in clinical development and is deleted for ICP34.5 and ICP47 containing an insertion of the gene encoding GM-CSF under CMV promoter control in place of ICP34.5 (ref. 29).
From a purification perspective, standard procedures used for the production of live attenuated viral vaccines are generally employed, including harvest of the supernatant from infected producer cells, size exclusion chromatography, ion-exchange chromatography, Benzonase treatment to remove contaminating DNA, sterile filtration, and filling of final product. To avoid centrifugation, use of tangential flow filtration can be employed for concentration and/or buffer exchange between steps, but if so care needs to be taken regarding the sheer forces exerted so as not to disrupt the complex virion, as is also the case for terminal sterile filtration if employed. Careful optimization of each of these steps is required. For characterization, western blotting to confirm the presence and relative abundance of key viral proteins can be used and product-specific PCR or Southern blot to confirm genome structure (Table 2). Potency assays will not only depend on the nature of any transgene but also seek to confirm oncolytic activity in appropriate cells. While these processes and procedures are generally robust, future improvements may seek to increase yields at each step and long-term stability at ambient or refrigerated temperatures.
Measles Viruses
Oncolytic measles viruses (MV) based on attenuated vaccine strains are currently under investigation in clinical trials (Table 1) as a promising modality of cancer treatment with the potential to induce immune-mediated tumor rejection. MV vaccine strains are oncolytic by preferentially entering tumor cells through CD46 (ref. 30), a membrane protein that is typically overexpressed in malignant cells.31
In contrast to using MV as a vaccine, oncolytic activity as an advanced therapy medicinal product depends on high concentration of infectious particles. While the size range of pleomorphic MV particles is often quoted as 100–300 nm32, in practice, MV must be treated as >1 µm particles that are extremely shear sensitive, to maximize recoveries and retain infectivity. Therefore, the entire production and purification process has to be done under gentle and aseptic GMP conditions.33 Clinical batches of MV are produced in Vero cells adapted to serum-free growth in cell factory multilayer vessels, resulting in 50% of the virus in the supernatant and 50% staying associated with the cells. The supernatant is clarified by filtration and treated with Benzonase to digest contaminating nucleic acids. MV particles are then concentrated and purified using tangential flow filtration and diafiltration, followed by a final passing through a clarifying filter prior to vialing and storage at < −65 °C.
Previously, various compounds and excipient formulations have been tested as stabilizers to protect the integrity of the viral envelope (i.e., viral infectivity) and also to inhibit aggregation of vp. MV formulated with a buffered sucrose solution containing additionally magnesium chloride showed enhanced physical stability providing virus longevity of more than 6 years, with stable infectivity of the sensitive agent.
Current batch preparations meet the requirements for clinical trials, but purity may be further improved. MV products are tested for identity, purity, potency, adventitious agents, contaminating host cell DNA, and proteins (Table 2). The ratio between total vp and ip is a critical parameter for the quality of virus preparations. Although a certain vp/ip ratio is not specified by the regulatory agencies, a value of ≈50 in MV preparations can be assumed on average. Residual DNA from host cells in the final product is one of the concerns in the manufacturing process of MV as an advanced therapy medicinal product. For the commercially produced MV vaccine, the WHO recommends values ≤100 pg per dose (i.e., 1,000 ip). But for the use of high doses of MV in cancer therapies (e.g., 109 ip for intratumoral injections), the limit for expectedly higher amounts of residual host DNA in the final product has to be coordinated with the regulating authorities.
Currently, several strategies are pursued to improve overall yield and purity of MV preparations. Cultivation using microcarriers or switching to suspension cells in bioreactors may lead to higher titers. Also, development of virus compatible, chromatographic procedures (ion exchangers) may be an option to contribute to the removal of non–particle-associated nucleic acids and proteins, thereby improving the quality of clinical MV batches.
Parvoviruses H-1PV
Several aspects of the biology of rodent protoparvoviruses (PVs), in particular H-1PV, make these agents attractive for the development of anticancer strategies (see Table 1, specific features). Some of these properties also impact on the procedures used for clinical-grade virus production, purification, and quality/quantity control. First of all, the low natural infectivity of PVs and their lack of association with human diseases minimize the precautions to be taken to protect the staff involved in virus manipulation. Furthermore, the high stability (and concomitant longevity) of infectious virions (tolerance to heating, extreme pH, and desiccation) makes their handling significantly easier.34
Besides these general factors, specific steps in virus batch preparation rely on physicochemical or biological features of PVs. (i) The compact physical (and genetic) structure of the virions (parvus = small) allows filtration to be used in purification and sterilization processes. (ii) Mature PVs are stable in the presence of lipid solvents which can thus be applied to virus release and purification. (iii) Full and empty PVs have distinct densities and can be isolated and concentrated through gradient centrifugation. (iv) PVs are among the most resistant pathogens to inactivation by gamma irradiation. Advantages could be taken of this resistance to inactivate microbial contaminants in PV samples. (v) Human cells can be used for efficient PV production, thereby avoiding the risk of batch contamination with animal microbes and immunogenic animal proteins. (vi) Producer cells can be adapted to suspension culture, raising the possibility of PV production upscaling in bioreactors. (vii) Through the establishment of master virus banks, clinical batches are obtained after infection, avoiding the need for transfection reagent testing of final products. (viii) PV formulation with Visipaque/Ringer solution allows computer tomography visualization of the inoculum, precise local delivery, and absence of backflow due to higher viscosity. Virus longevity for more than 4 years was demonstrated in this formulation, with little aggregation and stable infectivity (Leuchs B, et al., unpublished data).
Current PV batch preparation meets the requirements for clinical trials but still calls for further improvements. Special efforts are currently done to select producer cells adapted for large-scale cultivation in serum-free and optimized medium, using microcarrier or suspension bioreactors (e.g., wave or stirring). Since density centrifugation of large virus harvests is cumbersome, the development of chromatographic procedures with ion exchangers or membrane absorber is needed to optimize and shorten PV purification steps. This should contribute to improve the removal of cellular and non–particle-associated viral DNA and proteins and to enhance the specific infectivity of PV clinical batches.
Reoviruses: Reolysin (Pelareorep), A Unique Isolate of Reovirus T3D (Type 3 Dearing)
REOLYSIN, also known as pelareorep, is a proprietary isolate of the replication-competent reovirus type 3 Dearing, a non-enveloped double-stranded RNA virus that possess an intrinsic preference to replicate and subsequently lyse tumor cells with Ras pathway activation.35 The selective lysis of cells with an activated Ras pathway by reovirus arises from the inhibition of the autophosphorylation of dsRNA-activated protein kinase (PKR), thereby disabling the cells’ protective antiviral mechanism. Several groups have demonstrated that reovirus-induced lysis of tumor cells stimulates a potent antitumor immune response which is composed of both innate immune activation and adaptive immune responses.36–38
For the production of oncolytic reovirus in clinical applications, the cell culture process consists of thawing vials of human embryo kidney (HEK293S) cells and seeding in T-flasks with a proprietary medium supplemented with glutamine. Cell expansion continues using T flasks and/or increasing sizes of Erlenmeyer flasks, with passages every 3 to 3 days until a sufficient number of cells is achieved to seed a WAVE bioreactor in which further expansion occurs. Subsequently, the stirred-tank bioreactor is seeded, and the cells are further expanded until an optimal number of cells are present for infection. After achieving the desired HEK293S cell count, the infection step, in which virus is added to the bioreactor containing the HEK293S cells, takes place. Virus is harvested using a Triton lysis step, then a Benzonase treatment for degradation of residual nucleic acids, followed by purification of the lysate. The downstream purification process begins with chilling of the lysate and clarification through an 8.0-μm filter, followed by 3.0/0.8-μm filtration. After overnight storage, the clarified lysate is ultrafiltered and diafiltered through a hollow fiber filter, followed again by overnight storage. Two chromatography steps—ion exchange and gel permeation—are completed prior to final 0.22-μm filtration. Recovery of infectious particles by this methodology averages 40%.
Identity testing of pelareorep as a reovirus T3D strain has been confirmed by western blotting and confirmed in detail through genomic sequencing and comparison to GenBank sequences. As part of routine analytical testing on each lot of pelareorep produced, the identity of pelareorep is confirmed to be reovirus T3D by QPCR. A more specific identity test to confirm pelareorep identity is Sanger sequencing, which has recently been validated for use in the routine identity testing of pelareorep. The new Sanger sequencing method identifies five unique modifications in the L1 genome segment of reovirus T3D and thereby is specific to REOLYSIN.39
Drug substance testing is assayed for appearance, mycoplasma, adventitious viral contamination, identification, potency (virus titer), content (virus particle concentration), bioburden, residual HC DNA, residual HCP, and residual Benzonase (Table 2).
The greatest historical challenge in the production of the virus was related to deficiencies in the commercially available animal-component free cell culture medium that was used in early process development. This medium was optimized for the production of HEK293 suspension cells rather than the production of virus. This medium was further limiting in that it did not support cell growth beyond 2E6 cells/ml, contained phenol red which interfered with the anion exchange step, and allowed for the accumulation of ammonia which prevented reovirus uncoating, which in turn limited virus production in the bioreactor. To address these shortcomings, Oncolytics (Oncolytics Biotech., Calgary, Alberta, Canada) worked with third-party manufacturing experts to create an optimized medium that was able to optimize cell growth as well as virus production, principally by limiting ammonia production in the bioreactor. This leaner media improved the quality of the feedstock and improved recovery more than fourfold as compared to the commercially available medium.
Vaccinia Viruses
The extensive human safety data that is available for vaccinia viruses as a consequence of their more than 200 hundred year use as a vaccine for small pox infections makes this a very attractive platform as an oncolytic therapeutic. In addition, the virus can harbor and express in excess of 50 Kb of therapeutic transgenes and has multiple known mechanisms of action against tumors in both rodents and humans.40 Vaccinia viruses were the first to demonstrate consistent and convincing infection of tumor beds following i.v. administration41 and in a small randomized trial demonstrated some signs of survival benefit in a subset of hepatocellular carcinoma patients.42
Despite these encouraging results, there still exist several challenges to the widespread commercialization of the vaccinia OV platform even when it achieves clinical success in phase 3 studies. Like other OV platforms, large doses of viral product, well in excess of vaccine doses, need to be administered to patients in order to see effective delivery to tumors following i.v. administration.43 To achieve these doses, virus is best produced in adherent tumor cell cultures in serum-containing medium. To satisfy regulatory concerns, extensive testing of viral product to ensure that no oncogenic DNA is found in the final product needs to be done.
In addition, vaccinia remains largely cell associated and requires cell disruption followed by enzyme digestion steps to liberate the virus from cell debris and reduce host cell contaminants. An additional challenge to the manufacture of pharmaceutical-grade vaccinia virus is that its relatively large size prevents passing through “sterilizing filters” commonly of 0.2 µm or smaller pore size. This means that the entire manufacturing process must be done aseptically. Like many virus products, vaccinia is extremely stable when stored at −80 °C, but additional formulation studies need to be performed to create a pharmaceutical-grade product that can be routinely stored at −20 °C, 4 °C, or perhaps even at room temperature.
Further process development studies still need to be undertaken to optimize the production of oncolytic vaccinia viruses. Ideally, a serum-free suspension cell platform for producing high-titer virus preparations would be preferred. If adherent cell cultures remain required for satisfactory yields, high-intensity bioreactors like the macrofibre iCellis system or manufacturing cells grown on microcarriers should be explored.
Currently, downstream purification of vaccinia virus products has been limited to tangential flow filtration strategies, but the similar size of vaccinia to apoptotic bodies released from dying cells limits the ability to purify the virus from contaminating host protein and nucleic acids. An orthogonal purification strategy independent of particle size is preferred. Vaccinia virus has multiple isoforms, and one version called extra enveloped virus is thought to have the advantage of evading neutralizing antibodies.44 Current manufacturing processes focus on harvesting cell-associated viruses that would not include extra enveloped virus isoforms. Strategies that enrich and stabilize the somewhat delicate extra enveloped virus form of the virus could create products that could be more effectively delivered to immunized patients.
Synopsis and Future Perspectives
Robust and readily scalable manufacturing processes have been developed to produce various oncolytic viruses suitable as a drug for clinical studies. Starting from an established, GMP-certified master cell bank and a characterized master seed virus, the crude virus supernatant is generated mostly by infecting adherent producer cells at a low multiplicity of infection, then harvesting the OV when extensive cytopathic effect is formed. The purification process involves five steps common for almost all of the OV platforms presented here: (i) clarification to remove cellular debris; (ii) nuclease treatment to degrade host cell nucleic acids; (iii) ion-exchange/size exclusion chromatography for purification of the virus; (iv) ultracentrifugation or tangential flow ultrafiltration/diafiltration for concentration and buffer exchange; and (v) a terminal sterile filtration step, which may not be tolerated by every type of virus and therefore requires a totally aseptic production process instead.
Although the ultrafiltration/diafiltration and column chromatography steps are scalable using existing technologies, in order to scale up the infection process for licensed product manufacturing, it could be necessary to investigate bioreactor technology using adherent cells on microbeads or suspension cell cultures to support the production of OVs at desired higher titers. Ideally, cell growth would occur in serum-free medium to completely eliminate animal-derived components during GMP. Disposable single-use stirred tank bioreactors with high volumes would be suitable for virus manufacturing purposes. Downstream purification processes, especially separation from subcellular structures, may need optimization in some cases, depending on the size and density of the virus particle. Quality control is the part of GMP that is concerned with sampling, specifications, testing, documentation and release procedures. It ensures that the necessary and relevant tests are carried out and that all required materials are released for use only if their quality is satisfactory.
Before using a virus lot in clinical trials, it is necessary to submit an investigational new drug application to the regulatory authorities that contains all the information regarding production and testing of the clinical-grade OV, regarding its preclinical efficacy, biodistribution, and pharmacological/toxicological testing in laboratory animals, and the draft clinical protocol. It is highly recommended to keep the regulatory authorities informed regarding any difficulties encountered during lot release testing and to seek their input and guidance at all key steps in the clinical reagent development process. It may seem on the surface that replication-competent viruses as oncolytic agents could be uniformly evaluated in terms of manufacturing and approval for clinical studies. But release criteria basing on validated test methods to characterize identity, purity, potency, and safety of the OV products (Table 2) can vary taking the nature of the respective virus type into account due to its inherent, biologically specific properties like size, host species, tropism, etc. Thus, to find common ground, most release criteria of clinical OV platforms were derived from those defined in GMP specifications of viral vaccine drugs, most of them consisting of formulations with low amounts of vp sufficient for establishing the desired vaccination effect. However, current OV platforms require large doses of viral product, well in excess of vaccine doses, in order to see effective delivery to tumor sites and therapeutic effects. Higher virus concentrations often come along with accumulation of impurities like residual cellular host DNA. Eliminating this by more extensive purification procedures, virus particles may suffer resulting in a higher ratio of vp:ip, often depending on the nature of the virus type.
Deviations in the release criteria of the final product have to be coordinated with the local regulating authorities responsible for the site where the clinical study is planned to be conducted. As the authorities often define country-specific acceptance criteria, this might pose a problem for clinical trials intended as multicenter studies, especially when recruiting a large pool of patients in several continents for phase 3 trials. Therefore, a global harmonization of approval criteria and processes between the local regulatory authorities (e.g., Food and Drug Administration, Health Canada, European Medicines Agency, and International Council of Harmonization) could be a helpful development for future attempts to bring oncolytic virus platforms into the clinic at a faster and higher extent.
Acknowledgments
We thank Mark Federspiel for proofreading the MV manufacturing part. The H-1PV process development and the pre-clinical and clinical trial is supported by Oryx GmbH & Co. KG, Germany.
Matt Coffey is employee of Oncolytics Biotech Inc., Calgary, Alberta, Canada. Rob Coffin is employee of Replimune Ltd, Oxford, UK.
References
- (2015). First oncolytic viral therapy for melanoma. Cancer Discov 6:6. [DOI] [PubMed] [Google Scholar]
- Sheridan, C (2015). First oncolytic virus edges towards approval in surprise vote. Nat Biotechnol 33: 569–570. [DOI] [PubMed] [Google Scholar]
- Russell, SJ, Peng, KW and Bell, JC (2012). Oncolytic virotherapy. Nat Biotechnol 30: 658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, J and McFadden, G (2014). Viruses for tumor therapy. Cell Host Microbe 15: 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, HL, Kim, DW, DeRaffele, G, Mitcham, J, Coffin, RS and Kim-Schulze, S (2010). Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol 17: 718–730. [DOI] [PubMed] [Google Scholar]
- Lichty, BD, Breitbach, CJ, Stojdl, DF and Bell, JC (2014). Going viral with cancer immunotherapy. Nat Rev Cancer 14: 559–567. [DOI] [PubMed] [Google Scholar]
- Woller, N, Gürlevik, E, Ureche, CI, Schumacher, A and Kühnel, F (2014). Oncolytic viruses as anticancer vaccines. Front Oncol 4: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin, RS (2015). From virotherapy to oncolytic immunotherapy: where are we now? Curr Opin Virol 13: 93–100. [DOI] [PubMed] [Google Scholar]
- Bauzon, M and Hermiston, T (2014). Armed therapeutic viruses - a disruptive therapy on the horizon of cancer immunotherapy. Front Immunol 5: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach, CJ, Arulanandam, R, De Silva, N, Thorne, SH, Patt, R, Daneshmand, M et al. (2013). Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res 73: 1265–1275. [DOI] [PubMed] [Google Scholar]
- Miest, TS and Cattaneo, R (2014). New viruses for cancer therapy: meeting clinical needs. Nat Rev Microbiol 12: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, SJ, Federspiel, MJ, Peng, KW, Tong, C, Dingli, D, Morice, WG et al. (2014). Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc 89: 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, C, Oronsky, B, Scicinski, J, Fanger, GR, Stirn, M, Oronsky, A et al. (2015). Going viral: a review of replication-selective oncolytic adenoviruses. Oncotarget 6: 19976–19989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisher, M (2002). Biosafety and product release testing issues relevant to replication-competent oncolytic viruses. Cancer Gene Ther 9: 1056–1061. [DOI] [PubMed] [Google Scholar]
- Lusky, M (2005). Good manufacturing practice production of adenoviral vectors for clinical trials. Hum Gene Ther 16: 281–291. [DOI] [PubMed] [Google Scholar]
- Working, PK, Lin, A and Borellini, F (2005). Meeting product development challenges in manufacturing clinical grade oncolytic adenoviruses. Oncogene 24: 7792–7801. [DOI] [PubMed] [Google Scholar]
- Kovesdi, I and Hedley, SJ (2010). Adenoviral producer cells. Viruses 2: 1681–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau, I and Kamen, A (2003). Production of adenovirus vector for gene therapy. Biotechnol Adv 20: 475–489. [DOI] [PubMed] [Google Scholar]
- Kamen, A and Henry, O (2004). Development and optimization of an adenovirus production process. J Gene Med 6 (suppl. 1): S184–S192. [DOI] [PubMed] [Google Scholar]
- Lesch, HP, Heikkilä, KM, Lipponen, EM, Valonen, P, Müller, A, Räsänen, E et al. (2015). Process development of adenoviral vector production in fixed bed bioreactor: from bench to commercial scale. Hum Gene Ther 26: 560–571. [DOI] [PubMed] [Google Scholar]
- (2013). 23rd European Society for Animal Cell Technology (ESACT) meeting: better cells for better health. BMC Proc 7 (suppl. 6): O1–P116. [PubMed] [Google Scholar]
- Vellinga, J, Smith, JP, Lipiec, A, Majhen, D, Lemckert, A, van Ooij, M et al. (2014). Challenges in manufacturing adenoviral vectors for global vaccine product deployment. Hum Gene Ther 25: 318–327. [DOI] [PubMed] [Google Scholar]
- Iyer, P, Ostrove, JM and Vacante, D (1999). Comparison of manufacturing techniques for adenovirus production. Cytotechnology 30: 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestola, P, Peixoto, C, Silva, RR, Alves, PM, Mota, JP and Carrondo, MJ (2015). Improved virus purification processes for vaccines and gene therapy. Biotechnol Bioeng 112: 843–857. [DOI] [PubMed] [Google Scholar]
- Eglon, MN, Duffy, AM, O’Brien, T and Strappe, PM (2009). Purification of adenoviral vectors by combined anion exchange and gel filtration chromatography. J Gene Med 11: 978–989. [DOI] [PubMed] [Google Scholar]
- Shabram, PW, Giroux, DD, Goudreau, AM, Gregory, RJ, Horn, MT, Huyghe, BG et al. (1997). Analytical anion-exchange HPLC of recombinant type-5 adenoviral particles. Hum Gene Ther 8: 453–465. [DOI] [PubMed] [Google Scholar]
- Thomas, MA, Spencer, JF, La Regina, MC, Dhar, D, Tollefson, AE, Toth, K et al. (2006). Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res 66: 1270–1276. [DOI] [PubMed] [Google Scholar]
- Roizman, B (1979). The structure and isomerization of herpes simplex virus genomes. Cell 16: 481–494. [DOI] [PubMed] [Google Scholar]
- Liu, BL, Robinson, M, Han, ZQ, Branston, RH, English, C, Reay, P et al. (2003). ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 10: 292–303. [DOI] [PubMed] [Google Scholar]
- Dörig, RE, Marcil, A, Chopra, A and Richardson, CD (1993). The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75: 295–305. [DOI] [PubMed] [Google Scholar]
- Anderson, BD, Nakamura, T, Russell, SJ and Peng, KW (2004). High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res 64: 4919–4926. [DOI] [PubMed] [Google Scholar]
- Knipe, DM, and Howley, PM (2013). Fields Virology. Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA. [Google Scholar]
- Langfield, KK, Walker, HJ, Gregory, LC and Federspiel, MJ (2011). Manufacture of measles viruses. Methods Mol Biol 737: 345–366. [DOI] [PubMed] [Google Scholar]
- Cotmore, SF and Tattersall, P (2007). Parvoviral host range and cell entry mechanisms. Adv Virus Res 70: 183–232. [DOI] [PubMed] [Google Scholar]
- Strong, JE, Coffey, MC, Tang, D, Sabinin, P and Lee, PW (1998). The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J 17: 3351–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajani, K, Parrish, C, Kottke, T, Thompson, J, Zaidi, S, Ilett, L et al. (2016). Combination therapy with reovirus and anti-PD-1 blockade controls tumor growth through innate and adaptive immune responses. Mol Ther 24: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington, F, Steele, L, Prestwich, R, Harrington, KJ, Pandha, HS, Vidal, L et al. (2008). Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol 180: 6018–6026. [DOI] [PubMed] [Google Scholar]
- Prestwich, RJ, Errington, F, Ilett, EJ, Morgan, RS, Scott, KJ, Kottke, T et al. (2008). Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res 14: 7358–7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty, R, Tran, H, Fortin, Y, Yu, Z, Shen, SH, Kolman, J et al. (2014). Evaluation of homogeneity and genetic stability of REOLYSIN (pelareorep) by complete genome sequencing of reovirus after large scale production. Appl Microbiol Biotechnol 98: 1763–1770. [DOI] [PubMed] [Google Scholar]
- Kirn, DH and Thorne, SH (2009). Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer 9: 64–71. [DOI] [PubMed] [Google Scholar]
- Kim, JH, Oh, JY, Park, BH, Lee, DE, Kim, JS, Park, HE et al. (2006). Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther 14: 361–370. [DOI] [PubMed] [Google Scholar]
- Heo, J, Reid, T, Ruo, L, Breitbach, CJ, Rose, S, Bloomston, M et al. (2013). Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 19: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach, CJ, Burke, J, Jonker, D, Stephenson, J, Haas, AR, Chow, LQ et al. (2011). Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 477: 99–102. [DOI] [PubMed] [Google Scholar]
- Kirn, DH, Wang, Y, Liang, W, Contag, CH and Thorne, SH (2008). Enhancing poxvirus oncolytic effects through increased spread and immune evasion. Cancer Res 68: 2071–2075. [DOI] [PubMed] [Google Scholar]
- Cattaneo, R, Miest, T, Shashkova, EV and Barry, MA (2008). Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol 6: 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, BH, Hwang, T, Liu, TC, Sze, DY, Kim, JS, Kwon, HC et al. (2008). Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol 9: 533–542. [DOI] [PubMed] [Google Scholar]
- Hwang, TH, Moon, A, Burke, J, Ribas, A, Stephenson, J, Breitbach, CJ et al. (2011). A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol Ther 19: 1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, DG, Feng, X, DiFrancesco, LM, Fonseca, K, Forsyth, PA, Paterson, AH et al. (2013). REO-001: a phase I trial of percutaneous intralesional administration of reovirus type 3 dearing (Reolysin®) in patients with advanced solid tumors. Invest New Drugs 31: 696–706. [DOI] [PubMed] [Google Scholar]
- Gollamudi, R, Ghalib, MH, Desai, KK, Chaudhary, I, Wong, B, Einstein, M et al. (2010). Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs 28: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, MK, Breitbach, CJ, Moon, A, Heo, J, Lee, YK, Cho, M et al. (2013). Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci Transl Med 5: 185ra63. [DOI] [PubMed] [Google Scholar]
- Heo, J, Breitbach, CJ, Moon, A, Kim, CW, Patt, R, Kim, MK et al. (2011). Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: preclinical and clinical demonstration of combination efficacy. Mol Ther 19: 1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam, D, Patel, S, Nuovo, G, Gill, G, Selvaggi, G, Coffey, M et al. (2015). The combination of intravenous Reolysin and gemcitabine induces reovirus replication and endoplasmic reticular stress in a patient with KRAS-activated pancreatic cancer. BMC Cancer 15: 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, JC, Coffin, RS, Davis, CJ, Graham, NJ, Groves, N, Guest, PJ et al. (2006). A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 12: 6737–6747. [DOI] [PubMed] [Google Scholar]
- Senzer, NN, Kaufman, HL, Amatruda, T, Nemunaitis, M, Reid, T, Daniels, G et al. (2009). Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 27: 5763–5771. [DOI] [PubMed] [Google Scholar]
- Andtbacka, RH, Kaufman, HL, Collichio, F, Amatruda, T, Senzer, N, Chesney, J et al. (2015). Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 33: 2780–2788. [DOI] [PubMed] [Google Scholar]
- Thirukkumaran, CM, Nodwell, MJ, Hirasawa, K, Shi, ZQ, Diaz, R, Luider, J et al. (2010). Oncolytic viral therapy for prostate cancer: efficacy of reovirus as a biological therapeutic. Cancer Res 70: 2435–2444. [DOI] [PubMed] [Google Scholar]
- Forsyth, P, Roldán, G, George, D, Wallace, C, Palmer, CA, Morris, D et al. (2008). A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther 16: 627–632. [DOI] [PubMed] [Google Scholar]
- Kicielinski, KP, Chiocca, EA, Yu, JS, Gill, GM, Coffey, M and Markert, JM (2014). Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol Ther 22: 1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington, KJ, Karapanagiotou, EM, Roulstone, V, Twigger, KR, White, CL, Vidal, L et al. (2010). Two-stage phase I dose-escalation study of intratumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers. Clin Cancer Res 16: 3067–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal, L, Pandha, HS, Yap, TA, White, CL, Twigger, K, Vile, RG et al. (2008). A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res 14: 7127–7137. [DOI] [PubMed] [Google Scholar]
- Comins, C, Spicer, J, Protheroe, A, Roulstone, V, Twigger, K, White, CM et al. (2010). REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res 16: 5564–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolkema, MP, Arkenau, HT, Harrington, K, Roxburgh, P, Morrison, R, Roulstone, V et al. (2011). A phase I study of the combination of intravenous reovirus type 3 Dearing and gemcitabine in patients with advanced cancer. Clin Cancer Res 17: 581–588. [DOI] [PubMed] [Google Scholar]
- Karapanagiotou, EM, Roulstone, V, Twigger, K, Ball, M, Tanay, M, Nutting, C et al. (2012). Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res 18: 2080–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis, E, Markovic, SN, Suman, VJ, Nuovo, GJ, Vile, RG, Kottke, TJ et al. (2012). Phase II trial of intravenous administration of Reolysin(®) (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol Ther 20: 1998–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, M and Curiel, DT (2010). Current issues and future directions of oncolytic adenoviruses. Mol Ther 18: 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann, JK and Nettelbeck, DM (2012). Virus chimeras for gene therapy, vaccination, and oncolysis: adenoviruses and beyond. Trends Mol Med 18: 365–376. [DOI] [PubMed] [Google Scholar]
- Dorer, DE and Nettelbeck, DM (2009). Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev 61: 554–571. [DOI] [PubMed] [Google Scholar]
- Ketzer, P, Kaufmann, JK, Engelhardt, S, Bossow, S, von Kalle, C, Hartig, JS et al. (2014). Artificial riboswitches for gene expression and replication control of DNA and RNA viruses. Proc Natl Acad Sci USA 111: E554–E562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis, E, Atherton, PJ, Maurer, MJ, Knutson, KL, Dowdy, SC, Cliby, WA et al. (2015). Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res 75: 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis, E, Hartmann, LC, Cliby, WA, Long, HJ, Peethambaram, PP, Barrette, BA et al. (2010). Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res 70: 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geletneky, K, Huesing, J, Rommelaere, J, Schlehofer, JR, Leuchs, B, Dahm, M et al. (2012). Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer 12: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova, AL, Geletneky, K, Nüesch, JP and Rommelaere, J (2015). Tumor selectivity of oncolytic parvoviruses: from in vitro and animal models to cancer patients. Front Bioeng Biotechnol 3: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geletneky, K, Nüesch, JP, Angelova, A, Kiprianova, I and Rommelaere, J (2015). Double-faceted mechanism of parvoviral oncosuppression. Curr Opin Virol 13: 17–24. [DOI] [PubMed] [Google Scholar]
- Lang, FF, Bruner, JM, Fuller, GN, Aldape, K, Prados, MD, Chang, S et al. (2003). Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol 21: 2508–2518. [DOI] [PubMed] [Google Scholar]
- Tolcher, AW, Hao, D, de Bono, J, Miller, A, Patnaik, A, Hammond, LA et al. (2006). Phase I, pharmacokinetic, and pharmacodynamic study of intravenously administered Ad5CMV-p53, an adenoviral vector containing the wild-type p53 gene, in patients with advanced cancer. J Clin Oncol 24: 2052–2058. [DOI] [PubMed] [Google Scholar]