Abstract
Throughout the history of dialysis, four bioethical principles — beneficence, nonmaleficence, autonomy and justice — have been weighted differently based upon changing forces of technologic innovation, resource limitation, and societal values. In the 1960s, a committee of lay people in Seattle attempted to fairly distribute a limited number of maintenance hemodialysis stations guided by considerations of justice. As technology advanced and dialysis was funded under an amendment to the Social Security Act in 1972, focus shifted to providing dialysis for all in need while balancing the burdens of treatment and quality of life, supported by the concepts of beneficence and nonmaleficence. At the end of the last century, the importance of patient preferences and personal values became paramount in medical decisions, reflecting a focus on the principle of autonomy. More recently, greater recognition that health care financial resources are limited makes fair allocation more pressing, again highlighting the importance of distributive justice. The varying application and prioritization of these four principles to both policy and clinical decisions in the United States over the last 50 years makes the history of hemodialysis an instructive platform for understanding principlist bioethics. As medical technology evolves in a landscape of changing personal and societal values, a comprehensive understanding of an ethical framework for evaluating appropriate use of medical interventions enables the clinician to systematically negotiate and optimize difficult ethical situations.
Keywords: dialysis, ESRD, Beneficence, Bioethics, Humans, Patient Preference, quality of life, Social Justice, United States
Introduction
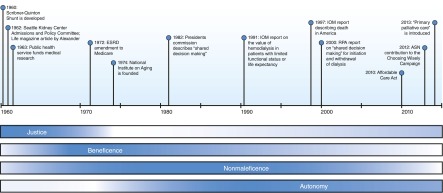
Though hemodialysis was conceived in the 1940s, maintenance dialysis only became feasible in 1960 when the Quinton–Scribner shunt allowed repeated vascular access (1). However, the technology itself does not determine which patients are most appropriately treated, and the indications for dialysis initiation would be vigorously debated for decades to come. The early days of dialysis will long be tied to the controversial life and death decisions of the Admissions and Policy Committee of the Seattle Artificial Kidney Center (2). Traditionally the protected realm of autonomous individual physicians, allocation of medical resources was for the first time in the hands of people without medical training, including philosophers, lawyers, and citizens, a transition that historian David Rothman and bioethics scholar Albert Jonsen said signaled the entrance of bioethics into medicine (3,4). The sociologist Judith Swazey noted, “Patient selection was certainly the most visible and therefore most discussed issue posed by the limited availability of chronic dialysis in the early 1960s, but it was by no means the only troubling matter that this procedure raised for medical technologies. The Seattle group was struggling … with a number of issues that at once were medical, moral, social, and would become major foci of those who wandered in and became known as bioethicists.” (5) As Swazey alludes, appropriate use of dialysis was a harbinger for many bioethical challenges now regularly encountered during the development of new technologies such as transplantation, extracorporeal membrane oxygenation, and artificial organs. Dilemmas related to ESRD feature prominently in Beauchamp and Childress’ 1979 text describing principlism, one of the first and still a dominant model of biomedical ethical analysis (6). Several alternative frameworks of ethical reasoning have been developed since, including casuistry, narrative-based ethics and virtue-based ethics (7). As a seminal model of ethical analysis, principlism lays the foundation for other strategies. We will examine the history of dialysis through the lens of principlism, elucidating the four principles — beneficence, nonmaleficence, autonomy and justice — as each come into focus over this chronology (Figure 1).
Figure 1.
Timeline of important events and publications related to the development of dialysis. Though there is significant overlap, the relative importance of each of the four principles varies over this course. ASN, American Society of Nephrology; IOM, Institute of Medicine; RPA, Renal Physicians Association.
Justice and Scarce Resources: The “Life or Death Committee”
The Seattle Admissions and Policy Committee
When the Seattle Artificial Kidney Center (later renamed Northwest Kidney Centers) opened in 1961, there were an estimated 5–20 candidates for maintenance dialysis per million people annually (8). With an estimated cost of at least $12,000 per patient each year and only three available machines, treating even that number would overwhelm the center’s capacity (8). The physician Medical Advisory Committee reduced the number of potential patients using medical suitability criteria including comorbidities (excluding those with long-standing hypertension, coronary artery disease, and vascular disease), ability to participate in care coordination, and age, as well as several nonmedical criteria (requiring residence in the Pacific Northwest and personal financial viability) (5). To choose among remaining medically appropriate candidates, the Admissions and Policy Committee was appointed, composed of seven citizens: a lawyer, clergyman, housewife, banker, labor leader, state official, and surgeon; chosen to represent broader societal values (8). The panel considered several methods of allocation, including random selection and “first-come, first-served,” before ultimately settling on social worth as the main criterion for selection. Social worth, in the committee’s assessment, derived from a combination of characteristics including age, sex, marital status, number of dependents, income, net worth, emotional stability, education, occupation, and potential for future societal contributions. During meetings, the committee debated the relative merits of individuals in terms of these qualities, but without a systematic form of evaluation. An article in Life Magazine by Shana Alexander brought this “Life or Death Committee” into the public awareness (2), where it received criticism for representing narrow, white middle class values (8). A scathing critique published in the UCLA Law Review noted, “the Pacific Northwest is no place for a Henry David Thoreau with bad kidneys.” (9)
Justice and an Absolutely Limited Resource
Faced with demand outstripping available dialysis machines and funding, the Seattle Artificial Kidney Center was placed in the unenviable position of being unable to provide for every patient in need. In this context, the principle of justice took a primary role in defining appropriate use of dialysis. Justice relates to the fair treatment of persons or groups. More specifically, distributive justice describes how resources, such as physician time, technology, and medications, are allocated to individuals and populations. The early experience of dialysis allocation raised several fundamental concerns regarding rationing of medical resources, or the withholding of a beneficial intervention from one individual or group while offering it to another. While it is tempting to simply claim that rationing dialysis or other medical resources is itself unethical, it becomes unavoidable when resources, such as a certain number of dialysis machines, cannot be subdivided and are consequently absolutely limited. If rationing must occur, the salient questions focus on the criteria of distribution, including who should decide on these criteria.
Although physicians traditionally serve as gatekeepers of medical resources, some are concerned that the act of rationing makes a doctor a “double agent” who compromises his duty as patient advocate when tasked with allocation of a limited resource (10). The Admissions and Policy Committee addressed this concern by taking some responsibility from the hands of physicians. Still, members of the Medical Advisory Committee played a large part in the initial selection process. It is not clear that physicians, who often have a unique perspective in understanding nuances of individual medical contexts, would or should totally abdicate this role (11).
Criteria of distribution generally fall into two broad categories. Egalitarian criteria prioritize equal treatment (i.e., free-market, random distribution by lottery, first-come first-served) while utilitarian criteria attempt to maximize benefit (i.e., likelihood or duration of medical benefit, potential or past contribution to society, merit) across a population. A precedent for using utilitarian criteria for medical decisions has been established in battlefield triage, during which prioritization is given to those most useful, for example, soldiers who would be able to return to battle (12). In the civilian context, the proper distribution criteria are less clear.
In the early days of dialysis, both the physician and layperson dialysis selection panels based their allocation decisions on various utilitarian criteria, including medical suitability and social worth. Social worth criteria have been extensively criticized for what, in retrospect, are subjective and potentially unfair discriminatory distinctions. Unlike in war, when a common goal of survival or victory unites different people, civilian life is composed of individuals with different value systems working towards varying goals. This made the Seattle Artificial Kidney Center’s task of defining fair social worth criteria a difficult, if not an impossible one. Another limitation of the panel’s strategy was the lack of an explicitly stated and consistent declaration of criteria (8). The “closed door” meetings concealed the process of allocating dialysis, which might easily mask discrimination. This was especially problematic as the panel, ostensibly chosen to represent a wide scope of society, was criticized for embodying a dominant subset of it.
Considerations of Beneficence and Nonmaleficence with Expansion of Dialysis
The Social Security Act Amendment of 1972
As dialysis technology improved, the treatment shifted from an experimental therapy to standard of care. For those with ESRD, dialysis and transplantation are potentially lifesaving and can alleviate the symptoms of advanced kidney disease. In 1972, after lobbying by health professionals, patients, and families, the U.S. Congress passed Public Law 92–603, with section 299I entitling ESRD patients to receive Medicare benefits (13,14). This immediately reduced financial limitations on dialysis centers, and over the next few years, enrollment accelerated beyond anything anticipated by legislators. Not only did the number of patients starting dialysis exceed initial expectations, but patient demographics also changed dramatically. Earlier candidates were younger than 45 years old and screened for comorbidities. With funding available through Medicare, a growing number of older patients with comorbidities began dialysis. This trend was concurrent with the rise of the antiageism movement and establishment of the National Institute on Aging, as well as the disabilities rights movement, culminating in the Americans with Disabilities Act (15,16). Within 5 years, utilization had grown to the point that Dr. Belding Scribner suggested the need for a “deselection committee” because the criteria for starting dialysis had become so liberal (8).
During the 1990s and early 2000s, several studies suggested that there were limits to the utility of dialysis in the elderly and those with comorbidities, for whom potential harm might outweigh benefit (17–20). The Institute of Medicine (IOM) responded with a call for clinical practice guidelines to suggest treatment strategies for dialysis patients “with limited survival possibilities and relatively poor quality of life.” (21) In 1997 and 2003, the IOM published accounts of the American experience of death, stressing the harms of focus on aggressive intervention in the last years of life, as well as encouraging further investigation into end-of-life issues (22,23). Other burdens on patients and their families were increasingly recognized, including time spent at dialysis facilities, polypharmacy, cost, and risks of infection. Given the complexity of this balance as well as a subjective component of quality of life, metrics such as quality-adjusted life years (QALYs) were developed in an attempt to quantify value of treatment (24).
Beneficence and Nonmaleficence: Balancing Benefit and Harm
After the Social Security Act of 1972, with technical limitations diminished and financial pressures lightened, the need for explicit consideration of justice in appropriate dialysis initiation became less prominent. Medical teams moved quickly to provide treatment for thousands of patients expected to die without maintenance dialysis, only later recognizing the importance of associated risks and burdens. The principle of beneficence describes a health care practitioner’s obligation to actively improve the wellbeing of a patient. The concept of nonmaleficence describes the reciprocal imperative to avoid harm.
The undeniable benefit of survival offered by dialysis provided a strong argument for beneficence in initiating treatment outweighing all other considerations, so allegiance to this principle initially dominated. In part because of a growing sense of a technologic imperative to provide all care technically possible (25), dialysis became the medical default. It was thought to be “morally unjustifiable to deny dialysis to a patient with ESRD.” (8) The mindset shifted as the medical community recognized that not all patients were as likely to experience a survival benefit. Though it has been decreasing, the annual unadjusted mortality of patients with ESRD continues to be 18% (26). An IOM report in 1991 questioned the value of dialysis in patients with limited functional status or life expectancy (27). Even among those who gain longevity, the toll of treatment on quality of life sometimes outweighed the benefit, highlighting a role for the concept of nonmaleficence.
It is difficult to compare the relative value of outcomes such as longevity, cardiac events, and hospitalizations. Outcomes that are more subjective, although also potentially more important to patients, such as clarity of mind and energy, are even more difficult to quantify (28). QALYs and other related metrics attempt to put qualitatively different outcomes on a common scale. Although not without controversy, this analysis offers a systematic comparison of benefit and burden rather than relying on an intuitive gestalt.
A further challenge in applying these principles is the difficulty in defining the focus of benefit and harm. Medical knowledge and practice is increasingly based on randomized controlled trials utilizing disease-based outcomes such as cardiovascular events and decline in kidney function. The prized outcome, and one of the easiest to measure, is change in mortality. However, these targets may lead to a narrow perspective of beneficence, prioritizing longevity over other values such as comfort and independence which may be more important to individual patients (29). These complexities highlight the growing limitation of traditional concepts of beneficence and nonmaleficence as a basis for the decision to initiate dialysis. A more patient-centered approach focuses instead on the effect of treatment on an individual’s goals, values, and preferences in the context of their experience and symptoms (28,30).
Technology in Service of Individual Values
Shared Decision-Making for Complex Medical Choices
Years after the Social Security Act amendment made dialysis universally available, there remains a cultural sense that it should also be universally applied. Well meaning physicians present dialysis as a necessary step in advanced renal failure, neglecting discussion of alternatives (30,31). However, individual patients value longevity and quality of life differently. One study of patients with advanced CKD found that the majority would not choose prolonged life if it entailed significant pain and discomfort (32). Despite these observations, most patients are not offered alternative treatment options (33). If there is discussion of alternative options, it is late in the disease course, with approximately 50% of patients presented treatment options less than a month before, or even after, initiation of dialysis (33). Despite known high mortality rates, most patients and physicians do not discuss end-of-life care prior to initiation of dialysis, and the majority of patients regret their decision to start (32). For those who choose to initiate dialysis, patient experience varies greatly by modality. Options beyond treatment at a center three times weekly, such as home peritoneal dialysis or short daily or long nocturnal hemodialysis, can now be offered to appropriate patients (26). The future promises even more dramatic innovation (34). As these options differ greatly in their effect on daily life, the choice should be based largely on patient-specific values, which may include preservation of independence, amount of time spent on dialysis, need to travel, impact on family, and ability to continue important activities (35).
In response to these concerns, a patient-centered approach to the treatment of ESRD is gaining traction (36). The process of shared decision-making guides clarification of what matters most to patients and alignment of treatment to individual preferences (37). In 1982, the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research described shared decision-making as the ideal model for complex medical decisions (38,39). In 2000, and in a revision in 2010, the Renal Physicians Association (RPA) established a clinical practice guideline for deciding to start or withdraw dialysis using this model (40). More recently, the American Society of Nephrology identified shared decision-making for initiation of maintenance dialysis as a key recommendation for the American Board of Internal Medicine’s Choosing Wisely campaign to improve patient care and resource utilization (41). Important steps in shared decision-making include fully informing patients about diagnosis, prognosis, and treatment options and then forming decisions in the context of the patient’s preferences and values.
Respect for Autonomy
Respect for autonomy reflects the idea that a well informed and autonomous person is in the best position to make decisions that support their own values and preferences. The typical burdens and benefits relevant to the majority of patients may or may not apply to a particular individual, whose decisions are dependent on their own life goals and self-image. Especially in the advanced kidney disease population, coexisting disease and disability make medical decisions complex. Benefit of dialysis and particular modality depends heavily on patient values in addition to the clinical scenario. Shared decision-making is a model for individualizing treatments by respecting autonomy.
The role autonomy plays in a clinical scenario relates to how a health care team acts to uphold it, but also to whether a certain patient is capable of being autonomous. Beauchamp and Childress describe three requirements necessary for a person to have autonomy: intentionality, understanding of the situation and freedom from controlling influences (6). These criteria can be true to varying degrees, meaning a patient’s autonomy is situation-dependent. The initiation of dialysis brings a dramatic life change that is difficult to conceptualize in advance, limiting a patient’s ability to fully understand the decision. To the extent that they increase this understanding, discussion with physicians and interdisciplinary educational programs incorporating experienced patients, dialysis nurses, and other team members improves the ability to make informed decisions (42). A patient cannot exercise autonomy if they are not presented with the range of viable options, including medical management without dialysis and the variety of dialysis modalities. Intentionality, or the ability to recognize and act according to one’s own values, is also a key component of autonomy. Cognitive decline that may coexist with ESRD causes capacity for autonomous choice to diminish. Autonomy can be preserved by discussion of options earlier in the disease course. Advance care planning and discussion about preferences, as well as the designation of surrogate decision-makers can help promote autonomy. Given that surrogates bring their own values to a discussion, it is important to continue studying how they make decisions and strategies for encouraging substituted judgment (43). Palliative care is increasingly recognized as crucial in defining patient preference and identifying barriers to autonomous decision-making. Because this need is ubiquitous, all providers should be proficient in primary palliative care; the basic skills all clinicians need to elicit patient values, preferences, and goals and use shared decision-making to establish the appropriate treatment plan (44).
Even as autonomy is emphasized, other principles are recognized. Both the President’s Commission and the RPA note that focus on patient preference does not require physicians to offer dialysis if they feel that the risk outweighs the benefit, especially in cases of profound neurologic impairment (39,40,45).
Renewed Concerns of Justice in the Context of Limited Fiscal Resources
Economic Changes and ESRD Spending
In the late 1960s, approximately 9000 ESRD patients were being treated with maintenance dialysis at any particular time. When the United States legislature passed the amendment to the Social Security Act in 1972 authorizing Medicare payments for ESRD treatment, annual cost per patient was estimated at $15,000–$20,000 (46). Underlying passage of the amendment was a belief that dialysis would provide rehabilitation for a small number of citizens at a relatively low cost (47). This utilitarian argument was upended by costs quickly exceeding expectations. In the first year of the program, 16,000 patients were enrolled at a cost of $229 million, rising to 90,000 patients and $1.9 billion only 10 years later (48). In 2010, 7.9% of all Medicare outlays went to ESRD patients, who composed only 1.3% of beneficiaries (26). The unanticipated cost is largely due to the expanded population of patients, including the elderly and those with comorbidities, who were previously excluded from treatment (46). These expenditures are taken in the context of Medicare’s current cost of $583 billion, representing 3.5% of the gross domestic product, with a predicted increase to 6.9% by 2088 (49). The Affordable Care Act and new government-based insurance redirecting funds to cover millions of Americans will put new stresses on currently subsidized benefits like dialysis.
Justice and a Relatively Limited Resource
Change in the economic climate has led to scrutiny of health care spending, with an eye toward maximizing population health while still optimizing equitable distribution. When considering the just allocation of limited Medicare funds, the utility of dialysis for an individual is not merely compared with the utility to another patient with advanced kidney disease, but to payment for coronary stent placement, supporting cancer research, or instituting preventive health programs. Some calculations using QALYs suggest that dialysis provides relatively little value as compared with other health care measures (24,50). From an egalitarian perspective, it is unclear why ESRD should be uniquely supported when we are unable or unwilling to do the same for other chronic diseases such as heart failure, lung disease, and cancer (50–52). Questions such as “On what basis should financial resources be allocated?” and “Who should decide on this foundation?” are ethically complex. The strategy of rationing medical resources has developed a negative connotation, and the Affordable Care Act explicitly rejects it (53). However, others suggest that we are already implicitly rationing by “cherry picking” patients who show up to clinic appointments or are adherent to medical regimens, neglecting those who may fail to do so for reasons such as limited finances, cognitive disability, or comorbidity (54,55). As the early experience of the Seattle Artificial Kidney Center demonstrates, if rationing must play a role in allocation of limited resources, criteria need to be transparent and explicit to avoid unintentional discrimination. However, by denying that rationing must occur, we lose the impetus and opportunity to debate such criteria.
Conclusion
The history of development and dissemination of maintenance dialysis provides an example of how the four principles forming the basis of clinical ethics are variably emphasized as technology, resources, and societal values related to health care shift. No static hierarchy exists. Given this variability, one strategy to create more sustainable ethical solutions is to consider and address all four ethical principles as fully as possible. As an example, Medicare’s National Quality Strategy’s three aims of better care for the individual, better health for populations, and reduced healthcare costs (56), is a multipronged goal that can only be reached by addressing multiple, and sometimes conflicting, values. As medical technology evolves in a landscape of changing personal and societal ideals, clinicians and policymakers should be familiar with an ethical framework for evaluating appropriate initiation and allocation of medical interventions.
Disclosures
D.Y.L. receives some salary support from the Northwest Kidney Centers as their Palliative Care Medical Advisor.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Blagg C: Belding Scribner interviewed. Nephrology 4: 295–297, 1998 [Google Scholar]
- 2.Alexander S: They decide who lives, who dies: Medical miracle puts a moral burden on a small committee. Life. 1962, pp 102–104, 106, 108, 110, 115, 117, 118, 123, 124, 127 [Google Scholar]
- 3.Rothman D: Strangers at the Bedside: A History of How Law and Bioethics Transformed Medical Decision Making, New York, Aldine Transaction, 1991 [Google Scholar]
- 4.Jonsen AR: The Birth of Bioethics, New York, Oxford University Press, 1998 [Google Scholar]
- 5.Blagg C: Development of ethical concepts in dialysis: Seattle in the 1960s. Nephrology 4: 235–238, 1998 [Google Scholar]
- 6.Beauchamp T, Childress J: Principles of Biomedical Ethics, New York, Oxford University Press, 2009 [Google Scholar]
- 7.Jonsen AR: Clinical Ethics: A Practical Approach to Ethical Decisions in Medicine, New York, McGraw Hill, 2006 [Google Scholar]
- 8.Fox R, Swazey J: The Courage to Fail: A Social View of Organ Transplants and Dialysis, Chicago, IL, University of Chicago Press, 1974 [Google Scholar]
- 9.Sanders D, Dukerminier J: Medical advance and legal lag: Hemodialysis and kidney transplantation. UCLA Law Rev 15: 357–413, 1968 [Google Scholar]
- 10.Lauridsen S: Administrative gatekeeping—A third way between unrestricted patient advocacy and bedside rationing. Bioethics 23: 311–320, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Tilburt JC, Wynia MK, Sheeler RD, Thorsteinsdottir B, James KM, Egginton JS, Liebow M, Hurst S, Danis M, Goold SD: Views of US physicians about controlling health care costs. JAMA 310: 380–388, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winslow G: Triage and Justice, Berkley, CA, University of California Press, 1982 [Google Scholar]
- 13.Rettig RA: Special treatment—The story of Medicare’s ESRD entitlement. N Engl J Med 364: 596–598, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Rettig RA: The policy debate on patient care financing for victims of end-stage renal disease. Law Contemp Probl 40: 196–230, 1976 [PubMed] [Google Scholar]
- 15.Butler RN: Psychiatry and the elderly: An overview. Am J Psychiatry 132: 893–900, 1975 [DOI] [PubMed] [Google Scholar]
- 16. Americans with Disabilities Act, Pub. L. No. 101-336, 104 Stat. 328, 1990.
- 17.Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE: Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 22: 1955–1962, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Ifudu O, Mayers J, Matthew J, Tan CC, Cambridge A, Friedman EA: Dismal rehabilitation in geriatric inner-city hemodialysis patients. JAMA 271: 29–33, 1994 [PubMed] [Google Scholar]
- 19.Byrne C, Vernon P, Cohen JJ: Effect of age and diagnosis on survival of older patients beginning chronic dialysis. JAMA 271: 34–36, 1994 [PubMed] [Google Scholar]
- 20.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE: Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 361: 1539–1547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rettig RA, Levinsky NG: Kidney Failure and the Federal Government, Washington, DC, The National Academy of Sciences, 1991 [PubMed] [Google Scholar]
- 22.Field MJ, Cassel CK: Approaching Death: Improving Care at the End of Life: R726.8.A68 1997. Institute of Medicine; Committee on Care at the End of Life, Division of Health Care Services, Washington, DC, National Academy Press, 1997 [Google Scholar]
- 23. Lunney JR, Foley KM, Smith TJ, Gelband H, editors: Describing Death in America: What We Need to Know: R726.8.D475 2003. Institute of Medicine; National Cancer Policy Board, Washington, DC, National Academies Press, 2003. [PubMed]
- 24.Lee CP, Chertow GM, Zenios SA: An empiric estimate of the value of life: Updating the renal dialysis cost-effectiveness standard. Value Health 12: 80–87, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Fuchs VR: The growing demand for medical care. N Engl J Med 279: 190–195, 1968 [DOI] [PubMed] [Google Scholar]
- 26.U.S. Renal Data System: USRDS 2014 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014 [Google Scholar]
- 27.Rettig RA, Levinsky NG, editors: Kidney Failure and the Federal Government: RA645.K5157 1991. Institute of Medicine; Committee for the Study of the Medicare End-Stage Renal Disease Program, Washington, DC, National Academies Press, 1991 [PubMed] [Google Scholar]
- 28.Tinetti ME, Fried T: The end of the disease era. Am J Med 116: 179–185, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Thorsteinsdottir B, Swetz KM, Tilburt JC: Dialysis in the frail elderly—A current ethical problem, an impending ethical crisis. J Gen Intern Med 28: 1511–1516, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufman SR, Shim JK, Russ AJ: Revisiting the biomedicalization of aging: Clinical trends and ethical challenges. Gerontologist 44: 731–738, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufman SR, Shim JK, Russ AJ: Old age, life extension, and the character of medical choice. J Gerontol B Psychol Sci Soc Sci 61: S175–S184, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davison SN: End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 195–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehrotra R, Marsh D, Vonesh E, Peters V, Nissenson A: Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int 68: 378–390, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Rastogi A, Nissenson AR: Technological advances in renal replacement therapy: Five years and beyond. Clin J Am Soc Nephrol 4[Suppl 1]: S132–S136, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Morton RL, Snelling P, Webster AC, Rose J, Masterson R, Johnson DW, Howard K: Factors influencing patient choice of dialysis versus conservative care to treat end-stage kidney disease. CMAJ 184: E277–E283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Hare AM, Armistead N, Schrag WL, Diamond L, Moss AH: Patient-centered care: an opportunity to accomplish the “Three Aims” of the National Quality Strategy in the Medicare ESRD program. Clin J Am Soc Nephrol 9: 2189–2194, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barry MJ, Edgman-Levitan S: Shared decision making—Pinnacle of patient-centered care. N Engl J Med 366: 780–781, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Charles C, Gafni A, Whelan T: Shared decision-making in the medical encounter: What does it mean? (or it takes at least two to tango). Soc Sci Med 44: 681–692, 1997 [DOI] [PubMed] [Google Scholar]
- 39.President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research: Making Health Care Decisions, Washington, DC, US Government Printing Office, 1982, pp 30–44 [Google Scholar]
- 40.Renal Physicians Association: Shared decision-making in the appropriate initiation of and withdrawal from dialysis, 2nd Ed. Rockville, MD, Renal Physicians Association, 2010 [Google Scholar]
- 41.Williams AW, Dwyer AC, Eddy AA, Fink JC, Jaber BL, Linas SL, Michael B, O’Hare AM, Schaefer HM, Shaffer RN, Trachtman H, Weiner DE, Falk AR; American Society of Nephrology Quality, and Patient Safety Task Force: Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol 7: 1664–1672, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Kurella Tamura M, Li S, Chen SC, Cavanaugh KL, Whaley-Connell AT, McCullough PA, Mehrotra RL: Educational programs improve the preparation for dialysis and survival of patients with chronic kidney disease. Kidney Int 85: 686–692, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Hines SC, Glover JJ, Babrow AS, Holley JL, Badzek LA, Moss AH: Improving advance care planning by accommodating family preferences. J Palliat Med 4: 481–489, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Quill TE, Abernethy AP: Generalist plus specialist palliative care—Creating a more sustainable model. N Engl J Med 368: 1173–1175, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Moss AH: Ethical principles and processes guiding dialysis decision-making. Clin J Am Soc Nephrol 6: 2313–2317, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Levinsky NG, Rettig RA: The Medicare end-stage renal disease program. A report from the Institute of Medicine. N Engl J Med 324: 1143–1148, 1991 [DOI] [PubMed] [Google Scholar]
- 47.Himmelfarb J, Ikizler TA: Hemodialysis. N Engl J Med 363: 1833–1845, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Long HW: Medicare’s ESRD program, part 1: Dialysis. Physician Exec 15: 24–26, 1989 [PubMed] [Google Scholar]
- 49. Boards of Trustees for Medicare: 2014 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds: 49th Report, July 28, 2014. Office of the Actuary in the Centers for Medicare & Medicaid Services, Washington, DC, Department of Health and Human Services, 2014.
- 50.Andersen MJ, Friedman AN: The coming fiscal crisis: Nephrology in the line of fire. Clin J Am Soc Nephrol 8: 1252–1257, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Fleck LM: The costs of caring: Who pays? Who profits? Who panders? Hastings Cent Rep 36: 13–17, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Moskop JC: The moral limits to federal funding for kidney disease. Hastings Cent Rep 17: 11–15, 1987 [PubMed] [Google Scholar]
- 53. Patient Protection and Affordable Care Act, Pub. L. No. 111–148, 124 Stat. 119–1025, 2010.
- 54.Ross W: God panels and the history of hemodialysis in America: A cautionary tale. Virtual Mentor 14: 890–896, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Desai AA, Bolus R, Nissenson A, Chertow GM, Bolus S, Solomon MD, Khawar OS, Talley J, Spiegel BM: Is there “cherry picking” in the ESRD Program? Perceptions from a Dialysis Provider Survey. Clin J Am Soc Nephrol 4: 772–777, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Quality Strategy: 2014 Annual Progress Report to Congress: National Strategy for Quality Improvement in Health Care: September 24, 2014. Agency for Healthcare Research and Quality, Washington, DC, US Department of Health and Human Services, 2014 [Google Scholar]