Abstract
Dynorphin A (1–17), an endogenous opioid neuropeptide, can have pathophysiological consequences at high concentrations through actions involving glutamate receptors. Despite evidence of excitotoxicity, the basic mechanisms underlying dynorphin-induced cell death have not been explored. To address this question, we examined the role of caspase-dependent apoptotic events in mediating dynorphin A (1–17) toxicity in embryonic mouse striatal neuron cultures. In addition, the role of opioid and/or glutamate receptors were assessed pharmacologically using MK(+)801, a non-equilibrium N-methyl-D-aspartate (NMDA) antagonist; CNQX, a competitive α–amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA)/kainate antagonist; or (−)-naloxone, a general opioid antagonist. The results show that dynorphin A (1–17) (≥10 nM) caused concentration-dependent increases in caspase-3 activity that were accompanied by mitochondrial release of cytochrome c and the subsequent death of cultured mouse striatal neurons. Moreover, dynorphin A-induced neurotoxicity and caspase-3 activation were significantly attenuated by the cell permeable caspase inhibitor, Z-DEVD-FMK, further suggesting an apoptotic cascade involving caspase-3. AMPA/kainate receptor blockade significantly attenuated dynorphin A-induced cytochrome c release and/or caspase-3 activity, while NMDA or opioid receptor blockade typically failed to prevent the apoptotic response. Last, dynorphin-induced caspase-3 activation was mimicked by the ampakine CX546 [1-(1,4-Benzodioxan-6-ylcarbonyl)piperidine], which suggests that the activation of AMPA receptor subunits may be sufficient to mediate toxicity in striatal neurons. These findings provide novel evidence that dynorphin-induced striatal neurotoxicity is mediated by a caspase-dependent apoptotic mechanism that largely involves AMPA/kainate receptors.
Keywords: AMPA receptors, NMDA receptors, neurotoxicity, opioid receptors, drug abuse, CX546
INTRODUCTION
Dynorphin A (1–17) (dynorphin A) is an endogenous opioid peptide derived from the preprodynorphin gene (Civelli et al., 1985) that is antinociceptive at physiological concentrations through actions at κ opioid receptors (Herman and Goldstein, 1985; Skilling et al., 1992; Shukla and Lemaire, 1994; Xue et al., 1995; Carrion et al., 1998, 1999; Laughlin et al., 1997, 2001; Tan-No et al., 2001; Vanderah et al., 2000; Cheng et al., 2002) However, following drug abuse, neurotrauma and some neurodegenerative disorders, prodynorphin mRNA and peptide levels are often significantly elevated with pathophysiological consequences (Caudle and Maness, 2000; Dubner and Ruda, 1992; Hauser et al., 1999; Iadarola et al., 1988; Laughlin et al., 2001; Long et al., 1988; Shukla and Lemaire, 1994). At high concentrations or with prolonged exposure, dynorphin can modify neuronal death through actions at NMDA, AMPA/kainate, and/or opioid receptors (Bakshi and Faden, 1990; Bakshi et al., 1992; Chen et al., 1995a; 1995b; Goody et al., 2003; Hauser et al., 1999; Kolaj et al., 1995; Laughlin et al., 1997; Skilling et al., 1992; Tang et al., 1999; Walker et al., 1982). Dynorphin A peptide precursors can induce cytotoxicity through specific protein-protein interactions with intracellular targets (Tan-No et al., 2001). Despite the established neurotoxicity, the mechanisms (necrosis versus apoptosis) and particular signaling pathways by which dynorphin induces cell death are incompletely understood.
Apoptosis is an important cellular process in a variety of different biological systems in which cell death occurs without inflammatory processes. These include normal cell turnover in the immune system, embryonic development, metamorphosis, responses to environmental stress (Cohen et al., 1992; Ellis et al., 1991; Granerus and Engstrom, 1996; Jacobson et al., 1997), and in various disease and injury processes including spinal cord trauma (Ashkenazi and Dixit, 1998; Sastry and Rao, 2000; Springer et al., 1999). Studies in Caenorhabditis elegans showed that the ced-3 gene, encoding a cysteine protease, is a critical component of the cell death machinery (Yuan et al., 1993) and this cell death is inhibited by ced-9 (Horvitz 1999). This finding prompted the identification of a conserved family of cysteine proteases homologous to ced-3, termed caspases that were present in mammals. Caspases can be broadly classified into several subgroups, which include those that initiate apoptosis (such as caspases-8 and 9) and effector caspases that cause many of the biochemical events that are hallmarks of apoptosis (such as caspases-3, 6 and 7) (Creagh and Martin, 2001; Ravagnan et al., 2002; van Loo et al., 2002).
Mitochondrial dysfunction is one of the key determinants in signaling apoptotic cell death. When cytochrome c is released into the cytoplasm, it binds to a cytosolic protein, Apaf-1 (apoptosis activating factor-1), and in the presence of ATP recruits procaspase-9 to form the apoptosome, which cleaves and activates procaspase-3 (Alnemri et al., 1996; Beere et al., 2000; Goldstein et al., 2000; Mattson and Duan, 1999; Nicholson and Thornberry, 1997; Schuler et al., 2000).
Once activated the effects of caspase-3 are believed to be irreversible (Li et al., 1996, 1997). Our findings demonstrate that the toxicity induced by dynorphin A in striatal neurons occurs through a novel mechanism that involves the activation of AMPA/kainate receptors and the caspase-3 apoptotic cascade.
MATERIAL AND METHODS
Materials
Dulbecco’s Modified Eagle’s Medium (DMEM), F-12, B27, and antibiotic-antimycotic (penicillin/streptomycin/amphoterin) were purchased from Gibco/Life Technologies (Grand Island, NY, USA). Purified mouse anti-cytochrome c monoclonal antibody was obtained from BD PharMingen, (San Diego, CA, USA). Insulin, linoleic acid, and protein G beads were purchased from Sigma-Aldrich (St. Louis, MO, USA). (−)-Naloxone hydrochloride, MK(+)801, CNQX (6-Cyano-7-nitroquinoxaline-2,3-dione), CX546 [1-(1,4-Benzodioxan-6-ylcarbonyl)piperidine] and nor-binaltorphimine dihydrochloride (nor-BNI) were obtained from RBI (Natick, MA, USA). Ac-Asp-Glu-Val-Asp- 7-amino-4-methylcoumarin (Ac-DEVD-AMC) and the reversible aldehyde inhibitor, Ac-DEVD-CHO (Ac-Asp-Glu-Val-Asp-aldehyde) were obtained from Bachem Bioscience (King of Prussia, PA, USA) or from Molecular Probes (Eugene, OR, USA). Caspase-3 inhibitor-II (Z-DEVD-FMK) was purchased from Calbiochem-Novabiochem Corp. (La Jolla, CA, USA). Dynorphin A (1–17) was purchased from Peninsula Laboratories (San Carlos, CA, USA). BCA protein assay kits were purchased from Pierce (Rockford, IL, USA). Bio-Rad Ready gels (10% and 12%) and Kaleidoscope prestained standards were obtained from Bio-Rad Laboratories (Hercules, CA, USA). The ECL Western blotting analysis system containing peroxidase labeled anti-mouse and anti-rabbit antibodies was purchased from Amersham Pharmacia Biotech (Buckinghamshire, England). Pregnant ICR mice were obtained from Harlan-Sprague-Dawley (Indianapolis, Indiana, USA).
Mouse Striatal Neuronal Cultures and Measurement of Neuronal Viability
Striatal neuronal cultures from embryonic day 15 (E15) ICR mice were prepared as previously described (Goody et al., 2003). Fetal striata were dissected, dissociated, and grown for 5–7 days in vitro (DIV) prior to assay in serum-free DMEM/F-12 medium supplemented with 2% B-27, 1 μg/ml linoleic acid, 25-μg/ml insulin, and 1% antibiotic/antimycotic. Striatal neurons were seeded in identical densities (4 × 105 cells/ well) and grown on poly-D-lysine (0.1 mg/ml) coated 24-well plates at 35 °C in 5% CO2/ 95% air. Neuronal viability was assessed as described before (Goody et al., 2003). Briefly, coverslips were securely attached into 24-well Costar plates using Syl-Gard (Dow Corning, MI, USA) and the underside of wells scored to aid in locating the same neurons repeatedly. Time-lapse digital microscopic images of individual neurons were collected using a Spot 2 digital camera (Diagnostic Instruments, Sterling Heights, MI, USA) and a Nikon Diaphot inverted microscope with phase contrast optics and a 20X objective (Goody et al., 2003). Z-DEVD-FMK was dissolved in dimethyl sulfoxide to the final volume 0.05% v/v dimethyl sulfoxide in all treatment groups. Cells were pretreated with the cell-permeable caspase-3 inhibitor Z-DEVD-FMK for 4 h prior to exposure to dynorphin A (1–17).
Caspase-3 Activity
Caspase-3 protease activity was measured as previously described (Rigamonti et al., 2000). Embryonic mouse striatal neurons were continuously treated with dynorphin A (1–17) (10 μM) in the presence and absence of various antagonists and harvested in ice-cold harvesting buffer (25 mM HEPES, pH 7.5, 5 mM EDTA, 1 mM EGTA, 5 mM magnesium chloride, 10 mM sucrose, 5 mM dithiothreitol, 1% 3-[-(3-chloramidopropyl)dimethylammonio]-1-propanesulfonic acid (CHAPS), 10 μg/ml pepstatin, 10 μg/ml leupeptin and 1 mM PMSF). After freezing and thawing 3 times, the cell lysates were centrifuged for 10 min at 5000 rpm, and the supernatants were centrifuged at 10,000g for 60 min. The cell lysates thus obtained were stored at −80 °C. Lysates were incubated at 37 °C in a buffer containing 25 mM HEPES, pH 7.5, 10% sucrose, 0.1% CHAPS, and 1 mM dithiothreitol supplemented with 50 μM Ac-DEVD-7- amino-4-methylcoumarin (AMC) in 96-well Costar plates. As a negative control, Ac-DEVD-CHO, a caspase-3 inhibitor, was added to the cell lysates 30 min before incubation with caspase-3 substrate, Ac-DEVD-AMC. The increase in fluorescence after the cleavage of the fluorogenic AMC moiety was monitored using a Cytofluor 4000 fluorimeter (Perspective Biosystems, Framingham, MA, USA) at 360 nm excitation and 460 nm emission wavelengths. Caspase-3 activity was normalized to protein concentration and expressed as fluorescence units or units per milligram of total cytosolic protein.
Mitochondrial Cytochrome c Release
Release of cytochrome c from mitochondria was measured as previously described (Yang et al., 1997). After incubation of the striatal neurons with dynorphin A in the presence and absence of various antagonists, cells were harvested with ice-cold buffer A containing 20 mM HEPES-KOH, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, and 250 mM sucrose. Cell homogenates were centrifuged first at 750 g for 10 min at 4 °C and the supernatant was centrifuged at 12,000g for 30 min at 4 °C. The supernatant fraction and the pellet containing mitochondria resuspended in buffer A were frozen at −80 °C until assayed. The supernatant (cytosolic) fraction and the mitochondrial pellet fraction were used for the detection of cytochrome c. Equal amounts of cytosolic and mitochondrial proteins from control and treated cultures were immunoprecipitated with 1 μg of purified mouse anti-cytochrome c monoclonal antibody (clone.6H2.B4 mouse IgG from BD PharMingen) for 3 h at 4 °C and incubated 10 μl of a 50% slurry of protein G-agarose beads in PBS overnight at 4 °C. Immunoprecipitates were then washed three times with PBS and resuspended in sodium dodecyl sulfate (SDS)-sample buffer and electrophoresed on SDS-polyacrylamide gel (SDS-PAGE). Proteins were electrophoresed to blotting PVDF membranes and then incubated with primary monoclonal antibodies against cytochrome c (7H8.2C12 mouse IgG, PharMingen, 1:333 dilution) at room temperature for 3 h. Finally, proteins were visualized using a peroxidase-conjugated antibody to mouse IgG and a chemiluminescence detection system. After immunoblotting, the bands on the filter were scanned and analyzed (Scion Image; Frederick, MD, USA). For each treatment group, the optical densities of mitochondrial and cytosolic cytochrome c bands were expressed as a relative change from untreated control values (fold-change) for the same filter. Analysis of the semiquantitative immunoblot data showed that cytosolic cytochrome c levels were as a rule inversely proportional to mitochondrial levels. For this reason, only cytosolic cytochrome c levels were reported.
Protein Determination
Protein concentrations were determined by the BCA-method using a commercially available kit (Pierce Biotechnology, Inc.; Rockford, IL, USA).
Radioimmunoassay (RIA)
The procedure was described elsewhere (Christensson-Nylander et al., 1985). Briefly, cell culture medium mixed with acetic acid (1 M final) was run through SP-Sephadex ion exchange C-25 column. Leu-enkephalin-Arg6, dynorphin A (13–17) and dynorphin A were eluted in different fractions and detected by RIA of Leu-enkephalin-Arg6 and dynorphin A.
Mass spectrometry (MS)
For electrospray (ES) ionization tandem mass spectrometry (ESI MS/MS), peptide fractions were purified from medium of primary neuronal cultures incubated with 100 μM of dynorphin A for 4 and 72 h using SEP-PAC columns (C18, Waters Inc., Manchester, UK) and C18 ZipTips (Millipore). Medium mixed with trifluoroacetic acid (TFA; 1% final) was loaded onto SEP-PAC columns, equilibrated with 1% TFA, and after washing with 1% TFA and 30% acetonitrile/1 % TFA, peptides were eluted with 60% acetonitrile/1 %TFA, dried and loaded onto ZipTips in 0.1% TFA. The ZipTips were preliminary activated and equilibrated using 10 μl 70% acetonitrile/0.1% TFA two times, 10 μl 50% acetonitrile/0.1% TFA twice and finally twice with 10 μl 0.1% TFA. Samples were loaded onto the ZipTip by pipetting 20 times and washed using 10 μl 0.1% TFA twice. Peptides were eluted with 60% acetonitrile/1% acetic acid.
The mass spectrometer (Q-TOF, Micromass, Manchester, UK) was equipped with an orthogonal sampling ES-interface (Z-spray, Micromass). Samples were introduced via gold-coated nano-ES needles (Protana). A capillary voltage of 800–1000 V was applied together with a cone voltage of 40–45 V and a collision energy of 4.2 eV. The sample aerosol was desolvated in a stream of nitrogen. During collision-induced dissociation (CID) the collision energy was in the range of 15–30 eV and argon was used as the collision gas. Data was acquired for samples incubated for 4 h and 72 h. Incubations were conducted with or without dynorphin A (100 μM).
Statistical Analysis
Data were reported as the mean ± SEM. Significant overall differences among experimental groups were assessed using ANOVA (STATISTICA, version 6, StatSoft, Inc., Tulsa, OK). When overall differences were noted (P < 0.05), individual group differences were compared post-hoc using Duncan’s test. A nonparametric, Kruskal-Wallis ANOVA was used when non-homogenous variances were detected across experimental groups (as determined using Levene’s test) and subsequent comparisons between individual dynorphin vs. dynorphin plus antagonist-treated groups were made using the Mann-Whitney U test.
RESULTS
Dynorphin Increases Caspase-3 Activity
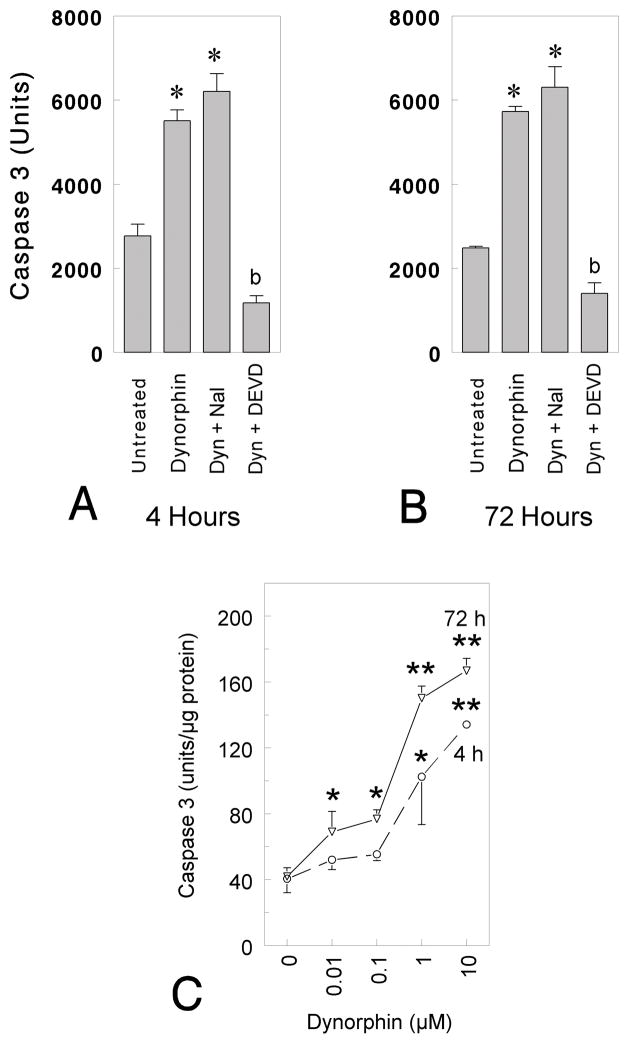
To address whether caspase-3 might contribute to dynorphin A-induced apoptosis, mouse striatal neurons were incubated with dynorphin A in the presence or absence of the caspase-3 inhibitor Ac-DEVD-CHO (Fig. 1A and B; DEVD). Caspase-3 activity was measured by monitoring the release of the fluorescent AMC moiety from the caspase-3 specific substrate Ac-DEVD-AMC. Cells were harvested with lysis buffer, caspase-3 activity was measured in lysates containing 25 μg protein and expressed as total units (Fig. 1A,B) or fluorescence units/μg protein (Fig. 1C). The generation of fluorescent product was completely blocked by the addition of the inhibitor Ac-DEVD-CHO (1 μM) (DEVD) to lysates derived from 5 DIV neuronal cultures, suggesting that the fluorometric assay was selective for caspase-3 (Fig. 1A and B; *P < 0.01 vs. untreated neurons; post-hoc Duncan’s test). Lastly, some caspase-3 activity was evident when comparing untreated cultures to cultures treated with dynorphin A plus DEVD (or DEVD alone—data not shown) suggesting that caspase-3 was tonically active at low levels [bP < 0.05 vs. untreated, dynorphin A (1–17) (10 μM), or dynorphin A (1–17) (10 μM) + naloxone treated neurons; post-hoc Duncan’s Test; Fig. 1A–B]. Caspase-3 activity was nominal following treatment with DEVD alone at 4 h (136 ± 46 units) or 72 h (270 ± 62 units) (data not shown) and significantly less than exposure to dynorphin A plus DEVD. Caspase-3 activity shown in Fig. 1A–B and following treatment with DEVD alone was measured in 25 μg protein samples and expressed as total units.
Fig. 1.
Dynorphin A (1–17)-induced changes in caspase-3 activation. A–B: Striatal neurons were grown for 5 days in culture and incubated with 10 μM dynorphin A (1–17) (Dyn) for 4 h (A) or 72 h (B) in the absence and presence of 10 μM (−)naloxone (Nal) or 1 μM Ac-DEVD-CHO (DEVD). Cells were harvested with lysis buffer, caspase activity was measured in lysates containing 25 μg protein and expressed as total units (A,B) or fluorescence units (C). C: Striatal neurons were grown for 7 days in vitro before incubation with varied concentrations of dynorphin A (1–17) for 4 or 72 h. Cells were harvested in lysis buffer and caspase-3 activity was measured as described above (C). Results are the mean ± the SEM from n = 4–6 experiments; *P < 0.05 or **P < 0.01 versus untreated neurons; bP < 0.05 versus untreated, dynorphin A (1–17) (10 μM), or dynorphin A (1–17) (10 μM) + naloxone treated neurons.
We then studied the time-course and concentration-dependence of dynorphin A-induced activation of caspase-3 in striatal neurons. Marked DEVD-cleaving activity was detected 4 h following treatment with dynorphin A, while maximal activation was achieved at 72 h. Dynorphin A caused concentration-dependent increases in caspase-3 activity (Fig. 1C; *P < 0.05 or **P < 0.01 vs. untreated neurons; post-hoc Duncan’s test). Maximal caspase-3 activation occurred following exposure to 10 μM dynorphin A (Fig. 1C).
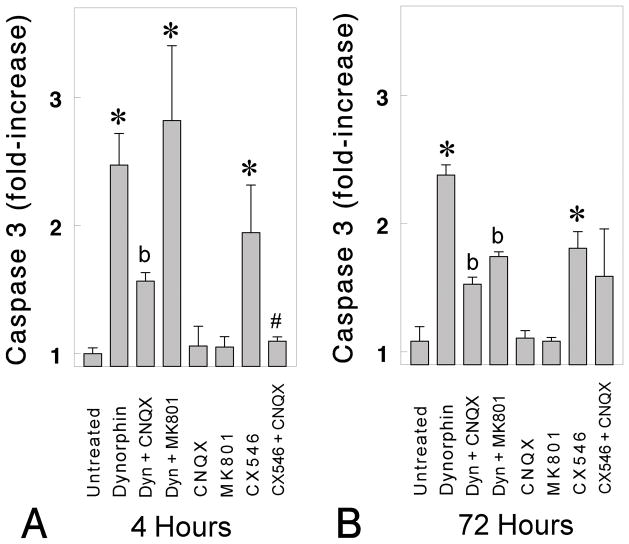
Dynorphin-induced caspase-3 activation (Fig. 2A–2B;*P < 0.05 vs. untreated neurons) was significantly inhibited by CNQX (10 μM), suggesting the involvement of AMPA/kainate receptors (Fig. 2A–2B;bP < 0.05 vs. dynorphin-treated neurons). By contrast, dynorphin-induced increases in caspase-3 were unaffected by (−)-naloxone (10 μM) (Fig. 1A–B) and partially attenuated by MK801 but only at 72 h following antagonist exposure (Fig. 2B). Findings that the broad acting μ, δ and κ opioid receptor antagonist naloxone did not attenuate dynorphin-induced caspase-3 activity, suggested that the caspase-3 increases were not mediated by opioid receptors (Fig. 1A–B). Similarly, the failure of MK801 to attenuate dynorphin-induced caspase-3 increases at 4 h suggests NMDA receptors are also not involved. Some MK-801-induced attenuation was noted at 72 h (Fig. 2B) suggesting that NMDA receptor signaling contributed to a component of the caspase-3 response. Importantly, the antagonists CNQX (10 μM) or MK 801 (10 μM), alone did not activate caspase-3 at 4 h or 72 h. Lastly, the ampakine CX546 alone caused significant increases in caspase-3 activity at 4 and 72 h (Fig. 2). The effects of CX546 (500 nM) were significantly attenuated by coadministering CNQX at 4 h (Fig. 2A; #P < 0.05 vs. CX546-treated neurons) but only partially reduced by CNQX at 72 h (Fig. 2B). The ability of CX546 to mimic dynorphin further supports the notion that AMPA receptors can mediate an apoptotic response in striatal neurons (Goody et al., 2003). Nonparametric analysis was used to test differences among the groups in Fig 2A because the variances were non-homogenous, while ANOVA and post-hoc Duncan’s tests were used to analyze the data in Fig 2B.
Fig. 2.
Dynorphin A (1–17)-induced changes in caspase-3 activation. Striatal neurons grown for 7 days in culture were incubated for 4 h (A) or 72 h (B) with dynorphin A (1–17) (10 μM) in the presence or absence of CNQX (10 μM), MK (+)801 (10 μM), and/or CX546 [1-(1,4-Benzodioxan-6-ylcarbonyl)piperidine]. Cells were harvested with lysis buffer; caspase activity was measured in 4–5 experiments and expressed as units per milligram of protein; *P < 0.05 versus untreated neurons; bP < 0.05 versus untreated or dynorphin A (1–17) treated neurons; #P < 0.05 versus CX546 (10 μM) treated neurons (A).
Mitochondrial Release of Cytochrome c
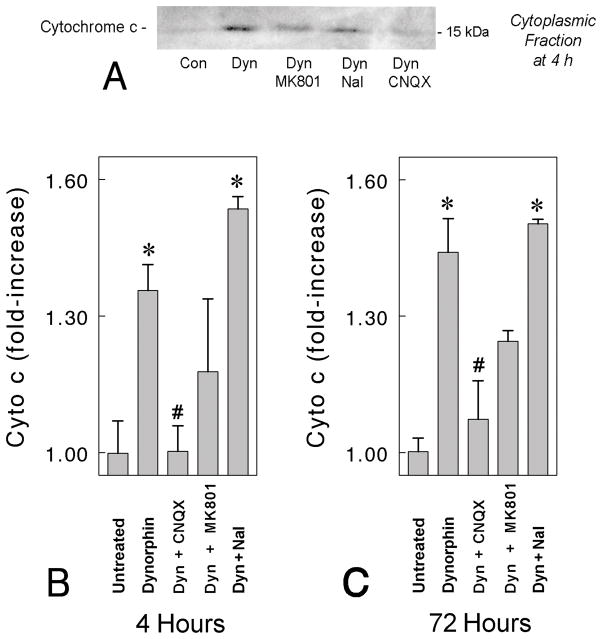
Western blot analysis showed that dynorphin A (1–17) significantly elevated the level of cytochrome c released from mitochondria in mouse striatal neuron cultures at 4 and 72 h (Fig. 3). The cytosol from dynorphin treated neuronal cultures contained significantly higher amounts of cytochrome c than vehicle-treated controls. Our results indicate that mitochondrial release of the apoptotic protein cytochrome c into the cytosol occurs as early as 4 h after exposure to dynorphin, and that the cytosolic levels of cytochrome c remain elevated at 72 h following continuous exposure to dynorphin A.
Fig. 3.
Dynorphin A (1–17) induced increases cytochrome c in the cytosolic fraction. Mouse striatal neurons were treated with dynorphin A (1–17) for 4 hr and 72 hr in the absence and presence of 10 μM (−)naloxone, 10 μM MK(+)801, or 10 μM CNQX,. Cytosolic fractions were separated from mitochondria as described in materials and methods. Equal amounts of cytosolic and mitochondrial protein (50 μg) were loaded in sample buffer and separated on 12% SDS-PAGE. The intensity of cytochrome c signal was compared to untreated control cultures from n = 4 experiments; *P < 0.05 versus untreated neurons; #P < 0.05 versus dynorphin A (1–17) (10 μM) treated neurons.
Concurrent incubation of striatal neurons with dynorphin (10 μM) in the presence of (−)-naloxone (10 μM) or MK 801 (10 μM) did not interfere with cytochrome c increases in the cytosol at 4 h or 72 h exposure (*P < 0.01 vs. untreated neurons; post-hoc Duncan’s test), while CNQX markedly attenuated dynorphin-induced increases in cytosolic cytochrome c at 4 or 72 h (Fig. 3B–C; #P < 0.05 vs. dynorphin-treated neurons). Treatment of antagonists, CNQX (10 μM), naloxone (10 μM) or MK 801 (10 μM) for 4 h or 72 h did not mediate the release of mitochondrial cytochrome c into the cytosol (data not shown).
Dynorphin Toxicity in Mouse Striatal Neurons
As previously demonstrated using time-lapse photography (Goody et al., 2003), dynorphin A (1–17) causes significant death of striatal neurons at 48 h following exposure. Significant neuronal losses are seen with 100 nM or 10-μM dynorphin A (1–17) and maximal toxicity is evident with 10-μM dynorphin, which mimics a 100-μM concentration (Hauser, unpublished observations).
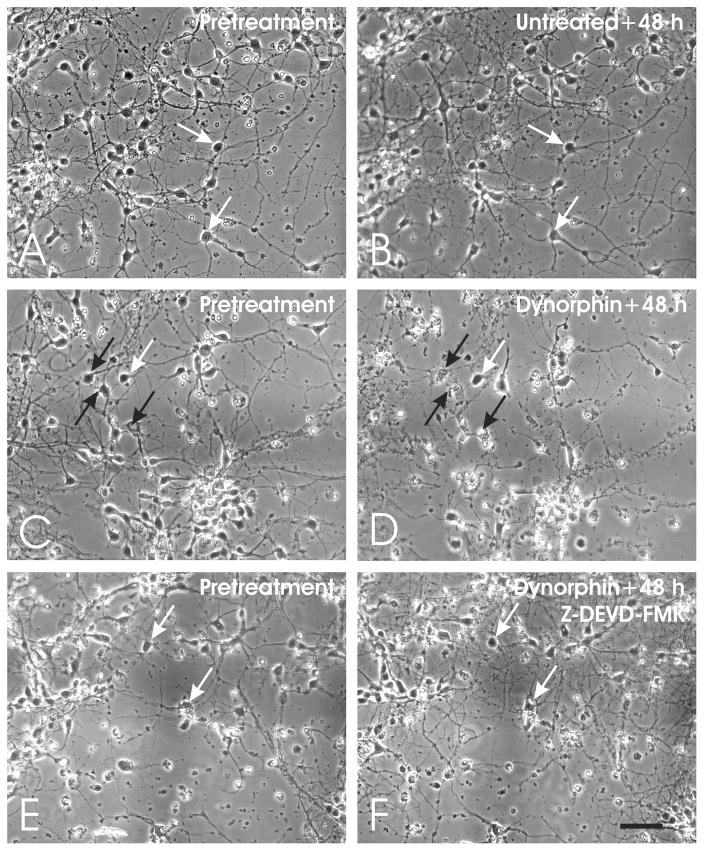
To experimentally assess whether caspases were mediating dynorphin A (1–17)-induced neuronal death, striatal neuron cultures were continuously exposed to high (10 μM) concentrations of dynorphin A (1–17) for 72 h with or without the caspase inhibitor Z-DEVD-FMK (Figs. 4 and 5). Dying neurons were identified by the fragmentation and destruction of the cell body and neurites (Fig. 4). As expected from previous work, dynorphin A (1–17) significantly increased neuronal losses. Importantly, the caspase inhibitor could overcome the effects of maximally toxic concentrations of dynorphin at 48 h, although the range of concentrations in which Z-DEVD-FMK achieved this effect was quite narrow (Fig. 5). Dynorphin A (1–17; 10 μM; *P < 0.05 versus untreated neurons; Duncan’s test) neurotoxicity was significantly attenuated by a 30 μM concentration of Z-DEVD-FMK (#P < 0.05 versus dynorphin; Duncan’s test), while a 10 μM concentration was ineffective and a 50 μM concentration was intrinsically toxic at 72 h (data not shown).
Fig. 4.
Time-lapse digital micrographs of striatal neurons in untreated, dynorphin A (1–17)-treated, or dynorphin A (1–17) + caspase inhibitor Z-DEVD-FMK-treated cultures. Untreated cultures showed few degenerating neurons (A–B), while exposure to dynorphin A (1–17) (10 μM) caused significant toxicity (C–D) that could be attenuated by Z-DEVD-FMK (30 μM) (E–F); non-degenerating neurons (white arrows), degenerating neurons (black arrows), scale bar = 25 μm.
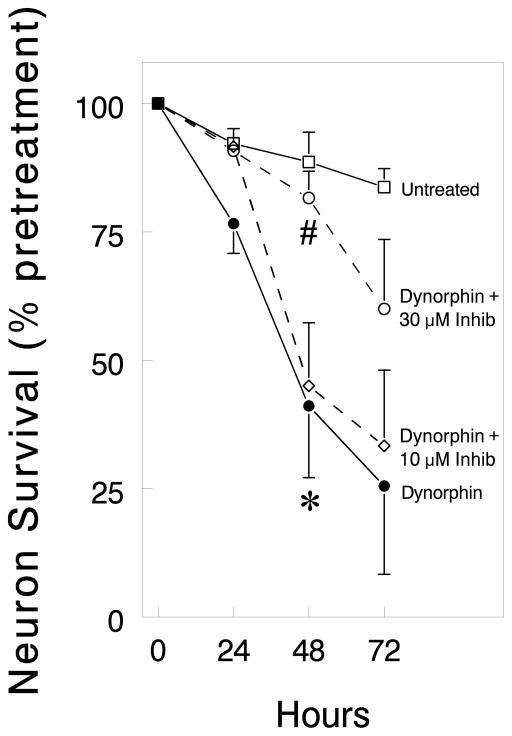
Fig. 5.
Striatal neuron survival in untreated or dynorphin A (1–17) (10 μM)-treated cultures with or without the caspase inhibitor Z-DEVD-FMK. Exposure to dynorphin A (1–17) caused significant toxicity that was attenuated by 30 μM, but not 10 μM, Z-DEVD-FMK. Data are the mean ± SEM from n = 4 experiments; *P < 0.05 versus untreated or dynorphin A (10 μM) + Z-DEVD-FMK (30 μM) exposed neurons; #P < 0.05 versus dynorphin A (10 μM) exposed neurons.
Peptide Loss
Degradation of dynorphin A in neuronal cultures and adsorption of peptide on poly-lysine coated plastic wells used for cell cultivation was analyzed by specific RIA of dynorphin A and its products Leu-enkephalin-Arg6 and dynorphin A (14–17) (Table 1), and by mass-spectrometry (Fig. 6). Both methods demonstrated that a substantial proportion (50–80%) of dynorphin A added into the medium at 100 μM concentrations was converted into shorter peptides after 72 h incubation. The remaining 20–50% dynorphin A was present in the medium in the native form.
Table 1.
Dynorphin A conversion into Arg6-Leu-enkephalin and dynorphin A(14–17)α
| Incubation time
|
Conversionβ
|
|
|---|---|---|
| Leu-enkephalin-Arg6 | dynorphin A(14–17) | |
| 4 h | 1.9% | 0.72% |
| 72 h | 56.5% | 3.2% |
100 μM dynorphin A was added into the medium of striatal neuron cultures at 7 days in vitro and analyzed after 4 h or 72 h. The background peptide levels were at least 1000 fold lower in cell culture medium incubated without dynorphin A. No Leu-enkephalin-Arg6 and dynorphin A (14–17) were detected in the medium immediately after dynorphin A was added.
Total amount of dynorphin A plus Leu-enkephalin-Arg6 or dynorphin A plus dynorphin A (14–17), was taken as 100%.
Fig. 6.
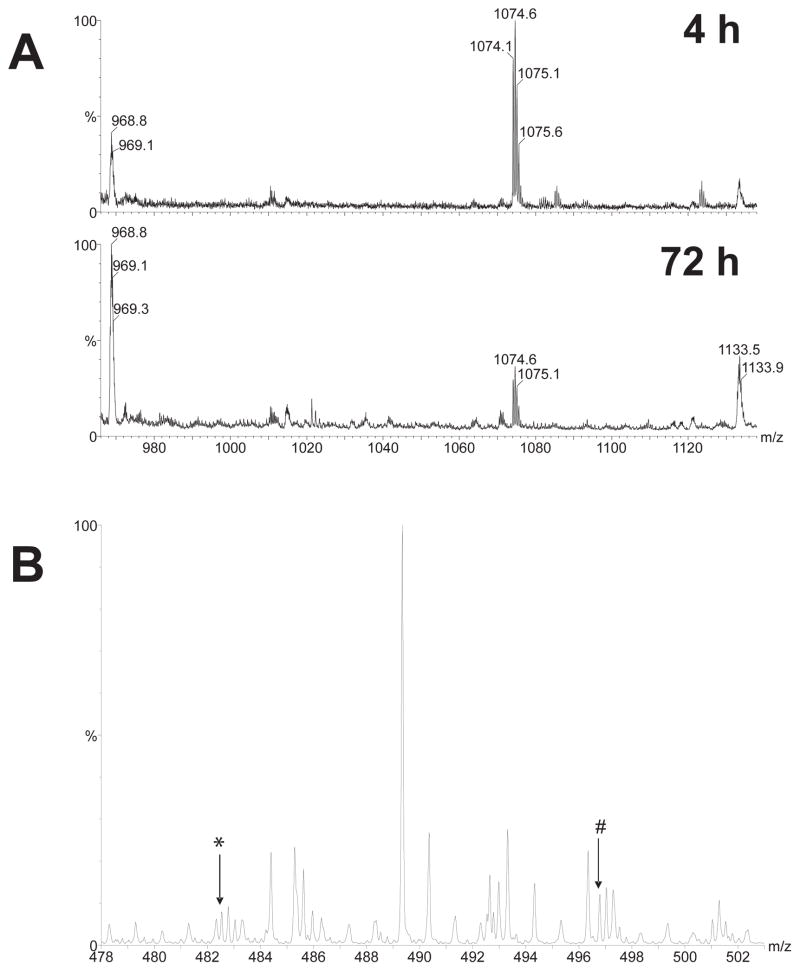
Mass spectra showing changes in dynorphin A and its derived products at 4 h and 72 h after incubating 100 μM dynorphin A in striatal neuron cultures. Signals at about 968 m/z originating from the cell culture medium were used as an internal standard to assess the degradation of dynorphin A (A). Dynorphin A was detected as a doubly charged component with a monoisotopic mass at 1074.1 m/z. After 72 h, approximately 80% of the dynorphin A was degraded compared to the amount present at 4 h. The mass spectra indicated that the larger degradation products of dynorphin A were N-terminally truncated derivatives of the parent peptide (B). The signals at 482.5 and 496.8 m/z (arrows labelled * and # respectively), correspond to quadruply charged peptides with masses corresponding to the dynorphin A partial sequences GGFLRRIRPKLKWDNQ and GFLRRIRPKLKWDNQ. These peaks were absent in control medium of primary neurons incubated without dynorphin A.
A doubly charged peptide at 1074 m/z corresponding to dynorphin A (2146 Da, neutral mass) was detected in the medium both after 4 h and 72 h of incubation at dynorphin A 100 μM concentration (Fig. 6). About 80 % of the intact dynorphin A was degraded after 72 h of incubation (Fig. 6A). N-terminally truncated species corresponding to the sequences dynorphin A (2–17) and dynorphin A (3–17) were detected (Fig. 6B). These peaks were absent in control medium of primary neurons incubated without dynorphin A. ESI MS/MS using CID with argon confirmed that the component corresponding to the peak at 1074 m/z was Dynorphin A.
To assess peptide loss due to adsorption, dynorphin A (100 μM) was incubated in poly-lysine coated plastic 12-well plates for 4 hours in Dulbecco’s Modified Eagle’s Medium (DMEM). Final concentration was measured using dynorphin A RIA and compared to the level of dynorphin A in stock solution. Adsorption did not exceed 20%.
DISCUSSION
Our results indicate that dynorphin kills neurons by triggering an apoptotic pathway that involves cytochrome c release and caspase-3 activation. Moreover, the present findings indicate that proapoptotic events are activated through glutamatergic, but not opioid, receptors, and further support the novel involvement of AMPA/kainate receptors in dynorphin-induced striatal neurotoxicity (Goody et al., 2003). Our previous studies found that dynorphin can modulate death in spinal cord or striatal neurons through events mediated by opioid and non-opioid receptors, but failed to elucidate the potential mechanisms (e.g., necrosis or apoptosis) or underlying pathways involved.
Caspases are known to participate in many apoptotic events including DNA fragmentation, chromatin condensation, membrane blebbing, cell shrinkage, and disassembly into membrane-enclosed vesicles known as apoptotic bodies (Dubner and Ruda, 1992; Lily et al., 2001; Parish et al., 2001; Yang et al., 1997). Our results in mouse striatal neurons are consistent with previous reports in other model systems indicating that caspase activation precedes apoptosis (Armstrong et al., 1997; Eldadah et al., 1997; Garrido et al., 2001; Padmanabhan et al., 1999; Thornberry and Lazebnik, 1998). In addition, the ability of caspase inhibitors or caspase gene disruption to enhance viability further supports the involvement of caspases in apoptosis (D’Mello et al., 1998; Jacobson et al., 1997; Oberdoerster and Rabin, 1999; Taylor et al., 1997). The present study showed the caspase-3 inhibitor Z-DEVD-FMK (30 μM) markedly, but not completely, attenuated dynorphin A (1–17)-induced death, indicating the involvement of caspase-3 in apoptotic death. Whether the inability to prevent neuronal death completely relates to the Z-DEVD-FMK dosages used (10 or 30 μM) or the involvement of an alternative apoptotic pathway such as endonuclease G remains uncertain. Alternative effectors that might induce apoptosis independently of caspase-3 include the mitochondrial factor Omi/HtrA2, apoptosis inducing factor, caspase-2, or endonuclease G (Ravagnan et al., 2002; van Loo et al., 2002). It is noteworthy that a 50 μM concentration of Z-DEVD-FMK was intrinsically toxic (Hauser, unpublished observations), while 10 μM had no significant effect on neuronal survival, suggesting optimal caspase inhibition occurs within a very narrow concentration range.
Caspase-3 activation was accompanied by mitochondrial release of cytochrome c into the cytoplasm. Cytochrome c release is a central event in the mitochondrial death pathway (Kluck et al., 1997; Kroemer et al., 1997; Li et al., 1997; Schimmer et al., 2001). Cytochrome c release can additionally accompany non-mitochondrial apoptotic induction such as Fas-mediated apoptosis (Krippner et al., 1996; Adachi et al., 1997; Ravagnan et al., 2002; van Loo et al., 2002), although it is uncertain whether cytochrome c precedes or accompanies other apoptotic signaling cascades. Our results indicate that mitochondrial release of cytochrome c in dynorphin A (1–17)-induced striatal neurotoxicity is accompanied by the activation of caspase-3.
Dynorphin A stability was observed at 4 h and 72 h to assess the extent that the peptide would undergo biotransformation, degradation, or adsorption in our cell culture conditions. Dynorphin A incubated with primary neuronal cultures was degraded into short N- and C-terminal fragments Leu-enkephalin-Arg6 and dynorphin A (14–17) detected by RIA, and also into longer peptides dynorphin A (2–17) and dynorphin A (3–17), detected by mass-spectrometry. Many of the C-terminal fragments detected (including 2–17 and 3–17) are intrinsically neurotoxic (Hauser et al., 2001). Importantly, a substantial portion of dynorphin A remained unmodified and intact after 4 h (80–90%) and 72 h (20–50%) of incubation, which is likely to be sufficiently excitotoxic to signal sustained neuronal death during the 72 h incubation period.
Collectively, the present findings show a tight correlation between increases in cytosolic cytochrome c levels, caspase-3 activation, and subsequent cell death. The prolonged period in which cytochrome c levels and caspase-3 activation is increased in our primary, neuron-enriched cultures (i.e., 72 h) may not result from sustained caspase activity within the same cell. Instead, a subset of striatal neurons may show delayed susceptibility because of subtle phenotypic or maturational differences in the receptors or effectors mediating the apoptotic response.
Previous studies have shown that dynorphin A (1–17) and dynorphin A-derived peptides modulate neurotoxicity by activating multiple receptor types and signaling pathways (Hauser et al., 1999). These include actions at κ-opioid and NMDA receptors in spinal cord neurons (Hauser et al., 1999), AMPA/kainate receptors in striatal neurons (Goody et al., 2003); and direct protein-protein interactions that may involve p53 or other key intracellular targets (Tan-No et al., 2001). Our present findings support the notion that dynorphin induces apoptosis through excitotoxic actions at AMPA/kainate receptors, since their blockade significantly attenuated dynorphin-induced caspase-3 activation. In addition, the ability of the ampakine CX546 to mimic the effects of dynorphin A (1–17) suggests that AMPA receptors alone may be involved, although more definitive evidence for the involvement of particular glutamate receptor types will likely require molecular-genetic approaches to more selectively target particular receptor subunits. Alternatively, the inability of CNQX to block completely some aspects of dynorphin-induced apoptosis suggests that a component of the toxicity not being mediated through AMPA/kainate receptors (Goody et al., 2003). Although Ca2+ impermeant GluR2 subunits can be expressed (Stefani et al., 1998; Goody et al., 2003), medium spiny neurons nevertheless can reportedly flux Ca2+ in response to AMPA receptor activation (Stefani et al., 1998), suggesting non-GluR2-AMPA-receptor containing complexes may be co-expressed by the same or a distinct subsets of striatal neurons. Alternatively, some of the neurotoxic effects of dynorphin, especially in mature striatal neurons, might be mediated through NMDA receptors. For example, NMDA receptor involvement (via release of Mg2+ blockade) might be secondary to AMPA/kainate receptor activation and neuronal depolarization (Kolaj et al., 1996; Goody et al., 2003). Structure-activity studies indicate that N-terminal fragments of dynorphin act as selective opioid receptor agonists, while the C-terminal domains are non-opioid and mediate deleterious effects (Walker et al., 1982; Cavicchini et al., 1989; Shippenberg et al., 2000; Hauser et al., 2001; Liu et al., 2001; Goody et al., 2003). In addition, dynorphin can reportedly induce glutamate release (Skilling et al., 1992), although dynorphin typically inhibits its release presynaptically (Wagner et al., 1992; Maneuf et al., 1995; Herrera-Marschitz and Terenius, 1999). Alternatively, dynorphin might affect key intracellular proteins through direct protein-protein interactions (Tan-No et al., 2001; Yakoveleva et al., 2001).
There are conflicting reports that dynorphin can mediate both neuroprotective and neurodegenerative effects (Dubner and Ruda, 1992; Iadarola et al., 1988; Long et al., 1988; Shukla and Lemaire, 1994). Thus, insights into pathways that signal both neuroprotection and apoptosis are vital toward understanding the role of dynorphin in neuroplasticity, neurotrauma, and neurodegenerative disorders. Opioid receptor activation can have differing effects depending on the particular opioid receptor type involved and the nature of its coupling to specific intracellular effectors (Hauser and Mangoura 1998). Opioid receptors are highly promiscuous in their ability to couple to second messenger systems, and the nature of this interaction can vary in cell type, region specific and age-dependent manners (Hauser and Mangoura 1998). In spinal cord neurons, κ-opioid receptor blockade with nor-BNI or naloxone exacerbates dynorphin-induced excitotoxic insult, suggesting that κ receptor stimulation imparts considerable neuroprotection (Hauser et al., 1999). Unlike the observations in spinal cord neurons (Hauser et al., 1999), we found opioid receptor blockade did not exacerbate dynorphin-induced proapoptotic events in striatal neurons in the present study, which agrees with viability measures in previous studies (Goody et al., 2003). To the contrary, preliminary findings (not included herein) using the selective κ receptor antagonist, nor-BNI, attenuated proapoptotic signaling; however, this only occurred at high concentrations, suggesting nor-BNI was no-longer acting selectively (Castanas et al., 1986; Pasternak 1988; Tiberi and Magnan, 1990). Lastly, (−)-naloxone, which blocks κ receptors in addition to μ and δ opioid receptors, failed to attenuate dynorphin A-induced cytochrome c release or caspase-3 activation. This agrees with studies in striatal neurons showing their viability is unaffected by (−)-naloxone (Goody et al., 2003). Apparent differences between spinal cord and striatal neurons in the neuroprotective effects of κ opioid receptor activation suggest that there are fundamental differences in the response of each neuronal type to dynorphin.
Taken together, our findings suggest that dynorphin A (1–17) per se can induce apoptosis in striatal neurons, and additionally suggest that apoptosis is induced through non-opioid, AMPA receptors and involves cytochrome c and caspase-3. Pathophysiologic changes in dynorphin production might contribute to disruptions in striatal function that occur with chronic opioid or psychostimulant abuse and/or withdrawal, or with neurodegenerative diseases such as HIV dementia (Maneuf et al., 1995; Berger and Arendt, 2000; Nestler, 2001; Nath et al., 2002; Goody et al., 2003). Interestingly, psychostimulant abuse, which increases dynorphin levels and may cause neurodegenerative changes in humans (Little et al., 2003), exacerbates the neurocognitive impairment seen with neuro-AIDS (Nath et al., 2002). Studies, in progress, are exploring whether pathophysiological changes in dynorphin biogenesis might contribute to the neuropathogenesis of HIV encephalopathy in the striatum.
Acknowledgments
This work was supported by NIH grant DA13559, and by grants from the Swedish Science Research Council (12190) and the AFA Foundation to GB. The editorial assistance of Ms. Sarah E. Lutz is gratefully acknowledged.
Abbreviations used
- Ac-DEVD-AMC
Ac-Asp-Glu-Val-Asp- 7-amino-4-methylcoumarin
- Ac-DEVD-CHO
Ac-Asp-Glu-Val-Asp-aldehyde
- AIDS
acquired immunodeficiency syndrome
- AMPA
α–amino-3-hydroxy-5-methylisoxazole-4-propionate
- Apaf-1
apoptosis activating factor-1
- CHAPS
3-[-(3-chloramidopropyl)dimethylammonio]-1-propanesulfonic acid
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CID
collision-induced dissociation
- CX546
1-(1,4-Benzodioxan-6-ylcarbonyl)piperidine or ampakine
- DIV
days in vitro
- DMEM
Dulbecco’s minimal essential medium
- Dyn
dynorphin A (1–17)
- ES
electrospray
- ESI MS/MS
electrospray ionization tandem mass spectrometry
- GluR2
ionotropic glutamate receptor subunit 2
- HIV
human immunodeficiency virus
- MS
Mass spectrometry
- Nal
naloxone
- NMDA
N-methyl-D-aspartate
- nor-BNI
nor-binaltorphimine
- PMSF
phenylmethylsulfonyl fluoride
- RIA
radioimmunoassay
References
- Adachi S, Cross AR, Babior BM, Gottlieb RA. Bcl-2 and the outer mitochondrial membrane in the inactivation of cytochrome c during Fas-mediated apoptosis. J Biol Chem. 1997;272:21878–21882. doi: 10.1074/jbc.272.35.21878. [DOI] [PubMed] [Google Scholar]
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171–171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Aja TJ, Hoang KD, Gaur S, Bai X, Alnemri ES, Litwack G, Karanewsky DS, Fritz LC, Tomaselli KJ. Activation of the CED3/ICE-related protease CPP32 in cerebellar granule neurons undergoing apoptosis but not necrosis. J Neurosci. 1997;17:553–562. doi: 10.1523/JNEUROSCI.17-02-00553.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Bakshi R, Ni RX, Faden AI. N-Methyl-D-aspartate (NMDA) and opioid receptors mediate dynorphin-induced spinal cord injury: Behavioral and histological study. Brain Res. 1992;580:255–264. doi: 10.1016/0006-8993(92)90952-6. [DOI] [PubMed] [Google Scholar]
- Bakshi R, Faden AI. Competitive and non-competitive NMDA antagonists limit dynorphin A-induced rat hindlimb paralysis. Brain Res. 1990;507:1–5. doi: 10.1016/0006-8993(90)90512-a. [DOI] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Mosser DD, Cain K, Kuwana T, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Berger JR, Arendt G. HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol. 2000;14:214–221. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- Carrion AM, Mellstrom B, Naranjo JR. Protein kinase A-dependent derepression of the human prodynorphin gene via differential binding to an intragenic silencer element. Mol Cell Biol. 1998;18:6921–6929. doi: 10.1128/mcb.18.12.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion AM, Link WA, Ledo F, Mellstrom B, Naranjo JR. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398:80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- Castanas E, Blanc D, Bourhim N, Cupo A, Cantau P, Giraud Reassessment of opioid binding sites in the rat brain. Neuropeptides. 1986;7:369–380. doi: 10.1016/0143-4179(86)90030-2. [DOI] [PubMed] [Google Scholar]
- Caudle RM, Maness AJ. Dynorphin: friend or foe? Pain. 2000;87:235–239. doi: 10.1016/S0304-3959(00)00360-2. [DOI] [PubMed] [Google Scholar]
- Cavicchini E, Candeletti S, Spampinto S, Ferri S. Hypothermia elicited by some prodynorphin-derived peptides: opioid and non-opioid actions. Neuropeptides. 1989;14:45–50. doi: 10.1016/0143-4179(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Cheng H-YM, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, Sarosi I, Woodgett JR, Oliveira-dos-Santos AJ, Ikura M, van der Kooy D, Salte MW, Penninger JM. DREAM is a critical transcriptional repressor for pain modulation. Cell. 2002;108:31–43. doi: 10.1016/s0092-8674(01)00629-8. [DOI] [PubMed] [Google Scholar]
- Chen L, Gu Y, Huang LY. The mechanism of action for the block of NMDA receptor channels by the opioid peptide dynorphin. J Neurosci. 1995a;15:4602–4611. doi: 10.1523/JNEUROSCI.15-06-04602.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Gu Y, Huang LY. The opioid peptide dynorphin directly blocks NMDA receptor channels in the rat. J Physiol. 1995b;482:575–523. doi: 10.1113/jphysiol.1995.sp020541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensson-Nylander I, Nyberg F, Ragnarsson U, Terenius L. A general procedure for analysis of proenkephalin B derived opioid peptides. Regul Peptides. 1985;11:65–76. doi: 10.1016/0167-0115(85)90032-1. [DOI] [PubMed] [Google Scholar]
- Civelli O, Douglass J, Goldstein A, Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci (USA) 1985;82:4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JJ, Duke RC, Fadok VA, Sellins KS. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- Creagh EM, Martin SJ. Caspases: Cellular demolition experts. Biochem Soc Trans. 2001;29:696–701. doi: 10.1042/0300-5127:0290696. [DOI] [PubMed] [Google Scholar]
- Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- D’Mello SR, Aglieco F, Roberts MR, Borodezt K, Heycock JW. A DEVD-inhibited caspase other than CPP32 is involved in the commitment of cerebellar granule neurons to apoptosis induced by K+ deprivation. J Neurochem. 1998;70:1809–1818. doi: 10.1046/j.1471-4159.1998.70051809.x. [DOI] [PubMed] [Google Scholar]
- Eldadah BA, Yakolev AG, Faden AI. The role of CED-3-related cysteine proteases in apoptosis of cerebellar granule cells. J Neurosci. 1997;17:6105–6113. doi: 10.1523/JNEUROSCI.17-16-06105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RE, Yuan JY, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Granerus M, Engstrom W. Growth factors and apoptosis. Cell Proliferation. 1996;29:309–314. doi: 10.1111/j.1365-2184.1996.tb01582.x. [DOI] [PubMed] [Google Scholar]
- Garrido R, Mattson MP, Hennig B, Toborek M. Nicotine protects against arachidonic-acid-induced caspase activation, cytochrome c release and apoptosis of cultured spinal cord neurons. J Neurochem. 2001;76:1395–1403. doi: 10.1046/j.1471-4159.2001.00135.x. [DOI] [PubMed] [Google Scholar]
- Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nature Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- Goody RJ, Martin KM, Goebel SM, Hauser KF. Dynorphin A toxicity in striatal neurons via an AMPA/kainate receptor mechanism. Neuroscience. 2003;116:807–816. doi: 10.1016/s0306-4522(02)00563-8. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Knapp PE, Turbek CS. Structure-activity analysis of dynorphin A toxicity in spinal cord neurons: Intrinsic neurotoxicity of dynorphin A and its carboxyl-terminal, non-opioid metabolites. Exp Neurol. 2001;168:78–87. doi: 10.1006/exnr.2000.7580. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Foldes JK, Turbek CS. Dynorphin A (1–13) neurotoxicity in vitro: Opioid and non-opioid mechanisms in mouse spinal cord neurons. Exp Neurol. 1999;160:361–375. doi: 10.1006/exnr.1999.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Mangoura DA. Diversity of the endogenous opioid system in development: novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation. Perspect Devel Neurobiol. 1998;5:437–449. [PubMed] [Google Scholar]
- Herman BH, Goldstein A. Antinociception and paralysis induced by intrathecal dynorphin A. J Pharmacol Exp Ther. 1985;232:27–32. [PubMed] [Google Scholar]
- Herrera-Marschitz M, Terenius L. Modulation of neurotransmitter release in the basal ganglia of the rat brain by dynorphin peptides. J Pharmacol Exp Ther. 1999;290:1307–1315. [PubMed] [Google Scholar]
- Horvitz HR. Genetic control of programmed cell death in the nematode Caenorrhabditis elegans. Cancer Res. 1999;59:S1701–1706. [PubMed] [Google Scholar]
- Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–326. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Kolaj M, Cerne R, Randic M. The opioid peptide dynorphin modulates AMPA and kainate responses in acutely isolated neurons from the dorsal horn. Brain Res. 1995;671:227–244. doi: 10.1016/0006-8993(94)01333-d. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- Krippner A, Matsuno-Yagi A, Gottlieb RA, Babior BM. Loss of function of cytochrome c in Jurkat cells undergoing Fas-mediated apoptosis. J Biol Chem. 1996;271:21629–21636. doi: 10.1074/jbc.271.35.21629. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Laughlin TM, Larson AA, Wilcox GL. Mechanisms of induction of persistent nociception by dynorphin. J Pharmacol Exp Ther. 2001;299:6–11. [PubMed] [Google Scholar]
- Laughlin TM, Vanderah TW, Lashbrook J, Nichols ML, Ossipov M, Porreca F, Wilcox GL. Spinally administered dynorphin A produces long-lasting allodynia: Involvement of NMDA but not opioid receptors. Pain. 1997;72:253–260. doi: 10.1016/s0304-3959(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Li GL, Brodin G, Farooque M, Funa K, Holtz A, Wang WL, Olsson Y. Apoptosis and expression of Bcl-2 after compression trauma to rat spinal cord. J Neuropathol Exp Neurol. 1996;55:280–289. doi: 10.1097/00005072-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and ATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Lily Y, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- Little KY, Krolewski DM, Zhang L, Cassin BJ. Loss of striatal vesicular monoamine transporter protein (VMAT2) in human cocaine users. Am J Psychiatry. 2003;160:47–55. doi: 10.1176/appi.ajp.160.1.47. [DOI] [PubMed] [Google Scholar]
- Liu B, Qin L, Yang SN, Wilson BC, Liu Y, Hong JS. Femtomolar concentrations of dynorphins protect rat mesencephalic dopaminergic neurons against inflammatory damage. J Pharmacol Exp Ther. 2001;298:1133–141. [PubMed] [Google Scholar]
- Long JB, Petras JM, Mobley WC, Holaday JW. Neurological dysfunction after injection of dynorphin A (1–13) in the rat: II. Nonopioid mechanisms mediate loss of motor, sensory and autonomic function. J Pharmacol Exp Ther. 1988;246:1167–1174. [PubMed] [Google Scholar]
- Mattson MP, Duan W. “Apoptotic” biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:152–166. [PubMed] [Google Scholar]
- Maneuf YP, Mitchell IJ, Crossman AR, Woodruff GN, Brotchie JM. Functional implications of kappa opioid receptor-mediated modulation of glutamate transmission in the output regions of the basal ganglia in rodent and primate models of Parkinson’s disease. Brain Res. 1995;683:102–108. doi: 10.1016/0006-8993(95)00358-w. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos WF, Prendergast M, Cass WA, Turchan J. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31:S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Oberdoerster J, Rabin RA. Enhanced caspase activity during ethanol-induced apoptosis in rat cerebellar granule cells. Eur J Pharmacol. 1999;385:273–282. doi: 10.1016/s0014-2999(99)00714-1. [DOI] [PubMed] [Google Scholar]
- Padmanabhan J, Park DS, Greene LA, Shelanski ML. Role of cell cycle regulatory proteins in cerebellar granule neuronal apoptosis. J Neurosci. 1999;19:8747–8756. doi: 10.1523/JNEUROSCI.19-20-08747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish J, Li L, Klotz K, Ledwich D, Wang X, Xue D. Mitochondrial endonuclease G is important for apoptosis in C. elegans. Nature. 2001;412:90–94. doi: 10.1038/35083608. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Multiple morphine and enkephalin receptors and the relief of pain. JAMA. 1988;259:1362–1367. [PubMed] [Google Scholar]
- Ravagnan L, Roumier T, Kroemer G. Mitochondria, the killer organelles and their weapons. J Cell Physiol. 2002;192:131–137. doi: 10.1002/jcp.10111. [DOI] [PubMed] [Google Scholar]
- Rigamonti D, Bauer JH, De-Fraja C, Conti L, Sipione S, Sciorati C, Clementi E, Hackam A, Hayden MR, Li Y, Cooper JK, Ross CA, Govoni S, Vincenz C, Cattaneo E. Wild-type Huntingtin protects from apoptosis upstream of caspase-3. J Neurosci. 2000;20:3705–3713. doi: 10.1523/JNEUROSCI.20-10-03705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry PS, Rao KS. Apoptosis and the nervous system. J Neurochem. 2000;74:1–20. doi: 10.1046/j.1471-4159.2000.0740001.x. [DOI] [PubMed] [Google Scholar]
- Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem. 2000;275:7337–7342. doi: 10.1074/jbc.275.10.7337. [DOI] [PubMed] [Google Scholar]
- Shukla VK, Lemaire S. Non-opioid effects of dynorphins: Possible role of the NMDA receptor. Trends Pharmacol Sci. 1994;15:420–424. doi: 10.1016/0165-6147(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Skilling SR, Sun X, Kurtz HJ, Larson AA. Selective potentiation of NMDA-induced activity and release of excitatory amino acids by dynorphin: possible roles in paralysis and neurotoxicity. Brain Res. 1992;575:272–278. doi: 10.1016/0006-8993(92)90090-v. [DOI] [PubMed] [Google Scholar]
- Spampinto S, Candeletti S. Characterization of dynorphin A-induced antinociception at spinal cord. Eur J Pharmcol. 1985;110:21–30. doi: 10.1016/0014-2999(85)90024-x. [DOI] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nature Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- Schimmer AD, Hedley DW, Penn LZ, Minden MD. Receptor-and mitochondrial-mediated apoptosis in acute leukemia: a translational view. Blood. 2001;98:3541–3553. doi: 10.1182/blood.v98.13.3541. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Funada M, Schutz CG. Dynorphin A (2–17) attenuates the unconditioned but not the conditioned effects of opiate withdrawal in the rat. Psychopharmacology (Berl) 2000;151:351–358. doi: 10.1007/s002130000475. [DOI] [PubMed] [Google Scholar]
- Stefani A, Chen Q, Flores-Hernandez J, Jiao Y, Reiner A, Surmeier DJ. Physiological and molecular properties of AMPA/Kainate receptors expressed by striatal medium spiny neurons. Dev Neurosci. 1998;20:242–252. doi: 10.1159/000017318. [DOI] [PubMed] [Google Scholar]
- Tan-No K, Cebers G, Yakovleva T, Hoon Goh B, Gileva I, Reznikov K, Aguilar-Santelises M, Hauser KF, Terenius L, Bakalkin G. Cytotoxic effects of dynorphins through nonopioid intracellular mechanisms. Exp Cell Res. 2001;269:54–63. doi: 10.1006/excr.2001.5309. [DOI] [PubMed] [Google Scholar]
- Tang Q, Gandhoke R, Burritt A, Hruby VJ, Porreca F, Lai J. High-affinity interaction of (des-Tyrosyl)dynorphin A(2–17) with NMDA receptors. J Pharmacol Exp Ther. 1999;291:760–765. [PubMed] [Google Scholar]
- Taylor J, Gatchalian CL, Keen G, Rubin LL. Apoptosis in cerebellar granule neurons: Involvement of interleukin-beta converting enzyme-like protease. J Neurochem. 1997;68:1598–1605. doi: 10.1046/j.1471-4159.1997.68041598.x. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Tiberi M, Magnan J. Quantitative analysis of multiple κ-opioid receptors by selective and nonselective ligand binding in guinea pig spinal cord: resolution of high and low affinity states of κ2 receptors by a computerized model-fitting technique. Mol Pharmacol. 1990;37:694–703. [PubMed] [Google Scholar]
- Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, Zhang ET, Malan TP, Jr, Ossipov MH, Lai J, Porreca F. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 2000;20:7074–7079. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ, Vandenbeele P. The role of mitochondrial factors in apoptosis: a Russian roulette with more that one bullet. Cell Death Differ. 2002;9:1031–1042. doi: 10.1038/sj.cdd.4401088. [DOI] [PubMed] [Google Scholar]
- Wagner JJ, Caudle RM, Chavkin C. Kappa-opioids decrease excitatory transmission in the dentate gyrus of the guinea pig hippocampus. J Neurosci. 1992;12:132–141. doi: 10.1523/JNEUROSCI.12-01-00132.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Moises HC, Coy DH, Baldrighi G, Akil H. Nonopiate effects of dynorphin and des-Tyr-dynorphin. Science. 1982;218:1136–1138. doi: 10.1126/science.6128791. [DOI] [PubMed] [Google Scholar]
- Xue JC, Yu YX, Han JS, Jen MF. Comparative study of the analgesic and paralytic effects induced by intrathecal dynorphin A in rats. Int J Neurosci. 1995;82:83–93. doi: 10.3109/00207459508994292. [DOI] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T-I, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–113. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Yakovleva T, Pramanik A, Kawasaki T, Tan-No K, Gileva I, Lindegren H, Langel U, Ekstrom TJ, Rigler R, Terenius L, Bakalkin G. p53 latency: C-terminal domain prevents binding of p53 core to target but not to nonspecific DNA sequences. J Biol Chem. 2001;276:15650–15658. doi: 10.1074/jbc.M100482200. [DOI] [PubMed] [Google Scholar]