Abstract
Background and objectives
Disorders of mineral metabolism are more common in African Americans with CKD than in European Americans with CKD. Previous studies have focused on the differences in mineral metabolism by self-reported race, making it difficult to delineate the importance of environmental compared with biologic factors.
Design, setting, participants, & measurements
In a cross-sectional analysis of 3013 participants of the Chronic Renal Insufficiency Cohort study with complete data, we compared markers of mineral metabolism (phosphorus, calcium, alkaline phosphatase, parathyroid hormone, fibroblast growth factor 23, and urine calcium and phosphorus excretion) in European Americans versus African Americans and separately, across quartiles of genetic African ancestry in African Americans (n=1490).
Results
Compared with European Americans, African Americans had higher blood concentrations of phosphorus, alkaline phosphatase, fibroblast growth factor 23, and parathyroid hormone, lower 24-hour urinary excretion of calcium and phosphorus, and lower urinary fractional excretion of calcium and phosphorus at baseline (P<0.001 for all). Among African Americans, a higher percentage of African ancestry was associated with lower 24-hour urinary excretion of phosphorus (Ptrend<0.01) in unadjusted analyses. In linear regression models adjusted for socio-demographic characteristics, kidney function, serum phosphorus, and dietary phosphorus intake, higher percentage of African ancestry was significantly associated with lower 24-hour urinary phosphorus excretion (each 10% higher African ancestry was associated with 39.6 mg lower 24-hour urinary phosphorus, P<0.001) and fractional excretion of phosphorus (each 10% higher African ancestry was associated with an absolute 1.1% lower fractional excretion of phosphorus, P=0.01).
Conclusions
A higher percentage of African ancestry was independently associated with lower 24-hour urinary phosphorus excretion and lower fractional excretion of phosphorus among African Americans with CKD. These findings suggest that genetic variability might contribute to racial differences in urinary phosphorus excretion in CKD.
Keywords: chronic kidney disease; mineral metabolism; African Americans; Cohort Studies; Fibroblast Growth Factors; Humans; parathyroid hormone; Phosphorus; Renal Insufficiency, Chronic
Introduction
Disorders of mineral metabolism are common among patients with CKD. Compared with European Americans with CKD, African Americans with CKD have a higher prevalence of hypocalcemia, hyperphosphatemia, secondary hyperparathyroidism and vitamin D deficiency, independent of kidney function and other clinical characteristics (1). Factors that are likely to contribute to the increased burden of disordered mineral metabolism in African Americans with CKD compared with European Americans with CKD include differences in diet, physical activity, access to health care, and biologic variability in bone and mineral homeostasis (2–4). Disentangling the importance of biologic factors from environmental factors in disordered mineral metabolism is challenging. However, genetic admixture analysis is one method that can be used to investigate the degree to which observed differences in a given trait are due to genetic (biologic) factors (5).
In this study, we used estimates of genetic African ancestry derived from genetic admixture analysis among African Americans enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study (a prospective study of people with CKD) to test the hypothesis that a higher percentage of African ancestry was independently associated with abnormalities in mineral metabolism markers. Furthermore, we examined the extent to which these associations might explain differences in mineral metabolism in African Americans compared with European Americans.
Materials and Methods
The CRIC study is an ongoing prospective observational cohort study of patients with mild to moderate CKD that was established to examine risk factors for chronic kidney and cardiovascular disease progression (6,7). A total of 3939 participants aged 21–74 years were initially enrolled from seven clinical centers (representing 13 recruiting sites) located in Ann Arbor, Michigan; Baltimore, Maryland; Chicago, Illinois; Cleveland, Ohio; New Orleans, Louisiana; Philadelphia, Pennsylvania; and Oakland, California. All participants completed a baseline visit, during which demographic characteristics, medical history, diet history, current medications, anthropomorphic measurements, and plasma, urine, and DNA samples were obtained. The study protocol was approved by the institutional review board at each of the recruiting sites, and all participants provided written informed consent.
Participants were asked to report their race as black, white, or other, and their ethnicity as either Hispanic or non-Hispanic. Diabetes was defined as having an established medical history of diabetes, current or previous use of diabetes medications, or documented laboratory evidence of diabetes (i.e., two episodes of a random plasma glucose >200 mg/dL in conjunction with classic symptoms or a fasting plasma glucose level >126 mg/dL). Annual household income, highest level of education achieved, and employment status were used as indexes of socioeconomic status.
Laboratory and Dietary Characteristics
All laboratory characteristics reported in this study were from the baseline study visit and were measured at a single laboratory. Serum and urine phosphorus, calcium, and creatinine and serum total alkaline phosphatase were measured with standard assays. Parathyroid hormone (PTH) concentrations were measured using an intact assay (Scantibodies, Santee, California). Fibroblast growth factor 23 (FGF23) concentrations were measured using a second-generation, carboxy-terminal assay (Immutopics, Santa Clara, California). eGFR was calculated using the CRIC study equation (8). Urine fractional excretion of phosphorus and calcium were calculated as follows: ([urine analyte × serum creatinine] ÷ [serum analyte × urine creatinine]) × 100%.
The National Cancer Institute Diet History Questionnaire (DHQ) was used to assess diet (9). The DHQ is a food frequency questionnaire designed to assess usual dietary intake by recording the frequency of consumption and portion size eaten for 124 food items over the preceding year. DHQs were analyzed for daily nutrient intake using DietCalc software (http://appliedresearch.cancer.gov/DHQ/dietcalc).
Estimation of Percentage Genetic African Ancestry
Genetic admixture analysis is one method used to investigate the degree to which observed differences in a given trait are due to genetic (biologic) versus environmental exposures. This technique relies on the principle that individuals can be classified into gradations of commonly identified ethnic groups (e.g., European, African) by using ancestry-informative single nucleotide polymorphisms to quantify the proportion of an individual’s genome that is of a given ancestral origin (10). These data can then be utilized to examine the potential associations between a specific continental ancestral group, for example, within an admixed group such as African Americans, and phenotypes of interest. The finding of such an association between percentage African ancestry within the same ethnic group (i.e., black) would then strongly support a genetic basis for the differential expression of a trait among racial groups.
A total of 1348 HapMap3-based ancestry-informative markers (AIMs) that were available on the IBC Illumina chip array panel were selected to determine percentage genetic African ancestry in African American participants (a full listing of markers used can be found in Supplemental Table 1). Quality control metrics for the Illumina based BeadChip genotype results included testing for Hardy–Weinberg Equilibrium, sex concordance, excess heterozygosity, call rates, and relatedness, resulting in 1053 AIMs that were used for this analysis. An admixture model was employed using the software Structure to derive global European and African continental genetic ancestry measures, as previously reported (11). Previous studies have established that even small subsets of fewer than 300 AIMs can reliably correlate ancestry to matching reference panels (r=0.99) (12,13). We found 96.7% and 98.8% concordance rate between self-reported white and black race with our genotype-derived European and African ancestry classifications, respectively (11).
Statistical Analyses
Participant characteristics at the time of entry into the CRIC study were compared between African Americans and European Americans overall using t tests or the Wilcoxon rank sum test for continuous variables as appropriate, and chi-squared tests for categorical variables. Among African Americans, characteristics were also compared across quartiles of percentage African ancestry using linear tests of trend for normally distributed continuous variables, the Kruskal–Wallis test for non-normally distributed continuous variables and Cochran–Armitage tests of trend for categorical variables. Generalized linear models were used to examine the association between percentage African ancestry and markers of mineral metabolism. Multivariable models were fitted to adjust for potential confounders and factors significantly (P<0.05) associated with percentage African ancestry on univariate analysis. As the values for alkaline phosphatase, FGF23, PTH, 24-hour urine calcium excretion, and fractional excretion of calcium were not normally distributed, models were fitted using natural log-transformed values, which were then transformed back into the conventional scale for clarity of presentation. In the primary analyses, we excluded individuals with implausible values for 24-hour urinary creatinine excretion of <350 or >3500 mg/day. In sensitivity analyses, we modeled urine mineral excretion as 24-hour urine phosphorus or calcium excretion indexed to urine creatinine excretion (expressed as milligram of phosphorus or calcium excretion per gram of creatinine excretion) and examined models excluding individuals with very low eGFR (<30), and also used stricter criteria for excluding individuals with low 24-hour urine creatinine excretion (<700 mg/day versus ≤350 mg/day). Two-tailed P values <0.05 were considered statistically significant in all analyses. SAS version 9.4 statistical software (SAS Institute, Cary, NC) was used to conduct all analyses.
Results
Study Population
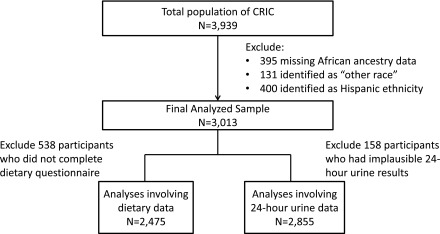
Of the 3939 CRIC study participants, we excluded 395 participants with missing data on percent African ancestry, 131 participants who identified themselves as “other race,” and 400 participants who identified themselves as being of Hispanic ethnicity (whether or not they self-reported black race), leaving 3013 participants in the final analyzed sample (1523 European Americans and 1490 African Americans by self-reported race; Figure 1). Of these, 538 participants (18%) did not complete dietary questionnaires or had questionnaires excluded because of extreme values for total energy intake (i.e., <600 kcal or >4000 kcal for women and <800 kcal or >5000 kcal for men), leaving 2475 participants available for analyses of dietary data. For analyses involving 24-hour urine data, 158 individuals (5%) were excluded because of implausible 24-hour urine results defined as total urine creatinine excretion <350 mg or >3500 mg per 24 hours.
Figure 1.
Flow diagram indicating derivation of final analyzed study sample.
Table 1 compares the demographic, clinical, and laboratory characteristics of participants by self-reported race (African Americans versus European Americans) and by quartiles of percent African ancestry; the latter only in participants who self-reported African-American race. The mean percent African ancestry in African-American participants was 77.6±9.1%. Compared with European Americans, African Americans were younger, more likely to be female, had higher body mass index (BMI) and waist circumference, were more likely to be current smokers, had higher systolic and diastolic blood pressure, were more likely to have a history of diabetes, cardiovascular disease and hypertension, were more likely to have lower annual family income and lower educational achievement, and had lower eGFR and higher urine albumin-to-creatinine ratio. Lower educational achievement and lower annual income were significantly associated with a higher percentage of African ancestry in participants who self-reported African American race. There were no differences in eGFR or urine albumin-to-creatinine ratio across quartiles of African ancestry.
Table 1.
Baseline characteristics among self-reported European American and African-American participants in the CRIC study
| European American | African Americana | P valuef | |||||
|---|---|---|---|---|---|---|---|
| Variables | n=1523 | Overall n=1490 | Quartile 1b n=373 | Quartile 2c n=368 | Quartile 3d n=373 | Quartile 4e n=376 | |
| Age | 58.5±10.9g | 57.6±10.6 | 57.9±11.2 | 57.0±10.5 | 56.5±10.4 | 58.8±10.2 | 0.37 |
| Female sex (%) | 40h | 51 | 50 | 50 | 55 | 51 | 0.46 |
| Body mass index (kg/m2) | 31.3±7.5h | 33.4±8.2 | 33.0±7.4 | 33.2±8.1 | 34.7±8.9 | 32.9±8.4 | 0.48 |
| Waist circumference (cm) | 105.7±17.6h | 108.0±18.0 | 108.3±17.1 | 107.8±18.7 | 109.9±18.5 | 106.0±17.6 | 0.27 |
| eGFR (ml/min per 1.73m2) | 47.6±17.0h | 43.8±16.4 | 44.5±15.6 | 43.5±16.5 | 43.3±17.0 | 44.0±16.4 | 0.65 |
| UACR (mg/g) | 25.3 (6.2, 212.2)h | 74.1 (10.9,513.5) | 75.0 (9.0, 494.4) | 84.9 (13.1, 554.4) | 70.5 (9.3, 699.9) | 68.6 (12.9,407.0) | 0.77 |
| Co-morbidities (%) | |||||||
| Diabetes | 41h | 52 | 52 | 50 | 54 | 50 | 0.96 |
| CVD | 32h | 38 | 34 | 46 | 39 | 35 | 0.61 |
| Hypertension | 80h | 93 | 92 | 92 | 92 | 95 | 0.10 |
| SBP (mmHg) | 122.0±18.6h | 132.9±23.1 | 132.0±22.1 | 132.3±23.6 | 133.5±24.4 | 134.0±22.1 | 0.19 |
| DBP (mmHg) | 69.0±11.4h | 74.0±13.9 | 72.8±14.7 | 74.3±12.9 | 74.6±13.5 | 74.1±14.2 | 0.19 |
| Smoking (current, %) | 10h | 20 | 21 | 20 | 17 | 20 | 0.57 |
| Less than high school diploma (%) | 5h | 26 | 19 | 27 | 29 | 30 | <0.001 |
| Annual income ≤ $20,000/year (%) | 18h | 48 | 40 | 48 | 54 | 49 | 0.01 |
| Medication use (%) | |||||||
| Active vitamin D | 2g | 4 | 3 | 6 | 3 | 4 | 0.99 |
| Bisphosphonates | 4h | 2 | 2 | 2 | 2 | 2 | 0.48 |
| Binders (non-calcium) | 0.5 | 0.3 | 0.3 | 0.3 | 0.5 | 0 | 0.65 |
| Binders (calcium) | 7 | 6 | 6 | 6 | 6 | 6 | 0.88 |
Results are presented as mean±SD, median (interquartile range), or frequencies. CRIC, Chronic Renal Insufficiency Cohort; UACR, urine albumin-to-creatinine ratio; CVD, cardiovascular disease; SBP, systolic blood pressure; DBP, diastolic blood pressure.
African-American data is shown overall and also stratified by quartiles of percentage African ancestry.
Percentage African ancestry <73.6%.
Percentage African ancestry 73.6%–79.6%.
Percentage African ancestry 79.7%–83.8%.
Percentage African ancestry >83.8%.
P for trend across categories of percentage African ancestry among self-reported African Americans; linear test for trend or Wilcoxon rank sum test were used for continuous variables and Cochran–Armitage test for trend was used for categorical variables.
P<0.05 comparing European Americans to African Americans.
P<0.001 comparing European Americans to African Americans.
When comparing differences in diet by self-reported race, African Americans reported higher daily energy intake, lower percent energy intake from fat and protein, higher percent energy from carbohydrates, lower calcium intake per day, and lower daily phosphorus intake compared with European Americans (Table 2). Among African American participants, a higher percentage of African ancestry was associated with lower energy intake from fat and higher energy intake from carbohydrates. Calcium and phosphorus intake did not vary significantly among quartiles of African ancestry.
Table 2.
Diet characteristics among European American and African-American participants in the CRIC study
| European American | African Americana | P valuef | |||||
|---|---|---|---|---|---|---|---|
| Variables | n=1354 | Overall n=1121 | Quartile 1b n=284 | Quartile 2cn=285 | Quartile 3d n=283 | Quartile 4e n=269 | |
| Kilocalories per day | 1808±761g | 1904±902 | 1844±858 | 1986±930 | 1915±913 | 1870±904 | 0.97 |
| Calories fat, % | 35±8h | 33±8 | 34±8 | 33±8 | 33±9 | 32±9 | 0.002 |
| Calories protein, % | 16±4h | 15±4 | 15±4 | 15±4 | 15±4 | 14±4 | 0.06 |
| Calories carbohydrates, % | 48±10h | 53±12 | 51±10 | 52±11 | 54±12 | 54±13 | 0.001 |
| Calcium (mg/day) | 649 [456,908]h | 577 [402,837] | 574 [396,851] | 592 [423,810] | 578 [387,837] | 558 [389,808] | 0.55 |
| Phosphorus (mg/day) | 1099 [814,1463]h | 1013 [703,1388] | 1007 [713,1365] | 1051 [742,1461] | 993 [681,1415] | 1006 [674,1299] | 0.26 |
Results are presented as mean±SD and median (interquartile range). CRIC, Chronic Renal Insufficiency Cohort.
African-American data is shown overall and also stratified by quartiles of percentage African ancestry.
Percentage African ancestry <73.6%.
Percentage African ancestry 73.6%–79.6%.
Percentage African ancestry 79.7%–83.8%.
Percentage African ancestry >83.8%.
P for linear trend or Kruskal–Wallis test across the quartiles of African ancestry among African Americans.
P<0.05 comparing European Americans to African Americans.
P<0.001 comparing European Americans to African Americans.
Self-reported Race, Percent African Ancestry and Markers of Mineral Metabolism
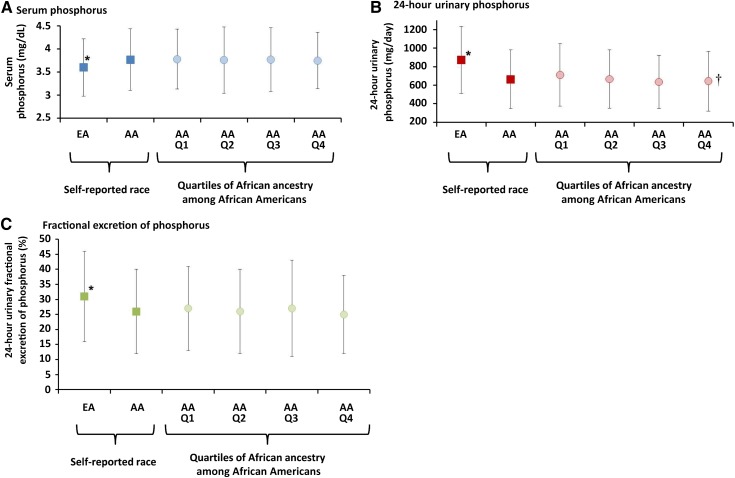
African Americans had higher blood concentrations of phosphorus, alkaline phosphatase, FGF23, and PTH, lower 24-hour urinary excretion of calcium and phosphorus, and lower urinary fractional excretion of calcium and phosphorus compared with European Americans (Table 3), as has been reported previously (1,14,15). In participants who self-reported African-American race, mean 24-hour urinary phosphorus excretion was lower with higher percentage of African ancestry (Ptrend<0.01). Mean urinary fractional excretion of phosphorus was also lower with higher percentage of African ancestry, but this difference was not statistically significant (P=0.13). The remaining markers of mineral metabolism did not vary significantly according to percentage of African ancestry. Differences in serum phosphorus, 24-hour urinary phosphorus excretion and fractional excretion of phosphorus by self-reported race and percentage African ancestry are depicted graphically in Figure 2.
Table 3. .
Markers of mineral metabolism among European American and African-American participants in the CRIC study
| European American | African Americana | P valuef | |||||
|---|---|---|---|---|---|---|---|
| Variables | n=1523 | Overall n=1490 | Quartile 1b n=373 | Quartile 2c n=368 | Quartile 3d n=373 | Quartile 4e n=376 | |
| Phosphorus (mg/dL) | 3.60±0.62g | 3.77±0.67 | 3.78±0.65 | 3.76±0.72 | 3.77±0.69 | 3.75±0.61 | 0.57 |
| Calcium (mg/dL) | 9.23±0.47 | 9.21±0.52 | 9.19±0.52 | 9.23±0.52 | 9.20±0.54 | 9.21±0.50 | 0.64 |
| Alkaline phosphatase (U/L) | 80.7 (79.4, 82.0)g | 92.1 (90.6, 93.6) | 90.2 (87.2, 93.3) | 91.6 (88.5, 94.8) | 92.6 (89.6, 95.8) | 94.0 (90.8, 97.2) | 0.09 |
| FGF23 (RU/ml) | 149.8 (144.1, 155.7)g | 164.4 (158.1, 171.0) | 162.4 (149.7, 176.2) | 170.9 (157.4, 185.6) | 165.9 (152.9, 180.0) | 158.8 (146.4, 172.2) | 0.60 |
| PTH (pg/ml) | 46.3 (44.7, 48.0)g | 68.6 (66.2, 71.0) | 65.2 (60.5, 70.4) | 69.9 (64.8, 75.5) | 70.3 (65.2, 75.8) | 68.9 (64.0, 74.3) | 0.32 |
| Urine calciumh (mg/day) | 52.2 (49.4, 55.2)g | 28.6 (27.0, 30.3) | 31.4 (27.8, 35.5) | 27.1 (24.0, 30.7) | 26.0 (23.0, 29.3) | 30.2 (26.7, 34.1) | 0.56 |
| FECa (%)h | 0.69 (0.66, 0.73)g | 0.45 (0.43, 0.48) | 0.47 (0.42,0.53) | 0.43 (0.38, 0.48) | 0.43 (0.38, 0.48) | 0.48 (0.43, 0.54) | 0.92 |
| Urine phosphorus (mg/day)h | 871.6±360.5g | 664.0±317.5 | 711.4±337.6 | 666.6±314.6 | 634.6±289.3 | 643.7±322.5 | 0.002 |
| FEPO4 (%)h | 31±15g | 26±14 | 27±14 | 26±14 | 27±16 | 25±13 | 0.13 |
Results are presented as arithmetic mean±SD or geometric means (95% confidence intervals). FGF23, fibroblast growth factor 23; PTH, parathyroid hormone; FECa, urine fractional excretion of calcium; FEPO4, urine fractional excretion of phosphorus.
African-American data is shown overall and stratified by quartiles of percentage African ancestry.
Percentage African ancestry <73.6%.
Percentage African ancestry 73.6%–79.6%.
Percentage African ancestry 79.7%–83.8%.
Percentage African ancestry >83.8%.
P for trend across the quartiles of African ancestry among African Americans.
P<0.001 comparing European Americans to African Americans.
Analyses restricted to 2855 participants with plausible 24-hour urine data (1462 European Americans, 1393 African Americans).
Figure 2.
Differences in markers of phosphorus metabolism by self-reported race and across quartiles of African ancestry among African Americans. Mean (SD) values of (A) serum phosphorus (mg/dL), (B) 24-hour urinary phosphorus (mg/day), and (C) fractional excretion of phosphorus (%) in European Americans (EA) compared with African Americans (AA), and across quartiles of percentage genetic African ancestry among African Americans (Q1–Q4). The range of percentage African ancestry in each quartile is as follows: Q1 <73.6%; Q2 73.6%–79.6%; Q3 79.7%–83.8%; Q4: >83.8%. *P<0.05 comparing European Americans to African Americans; †Ptrend <0.05 across Q1–Q4 African Americans.
Percentage African ancestry was inversely associated with 24-hour urinary phosphorus excretion after adjustment for age, female sex and baseline eGFR (P<0.001) and remained significant after further adjustment for BMI, diabetes, measures of socioeconomic status, dietary phosphorus intake, and serum phosphorus concentrations (P<0.001; Table 4). In the fully adjusted model, each additional 10% of African ancestry was associated with 39.6 mg lower urinary phosphorus excretion per day. After adjustment for age, female sex, and eGFR, the inverse association of African ancestry with urinary fractional excretion of phosphorus became statistically significant (P<0.01). The magnitude and statistical strength of this association did not change meaningfully after further adjustment for BMI, diabetes, socioeconomic status, diet phosphorus intake and serum phosphorus concentrations. In the final model, each additional 10% of African ancestry was associated with an absolute 1.1% lower urinary fractional excretion of phosphorus (P=0.01). These results did not differ when urinary phosphorus excretion was expressed per gram of urine creatinine, or when we excluded individuals with very low eGFR (<30) or when we used stricter criteria for excluding individuals with low 24-hour urine creatinine excretion (data not shown).
Table 4. .
Multivariable-adjusted difference in 24-hour urinary phosphorus excretion and fractional excretion of phosphorus per 10% higher percentage African ancestry among African-American participants in the CRIC study
| 24-hour urine phosphorus excretion | Fractional excretion of phosphorus | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| Variables | mg/day (95%CI) | P value | mg/day (95%CI) | P value | % (95%CI) | P value | % (95%CI) | P value |
| African ancestry, per 10% higher | −36.4 (−19.0, −53.7) | <0.001 | −39.6 (−18.2, −60.9) | <0.001 | −1.0 (−0.2, −1.8) | 0.009 | −1.1 (−0.2, −2.0) | 0.01 |
| Age, per 1 year | −3.1 (−1.6, −4.7) | <0.001 | −2.4 (−0.4, −4.4) | 0.02 | −0.1 (−0.0, −0.2) | 0.007 | −0.2 (−0.1, −0.3) | <0.001 |
| Female sex | −136.3 (−104.3, −168.2) | <0.001 | −147.2 (−105.1, −189.2) | <0.001 | −4.9 (−3.4, −6.3) | <0.001 | −3.9 (−2.2, −5.7) | <0.001 |
| eGFR, 1 ml/min per 1.73 m2 | 2.7 (1.7,3.7) | <0.001 | 2.7 (1.3,3.9) | <0.001 | −0.4 (−0.3, −0.4) | <0.001 | −0.4 (−0.3, −0.5) | <0.001 |
| Body mass index, per 1 kg/m2 | 6.3 (3.7,8.9) | <0.001 | −0.1 (−0.0, −0.2) | 0.19 | ||||
| Diabetes | 11.2 (−31.3,53.6) | 0.61 | 1.3 (−0.4,3.1) | 0.14 | ||||
| Education < versus ≥ high school diploma | 28.6 (−22.5,79.7) | 0.27 | 2.7 (0.5,4.9) | 0.01 | ||||
| Annual income ≤ versus > $20,000/year | −73.5 (−30.4, −116.7) | <0.001 | −0.1 (−1.9,1.8) | 0.95 | ||||
| Dietary phosphorus, per 100 g/day | 7.4 (3.74,11.1) | <0.001 | 0.3 (0.2,0.5) | <0.001 | ||||
| Serum phosphorus, per 1 mg/dL | 45.8 (11.3,80.3) | 0.01 | −4.2 (−2.8, −5.7) | <0.001 | ||||
Analyses restricted to 1393 African-American participants with plausible 24-hour urine data.
Discussion
We found that greater African ancestry was independently associated with lower 24-hour urinary phosphorus excretion and lower fractional excretion of phosphorus among African Americans with CKD. Additionally, we observed similar differences in urinary phosphorus excretion when comparing African Americans to European Americans. These findings suggest that genetic variability may partly explain racial differences in urinary phosphorus excretion between African Americans and European Americans.
Previous studies have shown lower mean 24-hour urinary phosphorus excretion in African Americans compared with European Americans (16–19). However, self-reported race is tightly linked to socioeconomic disparities, such as inadequate access to healthy foods or medical care, which might confound these associations, making it difficult to determine to what extent previously observed racial differences in urine phosphorus excretion reflected biologic versus environmental effects. Our finding that greater African ancestry was independently associated with lower 24-hour urinary phosphorus excretion and fractional excretion of phosphorus suggest that racial differences in urinary phosphorus excretion are at least partially due to genetic variability and not simply due to differences in dietary intake.
In steady-state conditions, differences in 24-hour urine phosphorus excretion most likely represent differences in gastrointestinal phosphorus absorption. Importantly, daily phosphorus consumption did not differ across quartiles of African ancestry among African-American CRIC study participants. Furthermore, the significant association of higher African ancestry with lower 24-hour urine phosphorus excretion remained after adjustment for dietary intake of phosphorus, making it less likely that differences in phosphorus consumption could explain our finding. Biologic differences in gut phosphorus absorption seem more plausible. Although primarily absorbed through passive paracellular mechanisms, dietary phosphorus is also absorbed via sodium–phosphorus cotransporters through mechanisms actively regulated by vitamin D (20). A previous study showed lower gut calcium absorption in black women treated with oral calcitriol compared with white women treated with oral calcitriol (21), suggesting relative gut resistance to the action of calcitriol in black individuals. Since calcitriol also stimulates phosphorus absorption in the gut, it is possible that the inverse association of African ancestry with urinary phosphorus excretion represents biologic differences in the sensitivity of sodium–phosphorus cotransporters to the activated form of vitamin D in gut epithelial cells. Unraveling the reasons why individuals with higher African ancestry had lower 24-hour urinary phosphorus excretion is necessary to determine the potential clinical applications of our findings.
Urinary fractional excretion of phosphorus is a marker of renal tubular phosphorus processing. The primary hormones involved in regulating fractional excretion of phosphorus are PTH and FGF23. Thus, it is interesting that lower fractional excretion of phosphorus was associated with greater African ancestry in multivariable-adjusted models despite an absence of differences in PTH or FGF23 across quartiles of African ancestry. For any given level of PTH or FGF23, individuals with greater African ancestry had lower fractional excretion of phosphorus, suggesting that another explanation for our observations may be potential resistance to the phosphaturic stimuli of PTH and FGF23 at the level of the renal tubules. Though speculative, it is possible that these findings represent an adaptive advantage for conserving bone mineral density in African Americans that becomes maladaptive in states of phosphorus overload, such as CKD or high dietary phosphorus intake, and thereby impairs the excretion of excess phosphorus.
We did not observe a significant association of African ancestry with other markers of mineral metabolism among African Americans despite marked differences between African Americans and European Americans with regard to these analytes. Although these findings cannot rule out a genetic component to racial differences in these other markers of mineral metabolism, they suggest that factors such as diet, bone turnover, and diurnal variation, may play more important roles. This is consistent with a previous finding from the CRIC study in which differences in concentrations of serum phosphorus between African Americans and European Americans depended on socioeconomic status, indicating that environmental factors at least partly influence differences in systemic phosphorus processing by race (14).
This study had a number of advantages over previous studies, including standardized collection of baseline data, and a large, multiracial cohort of individuals with CKD together with estimates of genetic African ancestry and markers of mineral metabolism, enabling us to report novel associations of African ancestry with urinary phosphorus excretion. However, this study also has limitations. Multiple factors impact the measures of mineral metabolism analyzed in the current study. Thus, it is possible that associations of African ancestry with markers of mineral metabolism were missed because of insufficient power to detect relatively small measures of effect. Because of important variations in ancestry-informative markers among different African subgroups, the results of the current study might only be informative for the African subgroups genotyped in this study and might not be applicable to all African ancestry subgroups. Although associations of African ancestry with 24-hour urinary phosphorus excretion were robust to adjustment for diet phosphorus intake, the diet instrument used to capture estimated phosphorus intake in the CRIC study probably had incomplete ascertainment of total phosphorus intake in study participants because of the high prevalence of phosphorus-based food additives in the United States food supply. We did not have any information on Klotho expression, precluding us from examining whether differences in Klotho might account for differences in urinary phosphorus excretion according to African ancestry.
In summary, greater African ancestry was independently associated with lower 24-hour urinary phosphorus excretion and fractional excretion of phosphorus in African American participants of the CRIC study. These findings support the notion that, in addition to environmental factors, racial differences in urine phosphorus excretion observed in this and previous studies are partly due to genetic factors. Identifying causal genetic loci that could account for these differences should provide important new insights into the biologic factors underlying systemic phosphorus handling, particularly with respect to intestinal and renal phosphorus handling.
Disclosures
None.
Supplementary Material
Acknowledgments
Funding for the CRIC study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the University of Pennsylvania Clinical and Translational Research Center Clinical and Translational Science Award UL1 RR-024134, Johns Hopkins University UL1 RR-025005, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences component of the National Institutes of Health (NIH) and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research UL1RR024986, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, and Kaiser NIH/NCRR UCSF-CTSI UL1 RR-024131.
O.M.G. was supported by grants R01NS080850, R03DK095005 and a Strategically Focused Research Network Center on Health Disparities from the American Heart Association; M.W. was supported by R01DK076116, R01DK081374, R01DK094796, K24DK093723, U01DK099930 and R21DK100754 from the NIH, and a Strategically Focused Research Network Center on Health Disparities from the American Heart Association.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08020715/-/DCSupplemental.
References
- 1.Gutiérrez OM, Isakova T, Andress DL, Levin A, Wolf M: Prevalence and severity of disordered mineral metabolism in Blacks with chronic kidney disease. Kidney Int 73: 956–962, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bell NH, Yergey AL, Vieira NE, Oexmann MJ, Shary JR: Demonstration of a difference in urinary calcium, not calcium absorption, in black and white adolescents. J Bone Miner Res 8: 1111–1115, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Bryant RJ, Wastney ME, Martin BR, Wood O, McCabe GP, Morshidi M, Smith DL, Peacock M, Weaver CM: Racial differences in bone turnover and calcium metabolism in adolescent females. J Clin Endocrinol Metab 88: 1043–1047, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Malluche HH, Mawad HW, Monier-Faugere MC: Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res 26: 1368–1376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotimi CN, Jorde LB: Ancestry and disease in the age of genomic medicine. N Engl J Med 363: 1551–1558, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT, Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI, CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S: Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol 154: 1089–1099, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Peralta CA, Ziv E, Katz R, Reiner A, Burchard EG, Fried L, Kwok PY, Psaty B, Shlipak M: African ancestry, socioeconomic status, and kidney function in elderly African Americans: a genetic admixture analysis. J Am Soc Nephrol 17: 3491–3496, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT, Jr, Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ, AASK Study Investigators. CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodaman N, Aldrich MC, Smith JR, Signorello LB, Bradley K, Breyer J, Cohen SS, Long J, Cai Q, Giles J, Bush WS, Blot WJ, Matthews CE, Williams SM: A small number of candidate gene SNPs reveal continental ancestry in African Americans. Ann Hum Genet 77: 56–66, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosoy R, Nassir R, Tian C, White PA, Butler LM, Silva G, Kittles R, Alarcon-Riquelme ME, Gregersen PK, Belmont JW, De La Vega FM, Seldin MF: Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum Mutat 30: 69–78, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutiérrez OM, Anderson C, Isakova T, Scialla J, Negrea L, Anderson AH, Bellovich K, Chen J, Robinson N, Ojo A, Lash J, Feldman HI, Wolf M, CRIC Study Group : Low socioeconomic status associates with higher serum phosphate irrespective of race. J Am Soc Nephrol 21: 1953–1960, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J: Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest 76: 470–473, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor EN, Curhan GC: Differences in 24-hour urine composition between black and white women. J Am Soc Nephrol 18: 654–659, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Whalley NA, Moraes MF, Shar TG, Pretorius SS, Meyers AM: Lithogenic risk factors in the urine of black and white subjects. Br J Urol 82: 785–790, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Gutiérrez OM, Isakova T, Smith K, Epstein M, Patel N, Wolf M: Racial differences in postprandial mineral ion handling in health and in chronic kidney disease. Nephrol Dial Transplant 25: 3970–3977, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uribarri J: Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin Dial 20: 295–301, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Dawson-Hughes B, Harris SS, Finneran S, Rasmussen HM: Calcium absorption responses to calcitriol in black and white premenopausal women. J Clin Endocrinol Metab 80: 3068–3072, 1995 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.