Abstract
CD47 is a widely expressed integral membrane protein that serves as the counter-receptor for the inhibitory phagocyte receptor signal-regulatory protein-α (SIRPα) and as a signaling receptor for the secreted matricellular protein thrombospondin-1. Recent studies employing mice and somatic cells lacking CD47 have revealed important pathophysiological functions of CD47 in cardiovascular homeostasis, immune regulation, resistance of cells and tissues to stress, and chronic diseases of aging including cancer. With the emergence of experimental therapeutics targeting CD47, a more thorough understanding of CD47 signal transduction is essential. CD47 lacks a substantial cytoplasmic signaling domain, but several cytoplasmic binding partners have been identified, and lateral interactions of CD47 with other membrane receptors play important roles in mediating signaling resulting from the binding of thrombospondin-1. This review addresses recent advances in identifying the lateral binding partners, signal transduction pathways, and downstream transcription networks regulated through CD47 in specific cell lineages. Major pathways regulated by CD47 signaling include calcium homeostasis, cyclic nucleotide signaling, nitric oxide and hydrogen sulfide biosynthesis and signaling, and stem cell transcription factors. These pathways and other undefined proximal mediators of CD47 signaling regulate cell death and protective autophagy responses, mitochondrial biogenesis, cell adhesion and motility, and stem cell self-renewal. Although thrombospondin-1 is the best characterized agonist of CD47, the potential roles of other members of the thrombospondin family, SIRPα and SIRPγ binding, and homotypic CD47 interactions as agonists or antagonists of signaling through CD47 should also be considered.
Key terms: autophagy, nitric oxide, stem cells, radioresistance, Myc, integrin signaling, hydrogen sulfide, immune regulation
Introduction
The cell surface receptor that is currently best known by its immunological moniker CD47 was first identified as a component of the Rh blood group antigen complex on red blood cells and then isolated as a protein that co-purifies with the integrin αvβ3 (Lindberg et al., 1993, Mawby et al., 1994). CD47 was consequently named “integrin-associated protein” or IAP, which should not be confused with the unrelated inhibitors of apoptosis that share the same acronym (Soto-Pantoja et al., 2013a). CD47 was independently identified as a tumor antigen that is over-expressed in ovarian cancer (Campbell et al., 1992). Although early studies revealed that expression levels of this tumor marker correlate with progression of some cancers (Nishiyama et al., 1997), and CD47 antibodies were developed and used for ovarian tumor imaging and immunotherapy (Tibben et al., 1992, van Ravenswaay Claasen et al., 1994), the subsequent finding that CD47 is widely expressed at lower levels by most healthy cells diminished the interest in CD47 as cancer therapeutic and diagnostic tool (Soto-Pantoja et al., 2013a). However, in the past few years interest in CD47 as a therapeutic target for cancer has reemerged with reports that antibodies targeting this receptor stimulate macrophage-mediated clearance of tumors (Majeti et al., 2009, Chao et al., 2011, Willingham et al., 2012). Humanized antibodies recognizing CD47 have now been developed (Edris et al., 2012), and a clinical trial for treating cancer patients was recently opened (NCT02096770).
These therapeutic CD47 antibodies inhibit the interaction of CD47 with its counter-receptor SIRPα, which is highly expressed on macrophages. Binding to CD47 triggers inhibitory signaling through SIRPα that prevents macrophage phagocytosis of normal and cancer cells (Oldenborg et al., 2000). The CD47 antibodies enhance phagocytosis of cancer cells by relieving this inhibition. Based on the publicity surrounding these findings, the lay press and some scientific press now view CD47 as a passive “don’t eat me” flag on cancer cells that keeps the innate immune system at bay (Kershaw and Smyth, 2013, Unanue, 2013). The mechanisms by which SIRPα signaling controls phagocyte and other antigen-presenting cell functions are well studied, and readers are referred to several excellent reviews (Matozaki et al., 2009, Chao et al., 2012, Per-Arne, 2012, Burger et al., 2012, Barclay and Van den Berg, 2014). Here we focus instead on the signaling functions of CD47. Within the immunology community this has been referred to as “reverse signaling,” presumably SIRPα binding triggering a CD47 signal (Sarfati et al., 2008, Cant and Ullrich, 2001). However, a number of functions of CD47 that are independent of SIRPα have been identified by researchers in many cell types, and those studies are the primary focus of this review.
CD47 structure and interactions
CD47 is an integral membrane protein consisting of an N-terminal extracellular IgV domain derived from the immunoglobulin superfamily followed by a presenilin domain containing five membrane-spanning segments and ending in a short variably spliced cytoplasmic sequence (Fig. 1) (Soto-Pantoja et al., 2013a, Mushegian, 2002). A long range disulfide bond links Cys33 in the IgV domain to Cys263 in the last extracellular loop in the transmembrane domain and is essential for some signaling functions of CD47 (Rebres et al., 2001b). The IgV domain is N-glycosylated and modified with an O-linked glycosaminoglycan. The five N-glycosylation sites are not required for SIRPα binding (Subramanian et al., 2007), but the glycosaminoglycan modification is required for thrombospondin-1 (TSP1) signaling through CD47 (Kaur et al., 2011).
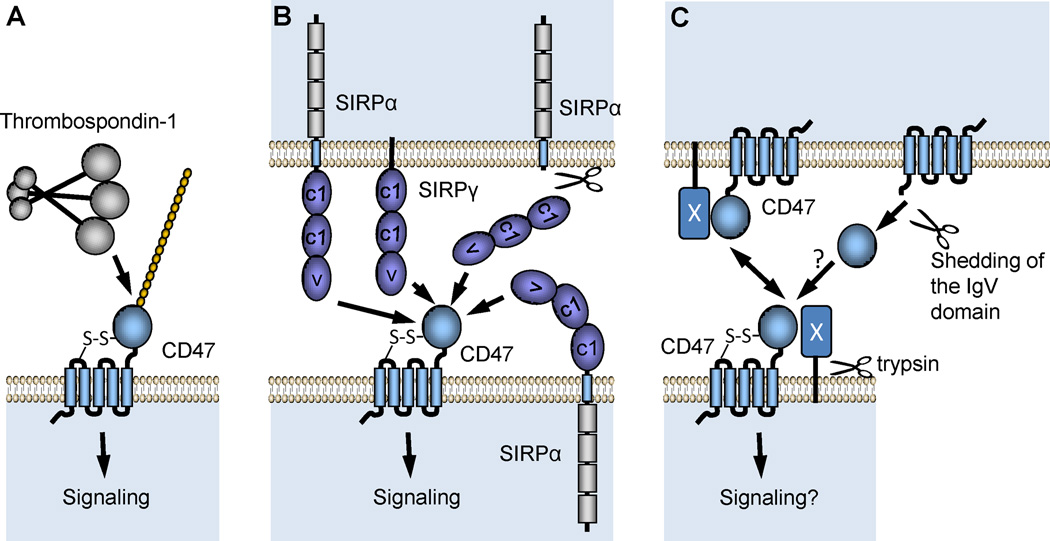
Fig. 1.
Topological arrangements of CD47 and its known extracellular ligands. (A) Thrombospondin-1 (TSP1) is a secreted protein that binds to a proteoglycan isoform of CD47 on the plasma membrane. Mutagenesis studies established that heparan sulfate modification of CD47 is required for high affinity interaction of TSP1 with CD47. (B) Two members of the signal recognition protein family (SIRPα and SIRPγ) bind to CD47 in a species-specific manner. These serve as counter-receptors for CD47 during cell-cell interactions, although evidence in smooth muscle cells suggests SIRPα can also interact in-cis with CD47 (Maile and Clemmons, 2003). Proteolytic cleavage of the extracellular domain of SIRPα can also generate a soluble ligand for CD47 (Umemori and Sanes, 2008). (C). Homotypic binding of CD47 to CD47 or binding to an unidentified trypsin-sensitive counter-receptor (X) can mediate cell-cell interactions (Rebres et al., 2005). The IgV domain of CD47 can be shed under some conditions (Maile et al., 2008b, Kaur et al., 2011), but the ability of this domain to engage in homotypic binding as a soluble CD47 ligand is not known.
The IgV domain of CD47 interacts with three classes of extracellular ligands. The major secreted ligand for CD47 is TSP1 (Fig. 1A). TSP1 is a homotrimeric glycoprotein, and its C-terminal signature domain mediates binding to CD47 (Isenberg et al., 2009a). At least two additional members of the thrombospondin family bind to CD47, albeit with much lower affinity. Human TSP1 controls CD47 signaling equally in human, bovine, porcine, rat, and murine cells, indicating that this interaction is not species-specific within mammals. The signal regulator protein family contains three members, and of these SIRPα and SIRPγ are known CD47 counter-receptors (Fig. 1B). Binding of CD47 to SIRPα is highly species-specific (Subramanian et al., 2006a). In contrast to the clearly defined signaling functions of SIRPα, SIRPγ is a transmembrane protein that lacks a cytoplasmic domain and, therefore, is thought to signal only indirectly by lateral association with other membrane proteins (Brooke et al., 2004, Matozaki et al., 2009). In addition to engaging CD47 in trans during cell-cell contact, lateral interactions between SIRPα and CD47 have also been proposed (Maile et al., 2008b, Maile and Clemmons, 2003), and proteolytic shedding of the extracellular domain of SIRPα may provide a second soluble signal-inducing ligand for CD47 (Umemori and Sanes, 2008). Cell-cell adhesion that requires CD47 but not any of its known ligands suggests that homotypic binding can also occur between the IgV domains of CD47 on opposing cells (Fig. 1C) (Rebres et al., 2005). This interaction may require an unidentified trypsin-sensitive protein (X) to mediate cell-cell adhesion, but the potential should be considered that this cell-cell interaction and homotypic binding of proteolytically shed CD47 IgV domain (Maile et al., 2010) or CD47 in exosomes (Kaur et al., 2014b) to cell surface CD47 could elicit CD47 signal transduction. However, direct evidence for CD47-CD47 binding and signaling resulting from homotypic CD47 binding is lacking.
To understand CD47 signaling, one must consider whether its several ligands can bind simultaneously, compete for binding, or allosterically regulate each other’s binding and signaling activities. Direct binding studies demonstrate that TSP1 inhibits SIRPα binding to cells expressing CD47 (Isenberg et al., 2009a). This data is consistent with competitive binding of TSP1 and SIRPα to a single site on CD47, steric inhibition of binding to distinct but proximal sites, or allosteric inhibition (Fig. 2A). Additional studies showed that a recombinant C-terminal domain of TSP1 yields similar inhibition (Isenberg et al., 2009a). Notably, the widely used CD47 blocking antibody B6H12 inhibits binding of both SIRPα and TSP1 to cell surface CD47 (Isenberg et al., 2009a). Therefore, this antibody cannot be used to distinguish which of these ligands is responsible for a given CD47 signal. A complex of the interacting Ig domains of CD47 and SIRPα has been crystallized (Fig. 2B) (Hatherley et al., 2008), but the location of the TSP1 binding site on CD47 remains unclear. Screening for adhesive peptides within the C-terminal domain of TSP1 identified two active sequences that share a VVM motif (Kosfeld and Frazier, 1993). One of these contains the TSP1 sequence 1016RFYVVMWK1024, and a modified peptide based on this sequence known as 4N1K (KRFYVVMWKK) was used to affinity label CD47(Gao et al., 1996) and as an immobilized ligand to affinity purify CD47 (Chung et al., 1997, Dorahy et al., 1997). Amino acid substitutions in the VVM motif in this peptide or both motifs in a recombinant C-terminal domain of TSP1 abolished its interaction with CD47 (McDonald et al., 2003). However, analysis of the crystal structure of the TSP1 domain containing this sequence found that the VVM motifs are not exposed to solvent and, therefore, are unlikely to directly mediate CD47 binding (Kvansakul et al., 2004). A conformation change in TSP1 that would expose one VVM motif was proposed but has not been experimentally verified (Floquet et al., 2008). In addition, a recombinant form of this TSP1 domain failed to bind to nonglycosylated recombinant IgV domain of CD47 (Adams et al., 2008). However, recent mutagenesis studies revealed that glycosylation of CD47 at Ser64 with a heparan sulfate chain is necessary for TSP1 signaling through CD47 in T cells (Kaur et al., 2011). Therefore, our current model is that high affinity binding of TSP1 involves interactions with this glycosaminoglycan and unidentified sites on the adjacent CD47 protein. Some parts of the active inhibitory peptides derived from TSP1 are exposed to solvent and may interact with CD47, but clarifying their function requires further study. Nonetheless, based on the location of the required Ser64 in CD47 and evidence that SIRPα binding does not require this CD47 glycosylation, we predict that the TSP1 binding site is located away from the known SIRPα binding site (Fig 2B).
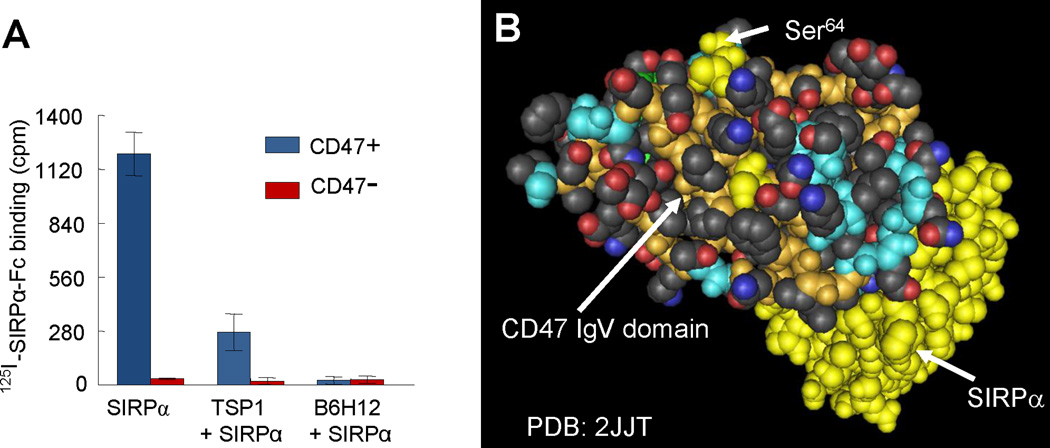
Fig. 2.
Evidence for the relationship between TSP1 and SIRPα binding sites on CD47. (A) Data from Isenberg et al (Isenberg et al., 2009a) demonstrates that TSP1 and the function-blocking CD47 antibody B6H12 inhibit binding of the radiolabeled extracellular domain of SIRPα to cells expressing CD47. Lack of binding to an isogenic cell line lacking CD47 confirms specificity of SIRPα binding. These competition data could indicate that TSP1 and SIRPα bind to overlapping sites on CD47 or that binding of these proteins to distinct sites on CD47 results in steric or allosteric inhibition of SIRPα binding by TSP1. (B) A space-filling projection of the crystal structure of the extracellular IgV domain of human CD47 (tan backbone with colored side chains) complexed with the terminal Ig domain of SIRPα (yellow). Mutagenesis established that post-translational modification of Ser64 on CD47 (yellow) is required for TSP1 signaling. This suggests that the TSP1 binding site is distinct from the SIRPα binding site on CD47.
Proximal signaling
Evidence for direct cytoplasmic signaling
Lacking a substantial cytoplasmic domain, efforts to identify proteins that directly interact with CD47 have utilized its cytoplasmic C-terminal peptide as bait for yeast two-hybrid screening alone or in combination with the transmembrane domain. The latter strategy identified BCL2/adenovirus E1B 19kDa-interacting protein-3 (BNIP3) as cytoplasmic binding partner of CD47 (Lamy et al., 2003) (Fig. 3B). Treatment of cells with the TSP1-derived peptide 4N1K but not with SIRPα-Fc induced translocation of BNIP3 to mitochondria, resulting in mitochondrial depolarization and cell death. Unfortunately, this result was not replicated using native TSP1. Readers are cautioned that the TSP1 peptide analog 4N1K has well-documented activities that are independent of CD47 (Tulasne et al., 2001, Barazi et al., 2002, Leclair and Lim, 2014), and the numerous published activities of 4N1K that have not been confirmed using native TSP1 or cd47-null cells must be viewed with skepticism. Therefore, the only conclusion that can be drawn from the above study is that SIRPα binding to CD47 does not trigger translocation of BNIP3. It also remains unclear whether CD47 regulates other signaling functions of BNIP3. Our recent report that CD47 limits protective autophagy in response to stress caused by ionizing radiation (Soto-Pantoja et al., 2012) suggests that CD47 signaling may regulate the complex functions of BNIP3 in autophagy and mitophagy (Vasagiri and Kutala, 2014, Ishihara et al., 2013, Hanna et al., 2012). This question deserves further investigation.
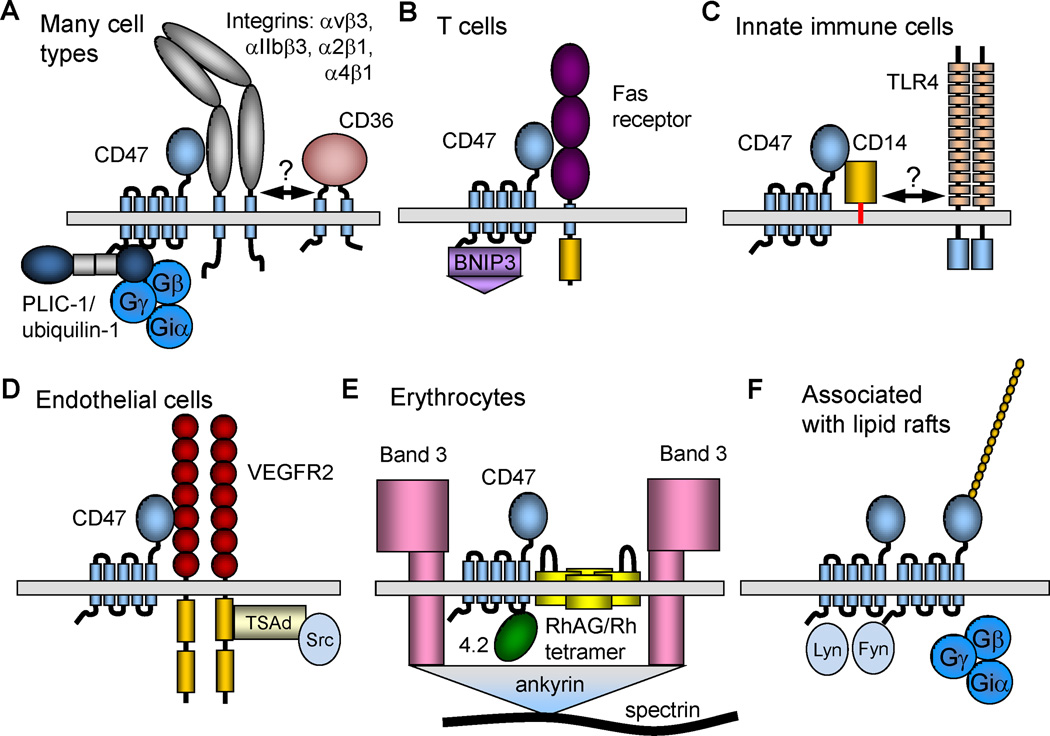
Fig. 3.
Supramolecular complexes containing CD47. (A) On a majority of cell types CD47 laterally associates with specific integrins. These integrins in turn may associate with other membrane proteins, potentially including CD36 in neuronal cells (Bamberger et al., 2003). However, readers are cautioned that the proposed CD36-α6β1-CD47 complex has not been directly demonstrated. PLIC1/ubiquilin-1 directly binds to the C-terminal cytoplasmic tail of CD47 and can recruit heterotrimeric G-proteins to CD47. (B) In T cells CD47 can laterally associate with Fas receptor. Yeast two-hybrid screening identified BNIP3 as another direct binding partner for the C-terminal tail of CD47. (C) In innate immune cells FRET studies identified lateral association of CD47 with the LPS co-receptor CD14. CD14 is also a component of the TLR4 signaling complex, but it is unclear whether CD447 can bind to CD14 while it is in the TLR complex. (D) In endothelial cells CD47 closely associates with VEGFR2. This complex also contains Src kinases, which associate with the cytoplasmic tail of VEGFR2 via TSAd (Shibuya, 2006). (E) Human erythrocytes lack integrins, and CD47 is found in a multiprotein complex that accounts for Rh-antigen activity. This complex also contains Band 3 and is anchored to the red cell cytoskeleton via ankyrin. Band 4.2 is required for maintaining CD47 in this complex. (F) Lateral oligomerization of CD47 has been reported in association with lipid rafts. Heterotrimeric G protein and the Src kinases Lyn and Fyn are enriched in this complex.
A second yeast two-hybrid screen used the IAP2 and IAP4 splice isoforms of the CD47 cytoplasmic tail as bait and identified two ubiquitin-related proteins, which were named protein-linking integrin-associated protein and cytoskeleton-1 (PLIC1, more widely known as ubiquilin-1) and PLIC2 (ubiquilin-2), as CD47 cytoplasmic binding partners (Wu et al., 1999). Over-expressing ubiquilin-1 limits integrin activation in a CD47-dependent manner and alters the vimentin intermediate filament cytoskeleton. Subsequent studies established that ubiquilin-1 binds Gβγ and thereby tethers heterotrimeric G proteins to CD47 (N’Diaye and Brown, 2003) (Fig. 3A). Ubiquilin-1 in this context inhibits chemotaxis signaled by the Gi-coupled receptor CXCR4 but does not alter Gs-mediated signal transduction. The same pathway was reported to mediate CD47− and Gβγ dimer-dependent activation of the PI3K/Akt pathway in astrocytoma cells but not in nontransformed cells (Sick et al., 2011). The Frazier lab had earlier shown that Giαβγ co-immunoprecipitates with CD47 in a pertussis toxin-sensitive manner (Frazier et al., 1999). Ligation of CD47 with 4N1K increases GTP binding to the membrane-associated G-protein complex and reduced downstream cAMP synthesis. Pertussis toxin sensitivity also implicates homotypic CD47 binding in regulating heterotrimeric G-protein signaling (Rebres et al., 2005).
Several additional activities of ubiquilin-1 may be relevant to known functions of CD47 signaling. Transgenic mice globally over-expressing ubiquilin-1 exhibit resistance to oxidative stress and ischemia/reperfusion injuries in the CNS (Liu et al., 2014), which parallels the resistance of cd47 null mice to cerebral ischemia (Jin et al., 2009). CD47 and ubiquilin-1 also share roles in regulation of cytoplasmic calcium levels. Ubiquilin-1 increased the ubiquitination of Orai1, which is a component of store-operated calcium entry channels (Lee et al., 2013b). Ubiquilin-1 decreases intracellular Ca2+ mobilization and downstream signaling by promoting the ubiquitination and lysosomal degradation of Orai1. Finally, ubiquilin-1 binds to the autophagy mediator LC3 via ubiquilin-4 (Lee et al., 2013a), and reducing ubiquilin-1 expression limits autophagosome formation (Rothenberg et al., 2010). However, no studies to date have addressed whether ubiquilin-1 mediates the corresponding effects of CD47 signaling on autophagy, stress resistance, and calcium signaling.
CD47 signaling through lateral associations
Given the limited number of known cytoplasmic interactions for CD47, its lateral interactions with other signaling receptors are believed to play important roles in its signal transduction, and several of these interactions are perturbed by ligand binding to CD47. As noted previously, CD47 was first isolated in a detergent-stable complex with the integrin αvβ3. This interaction requires the IgV domain of CD47 but not its transmembrane domain (Lindberg et al., 1996b). Specific associations of CD47 with αIIbβ3, α2β1, αLβ2, and α4β1 integrins have also been documented (Soto-Pantoja et al., 2013a). Lateral association with CD47 regulates the activation state of those integrins with which it associates, and activity of the CD47 IgV domain fused to a GPI anchor demonstrated that the IgV domain is sufficient to activate αvβ3 integrin (Lindberg et al., 1996b). Ligation of CD47 by CD47-binding peptides derived from TSP1 induces rapid activation of αvβ3 but not α3β1 in breast carcinoma cells that express both integrins (Chandrasekaran et al., 1999). Ligation of CD47 by the antibody B6H12 stimulates T cell adhesion mediated by α4β1 α2β1 and α5β1 integrins (Barazi et al., 2002). CD47 induces protein kinase A-dependent serine phosphorylation of the cytoplasmic domain of the α4 integrin subunit, and this requires Src family kinase activity (Brittain et al., 2004). A complex composed of CD47, α6β1 integrin, and CD36 has been proposed in microglia, but direct evidence such as co-immunoprecipitation was not presented, and the only evidence for CD47 function in this complex was inhibitory activity of the TSP1 peptide analog 4N1K (Bamberger et al., 2003). Regardless of whether CD36 and CD47 coexist in a physical complex, it is clear that some signals resulting from ligand binding to CD36 require CD47 (Isenberg et al., 2006, Miller et al., 2010b). Amyloid-β binding requires only CD36, but downstream regulation of NO/cGMP signaling by amyloid-β requires CD47 (Fig. 4). The fatty acid translocase activity of CD36 also regulates known targets of CD47 signaling (Isenberg et al., 2007b).
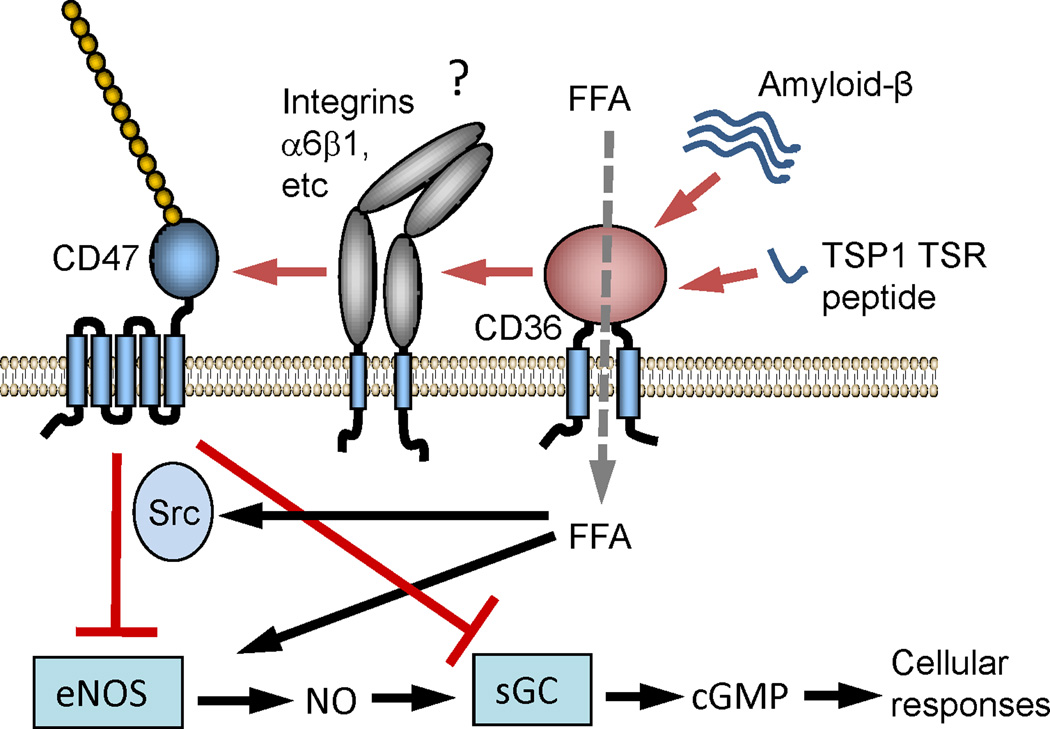
Fig. 4.
Functional crosstalk between CD36 and CD47 signaling. In vascular cells ligation of CD36 by amyloid-β or a peptide derived from the type 1 repeats of TSP1 inhibits uptake of free fatty acids via CD36, which regulates eNOS, and inhibits downstream NO/cGMP signaling. The latter pathway requires CD47, but the mechanism remains unclear. One possibility is the lateral association of CD36 with an integrin that also associates with CD47.
TSP1 has been reported to inhibit the activity of vascular endothelial growth factor (VEGF) to stimulate angiogenesis by several mechanisms including direct binding to VEGF, competing for VEGF binding to heparan sulfate proteoglycans on microvascular endothelial cells, and by inducing inhibitory signaling through the TSP1 receptors CD36 and CD47 (Gupta et al., 1999, Chu et al., 2013, Zhang et al., 2009, Kaur et al., 2010). Of these mechanisms, only inhibition of CD47 signaling has been validated at physiological sub-nanomolar concentrations of TSP1 (Isenberg et al., 2009b, Kaur et al., 2010). TSP1 signaling through CD47 redundantly inhibits the endothelial nitric oxide synthase (eNOS/NOS3)-sGC-cGK cascade downstream of VEGF (Isenberg et al., 2009b). In a thermal injury model and in T cells, CD47 signaling also inhibits the expression of VEGF and VEGFR2 (Soto-Pantoja et al., 2014a, Kaur et al., 2014a) , which results in increased capillary vessel density, improved tissue perfusion, and improved healing of second degree burns in cd47-null mice.
CD47 more broadly regulates VEGFR2 by directly associating with the receptor in endothelial cells and T lymphocytes (Kaur et al., 2010, Kaur et al., 2014a) (Fig. 3D). Evidence from FRET, immunoprecipitation, and microscopic co-localization techniques indicate that CD47 and VEGFR2 associate very closely, and this interaction is disrupted by TSP1 at physiological concentrations. We further showed that TSP1 and the CD47-binding peptide 7N3 (1102FIRVVMYEGKK1112) inhibit VEGFR2-Y1175 phosphorylation in human umbilical vein and microvascular endothelial cells. VEGFR2-Y1175 is essential for downstream signaling to regulate VEGFR2 trafficking, cell proliferation and cell adhesion and migration (Shibuya, 2006). This novel mechanism enables TSP1 to globally inhibit VEGF-induced VEGFR2 signaling.
TSP1 also inhibits tube formation and VEGFR2 phosphorylation in human endothelial progenitor cells (Qin et al., 2014). This inhibition by TSP1 is significantly attenuated by a CD47 function blocking antibody and by CD47 siRNA. This study confirms that TSP1 inhibits VEGF induced VEGFR2 downstream signaling in CD47-dependent manner.
Lateral interaction between VEGFR2 and CD47 is not limited to endothelial cells. CD47 and VEGFR2 also interact in T cells (Kaur et al., 2014a). In T cells TSP1 selectively inhibits the lateral association of VEGFR2 with the non-proteoglycan isoform of CD47, and inhibition of VEGFR2-Y1175 and Src-Y416 phosphorylation by TSP1 requires the presence of CD47. Remarkably, VEGF is unable to induce VEGFR2-Y1175 phosphorylation in the absence of CD47. Heparin/heparan sulfate binding enhances VEGF binding to and activation of VEGF receptors (Grunewald et al., 2010, Xu et al., 2011). The heparan sulfate modification of CD47 on many cell types including HUVEC and T cells may similarly enhance binding of VEGF to the cell surface and its presentation to VEGFR2.
In red blood cells CD47 is a component of the Rh blood group antigen complex (Fig. 3E). This complex links to spectrin in the cytoskeleton via ankyrin and contributes to maintaining red blood cell shape and ammonia transport (Van Kim et al., 2006). The function of CD47 in this complex remains unclear, but CD47 expression is reduced in individuals lacking specific components of the complex including the RhCE, band 4.2, and RhAG proteins (Mouro-Chanteloup et al., 2003, Cambot et al., 2013, Flatt et al., 2012). Individual variations in Rh expression also alter CD47 expression (Dahl et al., 2003). These CD47 interactions are also species-specific between mice and humans (Subramanian et al., 2006b). Notably, red blood cells lack integrins to complex with CD47, suggesting that this macromolecular complex may be unique to erythrocytes. However, other members of the Rh protein family are expressed in many cell types (Avent et al., 2006), and their potential interactions with CD47 merit further investigation.
A role for lateral homotypic association of CD47 with itself in signaling is suggested by characterization of antibodies originally classified as anti-CDw149 and currently as CD47R. These antibodies bound to a membrane complex containing CD47 in several cell types but not to CD47 on red blood cells (Drbal et al., 2000). Lateral clustering of CD47 on leukocytes but not erythrocytes was proposed to account for the selective binding of CDw149 antibodies to the former cells, although the different lateral binding partners of CD47 in these two cell types should also be considered. The CDw149 antibodies were found to be moderate affinity CD47 antibodies that selectively bind clustered but not monomeric CD47 on the cell surface. These lateral clusters of CD47 are enriched in Lyn and Fyn and trimeric G-proteins and appear to contain a detergent-resistant dimeric form of CD47 (Fig. 3F), although post-translational modification of monomeric CD47 should also be considered (Kaur et al., 2011).
Fas (CD95) was identified as a lateral binding partner of CD47 in T cells (Manna et al., 2005) (Fig. 3B). Activation of the Fas receptor on T cells by an antibody or by Fas ligand binding induced death only in cells that express CD47. Under basal conditions, CD47 weakly associates with Fas, but activation of Fas increases the lateral interaction as assessed by co-localization and co-immunoprecipitation studies. This interaction is necessary for signaling downstream of Fas including caspase-7 and PARP cleavage.
FRET studies established that the LPS coreceptor CD14 laterally associates with CD47 on human blood monocytes, and LPS binding to CD14 induced its dissociation from CD47 (Pfeiffer et al., 2001) (Fig. 3C). The association of CD14 with lipid rafts may contribute to its lateral interaction with CD47, which also associates peripherally with lipid rafts (Rebres et al., 2001a).
Extracellular signaling
CD47 can be released from cells by proteolytic cleavage of its IgV domain mediated by matrix metalloprotease-2 (Maile et al., 2008b, Maile et al., 2008a). This soluble CD47 fragment may bind to TSP1, SIRPα, or to intact CD47, but to date no signaling function for this fragment has been reported. On the other hand, several reports have shown that CD47 is present on extracellular vesicles (EVs), including exosomes released by platelets (Sadallah et al., 2011, Rho et al., 2013, Kim et al., 2012). CD47 bearing the glycosaminoglycan modification required for TSP1 signaling is present in supernatants of endothelial, vascular smooth muscle, CHO, and T cells (Kaur et al., 2011). These reports suggested that CD47 in EVs may have an unrecognized role in extracellular signaling.
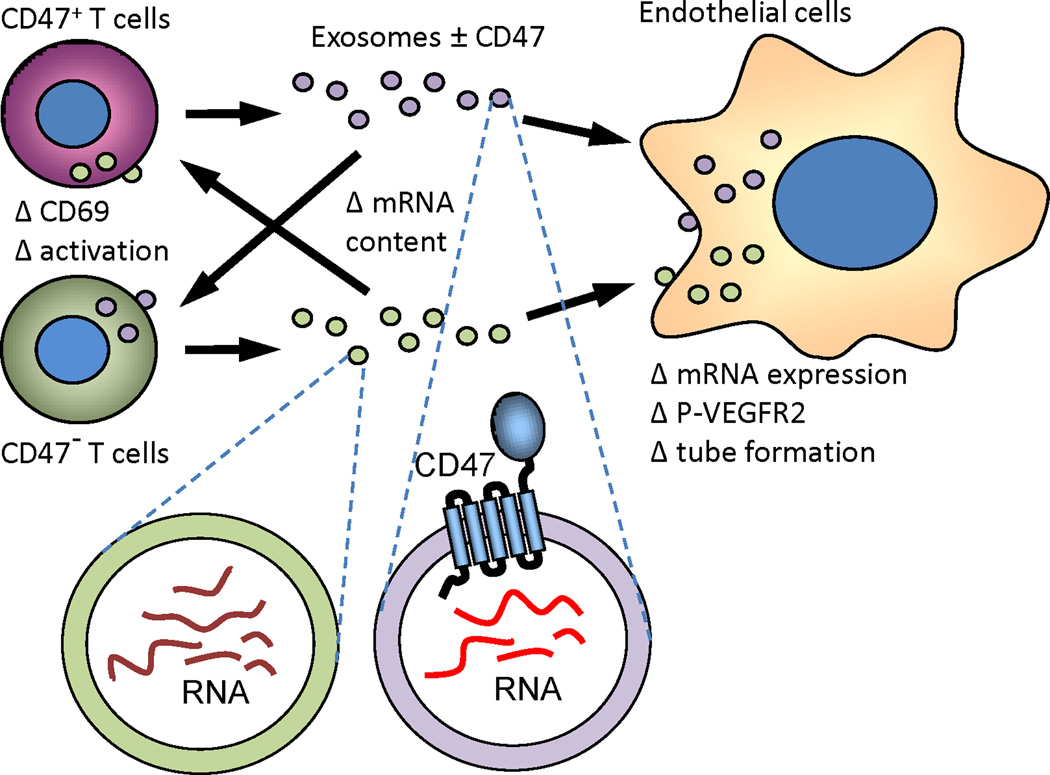
The first functional role of CD47 in EVs was recently reported (Kaur et al., 2014b). The presence of CD47 on EVs released by Jurkat T cells but not a somatic mutant lacking CD47 was confirmed using flow cytometry and western blot analysis. Exposure of Jurkat T cells to these EVs modulated the activity of TSP1 to inhibit TCR-dependent activation in CD47 dependent manner (Fig. 5). CD47-deficient EVs exhibited better uptake than those from parental Jurkat T cells, suggesting that CD47 may have role in uptake and transport of exosome contents into target cells. The role of CD47 on EVs was further examined using endothelial cells. Endothelial cells took up T cell-derived EVs, and these exosomes altered VEGFR2 phosphorylation and tube formation in CD47 dependent manner. The T cell derived EVs altered global gene expression of recipient endothelial cells in a CD47-dependent as well as -independent manner. These data suggest that EVs released by peripheral blood T cells mediate CD47-dependent communication with endothelial cells during T cell re-circulation and trans-endothelial migration. Future studies should examine the potential for incorporating CD47 into engineered exosomes to alter their functional activity.
Fig. 5.
Extracellular signaling by CD47. CD47 is released by cells in extracellular vesicles known as exosomes. Exosomes are taken up by endothelial cells and T cells and enable transfer of CD47 and the RNA cargo of exosomes to target cells that can alter gene expression and functional responses in the recipient cells in a CD47-dependent manner (Kaur et al., 2014b).
Downstream signaling
Unless noted otherwise, the signaling pathways discussed below are controlled by TSP1 binding to CD47. This does not imply that SIRPα or SIRPγ binding cannot alter CD47 signaling through some of the same pathways. Rather, this merely reflects a general lack of experimental data to test those hypotheses (Table 1).
Table 1.
Roles of specific ligands in CD47 signaling functions
| Signal | TSP1 peptides | TSP1 | SIRPα/SIRP-Fc | CD47/CD47-Fc |
|---|---|---|---|---|
| Integrin activation | + | + | − | ? |
| calcium | + | + | ? | ? |
| NO/cGMP | + | + | ? | ? |
| BNIP3 translocation | + | ? | − | ? |
| cAMP | + | + | ? | ? |
| autophagy | + | ? | ? | ? |
| Cell death | + | + | ? | ? |
| VEGFR2 activation | ? | + | ? | ? |
| Cell self-renewal: cMyc, Sox2, Klf4, Oct4 |
+ | + | ? | ? |
| Intercellular adhesion | + | + | + | + |
| Neurite and filopodia formation |
? | ? | + | ? |
| NADPH oxidase | ? | + | ? | ? |
| T cell activation | + | + | + | ? |
Integrin activation
Integrin activation was the first effector pathway identified for CD47 signaling. Based on their co-purification, this pathway was studied initially using αvβ3 integrin, which binds its ligand vitronectin only when induced to undergo a major conformation change that exposes its ligand binding site (Dong et al., 2012). Integrin activation can be quantified using conformation-dependent integrin antibodies and by measuring increased affinity of the integrins for soluble ligands. Functional integrin activation, assessed for αvβ3 by increased cell adhesion and/or spreading on immobilized vitronectin, also involves changes in integrin avidity, which is regulated by interactions with cytoskeletal elements that induce clustering of the integrin and favors multivalent interactions with an immobilized integrin ligand. Adhesion of cells to beads coated with vitronectin established that expression of CD47 is necessary for αvβ3-mediated but not α5β1-mediated binding of beads to cells (Lindberg et al., 1996b). The IgV domain of CD47 is sufficient to stimulate this multivalent interaction. Expressing CD47 in CD47-deficient OV10 cells increased binding of the activation-dependent antibodies LIBS1 and LIBS6 to αvβ3 (McDonald et al., 2004). This increase in integrin affinity is independent of membrane cholesterol, but depleting cholesterol reduced the ability of CD47 ligation to stimulate cell adhesion on vitronectin. These studies indicate that CD47 signaling regulates both the affinity and avidity of αvβ3 integrin.
Understanding the role of TSP1 in regulating CD47-mediated activation of αvβ3 integrin has been compromised by reliance on peptides as a TSP1 surrogate. Unfortunately, studies employing native TSP1 as a CD47 agonist to study integrin activation are limited by the fact that TSP1 itself is a ligand for several β1 integrins, and engaging some β1 integrins can lead to cross-activation of β3 integrins (Bernardi et al., 2006). One study using 4N1K to activate αvβ3 concluded that CD47-mediated integrin activation on platelets and a human B-lymphocytic cell line is independent of intracellular signaling (Fujimoto et al., 2003). TSP1 was not used as a CD47 ligand in this study, and as noted above, all studies relying exclusively on this peptide should be viewed with skepticism and may have no relevance to TSP1 signaling through CD47. Another study employing 4N1K to activate the related platelet integrin αIIbβ3 found that peptide ligation of CD47 stimulated phosphorylation of Lyn and Syk and their association with focal adhesion kinase (FAK). The phosphorylation of Syk was pertussis toxin sensitive, implicating a heterotrimeric G protein in mediating the integrin activating signal (Chung et al., 1997). 4N1K induced the recruitment of FAK to a membrane complex containing CD47, the integrin, and Src. Similarly, Src, Lyn, and SHP2 were implicated in αvβ3 activation (McDonald et al., 2004). Peptide 7N3, containing the other TSP1 VVM motif, similarly enhances αvβ3-mediated adhesion of cells on vitronectin, and this activity was also pertussis toxin-sensitive and induced tyrosine phosphorylation of FAK (Chandrasekaran et al., 1999, Sipes et al., 1999). In all of these studies, it is unclear which of the identified signaling responses are upstream versus downstream of integrin activation. Based on current understanding of integrin signaling, the FAK and Src kinase responses are probably downstream from integrin activation. Given the close physical association between CD47 and αvβ3 integrin, a ligand-induced conformation change in CD47, with some involvement of G-proteins, may be sufficient to activate the integrin.
CD47 ligation also induces activation of α4β1 and αLβ2 (LFA1) integrins (Barazi et al., 2002, Azcutia et al., 2013). In sickle red blood cells CD47-dependent activation of α4β1 was associated with a cAMP-independent PKA activity that phosphorylated the cytoplasmic tail of the α4 subunit (Brittain et al., 2004). Both soluble TSP1 and the 4N1K peptide activated α4β1-mediated adhesion of sickle red blood cells. A subsequent study showed that CD47-deficient T cells exhibit impaired adhesion on the LFA1 ligand ICAM1 and the α4β1 ligand VCAM1, but this was not due to defective integrin avidity regulation (Azcutia et al., 2013). Rather, CD47-deficient cells exhibited impaired integrin affinity activation by divalent cations as detected by activation-dependent antibody binding. This affinity regulation by CD47 occurred in intact cells but was lost following detergent solubilization and did not involve dissociation of CD47 from the integrin complex.
TSP1 and the peptide 4N1K increase platelet aggregation on an immobilized collagen substrate, accompanied by increased tyrosine phosphorylation (Chung et al., 1999). This activity requires expression of CD47 on the platelets and is mediated by α2β1 integrin. Co-immunoprecipitation suggested a physical interaction between CD47 and α2β1 integrin. Functional interaction between CD47 and α2β1 integrin was similarly reported in vascular smooth muscle cells (Wang et al., 1999). Ligation of CD47 in these cells stimulated chemotaxis in a soluble collagen gradient, which was mediated by Gi-dependent suppression of cAMP synthesis and inhibition of ERK activation.
CD47-induced cell epithelial cell spreading and cell-cell adhesion involves downstream activation of Src and mitogen-activated protein kinase kinase (MEK) (Shinohara et al., 2006). Based on lack of SIRPα co-localization, this does not involve interaction between CD47 and SIRPα. Further evidence that SIRP binding is not involved in integrin activation comes from the observation that the CD47 antibody B6H12 inhibits SIRPα binding but enhances melanoma and T cell adhesion on the αvβ3 ligand vitronectin and the α4β1 ligands NoC2 and VCAM1 (Barazi et al., 2002). Notably in the same study, B6H12 decreased the binding of soluble VCAM1 to cells despite increasing their adhesion. Therefore, some CD47 ligands can differentially regulate integrin affinity and avidity. It is unclear whether this activity extends to any physiological ligand of CD47.
Cytoplasmic calcium
Cytoplasmic calcium signaling has been consistently implicated in CD47 signal transduction, but many details of the mechanism remain unclear. CD47 selectively regulates integrin-dependent calcium influx in endothelial cells. B6H12 inhibited Ca2+ influx stimulated by an integrin ligand but not that stimulated by histamine (Schwartz et al., 1993). Because B6H12 is regarded as a function-blocking antibody for TSP1 binding, this data implies that TSP1/CD47 signaling positively regulates cytoplasmic calcium. Consistent with that model, recombinant C-terminal TSP1 containing its CD47 binding domain elevated cytoplasmic calcium in Jurkat T cells, and this in turn inhibited the activation of soluble guanylyl cyclase (sGC) by nitric oxide (NO) (Ramanathan et al., 2011). Cross-linking CD47 on the surface of rat brain endothelial cells also increased cytoplasmic calcium, and this involved increased Src kinase activity (Martinelli et al., 2013). In cardiac myocytes, treatment with the TSP1-derived CD47-binding peptide 7N3 increased cytoplasmic calcium (Sharifi-Sanjani et al., 2014). Notably, inhibition of the Na+/Ca2+ exchanger using amiloride prevented the 7N3-induced calcium flux, HDAC3 phosphorylation, and CAM-kinase II expression. Conversely, TSP1/CD47 signaling inhibited an ionomycin-stimulated calcium flux in endothelial cells (Bauer et al., 2010). This was consistent with the inhibition by TSP1of eNOS activation, which is also calcium dependent. It is unclear whether these disparate results reflect cell-specific or agonist-specific effects of CD47 signaling on cytoplasmic calcium flux.
Cyclic nucleotide signaling
As noted above, CD47 regulates cAMP synthesis via heterotrimeric G-protein signaling. Although the original studies employed the peptide 4N1K as the CD47 agonist (Frazier et al., 1999), other studies have verified that TSP1 and some CD47 antibodies can decrease cAMP levels in several cell types (Guo et al., 1998, Manna and Frazier, 2003, Yao et al., 2011). Conversely, cAMP levels are elevated in thbs1- and cd47-null vascular smooth muscle cells and skeletal and cardiac muscle tissues, and addition of exogenous TSP1 restores WT cAMP levels in thbs1-null cells (Isenberg et al., 2009c, Yao et al., 2011). Thus, it is clear that TSP1 signaling through CD47 acutely inhibits cAMP levels in several cell types. Some of this regulation involves G-protein mediated regulation of adenylyl cyclase. However, cGMP-regulated cAMP phosphodiesterases provide an additional pathway for CD47 signaling to regulate cAMP levels in some cell types (Yao et al., 2011).
Early studies in melanoma cells reported that TSP1 acutely decreases cellular cGMP levels (Guo et al., 1998). The elucidation of NO as the major activator of sGC in vascular cells prompted us to revisit this data. Physiological concentrations of TSP1 block NO-stimulated cGMP synthesis in endothelial cells, vascular smooth muscle cells, T cells, and platelets (Isenberg et al., 2009b). The inhibitory activity of TSP1 was lost in cells lacking CD47 but not in cells lacking the TSP1 receptor CD36. TSP1 also inhibited some pharmacological activators of sGC (Miller et al., 2010a), confirming that sGC is a target of CD47 signaling (Fig. 6). Some details of this signal transduction remain unclear, but a study in Jurkat T cells demonstrated that CD47-dependent elevation of cytoplasmic calcium levels inhibits sGC activation via phosphorylation by an unidentified kinase (Ramanathan et al., 2011).
Fig. 6.
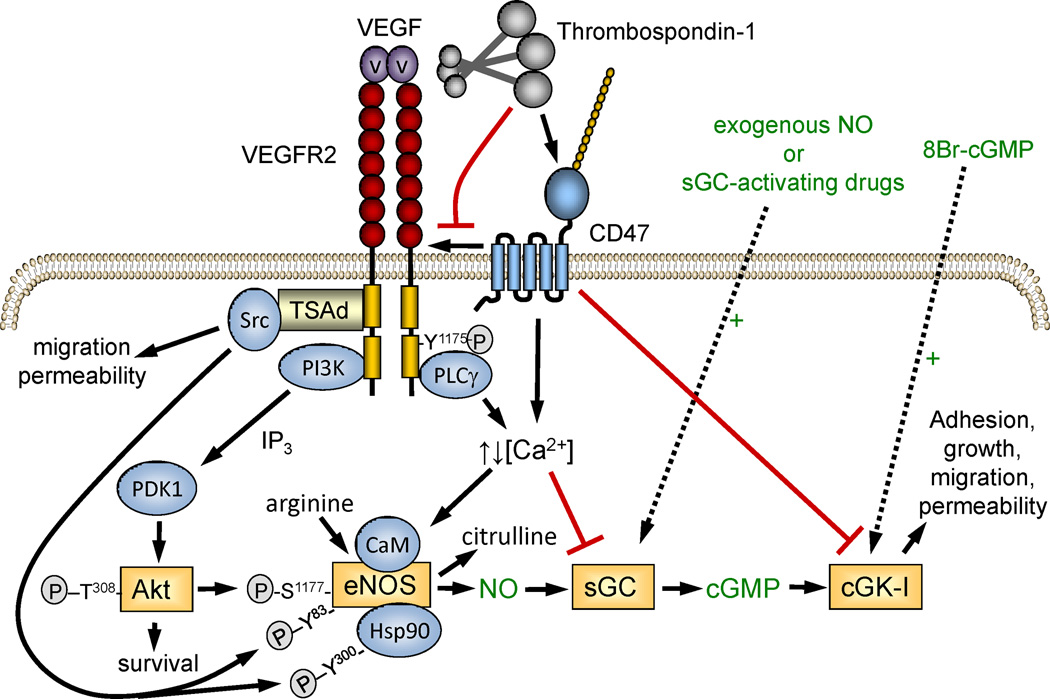
CD47 regulation of nitric oxide signaling in vascular cells. TSP1 signaling through CD47 redundantly inhibits NO signaling that the level of eNOS, sGC, and cGK. This blocks signaling initiated by upstream calcium- and phosphorylation-mediated activation of eNOS as well as activation by exogenous NO, sGC activating drugs or phosphodiesterase inhibitors, or the direct cGK activator 8-bromo-cGMP. By dissociating the lateral interaction of CD47 with VEGFR2, TSP1 further inhibits upstream signals that activate eNOS and globally inhibits NO-independent branches of the VEGFR2 signaling cascade.
Remarkably, sGC is not the only element of the NO/cGMP pathway that CD47 regulates (Fig. 6). Studies in platelets demonstrated that CD47 signaling also inhibits downstream signaling at the level of cGMP-dependent protein kinase (cGK). Activation of cGK by cell-permeable 8Br-cGMP was inhibited by TSP1 through CD47 (Isenberg et al., 2008d). Furthermore, TSP1/CD47 signaling was found to inhibit the activity of eNOS, the major NO biosynthetic enzyme in endothelial cells (Bauer et al., 2010). This was demonstrated by decreased conversion of radiolabeled arginine to citrulline, the other enzymatic product of eNOS. In the presence of calcium, calmodulin binds to and activates eNOS, and TSP1 limits the duration of calcium influx in endothelial cells. This contrasts with the inhibitory role of calcium at the level of sGC in T cells and suggests that the role of calcium in regulating the NO/cGMP cascade may be cell type-specific. Further work is needed to clarify whether calcium is the exclusive mediator of CD47 signaling in this context.
eNOS is also activated by Akt-mediated phosphorylation, and in endothelial cells this in turn is controlled by VEGF signaling through VEGFR2 (Fig. 6). We found that CD47 laterally associates with VEGFR2 in endothelial cells and T cells (Kaur et al., 2014a, Kaur et al., 2010). TSP1 binding to CD47 inhibits the ability of VEGF to induce activation of the tyrosine-kinase activity of VEGFR2, resulting in inhibition of downstream activation of Src and Akt, both of which regulate the activity of eNOS. Taken together, these studies establish that CD47 is a highly redundant regulator of the NO/cGMP cascade, controlling both upstream activation of NO biosynthesis and downstream effector functions in vascular cells. Furthermore, its regulation of VEGFR2 activation reveals that CD47 also regulates pro-angiogenic signaling pathways that are NO-independent.
Cell survival pathways
In the context of cellular stress CD47 plays roles both in cell death and survival. Deficiency of CD47 promotes cell survival from stress associated with ionizing radiation (Isenberg et al., 2008a, Maxhimer et al., 2009b). Mutational inactivation of CD47 in Jurkat T cells preserved cell viability, reduced cytotoxicity and preserved their proliferative capacity after exposure to ionizing radiation (Soto-Pantoja et al., 2012). Associated with the improved survival, increased autophagosome formation and expansion was detected by electron microscopy. To further characterize autophagosome formation, microtubule-associated protein 1A/1B–light chain 3 (LC3) expression was detected using a GFP-fusion construct, and increased LC3 puncta were observed in CD47-deficient Jurkat cells only after exposure to ionizing radiation. This indicated that the effect of CD47 deficiency in cell survival is mediated at least in part by the activation of autophagy.
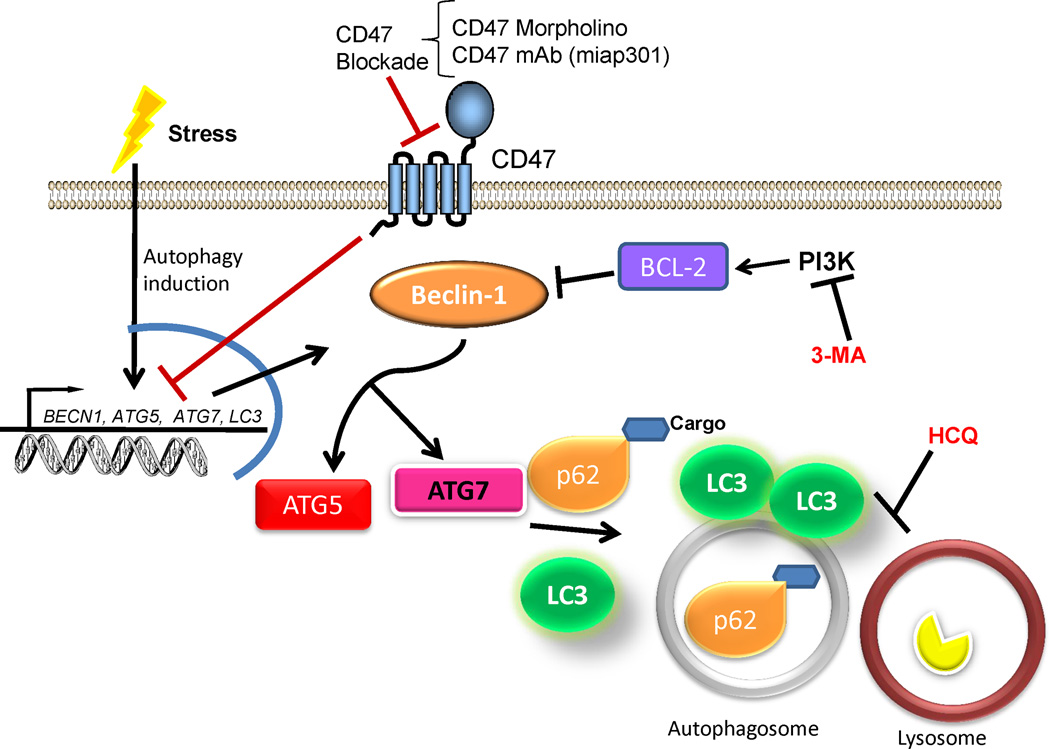
Autophagy is a homeostatic catabolic mechanism that is characterized by the formation of the autophagosome for the eventual degradation of cargo by fusion with a lysosome (Fig. 7). To confirm that deficiency of CD47 activates autophagy, autophagic flux was measured by determining the increase of LC3 lipidation and reduction in p62 sequestosome. p62 is an ubiquitin-binding scaffold that is degraded by autophagy and may serve to link ubiquitinated proteins to the autophagic machinery to enable their degradation in the lysosome. p62 accumulates when autophagy is inhibited, and decreased levels can be observed when autophagy is induced (Bjorkoy et al., 2009). Further experiments indicated that mRNA and protein expression of the BCL-2 interacting protein beclin-1 is up-regulated in CD47-deficient cells, indicating that CD47 governs at the earlier stages of autophagy. Moreover expression of the autophagy related proteins ATG5/7 is increased in the absence of CD47. As further proof that CD47 governs protective autophagy, treatment with 3-methyladenine, an inhibitor of phosphatidylinositol 3-kinase, and hydroxychloroquine, which raises lysosomal pH and inhibits autophagosome formation, reversed the protective effect. Knockdown of ATG5 and ATG7 using siRNAs also reversed the protective effect in CD47-deficient Jurkat cells. However, stable transfection of CD47-deficient Jurkat with a plasmid to re-express CD47 re-sensitized the cells to death by ionizing radiation and reversed the effect observed in autophagosome formation. These experiments confirm that the radioprotection observed in CD47-deficient Jurkat cells is due to the absence of CD47. Enhanced autophagy was also observed by knocking down CD47 using an anti-sense morpholino in wild type Jurkat cells and in vivo in mice prior to exposure to ionizing radiation (Soto-Pantoja et al., 2012, Soto-Pantoja et al., 2013b). In treated mice, increased autophagy was associated with reduced apoptosis as measured by TUNEL staining in lung, esophageal and intestinal tissues. Moreover, lung endothelial cells isolated from cd47-null mice show increased LC3 puncta after exposure to ionizing radiation indicating activation of autophagy in in vivo in knock out animals as a response to radiation.
Fig. 7.
CD47 regulation of autophagy. In the presence of cellular stress, blockade of CD47 activates gene expression of beclin-1 and autophagy related proteins (ATG) 5 and 7 that limit BCL-2 mediated pathways to induce cell death. The increase in beclin-1 ATG5 and ATG7 leads to increased autophagic flux by increasing expression of LC3 to form the autophagosome membrane and stimulates p62, which targets unwanted cargo to be degraded in the autophagosome. The radioprotective effect of CD47 is reversed by the administration of 3-methyladenine (3-MA), which inhibits early activation of autophagy, or the lysosomotropic agent hydroxychloroquine (HCQ), which inhibits autophagy at late stages of the pathway.
Others have reported that TSP1 activates autophagy in an H-Ras-transformed murine dermal fibroblast cell line (Kalas et al., 2013). However, activity of TSP1 was not examined and merely inferred by activity of the peptide 4N1K but not the analog 4N1GG at concentrations greater than 10 µM. The peptide 4N1K decreased viability of the Ras-transformed cells but not the parental fibroblast cell line. The cells did not show the hallmarks of apoptosis, but had increased LC3. The role of CD47 was further supported by activity of the mouse CD47 blocking antibody miap301. The conclusions from this study need to be explored further due to several limitations: autophagic flux was not measured, and autophagosome formation was inferred from acridine orange staining, which is not a specific stain for these structures.
Regulation of autophagy by CD47 was also reported in a model of transverse aortic constriction to simulate left ventricular heart failure (Sharifi-Sanjani et al., 2014). Wild type left ventricular heart tissue had elevated LC3 expression 7 days post transverse aortic constriction, but no increase in LC3 was seen post-injury in cd47-null mice subjected to the same protocol. Conversely, CD47 ligation in wild type mice by treatment with the function blocking antibody miap301 resulted in decreased protein expression of the autophagy markers LC3, ATG5 and ATG7. However, autophagic flux was not measured, and autophagosome formation was not assessed in this study. These results indicate that the presence of CD47 can enhance autophagy in injured heart muscle under conditions where TSP1 expression is also increased. Thus, the inhibitory activity of the CD47-blocking antibody is inferred to result from blocking TSP1-CD47 signaling, although an alternative hypothesis is that the antibody decreases the autophagy response by inhibiting inflammatory cell infiltration by blocking CD47-SIRPα interactions, and the autophagy response would be secondary to that inflammation. cd47-null mice have known deficits in recruitment of innate inflammatory cells.
Ligation of CD47 can also induce cell death. An apoptotic response induced by engagement of CD47 was observed in activated T cells treated with antibody Ab22 that reacts with the IgV domain of CD47 (Pettersen et al., 1999). This antibody also elicited a cell death response in Jurkat cells as determined by morphological cell structure, phosphatidylserine exposure on the cell surface, and measurement of cell death by TUNEL using flow cytometry. In the same study, CD47 antibodies B6H12 and 2D3 failed to induce a cell death response in culture. However, other studies demonstrate that CD47 interactions with immobilized B6H12 or antibody BRIC126 can induce an apoptotic response in B-chronic lymphocyte leukemia (Sick et al., 2012). Binding of the counter receptor SIRPα as a soluble Fc-fusion protein did not induce and apoptotic response (Lamy et al., 2003), but SIRPα bound onto the surface of beads induced apoptosis through CD47 in Jurkat T cells and the monocyte cell line U937 (Brooke et al., 2004). Similar results were found when assessing SIRPγ binding to CD47. TSP1 binding to CD47 can also elicit a cells death response in T cells. TSP1 plasma levels from human patients are correlated increased T cell apoptosis, which was also associated with increased CD47 expression before the development of T cell anergy. TSP1 engagement to CD47 also resulted in death of chronic lymphocytic leukemic B cells (Mateo et al., 2002).
Although different signaling pathways have been implicated in cell death induced by engaging CD47, most studies agree that the mechanism is independent of caspase activation. Cell death by ligation of CD47 involves the dynamin-related protein 1 (Drp1), which is known as a key activator of type II cell death (Bras et al., 2007). Plating on immobilized antibody B6H12 or treatment with soluble TSP1 induced Drp1 activation and translocation to the mitochondria, which caused a reduction in mitochondrial membrane potential, mitochondrial swelling, and other cell morphological changes such as the dilation of the Golgi complex and redistribution of the endoplasmic reticulum around the nucleus. Ligation of CD47 with 4N1K at an extremely high concentration (400 µM) induced death of Jurkat cells, associated in wild type Jurkat cells with the translocation of BNIP3 from CD47 to the mitochondria (Lamy et al., 2003). The same peptide did not induce death when antisense oligonucleotides were used to reduce BNIP3 expression or when similar treatments were performed in CD47-deficient Jurkat cells. Moreover, the up-regulation of BNIP3 was necessary for engaging CD47 by 4N1K to induce cell death in H-Ras transformed cells (Kalas et al., 2011), suggesting oncogenic transformation may be required for the sensitization of cells to death upon CD47 ligation.
Mitochondrial biogenesis
Transmission electron microscopic studies revealed that skeletal muscle in young thbs1-null and cd47-null mice contains a larger number and size of mitochondria than WT skeletal muscle (Frazier et al., 2011). The cd47-null mice correspondingly exhibited enhanced treadmill endurance, and biochemical studies demonstrated lower reactive oxygen species production and more efficient metabolism in the cd47-null mitochondria. Notably, this mitochondrial phenotype is tissue-specific and disappeared as the mice aged. Several CD47 signaling pathways may contribute to the mitochondrial phenotype. Expression of the major transcription factor for nuclear and mtDNA encoded mitochondrial genes, peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α), is elevated in cd47-null muscle, and expression of PGC-1α and mitochondrial biogenesis are known to be stimulated by NO (Nisoli and Carruba, 2006). cAMP-dependent protein kinase phosphorylation of the CD47 target Drp1 regulates its GTPase activity and mitochondrial fission (Chang and Blackstone, 2007) and could account for the increased mitochondrial size. cMyc levels also regulate mitochondrial biogenesis (Dang, 2013). Therefore, the elevated cMyc expression in cd47-null and thbs1-null tissues could also account for their increased mitochondrial densities.
cMyc and other stem cell transcription factors
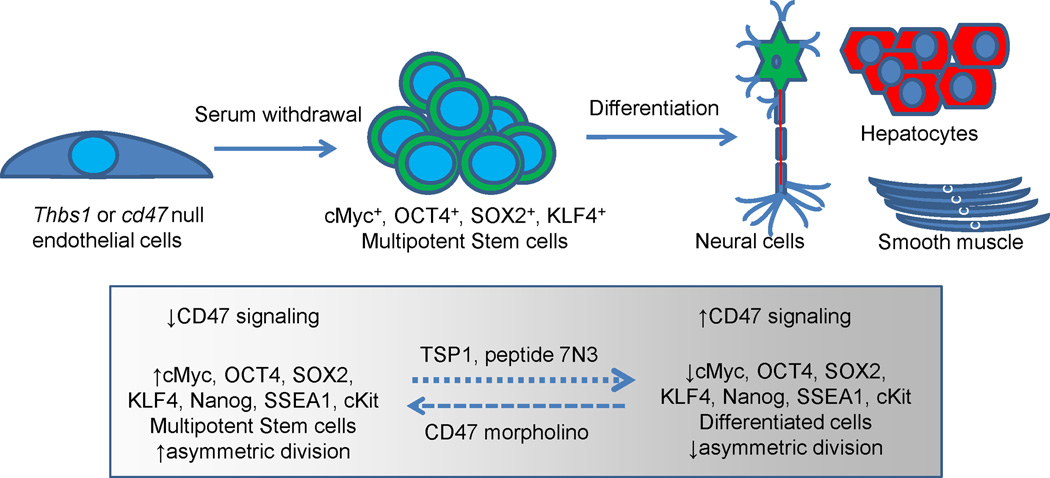
Differentiated eukaryotic cells can undergo a limited number of symmetric cell divisions before becoming senescent. By dividing asymmetrically, adult tissue stem cells preserve genomic integrity and enable the maintenance of viability and regenerative capacity of tissues in multicellular eukaryotes. The maintenance of stem cells is controlled by four major transcription factors: Oct3/4, Klf4, Sox2, and cMyc, also known as the Yamanaka factors (Takahashi and Yamanaka, 2006). Forced expression of these transcription factors in somatic cells can induce their conversion to induced pluripotent stem (iPS) cells. Thbs1-null mice have more circulating CD13+/VEGFR-2+/CD45−/CD117+ endothelial progenitor stem cells (EPCs) than wild type mice (Shaked et al., 2005). The increase in EPCs was suppressed by using drug targeting CD36, suggesting that this activity of TSP1 is mediated via CD36. On the other hand, EPCs express high levels of CD47, and knockdown enhanced their cell proliferation and angiogenic potential (Smadja et al., 2011). Recombinant human TSP1 inhibited the angiogenic potential of EPCs in vitro and was mediated by CD47 binding. Similarly, cd47- and thbs1-null mouse lung endothelial cells exhibit increased proliferative capacity, which is due to increased expression of the four Yamanaka transcription factors (Kaur et al., 2013). Upon withdrawal of serum, wild type endothelial cells rapidly become senescent, but the cd47-null endothelial cells continue proliferating and form embryoid body-like structures that can differentiate into the three germ layer cells with addition of appropriate growth factors (Fig. 8). Elevated numbers of SOX2high tissue stem cells were also found in vivo in cd47-null mice. TSP1 and the TSP1-derived CD47 binding peptide 7N3 down regulated expression of cMyc only in cells that express CD47. In contrast, cancer cells with dysregulated cMyc such as Burkitt’s lymphoma were insensitive to TSP1 or over-expression of CD47. This study demonstrated that CD47 in normal cells acts as repressor of stem cell self-renewal by limiting the expression of cMyc and other stem cell transcription factors, but this signaling becomes uncoupled in Myc-dependent cancers. These findings may explain how cancer cells can maintain high expression of CD47 to evade immune surveillance while simultaneously maintaining cancer stem cells/tumor initiating cells that sustain tumor growth.
Fig. 8.
CD47 signaling limits stem cell self-renewal. Withdrawal of serum from differentiated cd47-null cells induces their spontaneous reprogramming to multipotent stem cells that can differentiate along all embryonic lineages. Suppression of CD47 in wild type cells using a CD47 morpholino increases expression of stem cell transcription factors and increased asymmetric cell division, whereas treatment with TSP1 or a CD47-binding peptide derived from TSP1 suppresses the stem cell phenotype.
Hydrogen sulfide signaling
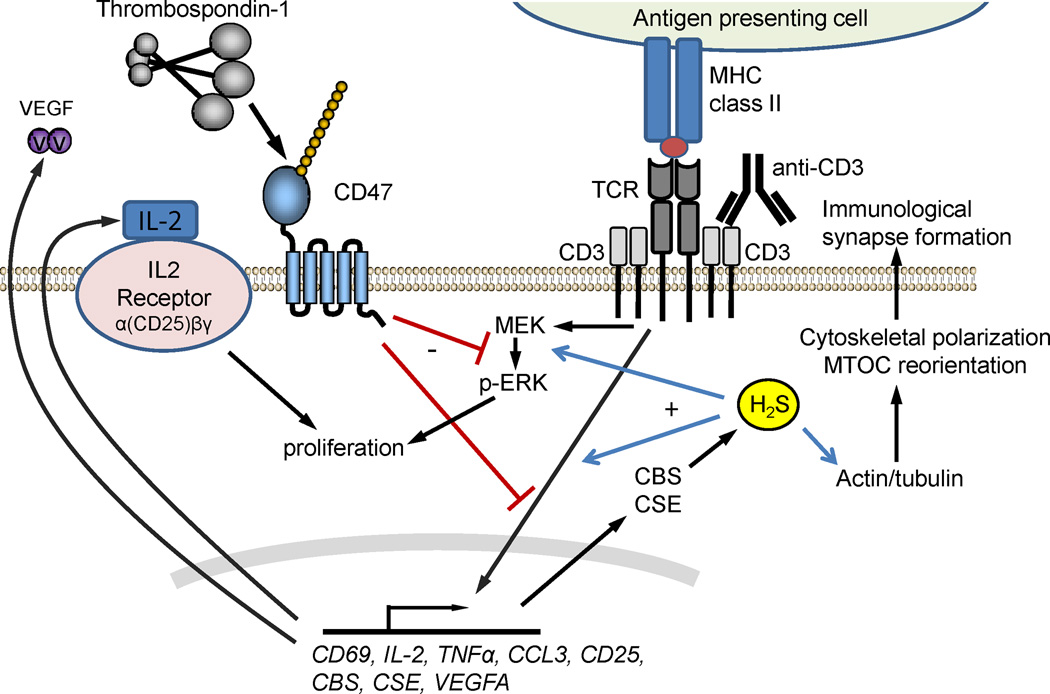
At high concentrations H2S is a toxic gas, but its biosynthesis is also important for physiological signaling in mammals. The toxicity of H2S involves inhibition of mitochondrial electron transport, but its physiological functions are mediated at least in part by sulfhydration of specific protein thiols and disulfides in a growing list of cellular proteins (Kolluru et al., 2013). At sub-micromolar concentrations H2S is a physiological enhancer of T cell activation (Miller et al., 2012). Treatment of anti-CD3- and anti-CD28-activated primary murine T cells and human Jurkat T cells with 300 nM NaHS enhanced the expression of CD69, CD25 and IL-2 under hypoxic conditions (1% O2) that limit the mitochondrial oxidation of H2S. H2S similarly enhanced antigen-dependent TCR activation of murine CD4+ OT-II T cells treated with OVA2 peptide and anti-CD28. H2S enhanced T cell adhesion, proliferation, and polarization of the actin cytoskeleton to form an immunological synapse. H2S also serves as an autocrine mediator of T cell activation because expression of the H2S biosynthetic enzymes cystathionine β–synthase (CBS) and cystathionine γ–lyase (CSE) was induced during T cell activation, and activation was attenuated if the expression of these enzymes was blocked using siRNA.
TSP1 inhibits T cell activation induced by TCR signaling (Li et al., 2001), and CD47 is essential for this inhibitory activity of TSP1 (Li et al., 2002, Kaur et al., 2011). Recently, TSP1 signaling through CD47 was demonstrated to inhibit H2S-stimulated T cell activation (Miller et al., 2013) (Fig. 9). Exogenous H2S further activated murine T cells lacking TSP1 more than wild type T cells that were stimulated using anti-CD3 plus anti-CD28, and exogenous TSP1 inhibited this activation. Naive and anti-CD3 and -CD28 activated thbs1-null cells also exhibited elevated expression of CBS and CSE. Thus, TSP1 regulates exogenous and endogenous H2S signaling to restrict T cell activation. Cd47-null murine T cells were resistant to the inhibitory activity of TSP1. Furthermore, H2S-induced ERK activation was inhibited by TSP1 in T cells derived from wild type mice but not mice lacking CD47. Therefore, TSP1 signaling via CD47 limits T cell activation at least in part by limiting H2S biosynthesis and signaling.
Fig. 9.
CD47 regulation of T cell activation and H2S signaling. T cells are activated by T cell receptor (TCR) signaling in response to recognition of antigens presented in the context of MHC or experimentally using an anti-CD3 antibody. TSP1 signaling through CD47 inhibits TCR signaling via several mechanisms including inhibiting induction of the H2S biosynthetic enzymes cystathionine β–synthase (CBS) and cystathionine γ–lyase (CSE). H2S produced by CBS and CSE increases actin polymerization and reorients the microtubule organizing center (MTOC) to enhance immunological synapse formation with antigen-presenting cells.
Other redox signaling pathways
TSP1 at 2 nM and the CD47-binding TSP1 peptide 7N3 at 10 µM stimulated superoxide production by vascular smooth muscle cells (Csanyi et al., 2012). CD47-blocking antibodies in mouse and rat cells/tissues prevented this induction, and siRNA knockdown confirmed that NADPH oxidase-1 (NOX1) mediated the superoxide production. TSP1 treatment of smooth muscle cells significantly increased serine phosphorylation of the p47phox subunit, and this was mediated by protein kinase C activation.
TSP1 at 10–20 nM induced the death of primary cortical mouse neurons (Xing et al., 2009). This was replicated by 4N1K at high concentrations that are probably nonspecific. Both TSP1 and the peptide increased the generation of ROS. Unfortunately, the evidence that CD47 induced this neuronal death and the involvement of caspase-3 activation was based solely on 4N1K peptide data. Furthermore, it should be considered that TSP1 at similar concentrations is known to activate NADPH oxidase in macrophages to produce superoxide via a CD47-independent mechanism (Martin-Manso et al., 2008).
CD47 signaling controlled by SIRPα binding
Several studies have indicated that SIRPα binding can regulate CD47 signaling. CD47 ligation by a CD47 antibody decreased IL-12-induced IFN-γ production in unfractionated T cells and purified CD4+ and CD8+ T cells cultured on immobilized anti-CD3 (Latour et al., 2001). Similarly, culturing in the presence of L cell fibroblasts transfected to express SIRPα inhibited IFN-γ production by purified T cells cultured for 5 days with anti-CD3, inhibited IL-12R expression, and down-regulated IL-12 responsiveness of activated CD4+ and CD8+ adult T cells without affecting their response to IL-2. Another study using immobilized CD47 antibody found that CD47 ligation in Jurkat T cells induces actin polymerization (Rebres et al., 2001a). This was mediated by recruitment of PKC-θ to the cytoskeleton. The same response was observed using immobilized SIRPα-Fc. Immobilized SIRPα-Fc also synergize with anti-CD3 to induce IL-2 expression. These studies provide evidence to support reverse SIRPα signaling via CD47, but the relevance to TSP1 signaling through CD47 under the same conditions is unclear.
SIRPα-Fc promoted neurite and filopodia formation by N1E-115 neuroblastoma cells in a Rac- and Cdc42-dependent manner (Miyashita et al., 2004). Over-expressing CD47 enhanced the activity of SIRPα-Fc. Inhibition by a β3 integrin antibody implicated this integrin in mediating Rac and Cdc42 signaling.
Signaling by homotypic CD47 binding
The potential signaling function of homotypic CD47 binding remains largely unexplored. Rac1 GTP loading was induced by CD47-dependent cell aggregation (Rebres et al., 2005). Further indirect evidence for a functional role of CD47 in cell-cell adhesion comes from its localization at epithelial and endothelial cell-cell contacts (Shinohara et al., 2006, Martinelli et al., 2013). Inhibitor studies implicated CD47-mediated regulation of the actin cytoskeleton via Src and mitogen-activated protein kinase kinase (MEK) in Madin-Darby canine kidney cells. However, a potential role of the counter-receptor SIRPα in the latter cell-cell interaction has not been excluded.
Signaling pathways downstream of CD47 where the CD47 ligand is not clearly identified
This category includes numerous publications in which activities of peptide 4N1K or CD47 antibodies were reported without confirming roles of TSP1 or SIRPα. In the former cases, involvement of CD47 signaling must also be questioned. Publications implicating TSP1 based on correlative data also require further verification. For example, expression of Drp1 mRNA and protein in B cells from chronic lymphocytic leukemia patients was found to correlate with susceptibility to cell death triggered by CD47 ligation by TSP1 or B6H12 (Bras et al., 2007). When cells were plated on immobilized B6H12, Drp1 redistributed from cytosol to mitochondria, and mitochondrial respiration was inhibited. In Jurkat T cells siRNA knockdown of Drp1 prevented death induced by the immobilized antibody. Although the role of Drp1 in the signal induced by immobilized CD47 antibody is clear in this study, the relevance of this pathway to cell death induced by TSP1 signaling through CD47 remains to be determined.
Several reported activities of CD47 “blocking” antibodies are difficult to reconcile with their known effects on TSP1 or SIRPα binding and suggest that these antibodies may also have agonist activities. For example, B6H12 inhibits binding of TSP1 to human CD47 (Isenberg et al., 2009a), and TSP1 binding to CD47 inhibits NO signaling in vascular endothelial cells. However, B6H12 used in the absence of added TSP1 inhibited NO-stimulated endothelial cell adhesion and proliferation in a similar manner as a recombinant C-terminal domain of TSP1, acting as an apparent agonist (Isenberg et al., 2006). B6H12 also activates specific integrins in a manner parallel to the activity of TSP1 (Barazi et al., 2002). Finally, B6H12 was reported to mimic the activities of TSP1 to induce regulatory T cell differentiation (Grimbert et al., 2006) and inhibit homotypic aggregation of U937 monocytic cells (Yamauchi et al., 2002). Thus, caution is required for interpreting activities of this anti-human CD47 blocking antibody.
Similarly, the anti-murine CD47 antibody miap301 inhibits SIRPα binding to CD47 (Oldenborg et al., 2001), but this antibody failed to enhance macrophage-mediated clearance of CD47-expressing tumor cells in vivo (Willingham et al., 2012). Antibody miap301 also blocks the effects of TSP1 on NO signaling and correspondingly increases tissue survival of ischemia and reperfusion injuries in mice (Isenberg et al., 2007c, Isenberg et al., 2008c, Isenberg et al., 2008b). However, intravenous injection of TSP1 or miap301 showed parallel hypertensive activities in mice, and the hypertensive activity of miap301 was observed even in thbs1-null mice (Bauer et al., 2010). Therefore, this murine CD47 “blocking” antibody appears to also exhibit agonist activities when it binds to CD47.
Regulation of CD47 signaling by trafficking and shedding
Evidence is accumulating that signaling of the CD47-associated receptor VEGFR2 is regulated both by intracellular trafficking and by shedding of the receptor from the cell surface (Swendeman et al., 2008, Zhang et al., 2013). This raises questions about the potential roles of intracellular trafficking and shedding of CD47 in its signaling. This topic is only beginning to be explored. One study in T cells implicated CD47 in the internalization of TSP1 via LDL receptor-related protein-1 (LRP1) and calreticulin (Li et al., 2006). Earlier studies found that a heparan sulfate proteoglycan is required for clearance of TSP1 by endothelial cells (Schon et al., 1992), suggesting that CD47 may play the role of the proteoglycan for presenting TSP1 to LRP1 in T cells. A role for CD47 in internalization of TSP1 is further supported by the observed accumulation of TSP1 in the extracellular matrix when cd47-null mice were subjected to a second degree thermal injury (Soto-Pantoja et al., 2014a). Potential signaling functions of intracellular CD47 remain to be defined. In addition, CD47-dependent clearance of TSP1 from the extracellular environment has the potential to inhibit TSP1 signaling through receptors other than CD47.
CD47 can be released from the cell surface either by proteolytic cleavage or exosome shedding (Kaur et al., 2014b). Although glucose-dependent proteolytic shedding of the IgV domain of CD47 was reported (Maile et al., 2008b), it remains unclear that proteolytic cleavage of the IgV domain necessarily eliminates all signaling through CD47 if the long range disulfide between this domain and the transmembrane domain remains intact. CD47 released in exosomes could represent a paracrine signaling molecule via several mechanisms (Fig. 5). CD47 could incorporate into target cells and signal in response to ligand binding as well as by delivery of the RNA contents of the exosome. Alternatively, CD47 on extracellular EVs could act as a competitive inhibitor by sequestering TSP1 or soluble SIRPα extracellular domain.
Pathophysiological functions of CD47 signaling
Cardiovascular pathophysiology
The NO/cGMP cascade is the major mediator of physiological CD47 signaling in vascular cells. Loss of this tonic inhibitory signaling in thbs1- and cd47-null mice increases NO synthesis and tissue responsiveness to NO, resulting in decreased pulse pressure in the thbs1-null, increased tissue perfusion, and increased resistance of platelets to prothrombotic stimuli (Isenberg et al., 2009c, Isenberg et al., 2008d). Consistent with this mode of action, intravenous injection of TSP1 results in an acute increase in blood pressure (Bauer et al., 2010). Under conditions of acute ischemic injury, blockade of CD47 signaling results in a rapid restoration of tissue perfusion by increasing NO/cGMP signaling in collateral vessels. TSP1 levels increase with aging, in patients with diabetes, reperfusion injury, and pulmonary hypertension, and NO/cGMP signaling consequently is suppressed. Thus, therapeutics that inhibit CD47 signaling could have broad utility.
Tissue self-renewal and stem cells
Several tissues in cd47-null mice have increased densities of Sox2+ stem cells (Kaur et al., 2013), but the pathophysiological consequences of this phenotype have not been defined. The ability of CD47 blockade to enhance tissue survival of stress caused by ischemic injuries and ionizing radiation may result in part from protection of stem cells. Further studies are needed to determine whether reprogramming tissue stem cells by therapeutic blockade of CD47 can be applied in regenerative medicine.
Ionizing radiation
Cd47- and thbs1-null mice are resistant to high dose local irradiation (Isenberg et al., 2008a). Wet desquamation and hair loss (alopecia) were reduced in irradiated hindlimbs of thbs1-null mice and almost completely absent in cd47-null mice. Viability and function of muscle tissue two months after irradiation were also preserved in hindlimbs of the thbs1- and cd47-null mice. Similar radioresistance could be obtained by treating wild type mice with agents to block TSP1 and or CD47 (Maxhimer et al., 2009b). Targeting CD47 in vivo using an antisense morpholino also protected hindlimb tissue after exposure to ionizing radiation. Blockade of CD47 in this model conferred protection to soft tissue as measured by TUNEL staining and preserved the proliferative capacity of hematopoietic precursors in bone marrow. The protection of tissue was associated with increased local tissue perfusion in mice that were administered the antisense morpholino, similar to what was observed in ischemia models. However, the radioprotection conferred by targeting CD47 is independent of the NO/cGMP pathway. Rather, autophagy activation is necessary for the radioprotective effect of CD47 blockade. siRNA-mediated silencing of ATG5 and ATG7 completely reverse the protective effect of CD47 deficiency in vitro (Soto-Pantoja et al., 2012). Targeting CD47 using the morpholino also increases survival of mice exposed to total body irradiation (Soto-Pantoja et al., 2013b). CD47 blockade preserved circulating blood cell numbers and preserved the viability of the radiosensitive esophageal and intestinal mucosa after irradiation. Tissue preservation was associated with reduced cell death and increased autophagy as measured by expression p62 in the same tissues, thus supporting that the radioprotection by CD47 blockade is mediated through an increase in protective autophagy.
Immunity and inflammation
CD47 has both positive and negative roles in immune regulation, and the functions of CD47 in systemic immune responses consequently depend on the specific challenge and nature of the immune response. Mice lacking CD47 are more sensitive to certain infectious agents (Lindberg et al., 1996a), but the milder response of the cd47-null can also be an advantage, as in the case of Staphylococcus aureus-induced arthritis (Verdrengh et al., 1999) and LPS-triggered acute lung injury and E. coli pneumonia (Su et al., 2008).
Cd47- and thbs1-null mice exhibit an enhanced delayed-type hypersensitivity response (Lamy et al., 2007), which is consistent with the inhibitory effects of CD47 signaling on T cell activation and migration (Li et al., 2001, Rebres et al., 2005). By reciprocal regulation of T cell production of immunostimulatory cytokines such as IL-2 and immunoinhibitory factors such as VEGF (Kaur et al., 2014a), CD47 signaling in T cells could also have systemic effects on immune responses. In addition to its roles in T cells, CD47 signaling in dendritic cells and NK cells can regulate immune responses (Mittal et al., 2010, Kim et al., 2008).
Suppression of CD47 signaling activates both innate and adaptive anti-tumor immunosurveillance. The former is currently believed to involve loss of inhibitory SIRPα signaling, but the latter is mediated by CD47 signaling in CD8+ T cells and may explain why blockade of CD47 sensitizes tumors to ionizing radiation (Soto-Pantoja et al., 2014b). Tumors of mice treated with antisense CD47 morpholino and ionizing radiation had increased CD8+ T cell infiltrates. Blockade of CD47 in effector T cells was sufficient to enhance antigen-dependent T cell cytolytic killing of fibrosarcoma cells. Consistent with CD47 having a primary function in the tumor microenvironment, further studies demonstrated that CD47-expressing B16 melanoma tumors grown in a cd47-null microenvironment were sensitized to ionizing radiation relative to the same tumors in a wild type host. Increased CD8 T cell infiltration was also found in irradiated tumors grown in cd47-null mice. Depletion of CD8 T cells in the fibrosarcoma and B16 melanoma tumor models reversed the anti-tumor effect of CD47 blockade and irradiation, indicating that CD8 T cells are essential to observe this anti-tumor effect. The enhanced tumor killing may be mediated by granzyme B, expression of which was increased in tumor of mice that were administered anti-sense CD47 morpholino. Moreover, CD47 expression inversely correlates with CD8 density in tumor sections from human melanoma patients, suggesting that decreased CD47 expression in the human tumor microenvironment similarly elicits an anti-tumor immune response.
Conclusions and perspectives
Although some investigators view CD47 as a passive cell surface protein that serves as a marker of self by engaging its signaling counter-receptor SIRPα, extensive evidence supports the primary function of CD47 as a signaling receptor that provides information to a cell about its neighboring cells and extracellular microenvironment. TSP1 in the extracellular matrix is a key regulator of CD47 signaling. The ability of thbs1-null mice to phenocopy the enhanced resistance of cd47-null mice to specific stresses provides strong genetic evidence that TSP1 signaling through CD47 mediates stress response signaling pathways. Targets of this CD47 signaling include the NO/cGMP pathway, H2S signaling, autophagy, mitochondrial biogenesis, and regulation of stem cell transcription factors. However, a few phenotypes of thbs1-null mice diverge from those of cd47-null mice. In some cases this may involve signaling through TSP1 receptors other than CD47, but further investigation of these divergent CD47 phenotypes could also reveal novel signaling functions of other CD47 ligands. At present, our understanding of how other extracellular and membrane binding partners of CD47 alter its intracellular signaling remains incomplete, and many important questions remain to be addressed (Table 1).
Unanswered questions
The macromolecular architecture of membrane complexes in the plasma membrane that contain CD47 need to be more clearly defined in different cell types and under specific stimulus conditions. How does the composition of these complexes control CD47 signaling? We and others have shown examples of proteins that dissociate from complexes containing CD47 in response to CD47 ligation. Do ligand-induced changes in the conformation of CD47 control these dynamics? Can TSP1 bind to several receptors in this complex simultaneously (e.g. CD47, integrins, and CD36) to elicit distinct signals?
CD47 appears to be relatively unstable on the plasma membrane. Studies have shown that CD47 traffics from the cell surface into extracellular vesicles that can transfer CD47 to other cells, and CD47 can be lost via proteolytic shedding. Some of the receptors that complex with CD47 such as VEGFR2 also traffic intracellularly. Does CD47 traffic with these, and through which compartments? Does CD47 regulate intracellular vesicle signaling while trafficking?
Historically CD47 was isolated as an integrin-associated protein, but beyond the ability of CD47 ligation to induce integrin activation we do not know the role of integrin association in CD47 signaling. Can CD47 signal in the absence of integrin association? Do specific signaling pathways have differential requirements for specific integrins that associate with CD47?
Is TSP1 the only agonist ligand for CD47? Current evidence indicates that TSP2 and TSP4 are lower affinity ligands of CD47 than TSP1 (Isenberg et al., 2009a), but physiological functions of these interactions have not been identified. SIRPα can be encountered by cell surface CD47 during cell-cell contact or as a result of proteolytic shedding of the SIRPα extracellular domain. Does monovalent or multivalent SIRPα binding to CD47 result in CD47 signaling? Alternatively, does binding of shed SIRPα act as a competitive antagonist of TSP1 signaling through CD47? Similarly, can homotypic binding of shed CD47 IgV domain to intact cell surface CD47 elicit agonist or antagonist effects either directly or by inhibiting TSP1 signaling?
Finally, does CD47 signal in the absence of any extracellular ligand? Different CD47 antibodies can enhance or inhibit specific cellular responses to CD47 signaling, e.g. T cell activation. Furthermore, we know that cd47-null murine and CD47-deficient human T cells exhibit defective activation signaling, but we do not know whether activation of CD47+ T cells requires CD47 ligation or whether the presence of unligated CD47 is sufficient to produce a tonic signal. Classic signaling receptors require binding of an agonist ligand, but it is unclear whether that paradigm is appropriate to understand the functions of CD47. Perhaps CD47 is primarily an accessory protein for the signaling receptors with which it laterally associates, and this association may be sufficient to mediate some physiological functions.
Implications for therapeutic targeting of CD47
CD47 signaling resulting from increased expression of TSP1 may play a central role in the pathophysiology of several major diseases of aging. Consequently, CD47 could be an effective therapeutic target in cardiovascular disease to increase blood flow to ischemic tissues. Animal studies further suggest that targeting CD47 could improve healing in diet-induced vasculopathy and ameliorate the effects of cardiac hypertrophy (Isenberg et al., 2007a, Sharifi-Sanjani et al., 2014). The protective activities of antibodies targeting CD47 in liver and kidney ischemia-reperfusion injury models suggest applications in organ transplantation (Isenberg et al., 2008b, Rogers et al., 2012, Lin et al., 2014). Antibodies targeting CD47 to block SIRPα signaling are entering clinical testing to enhance macrophage-mediated clearance of cancers, but the same humanized antibody may also block TSP1-induced signaling through CD47. In cancer, such antibodies could also enhance cytotoxic CD8 T cell anti-tumor responses when combined with radiation therapy (Soto-Pantoja et al., 2014b).
The ubiquitous expression of CD47 combined with its complex but incompletely understood signaling functions present challenges for developing CD47-targeted therapeutics. CD47 blocking antibodies are currently the most advanced towards clinical testing (Edris et al., 2012). Used systemically for cancer therapy, these antibodies will encounter a vast reservoir of CD47 on red blood cells (25,000 copies/cell (Tsai and Discher, 2008)) and on other critical vascular cells such as platelets and endothelium where CD47 plays important physiological roles by limiting NO signaling. Thus, anemia, thrombosis, and hypertension are all predicted side effects of therapeutic CD47 antibodies. Indeed, acute increases in mean arterial and diastolic blood pressure were demonstrated when mice were injected intravenously with a function-blocking mouse CD47 antibody (miap301) (Bauer et al., 2010). Local uses of CD47 antibodies to prevent or treat ischemia and ischemia/reperfusion injuries may avoid these complications and seem more promising (Maxhimer et al., 2009a, Lin et al., 2014).
Acute therapeutic modulation of CD47 expression by antisense therapeutics is one approach to avoid some of the vascular side effects of antibodies because mammalian red blood cells do not turn over their membrane proteins. Red blood cells have a long circulating lifetime and are predicted to exhibit decreased CD47 expression only with prolonged treatment with CD47 antisense therapies.
Ultimately small molecule modifiers of CD47 signaling will be needed. These have the potential to be selective antagonists or agonists of specific CD47 ligands and signaling pathways. Such agents that antagonize TSP1 binding without perturbing SIRPα binding could possess beneficial therapeutic activities without perturbing the “don’t eat me” signaling through SIRPα.
Acknowledgments
Declaration of Interest
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
References
- Adams JC, Bentley AA, Kvansakul M, Hatherley D, Hohenester E. Extracellular matrix retention of thrombospondin 1 is controlled by its conserved C-terminal region. J Cell Sci. 2008;121:784–795. doi: 10.1242/jcs.021006. [DOI] [PubMed] [Google Scholar]
- Avent ND, Madgett TE, Lee ZE, Head DJ, Maddocks DG, Skinner LH. Molecular biology of Rh proteins and relevance to molecular medicine. Expert Rev Mol Med. 2006;8:1–20. doi: 10.1017/S1462399406010969. [DOI] [PubMed] [Google Scholar]
- Azcutia V, Routledge M, Williams MR, Newton G, Frazier WA, Manica A, Croce KJ, Parkos CA, Schmider AB, Turman MV, Soberman RJ, Luscinskas FW. CD47 plays a critical role in T-cell recruitment by regulation of LFA-1 and VLA-4 integrin adhesive functions. Mol Biol Cell. 2013;24:3358–3368. doi: 10.1091/mbc.E13-01-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger ME, Harris ME, Mcdonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazi HO, Li Z, Cashel JA, Krutzsch HC, Annis DS, Mosher DF, Roberts DD. Regulation of integrin function by CD47 ligands. Differential effects on avb3 and a4b1 integrin-mediated adhesion. J Biol Chem. 2002;277:42859–42866. doi: 10.1074/jbc.M206849200. [DOI] [PubMed] [Google Scholar]
- Barclay AN, Van Den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res. 2010;88:471–481. doi: 10.1093/cvr/cvq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi B, Guidetti GF, Campus F, Crittenden JR, Graybiel AM, Balduini C, Torti M. The small GTPase Rap1b regulates the cross talk between platelet integrin alpha2beta1 and integrin alphaIIbbeta3. Blood. 2006;107:2728–2735. doi: 10.1182/blood-2005-07-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkoy G, Lamark T, Pankiv S, Overvatn A, Brech A, Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009;452:181–197. doi: 10.1016/S0076-6879(08)03612-4. [DOI] [PubMed] [Google Scholar]
- Bras M, Yuste VJ, Roue G, Barbier S, Sancho P, Virely C, Rubio M, Baudet S, Esquerda JE, Merle-Beral H, Sarfati M, Susin SA. Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol Cell Biol. 2007;27:7073–7088. doi: 10.1128/MCB.02116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JE, Han J, Ataga KI, Orringer EP, Parise LV. Mechanism of CD47-induced alpha4beta1 integrin activation and adhesion in sickle reticulocytes. J Biol Chem. 2004;279:42393–42402. doi: 10.1074/jbc.M407631200. [DOI] [PubMed] [Google Scholar]
- Brooke G, Holbrook JD, Brown MH, Barclay AN. Human lymphocytes interact directly with CD47 through a novel member of the signal regulatory protein (SIRP) family. J Immunol. 2004;173:2562–2570. doi: 10.4049/jimmunol.173.4.2562. [DOI] [PubMed] [Google Scholar]
- Burger P, De Korte D, Van Den Berg TK, Van Bruggen R. CD47 in Erythrocyte Ageing and Clearance - the Dutch Point of View. Transfus Med Hemother. 2012;39:348–352. doi: 10.1159/000342231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambot M, Mazurier C, Canoui-Poitrine F, Hebert N, Picot J, Clay D, Picard V, Ripoche P, Douay L, Dubart-Kupperschmitt A, Cartron JP. In vitro generated Rh(null) red cells recapitulate the in vivo deficiency: a model for rare blood group phenotypes and erythroid membrane disorders. Am J Hematol. 2013;88:343–349. doi: 10.1002/ajh.23414. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Freemont PS, Foulkes W, Trowsdale J. An ovarian tumor marker with homology to vaccinia virus contains an IgV-like region and multiple transmembrane domains. Cancer Res. 1992;52:5416–5420. [PubMed] [Google Scholar]
- Cant CA, Ullrich A. Signal regulation by family conspiracy. Cell Mol Life Sci. 2001;58:117–124. doi: 10.1007/PL00000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran S, Guo N, Rodrigues RG, Kaiser J, Roberts DD. Pro-adhesive and chemotactic activities of thrombospondin-1 for breast carcinoma cella are mediated by a3b1 integrin and regulated by insulin-like growth factor-1 and CD98. J. Biol. Chem. 1999;274:11408–11416. doi: 10.1074/jbc.274.16.11408. [DOI] [PubMed] [Google Scholar]
- Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, Park CY, Weissman IL, Majeti R. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374–1384. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Weissman IL, Majeti R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24:225–232. doi: 10.1016/j.coi.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LY, Ramakrishnan DP, Silverstein RL. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood. 2013;122:1822–1832. doi: 10.1182/blood-2013-01-482315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Gao AG, Frazier WA. Thrombspondin acts via integrin-associated protein to activate the platelet integrin aIIbb3. J Biol Chem. 1997;272:14740–14746. doi: 10.1074/jbc.272.23.14740. [DOI] [PubMed] [Google Scholar]
- Chung J, Wang XQ, Lindberg FP, Frazier WA. Thrombospondin-1 acts via IAP/CD47 to synergize with collagen in alpha2beta1-mediated platelet activation. Blood. 1999;94:642–648. [PubMed] [Google Scholar]
- Csanyi G, Yao M, Rodriguez AI, Al Ghouleh I, Sharifi-Sanjani M, Frazziano G, Huang X, Kelley EE, Isenberg JS, Pagano PJ. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol. 2012;32:2966–2973. doi: 10.1161/ATVBAHA.112.300031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl KN, Westhoff CM, Discher DE. Fractional attachment of CD47 (IAP) to the erythrocyte cytoskeleton and visual colocalization with Rh protein complexes. Blood. 2003;101:1194–1199. doi: 10.1182/blood-2002-04-1187. [DOI] [PubMed] [Google Scholar]
- Dang CV. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med. 2013:3. doi: 10.1101/cshperspect.a014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Mi LZ, Zhu J, Wang W, Hu P, Luo BH, Springer TA. alpha(V)beta(3) integrin crystal structures and their functional implications. Biochemistry. 2012;51:8814–8828. doi: 10.1021/bi300734n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorahy DJ, Thorne RF, Fecondo JV, Burns GF. Stimulation of platelet activation and aggregation by a carboxyl-terminal peptide from thrombospondin binding to the integrin-associated protein receptor. J Biol Chem. 1997;272:1323–1330. doi: 10.1074/jbc.272.2.1323. [DOI] [PubMed] [Google Scholar]
- Drbal K, Cerny J, Angelisova P, Hilgert I, Cebecauer M, Sinkora J, Horejsi V. CDw149 antibodies recognize a clustered subset of CD47 molecules associated with cytoplasmic signaling molecules. Tissue Antigens. 2000;56:258–267. doi: 10.1034/j.1399-0039.2000.560308.x. [DOI] [PubMed] [Google Scholar]
- Edris B, Weiskopf K, Volkmer AK, Volkmer JP, Willingham SB, Contreras-Trujillo H, Liu J, Majeti R, West RB, Fletcher JA, Beck AH, Weissman IL, Van De, Rijn M. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci U S A. 2012;109:6656–6661. doi: 10.1073/pnas.1121629109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt JF, Musa RH, Ayob Y, Hassan A, Asidin N, Yahya NM, Mathlouthi R, Thornton N, Anstee DJ, Bruce LJ. Study of the D-- phenotype reveals erythrocyte membrane alterations in the absence of RHCE. Br J Haematol. 2012;158:262–273. doi: 10.1111/j.1365-2141.2012.09149.x. [DOI] [PubMed] [Google Scholar]
- Floquet N, Dedieu S, Martiny L, Dauchez M, Perahia D. Human thrombospondin’s (TSP-1) C-terminal domain opens to interact with the CD-47 receptor: a molecular modeling study. Arch Biochem Biophys. 2008;478:103–109. doi: 10.1016/j.abb.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Frazier EP, Isenberg JS, Shiva S, Zhao L, Schlesinger P, Dimitry J, Abu-Asab MS, Tsokos M, Roberts DD, Frazier WA. Age-dependent regulation of skeletal muscle mitochondria by the thrombospondin-1 receptor CD47. Matrix Biol. 2011;30:154–161. doi: 10.1016/j.matbio.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier WA, Gao A-G, Dimitry J, Chung J, Brown EJ, Lindberg FP, Linder ME. The thrombospondin receptor integrin-associated protein (CD47) functionally couples to heterotrimeric Gi. J. Biol. Chem. 1999;274:8554–8560. doi: 10.1074/jbc.274.13.8554. [DOI] [PubMed] [Google Scholar]
- Fujimoto TT, Katsutani S, Shimomura T, Fujimura K. Thrombospondin-bound integrin-associated protein (CD47) physically and functionally modifies integrin alphaIIbbeta3 by its extracellular domain. J Biol Chem. 2003;278:26655–26665. doi: 10.1074/jbc.M302194200. [DOI] [PubMed] [Google Scholar]
- Gao A-G, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- Grimbert P, Bouguermouh S, Baba N, Nakajima T, Allakhverdi Z, Braun D, Saito H, Rubio M, Delespesse G, Sarfati M. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25− T cells in response to inflammation. J Immunol. 2006;177:3534–3541. doi: 10.4049/jimmunol.177.6.3534. [DOI] [PubMed] [Google Scholar]
- Grunewald FS, Prota AE, Giese A, Ballmer-Hofer K. Structure-function analysis of VEGF receptor activation and the role of coreceptors in angiogenic signaling. Biochim Biophys Acta. 2010;1804:567–580. doi: 10.1016/j.bbapap.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Guo N, Zabrenetzky VS, Chandrasekaran L, Sipes JM, Lawler J, Krutzsch HC, Roberts DD. Differential Roles of protein kinase C and pertussis toxin-sensitive G-binding proteins in modulation of melanoma cell proliferation and motility by thrombospondin-1. Cancer Res. 1998;58:3154–3162. [PubMed] [Google Scholar]
- Gupta K, Gupta P, Wild R, Ramakrishnan S, Hebbel RP. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis. 1999;3:147–158. doi: 10.1023/a:1009018702832. [DOI] [PubMed] [Google Scholar]
- Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson AB. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem. 2012;287:19094–19104. doi: 10.1074/jbc.M111.322933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatherley D, Graham SC, Turner J, Harlos K, Stuart DI, Barclay AN. Paired receptor specificity explained by structures of signal regulatory proteins alone and complexed with CD47. Mol Cell. 2008;31:266–277. doi: 10.1016/j.molcel.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD. Differential interactions of thrombospondin-1, −2, and −4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem. 2009a;284:1116–1125. doi: 10.1074/jbc.M804860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Hyodo F, Pappan LK, Abu-Asab M, Tsokos M, Krishna MC, Frazier WA, Roberts DD. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler Thromb Vasc Biol. 2007a;27:2582–2588. doi: 10.1161/ATVBAHA.107.155390. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem. 2007b;282:15404–15415. doi: 10.1074/jbc.M701638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer. 2009b;9:182–194. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Maxhimer JB, Hyodo F, Pendrak ML, Ridnour LA, Degraff WG, Tsokos M, Wink DADRD. Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am. J. Pathol. 2008a;173:1100–1112. doi: 10.2353/ajpath.2008.080237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, Roberts DD. Treatment of ischemia/reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery. 2008b;144:752–761. doi: 10.1016/j.surg.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Pappan LK, Romeo MJ, Abu-Asab M, Tsokos M, Wink DA, Frazier WA, Roberts DD. Blockade of thrombospondin-1-CD47 interactions prevents necrosis of full thickness skin grafts. Ann Surg. 2008c;247:180–190. doi: 10.1097/SLA.0b013e31815685dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009c;28:110–119. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007c;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood. 2008d;111:613–623. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M, Urushido M, Hamada K, Matsumoto T, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Horino T, Fujieda M, Fujimoto S, Terada Y. Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol. 2013;305:F495–F509. doi: 10.1152/ajprenal.00642.2012. [DOI] [PubMed] [Google Scholar]
- Jin G, Tsuji K, Xing C, Yang YG, Wang X, Lo EH. CD47 gene knockout protects against transient focal cerebral ischemia in mice. Exp Neurol. 2009;217:165–170. doi: 10.1016/j.expneurol.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalas W, Swiderek E, Rapak A, Kopij M, Rak J, Strzadala L. H-ras up-regulates expression of BNIP3. Anticancer Res. 2011;31:2869–2875. [PubMed] [Google Scholar]
- Kalas W, Swiderek E, Switalska M, Wietrzyk J, Rak J, Strzadala L. Thrombospondin-1 receptor mediates autophagy of RAS-expressing cancer cells and triggers tumour growth inhibition. Anticancer Res. 2013;33:1429–1438. [PubMed] [Google Scholar]
- Kaur S, Chang T, Singh SP, Lim L, Mannan P, Garfield SH, Pendrak ML, Soto-Pantoja DR, Rosenberg AZ, Jin S, Roberts DD. CD47 Signaling Regulates the Immunosuppressive Activity of VEGF in T Cells. J Immunol. 2014a;193:3914–3924. doi: 10.4049/jimmunol.1303116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Kuznetsova SA, Pendrak ML, Sipes JM, Romeo MJ, Li Z, Zhang L, Roberts DD. Heparan sulfate modification of the transmembrane receptor CD47 is necessary for inhibition of T cell receptor signaling by thrombospondin-1. J Biol Chem. 2011;286:14991–15002. doi: 10.1074/jbc.M110.179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits vascular endothelial growth factor receptor-2 signaling by disrupting its association with CD47. J Biol Chem. 2010;285:38923–38932. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Singh SP, Elkahloun AG, Wu W, Abu-Asab MS, Roberts DD. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells. Matrix Biol. 2014b;37:49–59. doi: 10.1016/j.matbio.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Soto-Pantoja DR, Stein EV, Liu C, Elkahloun AG, Pendrak ML, Nicolae A, Singh SP, Nie Z, Levens D, Isenberg JS, Roberts DD. Thrombospondin-1 Signaling through CD47 Inhibits Self-renewal by Regulating c-Myc and Other Stem Cell Transcription Factors. Sci Rep. 2013;3:1673. doi: 10.1038/srep01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw MH, Smyth MJ. Immunology. Making macrophages eat cancer. Science. 2013;341:41–42. doi: 10.1126/science.1241716. [DOI] [PubMed] [Google Scholar]
- Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, Hwang D, Kim KP, Kim DW. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839–849. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Lee JC, Lee JJ, Kim S, Lee SG, Park SW, Sung MW, Heo DS. Association of CD47 with natural killer cell-mediated cytotoxicity of head-and-neck squamous cell carcinoma lines. Tumour Biol. 2008;29:28–34. doi: 10.1159/000132568. [DOI] [PubMed] [Google Scholar]
- Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld MD, Frazier WA. Identification of a new cell adhesion motif in two homologous peptides from the COOH-terminal cell binding domain of human thrombospondin. J Biol Chem. 1993;268:8808–8814. [PubMed] [Google Scholar]
- Kvansakul M, Adams JC, Hohenester E. Structure of a thrombospondin C-terminal fragment reveals a novel calcium core in the type 3 repeats. Embo J. 2004;23:1223–1233. doi: 10.1038/sj.emboj.7600166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. J Immunol. 2007;178:5930–5939. doi: 10.4049/jimmunol.178.9.5930. [DOI] [PubMed] [Google Scholar]
- Lamy L, Ticchioni M, Rouquette-Jazdanian AK, Samson M, Deckert M, Greenberg AH, Bernard A. CD47 and the 19 kDa interacting protein-3 (BNIP3) in T cell apoptosis. J Biol Chem. 2003;278:23915–23921. doi: 10.1074/jbc.M301869200. [DOI] [PubMed] [Google Scholar]
- Latour S, Tanaka H, Demeure C, Mateo V, Rubio M, Brown EJ, Maliszewski C, Lindberg FP, Oldenborg A, Ullrich A, Delespesse G, Sarfati M. Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J Immunol. 2001;167:2547–2554. doi: 10.4049/jimmunol.167.5.2547. [DOI] [PubMed] [Google Scholar]
- Leclair P, Lim CJ. CD47-independent effects mediated by the TSP-derived 4N1K peptide. PLoS One. 2014;9:e98358. doi: 10.1371/journal.pone.0098358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Arnott D, Brown EJ. Ubiquilin4 is an adaptor protein that recruits Ubiquilin1 to the autophagy machinery. EMBO Rep. 2013a;14:373–381. doi: 10.1038/embor.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Jeon IS, Han NE, Song HJ, Kim EG, Choi JW, Song KD, Lee HK, Choi JK. Ubiquilin 1 interacts with Orai1 to regulate calcium mobilization. Mol Cells. 2013b;35:41–46. doi: 10.1007/s10059-013-2268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SS, Liu Z, Uzunel M, Sundqvist KG. Endogenous thrombospondin-1 is a cell surface ligand for regulation of integrin dependent T lymphocyte adhesion. Blood. 2006;108:3112–3120. doi: 10.1182/blood-2006-04-016832. [DOI] [PubMed] [Google Scholar]
- Li Z, Calzada MJ, Sipes JM, Cashel JA, Krutzsch HC, Annis D, Mosher DF, Roberts DD. Interactions of thrombospondins with a4b1 integrin and CD47 differentially modulate T cell behavior. J Cell Biol. 2002;157:509–519. doi: 10.1083/jcb.200109098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, He L, Wilson KE, Roberts DD. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J Immunol. 2001;166:2427–2436. doi: 10.4049/jimmunol.166.4.2427. [DOI] [PubMed] [Google Scholar]
- Lin Y, Manning PT, Jia J, Gaut JP, Xiao Z, Capoccia BJ, Chen CC, Hiebsch RR, Upadhya G, Mohanakumar T, Frazier WA, Chapman WC. CD47 Blockade Reduces Ischemia-Reperfusion Injury and Improves Outcomes in a Rat Kidney Transplant Model. Transplantation. 2014;98:394–401. doi: 10.1097/TP.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996a;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- Lindberg FP, Gresham HD, Reinhold MI, Brown EJ. Integrin-associated protein immunoglobulin domain is necessary for efficient vitronectin bead binding. J Cell Biol. 1996b;134:1313–1322. doi: 10.1083/jcb.134.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg FP, Gresham HD, Schwarz E, Brown EJ. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in alpha v beta 3-dependent ligand binding. J Cell Biol. 1993;123:485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lu L, Hettinger CL, Dong G, Zhang D, Rezvani K, Wang X, Wang H. Ubiquilin-1 protects cells from oxidative stress and ischemic stroke caused tissue injury in mice. J Neurosci. 2014;34:2813–2821. doi: 10.1523/JNEUROSCI.3541-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maile LA, Allen LB, Hanzaker CF, Gollahon KA, Dunbar P, Clemmons DR. Glucose regulation of thrombospondin and its role in the modulation of smooth muscle cell proliferation. Exp Diabetes Res. 2010;2010 doi: 10.1155/2010/617052. pii: 617052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maile LA, Capps BE, Miller EC, Aday AW, Clemmons DR. Integrin-associated protein association with SRC homology 2 domain containing tyrosine phosphatase substrate 1 regulates igf-I signaling in vivo. Diabetes. 2008a;57:2637–2643. doi: 10.2337/db08-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maile LA, Capps BE, Miller EC, Allen LB, Veluvolu U, Aday AW, Clemmons DR. Glucose regulation of integrin-associated protein cleavage controls the response of vascular smooth muscle cells to insulin-like growth factor-I. Mol Endocrinol. 2008b;22:1226–1237. doi: 10.1210/me.2007-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maile LA, Clemmons DR. Integrin-associated protein binding domain of thrombospondin-1 enhances insulin-like growth factor-I receptor signaling in vascular smooth muscle cells. Circ Res. 2003;93:925–931. doi: 10.1161/01.RES.0000101754.33652.B7. [DOI] [PubMed] [Google Scholar]
- Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Van Rooijen N, Weissman IL., Jr CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PP, Dimitry J, Oldenborg PA, Frazier WA. CD47 augments Fas/CD95-mediated apoptosis. J Biol Chem. 2005;280:29637–29644. doi: 10.1074/jbc.M500922200. [DOI] [PubMed] [Google Scholar]
- Manna PP, Frazier WA. The mechanism of CD47-dependent killing of T cells: heterotrimeric Gi-dependent inhibition of protein kinase A. J Immunol. 2003;170:3544–3553. doi: 10.4049/jimmunol.170.7.3544. [DOI] [PubMed] [Google Scholar]
- Martin-Manso G, Galli S, Ridnour LA, Tsokos M, Wink DADRD. Thrombospondin-1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity by differentiated U937 cells. Cancer Res. 2008;68:7090–7099. doi: 10.1158/0008-5472.CAN-08-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli R, Newton G, Carman CV, Greenwood J, Luscinskas FW. Novel role of CD47 in rat microvascular endothelium: signaling and regulation of T-cell transendothelial migration. Arterioscler Thromb Vasc Biol. 2013;33:2566–2576. doi: 10.1161/ATVBAHA.113.301903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo V, Brown EJ, Biron G, Rubio M, Fischer A, Deist FL, Sarfati M. Mechanisms of CD47-induced caspase-independent cell death in normal and leukemic cells: link between phosphatidylserine exposure and cytoskeleton organization. Blood. 2002;100:2882–2890. doi: 10.1182/blood-2001-12-0217. [DOI] [PubMed] [Google Scholar]
- Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 2009;19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Mawby WJ, Holmes CH, Anstee DJ, Spring FA, Tanner MJ. Isolation and characterization of CD47 glycoprotein: a multispanning membrane protein which is the same as integrin-associated protein (IAP) and the ovarian tumour marker OA3. Biochem J. 1994;304:525–530. doi: 10.1042/bj3040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD. Thrombospondin-1-CD47 blockade following ischemia reperfusion injury is tissue protective. Plast. Reconstr. Surg. 2009a;124:1880–1889. doi: 10.1097/PRS.0b013e3181bceec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, Wink DA, Isenberg JS, Roberts DD. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci. Transl. Med. 2009b;1:3ra7. doi: 10.1126/scitranslmed.3000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald JF, Dimitry JM, Frazier WA. An amyloid-like C-terminal domain of thrombospondin-1 displays CD47 agonist activity requiring both VVM motifs. Biochemistry. 2003;42:10001–10011. doi: 10.1021/bi0341408. [DOI] [PubMed] [Google Scholar]
- Mcdonald JF, Zheleznyak A, Frazier WA. Cholesterol-independent interactions with CD47 enhance alphavbeta3 avidity. J Biol Chem. 2004;279:17301–17311. doi: 10.1074/jbc.M312782200. [DOI] [PubMed] [Google Scholar]
- Miller TW, Isenberg JS, Roberts DD. Thrombospondin-1 is an inhibitor of pharmacological activation of soluble guanylate cyclase. Br J Pharmacol. 2010a;159:1542–1547. doi: 10.1111/j.1476-5381.2009.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Isenberg JS, Shih HB, Wang Y, Roberts DD. Amyloid-beta Inhibits No-cGMP Signaling in a CD36- and CD47-Dependent Manner. PLoS One. 2010b;5:e15686. doi: 10.1371/journal.pone.0015686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Kaur S, Ivins-O’keefe K, Roberts DD. Thrombospondin-1 is a CD47-dependent endogenous inhibitor of hydrogen sulfide signaling in T cell activation. Matrix Biol. 2013;32:316–324. doi: 10.1016/j.matbio.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Wang EA, Gould S, Stein EV, Kaur S, Lim L, Amarnath S, Fowler DH, Roberts DD. Hydrogen sulfide is an endogenous potentiator of T cell activation. J Biol Chem. 2012;287:4211–4221. doi: 10.1074/jbc.M111.307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R, Gonzalez-Gomez I, Prasadarao NV. Escherichia coli K1 promotes the ligation of CD47 with thrombospondin-1 to prevent the maturation of dendritic cells in the pathogenesis of neonatal meningitis. J Immunol. 2010;185:2998–3006. doi: 10.4049/jimmunol.1001296. [DOI] [PubMed] [Google Scholar]
- Miyashita M, Ohnishi H, Okazawa H, Tomonaga H, Hayashi A, Fujimoto TT, Furuya N, Matozaki T. Promotion of neurite and filopodium formation by CD47: roles of integrins, Rac, and Cdc42. Mol Biol Cell. 2004;15:3950–3963. doi: 10.1091/mbc.E04-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouro-Chanteloup I, Delaunay J, Gane P, Nicolas V, Johansen M, Brown EJ, Peters LL, Van Kim CL, Cartron JP, Colin Y. Evidence that the red cell skeleton protein 4.2 interacts with the Rh membrane complex member CD47. Blood. 2003;101:338–344. doi: 10.1182/blood-2002-04-1285. [DOI] [PubMed] [Google Scholar]
- Mushegian A. Refining structural and functional predictions for secretasome components by comparative sequence analysis. Proteins. 2002;47:69–74. [PubMed] [Google Scholar]
- N’diaye EN, Brown EJ. The ubiquitin-related protein PLIC-1 regulates heterotrimeric G protein function through association with Gbetagamma. J Cell Biol. 2003;163:1157–1165. doi: 10.1083/jcb.200307155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Tanaka T, Naitoh H, Mori C, Fukumoto M, Hiai H, Toyokuni S. Overexpression of integrin-associated protein (CD47) in rat kidney treated with a renal carcinogen, ferric nitrilotriacetate. Jpn J Cancer Res. 1997;88:120–128. doi: 10.1111/j.1349-7006.1997.tb00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119:2855–2862. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med. 2001;193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- Per-Arne O. Role of CD47 and Signal Regulatory Protein Alpha (SIRPalpha) in Regulating the Clearance of Viable or Aged Blood Cells. Transfus Med Hemother. 2012;39:315–320. doi: 10.1159/000342537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen RD, Hestdal K, Olafsen MK, Lie SO, Lindberg FP. CD47 signals T cell death. J Immunol. 1999;162:7031–7040. [PubMed] [Google Scholar]
- Pfeiffer A, Bottcher A, Orso E, Kapinsky M, Nagy P, Bodnar A, Spreitzer I, Liebisch G, Drobnik W, Gempel K, Horn M, Holmer S, Hartung T, Multhoff G, Schutz G, Schindler H, Ulmer AJ, Heine H, Stelter F, Schutt C, Rothe G, Szollosi J, Damjanovich S, Schmitz G. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur J Immunol. 2001;31:3153–3164. doi: 10.1002/1521-4141(200111)31:11<3153::aid-immu3153>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Qin Q, Qian J, Ge L, Shen L, Jia J, Jin J, Ge J. Effect and mechanism of thrombospondin-1 on the angiogenesis potential in human endothelial progenitor cells: an in vitro study. PLoS One. 2014;9:e88213. doi: 10.1371/journal.pone.0088213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S, Mazzalupo S, Boitano S, Montfort WR. Thrombospondin-1 and angiotensin II inhibit soluble guanylyl cyclase through an increase in intracellular calcium concentration. Biochemistry. 2011;50:7787–7799. doi: 10.1021/bi201060c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebres RA, Green JM, Reinhold MI, Ticchioni M, Brown EJ. Membrane raft association of CD47 is necessary for actin polymerization and protein kinase C theta translocation in its synergistic activation of T cells. J Biol Chem. 2001a;276:7672–7680. doi: 10.1074/jbc.M008858200. [DOI] [PubMed] [Google Scholar]
- Rebres RA, Kajihara K, Brown EJ. Novel CD47-dependent intercellular adhesion modulates cell migration. J Cell Physiol. 2005;205:182–193. doi: 10.1002/jcp.20379. [DOI] [PubMed] [Google Scholar]
- Rebres RA, Vaz LE, Green JM, Brown EJ. Normal ligand binding and signaling by CD47 (integrin-associated protein) requires a long range disulfide bond between the extracellular and membrane spanning domains. J Biol Chem. 2001b;13:13. doi: 10.1074/jbc.M106107200. [DOI] [PubMed] [Google Scholar]
- Rho J, Chung J, Im H, Liong M, Shao H, Castro CM, Weissleder R, Lee H. Magnetic nanosensor for detection and profiling of erythrocyte-derived microvesicles. ACS Nano. 2013;7:11227–11233. doi: 10.1021/nn405016y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NM, Thomson AW, Isenberg JS. Activation of Parenchymal CD47 Promotes Renal Ischemia-Reperfusion Injury. J Am Soc Nephrol. 2012;23:1538–1550. doi: 10.1681/ASN.2012020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg C, Srinivasan D, Mah L, Kaushik S, Peterhoff CM, Ugolino J, Fang S, Cuervo AM, Nixon RA, Monteiro MJ. Ubiquilin functions in autophagy and is degraded by chaperone-mediated autophagy. Hum Mol Genet. 2010;19:3219–3232. doi: 10.1093/hmg/ddq231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadallah S, Eken C, Martin PJ, Schifferli JA. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J Immunol. 2011;186:6543–6552. doi: 10.4049/jimmunol.1002788. [DOI] [PubMed] [Google Scholar]
- Sarfati M, Fortin G, Raymond M, Susin S. CD47 in the immune response: role of thrombospondin and SIRP-alpha reverse signaling. Curr Drug Targets. 2008;9:842–850. doi: 10.2174/138945008785909310. [DOI] [PubMed] [Google Scholar]
- Schon P, Vischer P, Volker W, Schmidt A, Faber V. Cell-associated proteoheparan sulfate mediates binding and uptake of thrombospondin in cultured porcine vascular endothelial cells. Eur J Cell Biol. 1992;59:329–339. [PubMed] [Google Scholar]
- Schwartz MA, Brown EJ, Fazeli B. A 50-kDa integrin-associated protein is required for integrin-regulated calcium entry in endothelial cells. J Biol Chem. 1993;268:19931–19934. [PubMed] [Google Scholar]
- Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, Rawn K, Voskas D, Dumont DJ, Ben-David Y, Lawler J, Henkin J, Huber J, Hicklin DJ, D’amato RJ, Kerbel RS. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Sharifi-Sanjani M, Shoushtari AH, Quiroz M, Baust J, Sestito SF, Mosher M, Ross M, Mctiernan CF, St Croix CM, Bilonick RA, Champion HC, Isenberg JS. Cardiac CD47 drives left ventricular heart failure through Ca2+-CaMKII-regulated induction of HDAC3. J Am Heart Assoc. 2014;3:e000670. doi: 10.1161/JAHA.113.000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Ohyama N, Murata Y, Okazawa H, Ohnishi H, Ishikawa O, Matozaki T. CD47 regulation of epithelial cell spreading and migration, and its signal transduction. Cancer Sci. 2006;97:889–895. doi: 10.1111/j.1349-7006.2006.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sick E, Boukhari A, Deramaudt T, Ronde P, Bucher B, Andre P, Gies JP, Takeda K. Activation of CD47 receptors causes proliferation of human astrocytoma but not normal astrocytes via an Akt-dependent pathway. Glia. 2011;59:308–319. doi: 10.1002/glia.21102. [DOI] [PubMed] [Google Scholar]
- Sick E, Jeanne A, Schneider C, Dedieu S, Takeda K, Martiny L. CD47 update: a multi-faceted actor in the tumour microenvironment of potential therapeutic interest. Br J Pharmacol. 2012;167:1415–1430. doi: 10.1111/j.1476-5381.2012.02099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipes JM, Krutzsch HC, Lawler J, Roberts DD. Cooperation between thrombospondin-1 type 1 repeat peptides and integrin avb3 ligands to promote melanoma cell spreading and focal adhesion formation. J. Biol. Chem. 1999;274:22755–22762. doi: 10.1074/jbc.274.32.22755. [DOI] [PubMed] [Google Scholar]
- Smadja DM, D’audigier C, Bieche I, Evrard S, Mauge L, Dias JV, Labreuche J, Laurendeau I, Marsac B, Dizier B, Wagner-Ballon O, Boisson-Vidal C, Morandi V, Duong-Van-Huyen JP, Bruneval P, Dignat-George F, Emmerich J, Gaussem P. Thrombospondin-1 is a plasmatic marker of peripheral arterial disease that modulates endothelial progenitor cell angiogenic properties. Arterioscler Thromb Vasc Biol. 2011;31:551–559. doi: 10.1161/ATVBAHA.110.220624. [DOI] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Kaur S, Miller TW, Isenberg JS, Roberts DD. Leukocyte surface antigen CD47. UCSD Molecule Pages. 2013a:2. [Google Scholar]
- Soto-Pantoja DR, Miller TW, Pendrak ML, Degraff WG, Sullivan C, Ridnour LA, Abu-Asab M, Wink DA, Tsokos M, Roberts DD. CD47 deficiency confers cell and tissue radioprotection by activation of autophagy. Autophagy. 2012;8:1628–1642. doi: 10.4161/auto.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Ridnour LA, Wink DA, Roberts DD. Blockade of CD47 increases survival of mice exposed to lethal total body irradiation. Sci Rep. 2013b;3:1038. doi: 10.1038/srep01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Shih HB, Maxhimer JB, Cook KL, Ghosh A, Isenberg JS, Roberts DD. Thrombospondin-1 and CD47 signaling regulate healing of thermal injury in mice. Matrix Biol. 2014a;37:25–34. doi: 10.1016/j.matbio.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Terabe M, Ghosh A, Ridnour LA, Degraff WG, Wink DA, Berzofsky JA, Roberts DD. CD47 in the Tumor Microenvironment Limits Cooperation between Antitumor T-cell Immunity and Radiotherapy. Cancer Res. 2014b doi: 10.1158/0008-5472.CAN-14-0037-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Johansen M, Looney MR, Brown EJ, Matthay MA. CD47 deficiency protects mice from lipopolysaccharide-induced acute lung injury and Escherichia coli pneumonia. J Immunol. 2008;180:6947–6953. doi: 10.4049/jimmunol.180.10.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Boder ET, Discher DE. Phylogenetic divergence of CD47 interactions with human signal regulatory protein alpha reveals locus of species specificity. Implications for the binding site. J Biol Chem. 2007;282:1805–1818. doi: 10.1074/jbc.M603923200. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Parthasarathy R, Sen S, Boder ET, Discher DE. Species- and cell type-specific interactions between CD47 and human SIRPalpha. Blood. 2006a;107:2548–2556. doi: 10.1182/blood-2005-04-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Tsai R, Sen S, Dahl KN, Discher DE. Membrane mobility and clustering of Integrin Associated Protein (IAP, CD47)--major differences between mouse and man and implications for signaling. Blood Cells Mol Dis. 2006b;36:364–372. doi: 10.1016/j.bcmd.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Swendeman S, Mendelson K, Weskamp G, Horiuchi K, Deutsch U, Scherle P, Hooper A, Rafii S, Blobel CP. VEGF-A stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk between VEGFR2 and ERK signaling. Circ Res. 2008;103:916–918. doi: 10.1161/CIRCRESAHA.108.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tibben JG, Massuger LF, Claessens RA, Schijf CP, Pak KY, Strijk SP, Kenemans P, Corstens FH. Tumour detection and localization using 99Tcm-labelled OV-TL 3 Fab’ in patients suspected of ovarian cancer. Nucl Med Commun. 1992;13:885–893. doi: 10.1097/00006231-199212000-00006. [DOI] [PubMed] [Google Scholar]
- Tsai RK, Discher DE. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180:989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulasne D, Judd BA, Johansen M, Asazuma N, Best D, Brown EJ, Kahn M, Koretzky GA, Watson SP. C-terminal peptide of thrombospondin-1 induces platelet aggregation through the Fc receptor gamma-chain-associated signaling pathway and by agglutination. Blood. 2001;98:3346–3352. doi: 10.1182/blood.v98.12.3346. [DOI] [PubMed] [Google Scholar]
- Umemori H, Sanes JR. Signal regulatory proteins (SIRPS) are secreted presynaptic organizing molecules. J Biol Chem. 2008;283:34053–34061. doi: 10.1074/jbc.M805729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue ER. Perspectives on anti-CD47 antibody treatment for experimental cancer. Proc Natl Acad Sci U S A. 2013;110:10886–10887. doi: 10.1073/pnas.1308463110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kim CL, Colin Y, Cartron JP. Rh proteins: key structural and functional components of the red cell membrane. Blood Rev. 2006;20:93–110. doi: 10.1016/j.blre.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Van Ravenswaay Claasen HH, Eggermont AM, Nooyen YA, Warnaar SO, Fieuren GJ. Immunotherapy in a human ovarian cancer xenograft model with two bispecific monoclonal antibodies: OV-TL 3/CD3 and OC/TR. Gynecol Oncol. 1994;52:199–206. doi: 10.1006/gyno.1994.1031. [DOI] [PubMed] [Google Scholar]
- Vasagiri N, Kutala VK. Structure, function, and epigenetic regulation of BNIP3: a pathophysiological relevance. Mol Biol Rep. 2014;41:7705–7714. doi: 10.1007/s11033-014-3664-x. [DOI] [PubMed] [Google Scholar]
- Verdrengh M, Lindberg FP, Ryden C, Tarkowski A. Integrin-associated protein (IAP)-deficient mice are less susceptible to developing Staphylococcus aureus-induced arthritis. Microbes Infect. 1999;1:745–751. doi: 10.1016/s1286-4579(99)80076-8. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Lindberg FP, Frazier WA. Integrin-associated protein stimulates alpha2beta1-dependent chemotaxis via Gi-mediated inhibition of adenylate cyclase and extracellular- regulated kinases. J Cell Biol. 1999;147:389–400. doi: 10.1083/jcb.147.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua MS, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PO, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, Van De Rijn M, Teng NN, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AL, Wang J, Zheleznyak A, Brown EJ. Ubiquitin-related proteins regulate interaction of vimentin intermediate filaments with the plasma membrane. Mol Cell. 1999;4:619–625. doi: 10.1016/s1097-2765(00)80212-9. [DOI] [PubMed] [Google Scholar]
- Xing C, Lee S, Kim WJ, Jin G, Yang YG, Ji X, Wang X, Lo EH. Role of oxidative stress and caspase 3 in CD47-mediated neuronal cell death. J Neurochem. 2009;108:430–436. doi: 10.1111/j.1471-4159.2008.05777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Fuster MM, Lawrence R, Esko JD. Heparan sulfate regulates VEGF165- and VEGF121-mediated vascular hyperpermeability. J Biol Chem. 2011;286:737–745. doi: 10.1074/jbc.M110.177006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Kuroki M, Imakiire T, Uno K, Abe H, Beppu R, Yamashita Y, Shirakusa T. Opposite effects of thrombospondin-1 via CD36 and CD47 on homotypic aggregation of monocytic cells. Matrix Biol. 2002;21:441–448. doi: 10.1016/s0945-053x(02)00036-7. [DOI] [PubMed] [Google Scholar]
- Yao M, Roberts DD, Isenberg JS. Thrombospondin-1 inhibition of vascular smooth muscle cell responses occurs via modulation of both cAMP and cGMP. Pharmacol Res. 2011;63:13–22. doi: 10.1016/j.phrs.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kazerounian S, Duquette M, Perruzzi C, Nagy JA, Dvorak HF, Parangi S, Lawler J. Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level. FASEB J. 2009;23:3368–3376. doi: 10.1096/fj.09-131649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lanahan AA, Simons M. VEGFR2 trafficking: speed doesn’t kill. Cell Cycle. 2013;12:2163–2164. doi: 10.4161/cc.25536. [DOI] [PMC free article] [PubMed] [Google Scholar]