Abstract
Gene therapy is a promising strategy for specific treatment of numerous gene-associated human diseases by intentionally altering the gene expression in pathological cells. A successful clinical application of gene-based therapy depends on an efficient gene delivery system. Many efforts have been attempted to improve the safety and efficiency of gene-based therapies. Nanoparticles have been proved to be the most promising vehicles for clinical gene therapy due to their tunable size, shape, surface, and biological behaviors. In this review, the clinical development of nanoparticles for gene delivery will be particularly highlighted. Several promising candidates, which are closest to clinical applications, will be briefly reviewed. Then, the recent developments of nanoparticles for clinical gene therapy will be identified and summarized. Finally, the development of nanoparticles for clinical gene delivery in future will be prospected.
Introduction
Gene therapy has drawn significant attention as a promising strategy for specific treatment of numerous gene-associated human diseases ranging from cancer, hemophilia, hypercholesterolemia, neurodegenerative diseases to autoimmune diseases.1–4 This strategy is to introduce genes into the target pathological tissues or cells by altering the expression of the endogenous genes to cure or prevent the progression of the related disease.5,6 Gene therapy has been widely studied in various areas, instead of conventional methods that usually fail to treat many diseases caused by genetic anomalies,7 and has become one of the most promising biomedical technologies for the clinical application.8,9 However, naked genetic molecules cannot be internalized efficiently by target cells because of their serum nuclease susceptibility, rapid renal clearance, phagocyte uptake, reduced uptake by target cells and toxic effect arose by immune response stimulation, which severely restricts their clinical application.10 With the developments of material sciences and the rapid progress of nanotechnology, nanosized materials for gene delivery have attracted worldwide attentions.11 Recently, some preliminary clinical trials of nanoparticles for gene delivery revealed promising effects.12,13 However, the development of safe, efficient, and controllable gene delivery nanoparticles for gene delivery is still now a bottleneck to successful clinical applications.14,15
Despite having been achieved some initiatory successes, the widespread clinical application of gene therapeutics for disease prevention and treatment meets many unavoidable challenges. The most important points are encapsulation efficiency, stability of nanoparticles, degradation in blood circulation, endocytosis by target cells, endosomal escape, delivery efficiency, and toxicity of pharmacology.16 To overcome these obstacles, many types of nanoparticles have been evaluated as gene carriers, which include lipid-based nanoparticles,17 polymer-based nanoparticles,18 and inorganic nanoparticles.19
In this review, we will particularly highlight the clinical development of nanoparticles for gene delivery rather than covering all the aspects of this field. Some promising candidates, which are closest to clinical applications in recent years, will be briefly reviewed. Then, the recent developments of nanoparticles for gene delivery will be identified and summarized in the clinical trials. Finally, the development of nanoparticles for clinical gene delivery in future will be prospected.
Production of Nanoparticles
With the great development of bioscience and nanotechnology, gene therapy shows an enormous potentiality in clinical application for many human serious incurable diseases.20 However, none of gene therapeutics based on nanoparticles has so far been approved by the US Food and Drug Administration (FDA). There are still several problems for the clinical application of nanoparticle-based gene therapy (Figure 1), including biodegradation and biocompatibility, aggregation in physiological fluids, nonspecific adsorption by nondesired tissues, less efficient extravasation to reach target tissues, cellular internalization, and endosomal escape.12
Figure 1.
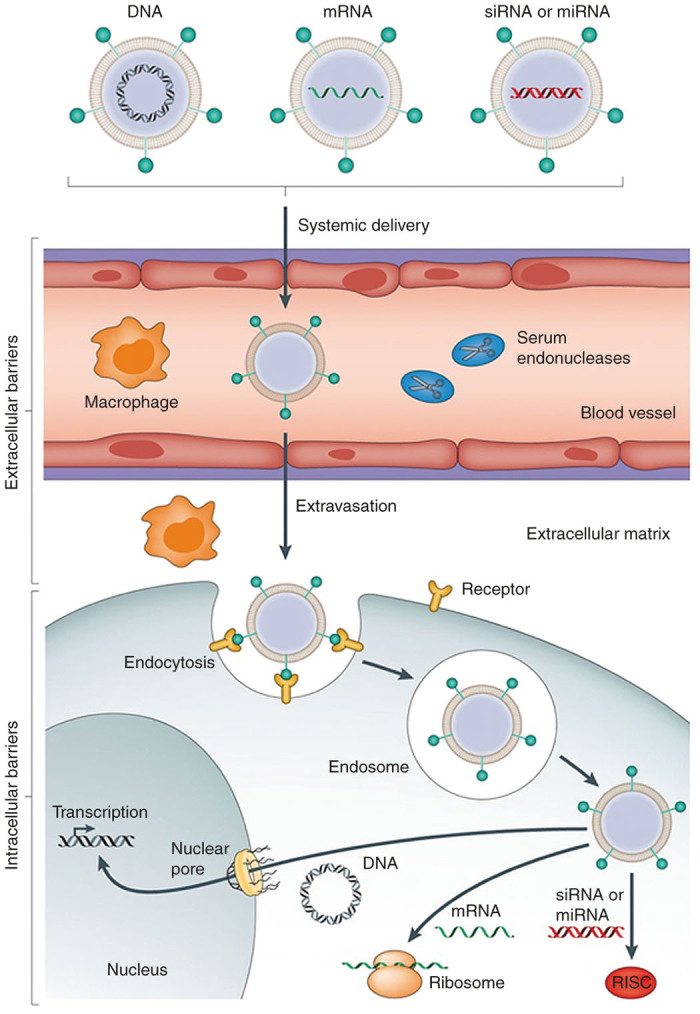
Schematization of nanoparticle-based gene therapy in vivo (Copyright 2014 Nature Publishing Group).
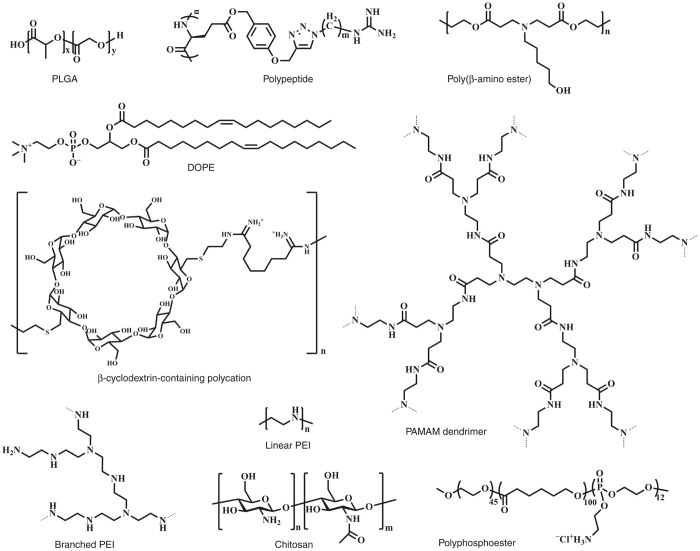
Biodegradation is the primary factor for the clinical application of nanoparticle-based gene delivery.21 Poly(lactic-co-glycolic acid) has been an FDA-approved biodegradable polymer since 1969. In recent years, poly(lactic-co-glycolic acid) has been explored as a gene vector due to its stable and able to protect DNA from degradation during circulation in vivo.22–24 DNA/RNA can be encapsulated in poly(lactic-co-glycolic acid) nanoparticles by double-emulsion solvent evaporation method. For more cellular endocytosis and efficient endosomal escape profile, poly(lactic-co-glycolic acid) nanoparticles are modified with biocompatible chitosan or other cationic components so that the nanoparticles possessed positive charges, thus achieving higher gene loading and transfection efficiency.25 Polypeptide-based cationic polymer is another candidate gene vector for clinical application. Cheng and colleagues26,27 prepared a series of cationic helical polypeptides and found that these polymers could overcome the efficiency–toxicity poor correlation of normal nanocarriers. Nature polymers, such as cyclodextrin and chitosan, have been intensively investigated due to their preferable biocompatibility.14,28 Cyclodextrins are generated during the bacterial digestion of cellulose and can form water-soluble complexes with small siRNA molecules. CALLA-01, a targeted nanoparticle system based on cyclodextrins, has been developed for the first in-human phase-1 clinical trial.8,29 Chitosan is another typical naturally polycation with the advantages of biocompatibility and biodegradability, which will be served as a very promising carrier for gene delivery.30 Lipid-based nanoparticles are one of the most extensively explored for gene delivery owing to their optimal properties, including high biocompatibility and close resemblance to the lipidic membranes, which facilitate their penetration into the cells.31,32 Many other biocompatible nanocarriers also attracted great attentions as gene delivery systems, including poly(β-amino ester)s,33 low-molecular-weight polyethylenimine,34 polyphosphoesters,35,36 disulfide cross-linked polymers,37 and polyamidoamine.38 Compared with the conventional gene delivery systems, the biodegradable and biocompatible nanoparticle-based systems (Figure 2) show improved formulation stability and safety, which are practically beneficial for the clinical gene therapy in future.
Figure 2.
The varieties of nanoparticles for the potential clinical gene therapy.
The aggregation and nonspecific adsorption by nondesired tissues are two serious problems which prohibit the clinical application of nanoparticle-based gene delivery, and those are mainly caused by the massive positive charges on the surface of cationic nanocarriers. PEGylation of these carriers is an essential strategy for reducing nonspecific interactions with serum proteins in the bloodstream and avoiding recognition by immune system components.39–41 Even for the neutral nanoparticles, they will quickly form large aggregates and are adsorbed by serum albumin in physiological salt concentrations without PEG, thus leading to rapid clearance by phagocytic cells and the reticuloendothelial systems.42,43 Therefore, PEGylated nanoparticles can extend blood circulation time and facilitate accumulation in targeted tissues. Shielding of the positive charges of cationic nanoparticles with polyanions is another strategy for stabilizing the nanoparticles, minimizing nonspecific interactions, and prolonging circulation time in vivo.44 There were numerous reports about the hydrophilic polymers modifying nanoparticles, which exhibited more steric stability with reduction in aggregation and breakdown during circulation.45,46
Clinical gene therapy using nanoparticles is also hampered by the lack of targeting ability when delivered into the desired diseased tissues.47,48 To overcome this drawback, the development of specific ligand receptor-mediated active targeting strategy for gene delivery system is critically required. When the ligands, such as antibody, protein, peptide and aptamer, interact to the receptors of targeted cells, the cellular uptake of nanoparticles will be further enhanced.41,49,50 To deliver nanoparticles effectively, the specific response of ligand-receptor pairs should be particularly strong and the target receptors should be overexpressed on target cells rather than normal cells.14,44 Passive targeting is exploited specially for the defective vascular architecture and the inefficient lymphatic drainage of tumors, which leads to the extravasation of nanoparticles into tumor tissues and enhances their retention in the interstitial space. Combined with the strategy mentioned above, PEGylation of nanoparticles is one of the most famous methods to improve the passive targeting by increasing the circulation time and avoiding possible serum aggregation in vivo.51 Stimulus-responsive nanoparticles can produce physical or chemical changes after exposing to diseased tissues or external signals including pH, temperature, light or magnetic field.52–54 Responsiveness towards internal or external stimuli makes it possible to tailor the time and site of gene therapy precisely, and greatly increase the possibility of clinical application for these nanoparticles.41
Effective cellular internalization of therapeutic genes is a critical process for the successful clinical application of nanoparticles for gene delivery. Although, several nanocarriers have been widely used to deliver therapeutic genes to the cells, it is still also urgent to improve the endocytosis of DNA/RNA to meet the ultimate goal of clinical application.55,56 Cell-penetrating peptides, with membrane translocation sequences or protein transduction domains, have been introduced on the surface of nanocarriers for gene therapy, which evolves quickly cellular uptake of the gene delivery system via direct translocation in addition to the endocytic way.57,58 In addition, to increase the endosomal escape activity of nanoparticles, endosomolytic agents with the ability to destabilize the endosomal membrane have been introduced.59 In order to further optimize gene delivery, novel nanocarriers were developed by combining both endosomolytic agents and cell-penetrating peptides.60 Harashima and colleagues61 reported a stearylated derivative INF-modified R8-MEND dual functional gene delivery system and achieved delectable results both in vitro and in vivo.
In addition, the production of nanoparticles for clinical gene therapy should be designed according to the practical needs in clinic (Table 1). Firstly, the functions of nanocarriers should be tailored by the types and mechanisms of nucleic acid determinants, such as DNA, mRNA, siRNA, and miRNA.12,62,63 Secondly, the construction of the nanocarriers should also be changed by the variety of genetic diseases including cancers, optic atrophy, hypercholesterolemia, and dry eye, etc.13 Finally, plentiful of administration routes including local and systemic approaches also provide the opportunities and challenges to the application of nanoparticle-based gene therapy.64,65
Table 1. Multifunctional nanoparticles for preclinical gene delivery studies.
| Functionalization | Delivery systems | Advantages |
|---|---|---|
| PEGylation | PEG-βCD; PEG-PEI | Enhanced of stabilization; prevention of protein absorption; improved circulation time39,40 |
| Targeting | RGD-HA-PEI-PBLG; R-PEG20C; transferrin-lipid | Enhanced gene target efficacy in vivo44,48,51 |
| Stimulus response | pH sensitive; light sensitive; redox sensitive | Enhanced gene target efficacy in vivo52–54 |
| Cell penetrating | p(DAHa-E/APIb) | Cross cell membrane; enhanced cellular uptake58 |
| Endosome escaping | (Arg)7-FI-PNA | Cross cell membrane; improved endosomal escaping59 |
| Nuclear localization | PC/pDNA/NLS; VKRKKKP-R8 | Nuclear localization62,63 |
βCD, β-cyclodextrin; NLS, nuclear localization sequence; PC, β-cyclodextrin and polyethylenimine; PEI, polyethylenimine; R-PEG20C, cRGD-PEG-PAsp(DET)-cholesteryl.
Clinical development of nanoparticles for gene delivery
Many clinical trials of gene therapy for preventing or treating genetic diseases are rapidly ongoing worldwide although none of the gene therapeutics based on nanoparticles have so far been approved by FDA.12
Anderson et al.66 conducted the first in-human clinical trial, which involved systemic administration of adenosine deaminase gene to a 4-year-old girl with severe combined immunodeficiency disease. An initial success of the trail was achieved, and then a research boom of gene therapy was set off worldwide. In 2000, Fischer and colleagues67 initiated a gene therapy trail for severe combined immunodeficiency-X1, an X-linked inherited disorder characterized by an early block in T and natural killer lymphocyte differentiation, based on the use of complementary DNA containing a retrovirus-derived vector and ex vivo infection of CD34+ cells. After a 10-month follow-up period, T, B, and NK cell counts and function of the two patients were comparable to those of age-matched controls. However, the two youngest boys revealed the symptoms of leukemia 3-years after gene therapy, which was mainly due to the retrovirus vector integration in proximity to the proto-oncogene and triggered deregulated premalignant cell proliferation with unexpected frequency.68 Thus, the broad application of gene therapy was restricted severely by the safety concerns aroused by viral vectors.69
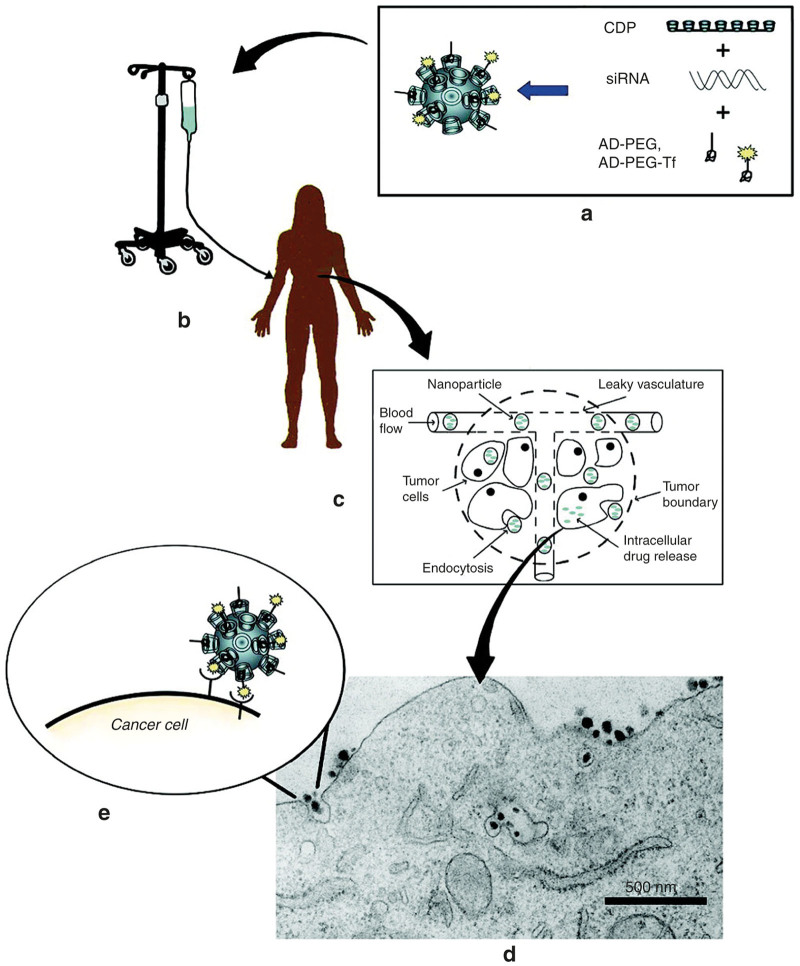
Recently, nanoparticles are being investigated to utilize as gene delivery systems to overcome the delivery barriers due to their properties associated with safety, nonimmunogenicity, controllability, and low cost.70 Davis and colleagues8 reported the first nanoparticle-based gene delivery system named CALAA-01 in a phase-1 clinical trials against cancers. CALAA-01 consists of siRNA targeting the M2 subunit of ribonucleotide reductase (RRM2), cyclodextrin containing polymer, PEG steric stabilization agent, and transferrin targeting ligand for binding to transferrin receptors upregulated on cancer cells.71 The results showed that this “drug” could deliver siRNA to melanoma cells by systemic administration and demonstrate potent antiproliferative activity across multiple types of cancer cells.29 (Figure 3)
Figure 3.
Schematic of mechanism proposal for CALAA-01. (a) Nanoparticles are assembled from a linear cyclodextrin-containing polymer (CDP), an adamantane-PEG conjugate (AD-PEG), a targeting component (transferrin, Tf) and the therapeutic gene (siRNA). (b) Nanoparticles are infused into patients. (c) The circulation of nanoparticles and their escape into tumors. (d) Receptor-mediated endocytosis. (e) Interactions between targeted nanoparticles and receptors on the surface of the cancer cell.29 (Copyright 2009 American Chemical Society).
Since then, many nanoparticle-based gene delivery systems have been developed for clinical trials.13 PEI has been carried out for local clinical gene therapy of various cancers (Table 2). However, the substantial cytotoxicity severely hampered its further application and a range of modifications to PEI had been investigated. Polyethylene glycol-polyethylenimine-cholesterol (PEG-PEI-cholesterol) was successfully developed as a gene delivery carrier for immunotherapy of epithelial ovarian by enhanced expression of cytokine interleukin-12.72 Lipid formation of nucleic acid shows the most clinical trials among nanoparticles for gene delivery. A phase-1 clinical trial for ALN-VSP, a lipid nanoparticle formulation encapsulating the siRNAs that can specially target the mRNA of vascular endothelial growth factor (VEGF siRNAs), was initiated by Alnylam Pharmaceuticals and showed that systemically therapy could induce the regression of liver metastases and improve the potentially sensitize of cancer cells to chemotherapy.73 ALN-TTR02 was an intravenously delivered lipid-based siRNA formulation in patients with TTR amyloidosis. The safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple doses of ALN-TTR02 were evaluated. The data showed that specific knockdown of up to 94% was achieved in serum TTR protein levels and the knockdown effect could sustain for 1 month. Furthermore, no infusion reactions were observed at the high dose of 0.3 mg/kg in the phase-2 study.74,75 Allovectin-7, which was consisted of DMRIE-DOPE and a plasmid DNA, had successfully passed phase-2 clinical trials, but failed to meet its endpoints in increasing percent DDR or overall survival in a phase-3 clinical trial for treatment of advanced metastatic melanoma and finally the development of this treatment had been abandoned.12,76 Nonetheless, various lipid-based formulations continued to be developed in clinic. (Table 2)
Table 2. Nanoparticle-based gene therapy under clinical evaluation.
| Delivery system | Product | Sponsor | Disease | Administration | Phase | Status | Gov identifier |
|---|---|---|---|---|---|---|---|
| PEI-based nanoparticles | BC-819/PEI | BioCancell | BC | Local | 2 | Active | NCT00595088 |
| BC-819 | BioCancell | OC | IP | 1/2 | Completed | NCT00826150 | |
| DTA-H19 | BioCancell | PN | Local | 1/2 | Completed | NCT00711997 | |
| EGEN-001 | Gynecologic Oncology Group | Cancer | IP | 2 | Active | NCT01118052 | |
| Lipid-based nanoparticles | TKM-080301 | National Cancer Institute | HM | IA | 1 | Completed | NCT01437007 |
| TKM-080301 | Tekmira Pharmaceuticals Corporation | HC | IV | 1/2 | Recruiting | NCT02191878 | |
| TKM-080301 | Tekmira Pharmaceuticals Corporation | NET; ACC | IV | 1/2 | Completed | NCT01262235 | |
| Atu027 | Silence Therapeutics GmbH | ASC | IV | 1 | Completed | NCT00938574 | |
| ALN-TTR02 | Alnylam Pharmaceuticals | TTR-A | IV | 2 | Completed | NCT01617967 | |
| DOTAP-Chol-fus1 | MD Anderson Cancer Center | LC | IV | 1 | Completed | NCT00059605 | |
| DCR-MYC | Dicerna Pharmaceuticals | ST; MM; NHL | IV | 1 | Recruiting | NCT02110563 | |
| DCR-MYC | Dicerna Pharmaceuticals | HC | IV | 1/2 | Recruiting | NCT02314052 | |
| ND-L02-s0201 Injection | Nitto Denko Corporation | EHF | IV | 1 | Recruiting | NCT02227459 | |
| PLGA-based nanoparticles | siG12D LODER | Silenseed | PC | Local | 2 | Active | NCT01676259 |
ACC, adrenocortical carcinoma; ASC, advanced solid cancer; BC, bladder cancer; EHF, extensive hepatic fibrosis; HC, hepatocellular carcinoma; HM, hepatic metastases; IA, intra-arterial; LC, lung cancer; MM, multiple myeloma; NET, neuroendocrine tumors; NHL, non-Hodgkins lymphoma; OC, ovarian cancer; PC,pancreatic cancer; PEI, polyethylenimine; PLGA, poly(lactic-co-glycolic acid); PN, pancreatic neoplasms; ST, solid tumors; TTR-A, transthyretin amyloidosis.
Recently, great attentions have been paid to the clinical application of combination approaches of gene with drug, radiotherapy, photodynamic therapy, or immunotherapy.13,48 A phase-1 study of the safety and biological activity of intraperitoneal GEN-1 (IL-12 plasmid formulated with PEG-PEI-cholesterol lipopolymer) administered in combination with standard neoadjuvant chemotherapy was carried out to diagnose with epithelial ovarian, fallopian tube, and primary peritoneal cancer (Table 3). A phase-2 study of the combination of intravenously administered SGT-53 (P53 gene therapy sponsored by SynerGene Therapeutics) and oral temozolomide was ongoing for the treatment of recurrent glioblastoma. SGT-53 nanodelivery system consisted of a cationic liposome, an antitransferrin receptor single chain antibody fragment and the wtp53 plasmid DNA.48 This trial will evaluate the nanoparticle delivery to tumor site, the induction of apoptosis in the tumor, antitumor activity, and safety (Table 3).
Table 3. Combination of gene therapy and chemotherapy under clinical evaluation.
| Delivery system | Product | Sponsor | Disease | Administration | Phase | Status | Gov identifier |
|---|---|---|---|---|---|---|---|
| PEG-PEI-cholesterol | EGEN-001 + carboplatin + docetaxel | EGEN | ON | IP | 1 | Completed | NCT00473954 |
| PEG-PEI-cholesterol | EGEN-001 + PLD | Gynecologic Oncology Group | R/POEC; FTC; PPC | IP | 1 | Recruiting | NCT01489371 |
| PEG-PEI-cholesterol | GEN-1 + SNC | Celsion | EOC; FTC; PPC | IP | 1 | Recruiting | NCT02480374 |
| Lipid | SGT-53 + nab-paclitaxel/gemcitabine | SynerGene Therapeutics | MPC | IV | 2 | Recruiting | NCT02340117 |
EOC, epithelial ovarian cancer; FTC, fallopian tube cancer; MPC, metastatic pancreatic cancer; ON, ovarian neoplasms; PEI, polyethylenimine; PLD, pegylated liposomal doxorubicin; PPC, primary peritoneal cancer; R/POEC, recurrent or persistent ovarian epithelial cancer; SNC, standard neoadjuvant chemotherapy.
Conclusion and outlook
In the past two decades, substantial nanoparticle-based gene therapies have been constructed in the treatment of various diseases due to the rapid development of nanotechnology and genomics.12 The clinical application of gene therapy is hindered by the challenges associated with its delivery system, including rapid degradation and clearance in circulation, insufficient half-life, nonspecific uptake, reduced uptake by target cells, inability to escape endosomes and toxic effect arose by immune response stimulation.12,77 Although several viral-based vectors have been used for clinical gene therapy due to their high transduction efficiency,78,79 they still face serious challenges, including adverse effects and high costs.80 Therefore, it is necessary to develop safe and effective nonviral gene delivery systems. Among them, nanoparticle-based delivery systems have shown their potential application in clinical gene therapy.13 Furthermore, many strategies have been introduced to improve the intelligences of nanoparticles for gene delivery systems, such as biodegraded, PEGylated, targeted, and modified with cell-penetrating peptides or endosomolytic agents.3,81,82
Since the first cyclodextrin-based phase-1 clinical trial for gene therapy was achieved in 2010, a number of nanoparticle-based gene delivery systems have been developed for clinical trials.13 Most of them consisted of cationic polymer for binding nucleic acids, PEG steric stabilization agent, and targeting ligand for binding to the receptors on target cells. However, some of the clinical trials finally failed to meet their endpoints and none of gene therapeutics based on nanoparticles has so far been approved by FDA. To our knowledge, the primary obstacle comes from the complexity of the disease and the precise interpretation of its pathogenesis by molecular biological approach will be a prerequisite for effective clinical gene therapy. Moreover, successful clinical gene therapy is also seriously restricted by the safety and effectiveness of gene delivery systems. Despite these difficult conditions, gene therapy still owned its great potentiality for preventing or treating genetic diseases.
Further clinical progress of nanoparticle-based gene therapy will be facilitated by additional biological insights and nanotechnology into the key rate-determining steps that limit the effective therapy. Moreover, structure–function relationships, anatomical barriers, nucleic acids stability and availability, immunoreactivity, delivery routes are all major clinical challenges. It is expected that gene therapy based on local administrations can reach its goals and be approved by FDA more easily due to foreseeable features. Immunotherapy based on gene delivery will be a very promising approach for the treatment of genetically related diseases by subcutaneous injection. However, the systemic application of DNA/RNA will be seriously hindered by the complicated in vivo microenvironment. Particularly, it is impossible to completely avoid the possible cross-reactivity between nucleic acid drug and all body cells, which will bring about unexpected side effects by systemic therapies. Based on it, a secure targeted delivery strategy will be especially necessary. Finally, combination approaches of nanoparticle-based gene therapy with drug, radiotherapy, photodynamic therapy, immunotherapy or others will be the one of the most emphasis in future clinical studies.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant numbers: 51520105004, 51303173, 51390484, 21474104, 51403205, 51203132, and 51222307), Jilin Province Science and Technology Development Program (20130521011JH), and the Project of National High Tech R&D Program (2014AA020708).
The authors declare no conflict of interest.
References
- Choi, YS, Lee, MY, David, AE and Park, YS (2014). Nanoparticles for gene delivery: therapeutic and toxic effects. Mol Cell Toxicol 10: 1–8. [Google Scholar]
- Chen, J, Jiao, ZX, Lin, L, Guo, ZP, Xu, CN, Li, YH et al. (2015). Polylysine-modified polyethylenimines as siRNA carriers for effective tumor treatment. Chin J Polym Sci 33: 830–837. [Google Scholar]
- Dizaj, SM, Jafari, S and Khosroushahi, AY (2014). A sight on the current nanoparticle-based gene delivery vectors. Nanoscale Res Lett 9: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y, Kim, YJ, Ji, M, Fang, J, Siriwon, N, Zhang, LI et al. (2014). Enhancing gene delivery of adeno-associated viruses by cell-permeable peptides. Mol Ther Methods Clin Dev 1: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapti, K, Chaanine, AH and Hajjar, RJ (2011). Targeted gene therapy for the treatment of heart failure. Can J Cardiol 27: 265–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando, M, Takahashi, Y, Yamashita, T, Fujimoto, M, Nishikawa, M, Watanabe, Y et al. (2014). Prevention of adverse events of interferon γ gene therapy by gene delivery of interferon γ-heparin-binding domain fusion protein in mice. Mol Ther Methods Clin Dev 1: 14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H, Li, Z and Si, J (2014). Nanocarriers in gene therapy: a review. J Biomed Nanotechnol 10: 3483–3507. [DOI] [PubMed] [Google Scholar]
- Davis, ME, Zuckerman, JE, Choi, CH, Seligson, D, Tolcher, A, Alabi, CA et al. (2010). Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464: 1067–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lächelt, U and Wagner, E (2015). Nucleic acid therapeutics using polyplexes: a journey of 50 years (and beyond). Chem Rev 115: 11043–11078. [DOI] [PubMed] [Google Scholar]
- Chen, J, Dong, X, Feng, T, Lin, L, Guo, Z, Xia, J et al. (2015). Charge-conversional zwitterionic copolymer as pH-sensitive shielding system for effective tumor treatment. Acta Biomater 26: 45–53. [DOI] [PubMed] [Google Scholar]
- Lee, H, Lytton-Jean, AK, Chen, Y, Love, KT, Park, AI, Karagiannis, ED et al. (2012). Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol 7: 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, H, Kanasty, RL, Eltoukhy, AA, Vegas, AJ, Dorkin, JR and Anderson, DG (2014). Non-viral vectors for gene-based therapy. Nat Rev Genet 15: 541–555. [DOI] [PubMed] [Google Scholar]
- Miele, E, Spinelli, GP, Miele, E, Di Fabrizio, E, Ferretti, E, Tomao, S et al. (2012). Nanoparticle-based delivery of small interfering RNA: challenges for cancer therapy. Int J Nanomed 7: 3637–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williford, JM, Wu, J, Ren, Y, Archang, MM, Leong, KW and Mao, HQ (2014). Recent advances in nanoparticle-mediated siRNA delivery. Annu Rev Biomed Eng 16: 347–370. [DOI] [PubMed] [Google Scholar]
- Zhang, B, Ma, XP, Sui, MH, Van Kirk, E, Murdoch, WJ, Radosz, M et al. (2015). Guanidinoamidized linear polyethyleneimine for gene delivery. Chin J Polym Sci 33: 908–919. [Google Scholar]
- Yue, YN and Wu, C (2013). Progress and perspectives in developing polymeric vectors for in vitro gene delivery. Biomater Sci 1: 152–170. [DOI] [PubMed] [Google Scholar]
- Pensado, A, Seijo, B and Sanchez, A (2014). Current strategies for DNA therapy based on lipid nanocarriers. Expert Opin Drug Deliv 11: 1721–1731. [DOI] [PubMed] [Google Scholar]
- Gao, S, Tian, H, Guo, Y, Li, Y, Guo, Z, Zhu, X et al. (2015). miRNA oligonucleotide and sponge for miRNA-21 inhibition mediated by PEI-PLL in breast cancer therapy. Acta Biomater 25: 184–193. [DOI] [PubMed] [Google Scholar]
- Tian, H, Guo, Z, Chen, J, Lin, L, Xia, J, Dong, X et al. (2012). PEI conjugated gold nanoparticles: efficient gene carriers with visible fluorescence. Adv Healthc Mater 1: 337–341. [DOI] [PubMed] [Google Scholar]
- Ibraheem, D, Elaissari, A and Fessi, H (2014). Gene therapy and DNA delivery systems. Int J Pharm 459: 70–83. [DOI] [PubMed] [Google Scholar]
- Lee, YS and Kim, SW (2014). Bioreducible polymers for therapeutic gene delivery. J Control Release 190: 424–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, X, Naguib, S and Wu, Z (2011). Recent advances of siRNA delivery by nanoparticles. Expert Opin Drug Deliv 8: 521–536. [DOI] [PubMed] [Google Scholar]
- Arora, S, Swaminathan, SK, Kirtane, A, Srivastava, SK, Bhardwaj, A, Singh, S et al. (2014). Synthesis, characterization, and evaluation of poly ( d,l -lactide-co-glycolide)-based nanoformulation of miRNA-150: potential implications for pancreatic cancer therapy. Int J Nanomed 9: 2933–2942. [Google Scholar]
- Zhou, J, Patel, TR, Fu, M, Bertram, JP and Saltzman, WM (2012). Octa-functional PLGA nanoparticles for targeted and efficient siRNA delivery to tumors. Biomaterials 33: 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, X, Shah, BA, Kotadia, NK, Li, J, Gu, H and Wu, Z (2010). The development and mechanism studies of cationic chitosan-modified biodegradable PLGA nanoparticles for efficient siRNA drug delivery. Pharm Res 27: 1285–1295. [DOI] [PubMed] [Google Scholar]
- Zheng, N, Yin, L, Song, Z, Ma, L, Tang, H, Gabrielson, NP et al. (2014). Maximizing gene delivery efficiencies of cationic helical polypeptides via balanced membrane penetration and cellular targeting. Biomaterials 35: 1302–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R, Zheng, N, Song, Z, Yin, L and Cheng, J (2014). The effect of side-chain functionality and hydrophobicity on the gene delivery capabilities of cationic helical polypeptides. Biomaterials 35: 3443–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y, Li, Z, Han, Y, Liang, LH and Ji, A (2010). Nanoparticle-based delivery system for application of siRNA in vivo. Curr Drug Metab 11: 182–196. [DOI] [PubMed] [Google Scholar]
- Davis, ME (2009). The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm 6: 659–668. [DOI] [PubMed] [Google Scholar]
- Lee, SJ, Lee, A, Hwang, SR, Park, JS, Jang, J, Huh, MS et al. (2014). TNF-α gene silencing using polymerized siRNA/thiolated glycol chitosan nanoparticles for rheumatoid arthritis. Mol Ther 22: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadinoto, K, Sundaresan, A and Cheow, WS (2013). Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm 85: 427–443. [DOI] [PubMed] [Google Scholar]
- Chitkara, D, Mittal, A and Mahato, RI (2015). miRNAs in pancreatic cancer: therapeutic potential, delivery challenges and strategies. Adv Drug Deliv Rev 81: 34–52. [DOI] [PubMed] [Google Scholar]
- Deng, X, Zheng, N, Song, Z, Yin, L and Cheng, J (2014). Trigger-responsive, fast-degradable poly(β-amino ester)s for enhanced DNA unpackaging and reduced toxicity. Biomaterials 35: 5006–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, X, Tian, H, Chen, L, Chen, J and Chen, X (2011). Biodegradable mPEG-b-P(MCC-g-OEI) copolymers for efficient gene delivery. J Control Release 152: 135–142. [DOI] [PubMed] [Google Scholar]
- Xu, WF, Wang, YD, Li, SY, Ke, ZY, Yan, YC, Li, S et al. (2015). Efficient gene and siRNA delivery with cationic polyphosphoramide with amino moieties in the main chain. RSC Adv 5: 50425–50432. [Google Scholar]
- Sun, TM, Du, JZ, Yan, LF, Mao, HQ and Wang, J (2008). Self-assembled biodegradable micellar nanoparticles of amphiphilic and cationic block copolymer for siRNA delivery. Biomaterials 29: 4348–4355. [DOI] [PubMed] [Google Scholar]
- Tai, Z, Wang, X, Tian, J, Gao, Y, Zhang, L, Yao, C et al. (2015). Biodegradable stearylated peptide with internal disulfide bonds for efficient delivery of siRNA in vitro and in vivo. Biomacromolecules 16: 1119–1130. [DOI] [PubMed] [Google Scholar]
- Xu, X, Jian, Y, Li, Y, Zhang, X, Tu, Z and Gu, Z (2014). Bio-inspired supramolecular hybrid dendrimers self-assembled from low-generation peptide dendrons for highly efficient gene delivery and biological tracking. ACS Nano 8: 9255–9264. [DOI] [PubMed] [Google Scholar]
- Mishra, S, Webster, P and Davis, ME (2004). PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol 83: 97–111. [DOI] [PubMed] [Google Scholar]
- Hong, JW, Park, JH, Huh, KM, Chung, H, Kwon, IC and Jeong, SY (2004). PEGylated polyethylenimine for in vivo local gene delivery based on lipiodolized emulsion system. J Control Release 99: 167–176. [DOI] [PubMed] [Google Scholar]
- Tian, H, Chen, J and Chen, X (2013). Nanoparticles for gene delivery. Small 9: 2034–2044. [DOI] [PubMed] [Google Scholar]
- Pack, DW, Hoffman, AS, Pun, S and Stayton, PS (2005). Design and development of polymers for gene delivery. Nat Rev Drug Discov 4: 581–593. [DOI] [PubMed] [Google Scholar]
- Ikeda, Y and Nagasaki, Y (2014). Impacts of PEGylation on the gene and oligonucleotide delivery system. J Appl Polym Sci 131: 40293 [Google Scholar]
- Tian, H, Lin, L, Chen, J, Chen, X, Park, TG and Maruyama, A (2011). RGD targeting hyaluronic acid coating system for PEI-PBLG polycation gene carriers. J Control Release 155: 47–53. [DOI] [PubMed] [Google Scholar]
- Oupicky, D, Ogris, M, Howard, KA, Dash, PR, Ulbrich, K and Seymour, LW (2002). Importance of lateral and steric stabilization of polyelectrolyte gene delivery vectors for extended systemic circulation. Mol Ther 5: 463–472. [DOI] [PubMed] [Google Scholar]
- Ballarín-González, B and Howard, KA (2012). Polycation-based nanoparticle delivery of RNAi therapeutics: adverse effects and solutions. Adv Drug Deliv Rev 64: 1717–1729. [DOI] [PubMed] [Google Scholar]
- Ku, SH, Kim, K, Choi, K, Kim, SH and Kwon, IC (2014). Tumor-targeting multifunctional nanoparticles for siRNA delivery: recent advances in cancer therapy. Adv Healthc Mater 3: 1182–1193. [DOI] [PubMed] [Google Scholar]
- Kim, SS, Rait, A, Kim, E, Pirollo, KF and Chang, EH (2015). A tumor-targeting p53 nanodelivery system limits chemoresistance to temozolomide prolonging survival in a mouse model of glioblastoma multiforme. Nanomedicine 11: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, CF, Liu, Y, Shen, S, Zhu, YH and Wang, J (2015). Targeting glucose uptake with siRNA-based nanomedicine for cancer therapy. Biomaterials 51: 1–11. [DOI] [PubMed] [Google Scholar]
- Akita, H, Ishiba, R, Togashi, R, Tange, K, Nakai, Y, Hatakeyama, H et al. (2015). A neutral lipid envelope-type nanoparticle composed of a pH-activated and vitamin E-scaffold lipid-like material as a platform for a gene carrier targeting renal cell carcinoma. J Control Release 200: 97–105. [DOI] [PubMed] [Google Scholar]
- Ge, Z, Chen, Q, Osada, K, Liu, X, Tockary, TA, Uchida, S et al. (2014). Targeted gene delivery by polyplex micelles with crowded PEG palisade and cRGD moiety for systemic treatment of pancreatic tumors. Biomaterials 35: 3416–3426. [DOI] [PubMed] [Google Scholar]
- Tian, H, Guo, Z, Lin, L, Jiao, Z, Chen, J, Gao, S et al. (2014). pH-responsive zwitterionic copolypeptides as charge conversional shielding system for gene carriers. J Control Release 174: 117–125. [DOI] [PubMed] [Google Scholar]
- Nishiyama, N, Iriyama, A, Jang, WD, Miyata, K, Itaka, K, Inoue, Y et al. (2005). Light-induced gene transfer from packaged DNA enveloped in a dendrimeric photosensitizer. Nat Mater 4: 934–941. [DOI] [PubMed] [Google Scholar]
- Cheng, R, Feng, F, Meng, F, Deng, C, Feijen, J and Zhong, Z (2011). Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release 152: 2–12. [DOI] [PubMed] [Google Scholar]
- de Figueiredo, IR, Freire, JM, Flores, L, Veiga, AS and Castanho, MARB (2014). Cell-penetrating peptides: a tool for effective delivery in gene-targeted therapies. Iubmb Life 66: 182–194. [DOI] [PubMed] [Google Scholar]
- Li, Y, Tian, H, Ding, J, Lin, L, Chen, J, Gao, S et al. (2015). Guanidinated thiourea-decorated polyethylenimines for enhanced membrane penetration and efficient siRNA delivery. Adv Healthc Mater 4: 1369–1375. [DOI] [PubMed] [Google Scholar]
- Farkhani, SM, Valizadeh, A, Karami, H, Mohammadi, S, Sohrabi, N and Badrzadeh, F (2014). Cell penetrating peptides: efficient vectors for delivery of nanoparticles, nanocarriers, therapeutic and diagnostic molecules. Peptides 57: 78–94. [DOI] [PubMed] [Google Scholar]
- Kim, TI, Rothmund, T, Kissel, T and Kim, SW (2011). Bioreducible polymers with cell penetrating and endosome buffering functionality for gene delivery systems. J Control Release 152: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi, T, Pankratova, S and Nielsen, PE (2005). Calcium ions effectively enhance the effect of antisense peptide nucleic acids conjugated to cationic tat and oligoarginine peptides. Chem Biol 12: 923–929. [DOI] [PubMed] [Google Scholar]
- Liou, JS, Liu, BR, Martin, AL, Huang, YW, Chiang, HJ and Lee, HJ (2012). Protein transduction in human cells is enhanced by cell-penetrating peptides fused with an endosomolytic HA2 sequence. Peptides 37: 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed, A, Masuda, T, Khalil, I, Akita, H and Harashima, H (2009). Enhanced gene expression by a novel stearylated INF7 peptide derivative through fusion independent endosomal escape. J Control Release 138: 160–167. [DOI] [PubMed] [Google Scholar]
- Hu, Q, Wang, J, Shen, J, Liu, M, Jin, X, Tang, G et al. (2012). Intracellular pathways and nuclear localization signal peptide-mediated gene transfection by cationic polymeric nanovectors. Biomaterials 33: 1135–1145. [DOI] [PubMed] [Google Scholar]
- Wang, HY, Chen, JX, Sun, YX, Deng, JZ, Li, C, Zhang, XZ et al. (2011). Construction of cell penetrating peptide vectors with N-terminal stearylated nuclear localization signal for targeted delivery of DNA into the cell nuclei. J Control Release 155: 26–33. [DOI] [PubMed] [Google Scholar]
- Borna, H, Imani, S, Iman, M and Azimzadeh Jamalkandi, S (2015). Therapeutic face of RNAi: in vivo challenges. Expert Opin Biol Ther 15: 269–285. [DOI] [PubMed] [Google Scholar]
- Xu, C, Wang, P, Zhang, J, Tian, H, Park, K and Chen, X (2015). Pulmonary codelivery of doxorubicin and siRNA by pH-sensitive nanoparticles for therapy of metastatic lung cancer. Small 11: 4321–4333. [DOI] [PubMed] [Google Scholar]
- Anderson, WF, Blaese, RM and Culver, K (1990). The ADA human gene therapy clinical protocol: points to consider response with clinical protocol, July 6, 1990. Hum Gene Ther 1: 331–362. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo, M, Hacein-Bey, S, de Saint Basile, G, Gross, F, Yvon, E, Nusbaum, P et al. (2000). Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288: 669–672. [DOI] [PubMed] [Google Scholar]
- Hacien-Bey-Abina, S (2003). LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302: 415–419. [DOI] [PubMed] [Google Scholar]
- Lehrman, S (1999). Virus treatment questioned after gene therapy death. Nature 401: 517–518. [DOI] [PubMed] [Google Scholar]
- Tian, H, Xiong, W, Wei, J, Wang, Y, Chen, X, Jing, X et al. (2007). Gene transfection of hyperbranched PEI grafted by hydrophobic amino acid segment PBLG. Biomaterials 28: 2899–2907. [DOI] [PubMed] [Google Scholar]
- Jain, KK (2014). Applications of Biotechnology in Oncology. Springer: New York. [Google Scholar]
- Alvarez, RD, Sill, MW, Davidson, SA, Muller, CY, Bender, DP, DeBernardo, RL et al. (2014). A phase II trial of intraperitoneal EGEN-001, an IL-12 plasmid formulated with PEG-PEI-cholesterol lipopolymer in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer: a gynecologic oncology group study. Gynecol Oncol 133: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabernero, J, Shapiro, GI, LoRusso, PM, Cervantes, A, Schwartz, GK, Weiss, GJ et al. (2013). First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov 3: 406–417. [DOI] [PubMed] [Google Scholar]
- Haussecker, D (2014). Current issues of RNAi therapeutics delivery and development. J Control Release 195: 49–54. [DOI] [PubMed] [Google Scholar]
- Wan, C, Allen, TM and Cullis, PR (2014). Lipid nanoparticle delivery systems for siRNA-based therapeutics. Drug Deliv Transl Res 4: 74–83. [DOI] [PubMed] [Google Scholar]
- Hersey, P and Gallagher, S (2014). Intralesional immunotherapy for melanoma. J Surg Oncol 109: 320–326. [DOI] [PubMed] [Google Scholar]
- Chen, Y, Gao, DY and Huang, L (2015). In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev 81: 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotterman, MA and Schaffer, DV (2014). Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet 15: 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau, A, Vandamme, C, Segovia, M, Devaux, M, Guilbaud, M, Tilly, G et al. (2014). Generation and in vivo evaluation of IL10-treated dendritic cells in a nonhuman primate model of AAV-based gene transfer. Mol Ther Methods Clin Dev 1: 14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer, AK, Duan, Z and Amiji, MM (2014). Nanodelivery systems for nucleic acid therapeutics in drug resistant tumors. Mol Pharm 11: 2511–2526. [DOI] [PubMed] [Google Scholar]
- Yang, XC, Niu, YL, Zhao, NN, Mao, C and Xu, FJ (2014). A biocleavable pullulan-based vector via ATRP for liver cell-targeting gene delivery. Biomaterials 35: 3873–3884. [DOI] [PubMed] [Google Scholar]
- Yang, YY, Hu, H, Wang, X, Yang, F, Shen, H, Xu, FJ et al. (2015). Acid-labile poly(glycidyl methacrylate)-based star gene vectors. ACS Appl Mater Interfaces 7: 12238–12248. [DOI] [PubMed] [Google Scholar]