Abstract
Objectives
To investigate associations between acute care and critical illness hospitalizations and performance on physical functional measures and activities of daily living (ADLs).
Design
Prospective cohort study.
Setting
Large health maintenance organization.
Participants
2926 Participants in Adult Changes in Thought, a study of aging enrolling dementia-free individuals aged 65 and older not living in a nursing home from 1994 to September 30, 2008 (N=2,926).
Measurements
The exposure of interest was hospitalization during study participation, subdivided by presence of critical illness. Outcomes included gait speed, grip strength, chair stand speed, and difficulty and dependence in performing ADLs measured at biennial visits.
Results
Median time between hospital discharge and the next study visit was 311 days (interquartile range (IQR) 151–501 days) after acute care hospitalization and 359 days (IQR 181–420 days) after critical illness hospitalization. Gait speed was slower after acute care (–0.05 m/s, 95% confidence interval (CI)=0.01–0.04 m/s slower, P< .001) and critical illness (–0.16 m/s, 95% CI=–0.22 to –0.10, P< .001). Grip was weaker after acute care hospitalization (–0.8 kg, 95% CI=–1.0 to –0.6, P<.001) but not significantly different after critical illness hospitalization. Chair-stand speed was slower after acute care hospitalization (–0.04 stands/s, 95% CVI=–0.05 to –0.04, P< .001) and critical illness hospitalization (–0.09, 95% CI=–0.15 to –0.03, P=.003). The odds of difficulty with (odds ratio (OR)=1.4, 95% CI=1.2–1.6, P<.001) or dependence in (OR=2.0, 95% CI=1.2–3.2, P=.006) one or more ADLs was higher after acute care hospitalization, as were the odds of difficulty with (OR=1.9, 95% CI=1.1–3.6, P=.03) or dependence in (OR=7.9, 95% CI=2.5–25.7, P=.001) one or more ADLs after critical illness.
Conclusion
In older adults, hospitalization, especially for critical illness, was associated with clinically relevant decline in gait and chair stand speed and strongly associated with difficulty with and dependence in ADLs.
Keywords: activities of daily living, critical illness, disability, long-term outcomes, gait speed
INTRODUCTION
Hospitalized older adults frequently experience declines in physical function during and after hospitalization, and several studies have demonstrated persistence of dysfunction as long as 12 months after discharge.1–7 Some longitudinal studies have included prehospitalization data, strengthening the evidence that illnesses and injuries leading to hospitalization can cause disability in older adults.8, 9 Prospective cohort studies demonstrate that survivors of critical illness frequently experience persistently abnormal health-related quality of life, a high burden of psychological symptoms, and impairments in cognitive and physical function, but few of these studies have been able to account for baseline or premorbid health status.10–14 One study that used longitudinal data discovered that older adults hospitalized with severe sepsis were at greater risk of new and persistent activity of daily living (ADL) disability, but performance-based physical function measures were not available to provide insights into the mechanisms of disability in these individuals.13 It is also not known to what degree severity of acute illness contributes to posthospitalization functional impairment or whether critical illness conveys additional risk of functional impairment beyond acute care hospitalization. Furthermore, inability to account for premorbid functional level creates the risk that some of the observed associations may be spurious.15 Better understanding of those at risk of functional impairment, and the nature of the impairments that develop, might allow for better design of interventional studies aimed at improving functional outcomes, which have thus far yielded largely disappointing results.16–18 A prospective, community-based longitudinal cohort study of aging was used to investigate the association between hospitalizations for acute care and for critical illness and performance on specific physical function measures and ability to perform ADL.
METHODS
Data from the Adult Changes in Thought (ACT) study, an ongoing prospective, population-based, longitudinal study of aging and dementia, were analyzed. The details of this study have been described elsewhere.19, 20 The ACT study population was created from a random sample of individuals aged 65 and older without dementia residing in the Seattle area, not living in a nursing home at baseline, and belonging to the Group Health Cooperative, a consumer-governed health maintenance organization (HMO). Participants were interviewed using structured questionnaires to obtain data on demographic characteristics, medical history, memory, and general functioning, such as ADL,. Participants also received a screen for cognitive function, the Cognitive Abilities Screening Instrument (CASI)21, and were evaluated using performance-based measures of physical function, including dominant-hand grip strength, a timed 10-foot walk test, and a timed chair stand test, at study visits occurring every 2 years. The original cohort was enrolled between 1994 and 1996, with 2,581 of 5,422 eligible individuals agreeing to participate.19 Between 2000 and 2002, an expansion cohort was recruited, adding 811 individuals. In recent years a continuous enrollment strategy has been used, with the goal of keeping the number of alive and at-risk individuals in the cohort at approximately 2,000; study visits through September 30, 2008, were included in the present study. Each study participant was evaluated approximately once every 2 years. The ACT study has an excellent index of completeness of follow-up 22 of greater than 95%. Participants with two or more study visits during which a valid cognitive screening score was obtained were eligible to be included in the present study.
Measures of Physical Function and Functional Disability
At biennial visits, subjects were evaluated using several tests of physical function: dominant-hand grip strength (kg), measured three times and averaged; time to walk 10 feet at usual pace, performed twice and averaged; and total time to stand five times from a chair successfully. Subjects were also queried regarding their ability to perform ADLs23, specifically, ambulating, bathing, transferring, dressing, feeding, and toileting. For each activity, subjects were asked whether they had no difficulty performing the task, could perform the task but did not do so, had some difficulty performing the task, had a lot of difficulty performing the task, or were unable to perform the task. For these analyses, responses were recategorized on a scale from 0 to 3 (able to do without difficulty, able to do with some difficulty, able to do with a lot of difficulty, unable to perform). An ADL composite score was also created by summing these scores for each of the six ADLs, generating a score from 0 to 18, with higher scores indicating more difficulty with or dependence in ADLs.
Exposure Definitions
Associations between hospitalizations that did not include a critical illness (acute care hospitalizations) and critical illness hospitalizations and the outcomes of interest were investigated. The present study linked data from this ongoing prospective cohort study with claims data from hospitalizations of study participants that were submitted to the HMO to which all study participants belonged. Diagnosis and procedure codes from all hospitalizations occurring during study participation were examined. Hospitalizations were identified as including critical illness by the presence of any one of a list of critical illness diagnosis and procedure codes from the International Classification of Diseases, Ninth Revision (On-line Appendix 1, Table 1). Acute care and critical illness hospitalizations were each coded using an indicator variable that changed after the relevant exposure such that visits before such a hospitalization were considered unexposed, and all visits after were considered exposed. In this way, exposures to acute care and critical illness hospitalizations were each considered in a time-dependent fashion, and individuals could contribute data from multiple study visits before and multiple study visits after hospitalization. Individuals who received one or more diagnosis codes for primary brain injury or any fractures during any hospitalization (On-line Appendix 1, Table 2) were censored at the time such a hospitalization occurred to reduce the risk of confounding any association between hospitalization and physical dysfunction.
Table 1.
Baseline Characteristics of Study Participants at Baseline Categorized According to Subsequent Hospitalization
| Characteristic | No Hospitalizations During Study, n=1,542 |
≥1 Acute Care Hospitalizations, n=1,340 |
≥1 Critical Illness Hospitalizations, n=44 |
P- Valuea |
|---|---|---|---|---|
| Age, mean±SD | 74.6±6.0 | 75.2±6.1 | 76.0±6.8 | .01 |
| Female, n (%) | 932 (60.4) | 775 (57.8) | 20 (45.5) | .07 |
| Nonwhite, n (%) | 178 (11.5) | 102 (7.6) | 2 (4.6) | .001 |
| High school graduate, n (%) | 1376 (89.2) | 1174 (87.6) | 32 (72.7) | .002 |
| Difficulty with activities of daily living, n (%) |
||||
| Bathing | 44 (2.9) | 68 (5.1) | 5 (11.4) | <.001 |
| Ambulating | 66 (4.3) | 90 (6.7) | 6 (13.6) | <.001 |
| Transferring | 202 (13.1) | 275 (20.5) | 10 (22.7) | <.001 |
| Dressing | 51 (3.3) | 71 (5.3) | 5 (11.4) | .002 |
| Feeding | 10 (0.6) | 10 (0.7) | 0 (0) | .75 |
| Toileting | 22 (1.4) | 20 (1.5) | 2 (4.5) | .22 |
| Time to walk 10 feet, seconds, mean±SDb |
3.7±2.1 | 3.8±1.4 | 4.2±1.3 | .01 |
| Dominant hand grip strength, kg, mean±SD c |
||||
| Women | 21.6±5.5 | 20.9±5.6 | 21.2±5.1 | .51 |
| Men | 36.1±7.8 | 35.5±8.1 | 34.2±8.1 | <.001 |
| Time to stand from seated position 5 times, seconds, mean±SDd |
14.7±4.7 | 15.0±5.0 | 14.3±4.3 | .25 |
| Exercise ≥ 3 times/wk, n (%) | 1,114 (72.2) | 958 (71.5) | 24 (54.6) | .04 |
| Recently spent time in a nursing home, n (%) |
10 (0.7) | 13 (1.0) | 1 (2.3) | .20 |
| Depressed, n (%)e | 143 (9.3) | 158 (11.8) | 5 (11.6) | .08 |
| Chronic conditions, n (%) | ||||
| Nonskin cancer | 255 (16.5) | 253 (18.9) | 6 (13.6) | .22 |
| Heart disease | 242 (15.7) | 302 (22.5) | 15 (34.1) | <.001 |
| Hypertension | 553 (35.9) | 545 (40.7) | 24 (54.6) | .003 |
| Congestive heart failure | 42 (2.7) | 64 (4.8) | 6 (13.6) | <.001 |
| Diabetes mellitus | 118 (7.7) | 152 (11.3) | 6 (13.6) | .002 |
| Stroke | 41 (2.7) | 44 (3.3) | 1 (2.3) | .59 |
| Parkinson’s disease | 7 (0.5) | 9 (0.5) | 0 (0) | .83 |
| RxRisk score, $, mean±SD f | 3,969.1±2,498.2 | 4,770.4±3,071.9 | 5,678.1±2,379.7 | <.001 |
| Cognitive Abilities Screening Instrument raw score, mean±SD (range 0–100) |
93.3±4.7 | 92.98±4.7 | 93.5±4.5 | .24 |
Test of difference in mean using Pearson chi-square for binary variables, Fisher exact test for binary variables with any cell size ≤10, and analysis of variance for continuous variables
Average of two timed walks; missing for 19 subjects in the group with no hospitalizations, 21 in the acute care hospitalization group, and 2 in the critical illness group
Average of up to three attempts; missing for 17 subjects in the group with no hospitalizations, 17 in the acute care hospitalization group, and 2 in the critical illness group
Missing for 116 subjects in the group with no hospitalizations, 152 in the acute care hospitalization group, and 9 in the critical illness group
Missing for 3 subjects in the group with no hospitalizations, 4 in the acute care hospitalization group, and 1 in the critical illness group
Calculation of RxRisk scores used estimated healthcare costs based on prescription drug fills for the 12-month period before a subject’s enrollment in ACT.
SD=standard deviation.
Table 2.
Difference in Follow-Up Physical Performance Measures According to Hospitalization Status
| Physical Performance Measure | After Acute Care Hospitalization |
After Critical Illness Hospitalization |
|---|---|---|
| Difference in Measurement (95% Confidence Interval) P-Value |
||
| Time to walk 10 feet, seconds | 0.8 (0.6–0.9) <.001 | 1.6 (1.0–2.2) <.001 |
| Adjusted difference | 0.4 (0.2–0.5) <.001 | 1.1 (0.5–1.7) <.001 |
| Dominant hand grip strength, kg | −2.9 (−3.2 to −2.6) <.001 | −3.3 (−4.6 to −1.9) <.001 |
| Adjusted difference | −0.8 (−1.0 to −0.5) <.001 | −1.3 (−2.5 to −0.02) .047 |
| Time to stand from seated position five times, seconds |
0.9 (0.6–1.2) <.001 | 0.3 (−1.6–2.3) .69 |
| Adjusted difference | 0.7 (0.4–0.9) <.001 | 0.13 (−1.9–2.1) .90 |
Linear regression with generalized estimating equations was used to account for repeated observations, specifying an exchangeable correlation matrix and robust variance estimates. The referent group was visits not occurring after any hospitalization.
Adjusted models included age at study visit, sex, an interaction term for age and sex, Cognitive Abilities Screening Instrument score <90, the relevant baseline physical performance measurement value, time since baseline visit, and the RxRisk adjustment score at baseline.
Adjusted gait time was significantly longer after critical illness than acute care (p=.01) hospitalization. There was no significant difference in adjusted grip strength or chair stand time between the critical illness and acute care groups at follow-up.
Statistical Analysis
Baseline differences between groups categorized according to future hospitalization status were evaluated using Pearson chi-square for binary variables, Fisher exact test for binary variables with any cell size of 10 or less, and analysis of variance for continuous variables. To assess the association between acute care or critical illness hospitalizations and physical function, multiple linear regression models were developed using population-averaged generalized estimating equations (GEEs).24 Separate models were created for grip strength, timed walk, and timed chair stands. An exchangeable correlation matrix was specified, and the robust standard error (Huber-White sandwich) estimator was used to allow for valid inference even if the working correlation matrix was incorrectly specified.25 The outcome variable for these models was the physical functional measure at follow-up study visits. The following variables were added to the models a priori based upon known associations between these predictors and physical function: age at study visit, sex, cognitive impairment (CASI score < 90), the relevant baseline physical performance measurement value, time since baseline visit, a positive depression screen (Center for Epidemiologic Studies Depression Scale score ≥10)26, and the burden of comorbid illness (using RxRisk27, a validated risk adjustment score developed at Group Health Cooperative using automated ambulatory pharmacy data) at baseline. Regression analyses were performed using complete case analysis.
To reduce the bias from potential differential missingness, time to perform the 10-foot walk was transformed into gait speed (m/s) (0.3048 m/10 feet /walk time in seconds); individuals unable to perform the test at a given study visit were given a value of 0 for that visit. Similarly, the time to perform five chair stands was transformed into chair stands/second; individuals unable to perform the test at a given study visit were given a value of 0 for that visit.
The association between hospitalization status and inability to perform each physical functional measure was explored using logistic regression with GEEs, adjusting for age at study visit, sex, CASI score, the relevant baseline physical performance measurement value, time since baseline visit, the RxRisk adjustment score at baseline, and the presence of depression.
Associations between acute care and critical illness hospitalizations and incident disability as measured according to ADLs were assessed for using logistic regression with GEEs. Separate models were created to evaluate for associations with difficulty with and dependence in one or more ADLs. Models were adjusted for sex, possible cognitive impairment (CASI<90), age at study visit, baseline ADL composite score, presence of coronary artery disease at baseline, presence of cerebrovascular disease at baseline, RxRisk score at baseline, time since baseline visit, and presence of depression.
To further explore the association between critical illness and decline in physical performance measures and functional independence, additional analyses were performed restricted to individuals experiencing a critical illness hospitalization during study participation using linear regression models with GEEs for each outcome including only a variable for time since study entry and an indicator of when the critical illness hospitalization occurred. In these analyses, each subject served as his or her own control.
All regression models were created using complete-case analyses, in that observations with missing values for any of the covariates in the model were excluded.
Statistical analyses were performed using Stata 13.1 (Stata Corp., College Station, TX). All reported p-values were two-sided, and results were considered statistically significant at the p<.05 level. The institutional review board of Group Health Cooperative approved the parent study and this substudy. All study participants provided written consent to ACT study participation at parent study enrollment.
RESULTS
Data were analyzed for 2,926 participants in the ACT study. Forty-four ACT subjects identified with one or more critical illness hospitalizations experienced a total of 49 critical illness hospitalizations and 98 acute care hospitalizations between their first and last study visits. The most common primary diagnoses for the critical illness hospitalizations were cardiac diagnoses, including myocardial infarction and congestive heart failure, present in 41% of those hospitalizations; respiratory diagnoses, including pneumonia and respiratory failure, in 27%; and nonpneumonia sepsis, in 18%. Subjects received invasive mechanical ventilation in 19 (39%) of these hospitalizations. One thousand three hundred forty subjects identified with one or more acute care hospitalization without any critical illness hospitalizations experienced 2,344 hospitalizations during study participation. One thousand five hundred forty-two subjects who were never hospitalized during study participation were identified. At the baseline study visit, subjects with one or more hospitalizations for critical illness during subsequent study participation were significantly more likely than those without any hospitalizations to be white, to have not graduated from high school, to have more ADL difficulties and poorer physical performance, to a have comorbid illness, and to be less likely to report regular exercise (Table 1). Baseline grip strength was similar across groups and similar to age- and sex-stratified normative values.28, 29 There were few missing values for grip strength and timed walk at the baseline visit, but timed chair stands were missing from the baseline visit for 7.5% of the never hospitalized group, 11.3% of those experiencing acute care hospitalization but never hospitalized for critical illness, and 20% of those being hospitalized for critical illness. Only individuals with nonmissing values for each functional measure of interest at baseline were included in analyses for that measure. Median time between hospital discharge and the next study visit was 311 days (interquartile range (IQR) 151–501 days) for those experiencing one or more acute care hospitalizations and 359 days (interquartile range 181–420 days) for those experiencing one or more critical illness hospitalizations.
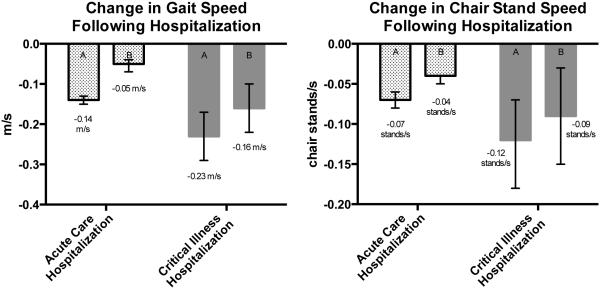
The adjusted time required to walk 10 feet was 0.4 seconds longer at visits after an acute care hospitalization (95% confidence interval (CI)=0.2–0.5, p<.001) and 1.1 seconds longer at visits after a critical illness hospitalization (95% CI=0.5–1.6, p<.001) (Table 2) than at visits not after a hospitalization. By comparison, the average yearly increase in the time it took to walk 10 feet in the whole cohort was only 0.06 seconds per year. Not only was the adjusted walk time longer in each hospitalization group than at visits not after hospitalization, the 0.7-second greater decline in adjusted walk time at follow-up visits after a critical illness hospitalization was statistically significantly different from that after an acute care hospitalization (p=.01). Hospitalization status was strongly associated with inability to perform the timed walk test at follow-up study visits. After adjustment for prehospitalization performance and other confounders, the odds of being unable to perform the timed walk was more than four times as high after acute care (OR=4.3, 95% CI=2.4–7.8, p<.001) and 10 times as high after critical illness (OR=10.1, 95% CI=2.7–38.1, p=.001) hospitalizations as at visits not after any hospitalization (Table 3). Therefore, data were reanalyzed, with a value of 0 assigned for gait speed (1/walk time) for individuals for whom study staff indicated inability to perform the timed walk at follow-up visits. Adjusted gait speed was found to be significantly slower after acute care (0.05 m/s slower, 95% CI=0.01–0.04 m/s slower, p< .001) and critical illness (0.16 m/s slower, 95% CI=0.10–0.22 m/s slower, p< .001) hospitalizations (Figure 1) than at visits not after hospitalization. This 0.11-m/s difference in adjusted gait speed at follow-up visits between the two hospitalization groups was statistically significant (p<.001).
Table 3.
Inability to Participate in Timed Walk and Timed Chair Stands According to Hospitalization Status
| Physical Performance Measure | Following Acute care Hospitalization |
Following Critical Illness Hospitalization |
|---|---|---|
| Odds Ratio (95% Confidence Interval) P-Value | ||
| Inability to perform timed walk | 4.3 (2.5–7.3) <.001 | 8.2 (1.7–38.1) <.007 |
| Adjusted | 4.6 (2.5–8.5) <.001 | 8.3 (1.6–43.2) .01 |
| Inability to perform timed chair stands |
3.2 (2.8–3.8) <.001 | 5.0 (2.8–9.0) <.001 |
| Adjusted | 2.1 (1.8–2.5) <.001 | 3.3 (1.7–6.3) <.001 |
Logistic regression with generalized estimating equations was used to account for repeated observations, specifying an exchangeable correlation matrix and robust variance estimates. The reference group was visits not after any hospitalization.
Adjusted models included age at study visit, sex, Cognitive Abilities Screening Instrument score < 90, the relevant baseline physical performance measurement value, time since baseline visit, the RxRisk adjustment score at baseline, and depression.
Figure 1.
Change in gait speed and chair-stand speed after hospitalization in (A) unadjusted and (B) adjusted analyses; error bars represent 95% confidence intervals. Adjusted for age at study visit, sex, Cognitive Abilities Screening Instrument score < 90, the relevant baseline physical performance measurement value, time since baseline visit, the RxRisk adjustment score at baseline, and depression. Baseline gait speed was 0.92 m/s in those never hospitalized (n=1,523), 0.87 m/s in those with one or more acute care hospitalizations (n=1,426), and 0.80 m/s in those with one or more critical illness hospitalizations (n=43). Baseline chair speed was 0.37 stands/s in those never hospitalized (n=1,426), 0.37 stands/s in those with one or more acute care hospitalizations (n=1,188), and 0.38 stands/s in those with one or more critical illness hospitalizations (n=35).
Adjusted dominant-hand grip strength was significantly weaker at visits after acute care (0.8 kg weaker, 95% CI=–1.0 to –0.5, p<.001) and critical illness (1.2 kg weaker, 95% CI=–2.5 to –0.2, p=.047) hospitalizations than at visits not after any hospitalization; the difference between the two hospitalization groups was not significant (p=.44). Adjusted performance on the timed chair stands was worse at visits after acute care hospitalization than at visits not after hospitalization (0.7 seconds longer, 95% CI=0.4–0.9, p<.001) but not significantly different at visits after critical illness hospitalization and visits not after hospitalization. As with the timed walk, hospitalization status was strongly associated with inability to perform the timed chair-stand test. The adjusted odds of being unable to perform timed chair stands was more than two times as high at visits after acute care hospitalizations (OR=2.1, 95% CI=1.8–2.5, p<.001) and more than three times as high at visits after critical illness (OR=3.3, 95% CI=1.7–6.3, p<.001) as at visits not after hospitalization. Therefore, data were reanalyzed with a value of 0 assigned for chair stand speed (1/chair-stand time) to individuals for whom study staff indicated inability to perform the timed chair-stand test at follow-up visits. In this analysis, the adjusted speed with which the timed chair-speed test was performed was significantly slower at study visits after acute care (0.04 chair-stands/s slower, 95% CI=–0.05 to –0.04, p<.001) and critical illness (0.09 chair-stands/s slower, 95% CI=–0.15 to –0.03, p=.003) hospitalizations than at visits not after any hospitalization (Figure 1); the difference between the two hospitalization groups was not statistically significant.
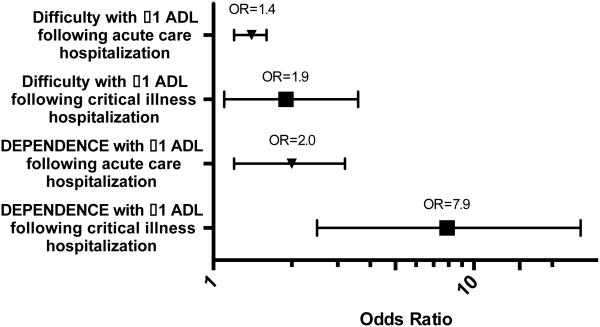
Subjects reported significant declines in functional independence after acute care and critical illness hospitalizations. At their final study visit before a critical illness hospitalization, none of the 44 subjects reported dependence in any ADLs. In the first study visit after a critical illness hospitalization, five of these subjects reported dependence in one or more ADLs, and none of these five had been diagnosed with dementia. The adjusted odds of reporting difficulty with one or more ADLs was 40% higher at visits after an acute care hospitalization (OR=1.4, 95% CI=1.2–1.6, p<.001) and nearly twice as high at visits after a critical illness hospitalization (OR=1.9, 95% CI=1.1–3.6, p=.03); this difference between the two hospitalization groups was not statistically significant (Figure 2). The adjusted odds of dependence in one or more ADLs were twice as high after acute care hospitalization (OR=2.0, 95% CI=1.2–3.2, p=.006) and nearly eight times as high after a critical illness hospitalization (OR=7.9, 95% CI=2.5–25.7, p=.001). The difference in the odds of dependence in one or more ADLs between these two hospitalization groups was highly statistically significant (p=.001).
Figure 2.
Odds of activity of daily living (ADL) difficulty and dependence at follow-up visits according to hospitalization status. Error bars represent 95% confidence intervals. Models included age at study visit, sex, Cognitive Abilities Screening Instrument score < 90, baseline ADL composite score, time since baseline visit, and the RxRisk adjustment score at baseline.
In models restricted to those experiencing one or more critical illness hospitalizations and accounting for expected decline over time, the occurrence of that critical illness hospitalization was associated with a decline of 0.11 chair stands/s (95% CI=0.18–0.03 slower, p=.004), a decline in gait speed of 0.12 m/s (95% CI=0.21–0.03 m/s slower, p=.01), and an increase in ADL composite score of 1.4 points (95% CI=0.20–2.54, p=.023) at subsequent study visits.
DISCUSSION
This study found associations between critical illness and acute care hospitalizations and subsequent decline in physical function and functional independence measured a median of 311 days after acute care hospitalizations and 359 days after critical illness hospitalizations. These associations are statistically and clinically significant and provide strong evidence of the burdens of critical illness survivorship that many older adults experience. In this population-based cohort, subjects who eventually experienced critical illness had a higher burden of chronic illness, worse physical function, and more difficulty with ADLs at baseline. Together, these findings provide evidence not only that debility may increase the likelihood of critical illness, but also that critical illness is associated with subsequent functional decline.
Acute care hospitalization and critical illness hospitalization were each associated with significant declines in gait speed after adjusting for likely confounders, and the decline in gait speed was significantly larger after critical illness hospitalization than acute care hospitalization. Gait speed in older adults reflects functional and physiological ability, and the decline in gait speed demonstrated in this study, particularly after hospitalizations for critical illness, was substantial and likely to have a significant effect. Gait speed has been shown to be a powerful predictor of future disability and survival in older adults, and changes in gait speed are associated with changes in quality of life.30–33 This decline in gait speed after critical illness of 0.16 m/s represents a substantial and meaningful change in gait speed, because the minimum clinically important difference for this measure is 0.1 m/s.34–37 Not only was gait slower after critical illness, but subjects were also much less likely to be able to perform the walk and timed chair-stand tests after hospitalization. This striking finding indirectly but powerfully demonstrates significant functional impairment.
Although grip strength declined significantly after acute care and critical illness hospitalizations, the small decline in grip strength observed in this study is well below the minimum clinically important difference and thus is unlikely to be clinically significant.38 Although a number of studies have demonstrated that lower grip strength is associated with accelerated decline in ADL disability, the prior evidence on the utility of handgrip strength as a surrogate for global muscle strength has been mixed.39–42 The contrast between the current findings for grip strength and those for gait speed and chair stand time after critical illness raises a question about whether critical illness affects proximal and distal muscle function differently, although given the small sample size, power limitation is an alternative explanation.43
There was a very strong association between acute care and critical illness hospitalizations and the development of difficulty with and dependence in ADL performance. Other studies have shown that ADL disability has been associated with postdischarge mortality, and all of the follow-up study visits occurred well beyond the 1-month window during which most postdischarge ADL recovery has been demonstrated to occur in older adults.44 Community-dwelling older survivors of critical illness are frequently discharged to skilled nursing and other care facilities, with them and their providers often planning for or assuming an eventual return to community living.45, 46 Functional ability is the primary determinant of the need for long-term care, and the strong association between critical illness and the acquisition of new, persistent functional disabilities seen in this study raises the possibility that many survivors might permanently lose the ability to reside outside of a care facility after their intensive care unit stay.47
Critical illness hospitalization was associated with significant declines in physical functional measures and ADL independence in analyses restricted to the group experiencing a critical illness hospitalization, after adjusting for time. This further supports a true association between the episode of critical illness and substantial functional decline. Although the group that went on to experience critical illness had worse physical performance and more comorbid illness at baseline, these findings provide evidence that these baseline characteristics alone do not explain the association, because in these analyses individuals were, in effect, serving as their own controls.
There are several important limitations to this study. Although the associations were statistically significant after adjustment for potential confounders, the existence of important unmeasured confounders remains possible. Because the interval between study visits was 2 years or more, there was time for the development of functional impairment between prior study visit and hospitalization, and thus the arrow of causation might actually be reversed. That is, functional decline may be leading to hospitalizations, and it is possible that greater functional decline might make critical illness more likely. A better understanding of the cause-and-effect relationship is important, particularly in trying to design interventions to improve these outcomes, although from another perspective, causation is less relevant because it remains true that older adults who are hospitalized, particularly those experiencing critical illness, are at significantly greater risk of functional impairment. The interval between hospitalization and follow-up visit means that initial postdischarge disability and recovery could not be fully evaluated, so subjects may have partially recovered from worse disability and performance between hospital discharge and study follow-up. Another limitation is that it was possible to evaluate functional outcomes only in survivors of hospitalization who remained in the study. It is possible that those with severe functional impairment might have been less likely to continue to participate in the study, leading to underestimation of the association between hospitalization and functional impairment. The fact that this prospective cohort study enrolled relatively healthy, community-dwelling older adults may also reduce the generalizability of these results to the general population. In particular, the HMO members who participate in this study are better educated and have higher socioeconomic status than the general population, and enrollment criteria resulted in a cohort with better baseline functional and cognitive status than the general population of older adults. These factors make it possible that the findings underestimate the functional impairment that survivors of acute care hospitalization or critical illness from the general population, who are more likely to have additional vulnerabilities such as poverty or disability before admission, experience. Finally, the definition of critical illness was intended to be specific rather than sensitive; it is likely that this definition misses many subjects whose hospitalization included time in an intensive care unit. If many critically ill individuals were misclassified into the acute care hospitalization group, it would likely introduce conservative bias and thus attenuate the observed differences between the two groups.
These findings identify hospitalization, and particularly critical illness hospitalization, as an important risk factor for clinically important, persistent functional impairment. This information underscores the importance of studying in-hospital interventions aimed at maintaining physical function, such as early mobility programs for critically ill individuals, as well as posthospitalization interventions, such as rehabilitation programs.48–50 It also alerts primary care physicians who care for individuals after hospitalization to pay particular attention to declines in physical function and development of new disability.
Supplementary Material
ACKNOWLEDGMENTS
Dr. Ehlenbach was supported by a Paul Beeson Career Development Award in Aging Research (K23AG038352) funded by the National Institute on Aging (NIA), the Atlantic Philanthropies, the John A. Hartford Foundation, the Starr Foundation and an anonymous donor. Dr. Larson and the ACT study are supported by the National Institutes of Health (NIH) (NIA U01AG006781–25). Dr. Curtis was supported by the National Heart, Lund, and Blood Institute (NHLBI) (K24HL068593–10) and National Center for Research Resources (R01NR005226–12). Dr. Hough was supported by the NHLBI (R01-HL096504) and the John A. Hartford Foundation.
Sponsor’s Role: The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Dr. Ehlenbach had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ehlenbach, Larson, Curtis, Hough. Acquisition of data: Larson. Analysis and interpretation of data: Ehlenbach, Larson, Curtis, Hough. Drafting of the manuscript: Ehlenbach, Hough. Critical revision of the manuscript for important intellectual content: Ehlenbach, Larson, Curtis, Hough. Statistical analysis: Ehlenbach. Obtained funding: Ehlenbach, Larson, Hough. Administrative, technical, or material support: Larson. Study supervision: Larson, Hough.
REFERENCES
- 1.Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med. 1982;16:1033–1038. doi: 10.1016/0277-9536(82)90175-7. [DOI] [PubMed] [Google Scholar]
- 2.Landefeld CS, Palmer RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332:1338–1344. doi: 10.1056/NEJM199505183322006. [DOI] [PubMed] [Google Scholar]
- 3.Sager MA, Rudberg MA, Jalaluddin M, et al. Hospital admission risk profile (HARP): Identifying older patients at risk for functional decline following acute medical illness and hospitalization. J Am Geriatr Soc. 1996;44:251–257. doi: 10.1111/j.1532-5415.1996.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 4.Sager MA, Franke T, Inouye SK, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med. 1996;156:645–652. [PubMed] [Google Scholar]
- 5.Wu AW, Yasui Y, Alzola C, et al. Predicting functional status outcomes in hospitalized patients aged 80 years and older. J Am Geriatr Soc. 2000;48:S6–S15. doi: 10.1111/j.1532-5415.2000.tb03142.x. [DOI] [PubMed] [Google Scholar]
- 6.Inouye SK, Wagner DR, Acampora D, et al. A predictive index for functional decline in hospitalized elderly medical patients. J Gen Intern Med. 1993;8:645–652. doi: 10.1007/BF02598279. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch CH, Sommers L, Olsen A, et al. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38:1296–1303. doi: 10.1111/j.1532-5415.1990.tb03451.x. [DOI] [PubMed] [Google Scholar]
- 8.Gill TM, Allore HG, Holford TR, et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 9.Gill TM, Allore HG, Gahbauer EA, et al. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304:1919–1928. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 12.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39:371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 13.Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwashyna TJ, Netzer G, Langa KM, et al. Spurious inferences about long-term outcomes: The case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841. doi: 10.1164/rccm.201109-1660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuthbertson BH, Rattray J, Campbell MK, et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: A pragmatic randomised controlled trial. BMJ. 2009;339:b3723. doi: 10.1136/bmj.b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott D, McKinley S, Alison J, et al. Health-related quality of life and physical recovery after a critical illness: A multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care. 2011;15:R142. doi: 10.1186/cc10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denehy L, Skinner EH, Edbrooke L, et al. Exercise rehabilitation for patients with critical illness: A randomized controlled trial with 12 months of follow-up. Crit Care. 2013;17:R156. doi: 10.1186/cc12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: A prospective cohort study. Arch Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, van Belle G, Kukull WB, et al. Predictors of functional change: A longitudinal study of nondemented people aged 65 and older. J Am Geriatr Soc. 2002;50:1525–1534. doi: 10.1046/j.1532-5415.2002.50408.x. [DOI] [PubMed] [Google Scholar]
- 21.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. discussion 62. [DOI] [PubMed] [Google Scholar]
- 22.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359:1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 23.Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 24.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 25.Diggle PJ, Heagerty P, Liang K, et al. Analysis of Longitudinal Data. Oxford University Press; New York: 2002. [Google Scholar]
- 26.Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 27.Fishman PA, Goodman MJ, Hornbrook MC, et al. Risk adjustment using automated ambulatory pharmacy data: The RxRisk model. Med Care. 2003;41:84–99. doi: 10.1097/00005650-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Mathiowetz V, Kashman N, Volland G, et al. Grip and pinch strength: Normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74. [PubMed] [Google Scholar]
- 29.Massy-Westropp NM, Gill TK, Taylor AW, et al. Hand grip strength: Age and gender stratified normative data in a population-based study. BMC Res Notes. 2011;4:127. doi: 10.1186/1756-0500-4-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fritz S, Lusardi M. White paper: “Walking speed: The sixth vital sign.”. J Geriatr Phys Ther. 2009;32:46–49. [PubMed] [Google Scholar]
- 32.Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38:2096–2100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 33.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 34.Tilson JK, Sullivan KJ, Cen SY, et al. Meaningful gait speed improvement during the first 60 days poststroke: Minimal clinically important difference. Phys Ther. 2010;90:196–208. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barthuly AM, Bohannon RW, Gorack W. Gait speed is a responsive measure of physical performance for patients undergoing short-term rehabilitation. Gait Posture. 2012;36:61–64. doi: 10.1016/j.gaitpost.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Palombaro KM, Craik RL, Mangione KK, et al. Determining meaningful changes in gait speed after hip fracture. Phys Ther. 2006;86:809–816. [PubMed] [Google Scholar]
- 37.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 38.Lang CE, Edwards DF, Birkenmeier RL, et al. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil. 2008;89:1693–1700. doi: 10.1016/j.apmr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taekema DG, Gussekloo J, Maier AB, et al. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 2010;39:331–337. doi: 10.1093/ageing/afq022. [DOI] [PubMed] [Google Scholar]
- 40.Giampaoli S, Ferrucci L, Cecchi F, et al. Hand-grip strength predicts incident disability in non-disabled older men. Age Ageing. 1999;28:283–288. doi: 10.1093/ageing/28.3.283. [DOI] [PubMed] [Google Scholar]
- 41.Ali NA, O’Brien JM, Jr., Hoffmann SP, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178:261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 42.Lee JJ, Waak K, Grosse-Sundrup M, et al. Global muscle strength but not grip strength predicts mortality and length of stay in a general population in a surgical intensive care unit. Phys Ther. 2012;92:1546–1555. doi: 10.2522/ptj.20110403. [DOI] [PubMed] [Google Scholar]
- 43.De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: A prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 44.Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gehlbach BK, Salamanca VR, Levitt JE, et al. Patient-related factors associated with hospital discharge to a care facility after critical illness. Am J Crit Care. 2011;20:378–386. doi: 10.4037/ajcc2011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kane RA, Kane R. Long-Term Care Principles, Programs and Policies. Springer Publishing; New York: 1987. [Google Scholar]
- 47.Unroe M, Kahn JM, Carson SS, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: A cohort study. Ann Intern Med. 2010;153:167–175. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adler J, Malone D. Early mobilization in the intensive care unit: A systematic review. Cardiopulm Phys Ther J. 2012;23:5–13. [PMC free article] [PubMed] [Google Scholar]
- 49.Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: A systematic review and meta-analysis. Crit Care Med. 2013;41:1543–1554. doi: 10.1097/CCM.0b013e31827ca637. [DOI] [PubMed] [Google Scholar]
- 50.Brummel NE, Jackson JC, Girard TD, et al. A combined early cognitive and physical rehabilitation program for people who are critically Ill: The Activity and Cognitive Therapy in the Intensive Care Unit (ACT-ICU) Trial. Phys Ther. 2012;92:1580–1592. doi: 10.2522/ptj.20110414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.